Abstract
Substance abuse still remains one of the major problems in the world today with millions of people abusing legal and illegal drugs. In addition, a billion people may also be infected with one or more infections. Both drugs of abuse and infections are associated with enormous burden of social, economic and health consequences. This paper briefly discusses a few medical consequences of drugs of abuse and infections such as human immunodeficiency virus, hepatitis C virus; psychiatric complications in hepatitis C infection; pharmacokinetic drug-drug interactions among medications used in the treatment of addiction and infections; and new drugs in development for the treatment of infections. Research is encouraged to study interactions between infections, drugs of abuse, and underlying pathophysiologic and molecular/genetic mechanisms of these interactions.
Keywords: Medical consequences, drug abuse, infections
Substance abuse and infections are two of the major problems in the world today with an estimated 200 million people abusing illegal drugs regularly (1) and an estimated one billion people living with one or more infections (2). In the US alone, according to the 2005 National Survey on Drug Use and Health (3), about 110 million Americans age 12 years and older (46%) used at least one illicit drug (e.g., amphetamines, cocaine, heroin, or marijuana) in their lifetime. An estimated 19 million people are current users of an illicit drug. There are an estimated 40 million people in the world living with human immunodeficiency virus (HIV) infection, about 200 million infected with hepatitis C virus (HCV), 500+ million people infected with tuberculosis (TB) and many more millions with various other bacterial and viral infections. An estimated 1 million people infected with HIV, and 4 million people infected with HCV live in the US. Both infections are prevalent among substance abusers. Injection drug use (IDU) directly and indirectly accounts for more than one-third (36%) of AIDS cases; of the 42,156 new cases of AIDS reported in the US in 2000, 11,635 (28%) were associated with injection drug use (4). About 80% to 90% of HIV positive IDUs may also infected with HCV (5). In addition to HIV and HCV, other viral and bacterial infections such as mycobacterial leading to tuberculosis (TB), sexually-transmitted infections, streptococcal and staphylococcal infections (leading to endocarditis) and others have all been reported in drug abusers (6).
Sociopolitical, economic, and health costs to the society from substance abuse and infections are enormous. Legal and illegal substance abuse alone costs the American society an estimated one-half a trillion dollars annually (7), while diabetes and cancer cost an estimated $132 billion (8) and $219 billion (9), respectively. Both drugs of abuse and infections such as HIV and HCV affect almost every physiological/biochemical system in the body. Thus, health effects may range between neuropsychiatric complications, anxiety and depressive disorders, cardiomyopathies, immune impairment, metabolic/endocrine disorders (lipodystrophy), and hepatic failure, to name a few. Because this subject of health consequences of drugs of abuse and infections is very wide and could not be covered in a mini-symposium at this ISAM conference, we decided to present only three aspects of consequences- a brief review of medical consequences in general (Jag H. Khalsa, Ph.D., NIDA, NIH), psychiatric conditions complicating hepatitis C infection in drug addicts (Glenn Treisman, MD, Johns Hopkins School of Medicine, Baltimore, MD, USA), pharmacokinetic drug interactions between medications used in the treatment of addiction (e.g., methadone, buprenorphine) and infections (antiretroviral medications) [Elinore McCance-Katz, MD, PhD, Virginia Commonwealth University College of Medicine, Richmond, VA, USA), and a brief review of drugs in development for the treatment of infections (Ellen Tedaldi, MD, Temple University Hospital, Philadelphia, PA, USA).
Medical Consequences: Brief Review and Research at NIDA
In general, stimulants such as cocaine and methamphetamine (‘met’, ‘speed’, or ‘ice’) increase the heart rate while constricting the blood vessels; in susceptible individuals, these two actions together set the stage for cardiac arrhythmias and strokes (10, Gold). Methamphetamine also causes serious hyperthermia, increases wakefulness and physical activity, creating the potential for a combination of activity and overheating that leads to convulsions and dangerous, sometimes lethal elevation of body temperature (10). Cocaine use decreases the blood flow to the brain, increases the heart rate, and elevates the blood components that promote clotting—effects that can lead to stroke or heart attack even in those not otherwise at risk for these serious cardiovascular events (10). NIDA-funded research also shows that chronic cocaine use is associated with left ventricular dysfunction (11) and increased calcium deposits in the coronaries (12) of HIV infected African-Americans, and that its use may also facilitate the entry of HIV into brain cells (13), possibly leading to HIV encephalopathy. The club drug methylene-dioxy-methamphetamine (MDMA), also known as ‘ecstasy’), which many users mistakenly believe to be safe, has caused malignant hyperthermia, permanent kidney damage, and death. MDMA also damages serotonin nerve fibers in the brain. Heroin can cause a life-threatening kidney renal condition called focal glomerulosclerosis (14). Opiate (heroin) use is associated with consequences ranging from nausea and constipation to renal, dental, and orofacial complications. PCP (phencyclidine, or ‘angel dust’) decreases heart rate and blood pressure, triggers violent aggression, and may trigger muscle contractions strong enough to break a bone (10). Marijuana, the most abused illicit drug in the world and often perceived by many as an innocuous drug, is associated with consequences ranging from memory, cognitive and motor problems in young and adult individuals to possible lung cancer in chronic marijuana smokers. The NIDA-published special supplement reviewed the most up-to-date research on clinical consequences of marijuana (15). Injecting drug use and drug use associated impulsive sexual activity further promotes acquisition and transmission of sexually-transmitted and blood-borne infections including life-threatening endocarditis, viral hepatitis, HIV/AIDS and STDs.
HIV, a blood-borne retrovirus that infects CD4 T-cell lymphocytes and macrophages, causes profound immunosuppression that eventually develops into full-blown AIDS. HIV infection results into flu-like syndrome consisting of fever, fatigue, pharyngitis, decreased CD4 T-cell lymphocytes, increased viral load, and finally progression to AIDS, the latter depending on factors such as the use of illicit drugs, opportunistic infections (OI) prophylaxis, and antiretroviral therapy. For about 5% of the individuals, the disease does not progress to AIDS, known as long-term non-progressors with a low viral load burden, strong virus-specific immune responses, and moderate viral attenuation (16). Since the virus may infect almost every organ, the effects of HIV infection may also range from immunosuppression to wasting and other metabolic/endocrine disorders, cardiomyopathy, nephropathy, neuroAIDS, and many other health consequences. The course of HIV infection and the development of AIDS are further complicated by metabolic and endocrine abnormalities secondary to the direct toxic effects of HIV, other OIs such as HCV, TB, STIs, neoplasms, and complications of drugs used during treatment.
Hepatitis C virus is another blood-borne pathogen that is easily transmitted through contaminated drug injection paraphernalia. Approximately 40% of chronic liver disease is related to HCV infection, making it the most common cause of chronic liver disease and the major reason for liver transplantation performed in the US. An estimated 8,000 to 10,000 persons with HCV-related liver cancer may die each year (17). Because HIV and HCV have common transmission pathways, coinfection is quite frequent, with prevalence as high as 90% of HIV-infected IDUs also infected with HCV in some countries in Central, South, and Southeast Asia and Eastern Europe (18, 19, 20); 50 to 75% in countries in Southeast Asia (21); 33% in St. Petersburg, Russia (22); and 50 to 55% in Australia (23). Recent reports from India show that there are an estimated 5 million people living with HIV infection (24; no numbers are available on co-infections with HIV/HCV).
During the acute phase of HCV infection, which is difficult to diagnose and which may last about six weeks, symptoms may include malaise, nausea, right upper quadrant pain, and jaundice. About 75 to 85% of these patients may become chronically infected. During the chronic phase of HCV infection, which may last several decades, symptoms may include nausea, anorexia, myalgia, and arthralgia, with fatigue being the most common complaint (25). Alcohol use and advanced age accelerate the disease progression of HCV infection, especially among men. Approximately 20% of these chronic patients will develop liver cirrhosis within 20 years, and 1 to 5% of them will die from HCV-related liver cancer. HCV infection is also associated with the development of diabetes mellitus among IDUs (26). Dual infections with HIV and HCV also occur from common routes of transmission and these individuals are at risk of developing chronic liver inflammation and hepatic cancer or liver failure requiring transplantation. Hepatic injury seems to occur in dual infections through the induction of a novel signaling pathway, that is cooperatively activated by specialized protein molecules, known as HCV E2 and HIV gp120, thereby providing a rationale for therapeutic interventions (27). NIDA supports a wide spectrum of research on epidemiology, natural history, underlying pathogenesis, prevention and treatment of HIV/HCV co-infections among drug abusers.
Contrary to the current perception among many clinicians, patients who enter clinical management and follow-up can adhere to complicated and lengthy medication regimens necessary to control these diseases. Completing the entire regimen is crucial to successful treatment and to prevent the growth of resistant viral or bacterial strains that can withstand currently available medications and potentially give rise to devastating epidemics. Although chronic drug abusers appear to comply with medication regimens, still a large percentage of them do not. Thus, we need to learn much more about both adapting medication regimens for drug abusers and techniques for increasing their adherence. Moreover, some illicit drugs and drug abuse medications can interact with medications used for treating diseases, resulting in possible loss of efficacy and adverse effects. This is further discussed below by Dr. McCance-Katz.
In a relatively new area of research at NIDA, research suggests that nutrition might be playing an important role in HIV disease progression. Drug abusers with inadequate nutrition, particularly with sub-optimal levels of anti-oxidant micronutrients such as selenium and zinc, may be at high risk of mortality if they are also co-infected with HIV (28). Clinical trials are underway to determine if supplementation with selenium, zinc, and other anti-oxidant micronutrients would slow the progression of HIV/AIDS disease. Daily supplementation with 200 ug of selenium increases the CD4 T cell lymphocyte counts and decreases the viral load in HIV-infected patients (29). This type of research would have worldwide implications, such that, in underdeveloped countries where poor people cannot afford expensive antiretroviral therapy, would benefit from inexpensive treatment modality to slow disease progression and improve the quality of life.
We hope that research on a wide spectrum of medical consequences of drug abuse and co-occurring infections will facilitate the design of effective intervention strategies. Research from studies of cardiovascular complications of cocaine and HIV will help in the design and utilization of noninvasive imaging techniques to better diagnose and treat heart disease in individuals using cocaine and other stimulants and research on the role of nutrition in immunity and disease progression will help in the design of effective alternative and inexpensive treatment modalities against HIV and other infectious diseases progression in drug abusers.
Psychiatric Conditions Complicating Hepatitis C
Hepatitis C and HIV are viral illnesses that continue to spread in an epidemic fashion among vulnerable populations, including those with mental illness and drug addictions. The vector for transmission Hepatitis C has become primarily a set of high-risk drug taking behaviors, with high risk sexual behaviors often related to drug use as the second most common vector (30). Because of this, psychiatric disorders, including substance abuse disorders themselves, as well as psychiatric conditions that complicate substance use behaviors, are now involved in maintaining the Hepatitis C epidemic. Major depression, severe mental illness, and personality disorders lead to high risk behaviors for Hepatitis C, and yet these disorders may be made worse by Hepatitis C and the medications used to treat it. Patients with chronic mental illness have elevated rates of Hepatitis C and have increased risk behaviors for infection (31). Major depression is a common co-morbidity in patients with Hepatitis C infection (32). In a VAMC sample of patients with Hepatitis C, 85% had major depression (33). Personality disorder has been scrutinized less, but is clearly associated with drug use, impulsivity, and self-destructive behaviors. These co-morbidities make Hepatitis C patients less able to seek help, more challenging for clinicians, and less able to obtain heath care (34).
Hepatitis C and HIV are both associated with addictions, and the ways in which addictions complicate these viral illnesses are complex. They contribute to risks directly, in that intravenous drug use with contaminated needles is the proximate source of viral exposure for most Hepatitis C patients and many HIV patients. But they also contribute indirectly, in that intoxicated persons are at far greater risks for impulsive behaviors and high risk sexual activities, and further, that high risk sexual behaviors are often the way drugs are obtained. Even further, intoxication may interfere with treatment adherence and lead to continued infection and treatment failure. The most common condition complicating Hepatitis C is substance abuse. Hepatitis C is common in intravenous drug users, with up to 90% of IV drug users infected in some populations (35). The use of IV drugs is associated with poorer outcomes in many illnesses, including bipolar disorder (36) and HIV. Hepatitis C patients also have poorer outcomes if they are ongoing drug users (25). Alcohol has been shown to be a risk factor for infection with Hepatitis C, probably through the indirect mechanisms described, but it is also associated with more rapid progression of Hepatitis C and more frequent development of Hepatic Carcinoma (37). Despite these ominous problems, patients with substance use disorders can be successfully treated for HIV and Hepatitis C if the clinical resources needed for treatment are provided (38-40).
The presence of major depression, bipolar disorder, and chronic mental illness have all been associated with substance use, increased high risk sexual behavior, and with other self destructive behaviors (for review see ref: 41) Depression is a particular problem for both HIV and Hepatitis C patients. In HIV and Hepatitis C, depression is a risk factor for infection and is associated with poor outcome (42, 43). Depression research is complex because of the incomplete agreement on the definition of the condition. We believe there are two common problems in patients with HIV and Hepatitis C, one of which is the expected decrease in mood associated with a potentially fatal chronic illness, while the other is the change in mood associated with a diseased limbic system that can occur in almost any patient. We have referred to the first condition as demoralization, while the second is generally referred to as major depression, but the older terms “reactive depression” and “endogenous depression” are often used to make the distinction.
Because research often relies on symptom scales, which may not distinguish these two conditions very well, interpretation of the research literature is complicated. The literature has shown that depression or depressive symptoms are present in the instances we will describe, but we will describe the conditions that are related to major depression, having written extensively about the distinction in both HIV (41) and Hepatitis C (42).
Depression may increase the risk of infection with Hepatitis C and HIV, and may interfere with treatment, as noted. It also may be associated directly with viral damage to the CNS or with CNS inflammation. It may therefore cause viral infection and also be a consequence of viral infection. Depression may also be produced by the medications used to treat HIV (particularly efavirenz, although there is debate about this) (44) and Hepatitis C (particularly interferon). Depression has also been a barrier to treatment, both because of the effect on patients, but also because of fears about the consequences of treating depressed patients with medications that may cause suicide or interfere with treatment. During the early use of interferon for hepatitis C, depression was considered a contraindication to treatment. The CNS consequences of HIV medications have been reviewed (45), but overall have been less problematic than those associated with Hepatitis C. The current standard of care for Hepatitis C includes the use of interferon, usually given as a polyethylene glycol polymer called “pegylated interferon” along with the drug ribavirin. Although there is a little bit of evidence to suggest that ribavirin may worsen depression, interferon is associated with a host of severe CNS side effects, most severe of which is frank psychosis, which may persist after discontinuation. Also common are depressive symptoms, apathy, fatigue, irritability, sleep disturbance (both hypersomnia and insomnia), confusion, inattention, anorexia, sexual dysfunction, and suicidal feelings (for review see ref # 43). It is our belief that these effects are immune mediator induced, and are similar to those changes that occur with steroids, and occur on the spectrum of mood disorders from mostly depressive to more rarely manic with occasional “mixed states” and variable severity. Treatments with antidepressants have been shown to be effective, and do not favor any specific class of medications. There is more debate about the use of antidepressant “prophylaxis” to try to prevent depressive symptoms, as these are a leading cause of treatment failure.
Treatment failure caused by psychiatric conditions is a common problem and a major contributor to mortality and morbidity of these conditions. More importantly, psychiatric conditions contribute to the epidemic spread of HIV and Hepatitis C. These conditions are treatable, and treatment is cost effective, yet we do not provide adequate psychiatric treatment resources for patients needing treatment for HIV and Hepatitis C, nor those at risk where psychiatric intervention might prevent infection. The effectiveness of treatment for the neuropsychiatric conditions that complicate these ominous epidemics compel us to develop more organized and effective treatments that integrate medical, psychiatric, and substance use treatment to help stem the tide of this epidemic.
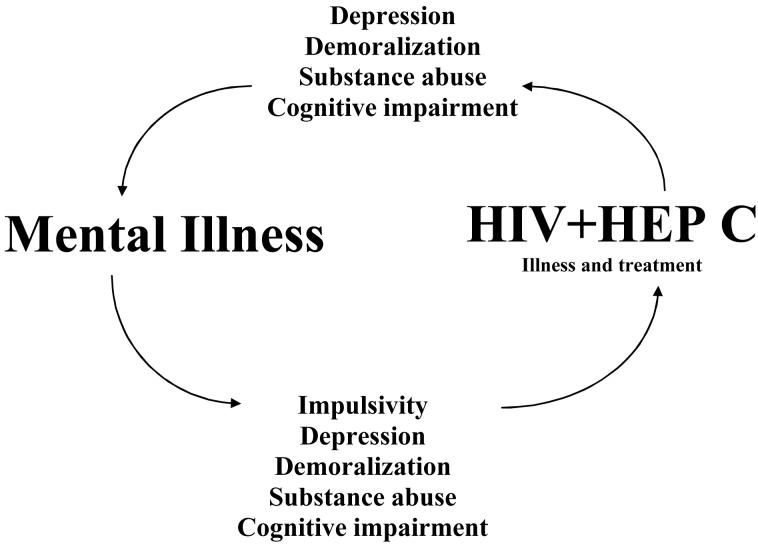
Psychiatric illness has several roles in the propagation of behaviorally transmitted viruses such as Hepatitis C and HIV. The ways in which various psychiatric disorders promote the transmission of infection, worsen the course of viral infection, interfere with prevention, and interfere with treatment are complex. They are briefly diagrammed in Figure 1. Patients with psychiatric disorders are at increased risk for acquiring and transmitting infections such as HIV, Hepatitis C/B, STI’s, and several other viruses, the conditions that spread by “risky behaviors”.
Figure 1.
Patients with psychiatric disorders at increased risk for acquiring HIV, Hepatitis C, HBV, STD’s, and other viruses; conditions spread by intimate contacts, and transmitted by “risky behaviors”.
Drug Interactions
The US Centers for Disease Control (CDC) estimates that approximately 27% of HIV infections are attributable to injection drug use and another 27% through high-risk heterosexual practices, with the remaining cases attributed to high risk sexual contact between men who have sex with men (MSM) (46). What the latter statistics do not address is how many of these high-risk sexual contacts were made in the context of drug abuse. Despite the large number of drug abusers with HIV disease, HAART is frequently underutilized in this population because of the difficulties experienced in obtaining adherence adequate to maintain viral suppression (47-50). Provision of drug abuse treatment is often a key component to successful treatment of HIV disease in this population. Multiple studies have shown that drug interactions between antiretrovirals and other medications having effects on cytochrome p450 enzymes can lead to altered therapeutic profiles, toxicities and side effects with drugs used for the treatment of HIV (51). This, in turn, may decrease adherence to medical treatment for HIV/AIDS (52) leading to lack of efficacy of HIV treatment, development of viral resistance to currently available therapies, and increased illicit drug abuse. Because opioid pharmacotherapies are the treatment of choice for opioid-addicted individuals with HIV/AIDS, it is essential that clinicians have a thorough understanding of possible drug interactions between opioids, specifically methadone and buprenorphine, and antiretroviral therapies in order to enhance the clinical care of drug users with HIV/AIDS.
For many years methadone has been the principal opioid medication for treatment of opioid dependence in patients with HIV/AIDS. It has been shown to have numerous interactions with antiretroviral therapies described below. Buprenorphine, an opioid partial agonist, has now become available for the treatment of opioid dependence. An important question is whether buprenorphine is associated with similar interactions as have been described for methadone with various antiretroviral medications.
An early study of the interaction of methadone and zidovudine was conducted as a result of the observation that some patients who were methadone-maintained and placed on zidovudine treatment for AIDS developed symptoms of malaise, dysphoria, and insomnia that were initially thought to be related to opiate withdrawal, but serum methadone concentrations were found to be therapeutic. This drug interaction study revealed a 41% increase in zidovudine exposure in methadone-maintained individuals. The study results indicated that methadone inhibited zidovudine glucuronidation resulting in delayed clearance (53). A subsequent question was whether the observed effect was a general opioid effect or whether there were differences in effect on zidovudine metabolism with other opioids. In a second drug interaction study, it was determined that the opioids, l-acetyl-methadol (LAAM) and buprenorphine were not associated with increased zidovudine plasma levels (54). From these studies it was concluded that drug interactions between opioids and antiretrovirals should be examined individually and that it might be possible to match medication treatments to opioid-dependent patients with HIV/AIDS.
Subsequently, drug interaction studies between methadone or buprenorphine and several HIV therapeutics have been undertaken (51, 55-56). Several adverse drug interactions between methadone and protease inhibitors were observed. Lopinavir/ritonavir was associated with opiate withdrawal in some study participants which appeared to be related to the ability of lopinavir to induce CYP450 3A4 and increase the rate of methadone metabolism. Ritonavir non-significantly increased methadone serum concentrations (57). Nelfinavir administration was associated with significantly decreased methadone concentrations (58), although this was rarely associated with opiate withdrawal. The non-nucleoside reverse transcriptase inhibitor, efavirenz, is associated with rapid onset of severe opiate withdrawal symptoms in methadone-maintained patients requiring rapid methadone dose increases of up to 50% (59). Such dose increases can also present a clinical issue when patients need to have their antiretroviral regimens changed and must undergo a rapid taper of methadone dose to avoid opiate toxicity. Delavirdine has been associated with significant increases in methadone serum concentrations, but no evidence of opioid toxicity was observed (60).
By contrast, the interaction of buprenorphine was examined with the same antiretroviral medications as studied with methadone. While some medications yielded pharmacokinetic interactions (ritonavir, efavirenz, delavirdine) (55-56), no clinically significant drug interactions were observed. The only medication for which a significant pharmacokinetic effect was observed was the protease inhibitor atazanavir and atazanavir in combination with ritonavir. This was manifest as significant increases in buprenorphine and norbuprenorphine plasma concentrations (61). Sedation was observed in three study participants receiving atazanavir/ritonavir, but no other toxic effects were observed, although in clinical practice patients should be observed for evidence of toxicity. Importantly, buprenorphine had no significant effect on any antiretroviral medications studied.
In summary, buprenorphine appears to have fewer adverse drug interactions with HIV therapeutics studied to date than methadone. Thus, there are now at least two opioid therapies that should be considered in the treatment of patients who have HIV disease and who are opioid-dependent. Buprenorphine may offer an alternative opioid therapy that could help to improve adherence to antiretroviral medications prescribed for the treatment of HIV disease in opioid-dependent patients.
New Drugs on the Horizon
New drug therapies to treat hepatitis C (HCV) and HIV infection are being developed with improved understanding of the molecular structures of the viruses themselves, the pathogenesis of infection and the specific immune responses needed to eradicate or control these infections. Interferon and ribavirin based therapies will continue to be a component of HCV therapy for the near future combined with other novel compounds directed at targets of viral replication, immunomodulation or cell entry. The goals of anti-HCV therapy are viral eradication for various genotypes, prevention of hepatic morbidity such as hepatocellular carcinoma and cirrhosis. Future antiretroviral therapies for HIV will include agents that focus on new classes of inhibitors of viral replication and cell binding. The new treatment choices in HIV will need to ensure effective and durable viral suppression especially against highly resistant virus strains, regimen tolerability and improved toxicity
The antiviral treatments available for hepatitis C and HIV continue to increase and offer the possibility of novel strategies for virologic control and improved clinical outcomes. For drug abusers who may be co-infected with both viruses, there are challenges with current therapies including tolerability, drug interactions and adherence among others. Currently, interferon based therapies with ribarvirin are the preferred agents for treatment of hepatitis C in the monoinfected and HIV/HCV co-infected patients. Although in monoinfected patients, overall rates of sustained virologic response (SVR) of up to 55% have been achieved with pegylated interferon alfa and daily oral ribavirin (62-63), in HIV co-infected patients, the SVR rates are in the range of 27-40% (64-66). In genotype 2, patients may not require a liver biopsy prior to treatment and duration of therapy may be adequate at 24 weeks compared to 48 weeks for other genotypes (67). In addition to HIV co-infection, other confounding factors that attenuate the response to interferon and ribavirin are e.g. African-American ethnicity, obesity, genotype 1 with high viral loads and liver transplantation (68-69). Even in centers with experience in treating hepatitis C and drug users, the number of individuals who actually initiate and complete HCV treatment is disappointingly small (70-71).
Targets for Hepatitis C Infection
There are at least two developments in hepatitis C research that facilitate the investigation of viral dynamics and novel therapeutic antiviral agents: the replicon system and the cell culture system with specialized hepatocytes (72-74). With the genomic structure and encoded proteins functions described, it is now possible to evaluate compounds for their effect on the viral replication and infectivity. The goals of antiviral therapy in hepatitis C are to limit viral replication, prevent new infection and enhance clearance of infected cells. Currently, drug candidates in various stages of development are: (1) Derivative of interferon, such as albuferon (75-76), and derivatives of ribavirin such as viramadine or taibivirin); (2) Drugs directed against components of HCV genome (e.g., protease inhibitors such as telaprevir or VX-950; SCH 503034; nucleoside HCV RNA polymerase inhibitors such as valopicitabine (NM283), R1626 and HCV-796); and (3) Immune modulators including vaccines (such as isatoribine, a Toll-like receptor agonist that stimulates the natural immune response to a pathogen such as hepatitis C; and CPG 10101, a TLR9 receptor agonist that acts as an antiviral and Th1 immune enhancer.
Targets for HIV Infection
Currently there are at least 26 FDA approved medications for HIV/AIDS. These belong to four classes of drugs and include: nucleoside reverse transcriptase inhibitors (NRTI’s), non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs) and one fusion inhibitor (FI). There are new formulations of drugs to reduce pill burden such as a single pill daily (emcitritabine/tenofovir/efavirenz) or newer agents in a class that may suppress resistant virus strains e.g. darunavir, a PI with excellent virologic activity against HIV with multiple resistance mutations.
Metabolic complications e.g. dyslipidemia whether from chronic viremia or antiretroviral drugs themselves increase the secondary morbidity of HIV infection. Patients have to take additional medications such as lipid lowering drugs to avoid long term cardiovascular consequences. As the HIV population ages, it is expected that patients will develop age-related chronic conditions of cardiac and renal disease, malignancy, etc. whether they develop them prematurely or with greater morbidity.
Other newer therapeutic agents in development include the following: (1). Entry inhibitors such as CXCR4 (AMD 070) or the CCR5 receptor blockers (maraviroc and vicriviroc); (2) integrase inhibitors such as MK-0518 and GS-9137 and (3) maturation inhibitor such as PA-457 are in various stages of development. These new agents may provide options for both naïve and treatment experienced HIV infected patients. These drugs may be sequenced or combined to minimize toxicities complicating earlier treatment combinations or to overcome acquired drug resistance during acute infection or on treatment. While it is encouraging that drug development offers the potential for new agents to treat hepatitis C or HIV, there are daunting issues for populations infected with these viruses including drug users. In addition to therapeutic efficacy and durability, the drugs have to be used strategically to improve adherence and tolerability. In the absence of preventive or therapeutic vaccines for hepatitis C or HIV, the intersections of human behavior, genetics and pharmacology will provide ongoing challenges to the management of infected persons.
In summary, drug abuse and infections such as HIV and HCV are associated with a wide variety of medical and health consequences including neuropsychiatric complications such as anxiety disorders, severe depression, and suicidal attempts. Although treatment of drug addiction and dual infections of HIV and HCV is complex, it is achievable with integrated programs of health care for dually infected drug addicts. The problem of drug interactions that appeared between HIV antiretrovirals and methadone seems to be less with the newly approved buprenorphine. Future research will show whether similar interactions would occur between buprenorphine or methadone and newer drugs that are being developed for the treatment of HIV and HCV. It is also anticipated that the newer antiretroviral medications would have lesser neuropsychiatric complications or pharmacokinetic interactions.
Contributor Information
Jag H. Khalsa, National Institute on drug Abuse, National Institutes of Health, Bethesda, Maryland
Glenn Treisman, Johns Hopkins University School of Medicine, Baltimore, Maryland
Elinore McCance-Katz, Virginia Commonwealth University College of Medicine, Richmond, Virginia
Ellen Tedaldi, Temple University Hospital, Philadelphia, Pennsylvania
References
- 1.United Nations Office on Drugs and Crime . World Drug Report 2006. 1,2. United Nations Office on Drugs and Crime; New York: 2006. ISBN 92-1-148214-7. http://www.unodc.org. [Google Scholar]
- 2.United Nations AIDS (UNAIDS) 2006 Report on the global epidemic of AIDS: Executive Summary; UNAIDS/06-20E, English. Joint United Nations Programme on HIV/AIDS/WHO; 2006. ISBN 929-1735-116. http://www.unaids.org. [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration (SAMHSA) Overview of Findings from the 2005 National Survey on Drug Abuse and Health, Office of Applied Studies, NSDUH Series H-24. DHHS Publications; Rockville, MD: 2006. http://oas.samhsa.gov. [Google Scholar]
- 4.Gayle H. An overview of the global HIV/AIDS epidemic, with a focus on the United States. AIDS. 2000;14(suppl2):S8–S17. [PubMed] [Google Scholar]
- 5.Thomas D. Hepatitis C and Human Immunodeficiency Virus Infection. Hepatology. 2002;36:S201–S209. doi: 10.1053/jhep.2002.36380. [DOI] [PubMed] [Google Scholar]
- 6.Contoreggi C, Rexroad VE, Lange WR. Current management of infectious complications in the injecting drug user. J Subs Abuse Treatment. 1998;15(2):95–106. doi: 10.1016/s0740-5472(97)00048-2. [DOI] [PubMed] [Google Scholar]
- 7.Office of National Drug Policy . The Economic Costs of Drug Abuse in the United States: 1992-2002. Executive Office of the President; Washington, DC: 2004. Publication No. 207303. [Google Scholar]
- 8.American Diabetes Association Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26:917–932. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- 9.American Cancer Society . Cancer costs. Cancer Society; 2007. http://www.cancer.org. [Google Scholar]
- 10.Gold MS. In: Clinical aspects of substance abuse: A comprehensive text book. 3rd Edition Lewinson, editor. Wilkinson and Willey; Baltimore: 1997. [Google Scholar]
- 11.Ren S, Tong W, Lai H, Osman NF, Pannu H, Lai S. Effect of long-term cocaine use on regional left ventricular function as determined by magnetic resonance imaging. Am J Cardiol. 2006 Apr 1;97(7):1085–8. doi: 10.1016/j.amjcard.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 12.Lai S, Lima JA, Lai H, Vlahov D, Celentano D, Tong W, Bartlett JG, Margolick J, Fishman EK. Human immunodeficiency virus 1 infection, cocaine, and coronary calcification. Arch Intern Med. 2005;165(6):690–5. doi: 10.1001/archinte.165.6.690. [DOI] [PubMed] [Google Scholar]
- 13.Nair MP, Schwartz SA, Mahajan SD, Tsiao C, Chawda RP, Whitney R, Don Sykes BB, Hewitt R. Drug abuse and neuropathogenesis of HIV infection: role of DC-SIGN and IDO. J Neuroimmunol. 2004;157(12):56–60. doi: 10.1016/j.jneuroim.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 14.do Samiero Fania M, Sampaio S, Fania V, Carvalho E. Nephropathy associated with heroin abuse in Caucasian patients. Nephron Dial Transplant. 2003;113:2308–2313. doi: 10.1093/ndt/gfg369. [DOI] [PubMed] [Google Scholar]
- 15.Khalsa JH, Genser S, Francis H, Martin B. Clinical consequences of marijuana. J Clin Pharmacol. 2002;42(11):7s–11s. doi: 10.1002/j.1552-4604.2002.tb05997.x. [DOI] [PubMed] [Google Scholar]
- 16.Cao Y, Qin L, Zhang L, Safrit J, Ho DD. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. New Engl J Medicine. 1995;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 17.Gunn RA, Murray PJ, Ackers ML, Hardison WG, Margolis HS. Screening for chronic hepatitis B and C virus infections in an urban sexually transmitted disease clinic: rationale for integrating services. Sex Transm Dis. 2001;8(3):166–70. doi: 10.1097/00007435-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Garfein RS, Doherty MC, Monterroso ER, Thomas DL, Nelson KE, Vlahov D. Prevalence and incidence of hepatitis C virus infection among young adult injection drug users. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(suppl 1):11–19. doi: 10.1097/00042560-199802001-00004. [DOI] [PubMed] [Google Scholar]
- 19.Law MG, Hepatitis C Virus Projections Working Group Modelling the hepatitis C virus epidemic in Australia. J Gastroenterol Hepatol. 1999;14:1100–1107. doi: 10.1046/j.1440-1746.1999.02014.x. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald MA, Wodak AD, Dolan KA, van Beek I, Cunningham PH, Kaldor JM, Collaboration of Australian NSPs Hepatitis C virus antibody prevalence among injecting drug users at selected needle and syringe programs in Australia, 1995-1997. Med J Aust. 2000;172:57–61. doi: 10.5694/j.1326-5377.2000.tb139199.x. [DOI] [PubMed] [Google Scholar]
- 21.Quaglio G, Lugoboni F, Pajusco B. Factors associated with hepatitis C virus infection in injection and noninjection drug users in Italy. Clin Infect Dis. 2003;37:33–40. doi: 10.1086/375566. [DOI] [PubMed] [Google Scholar]
- 22.Thomas DL, Vlahov D, Solomon L. Correlates of hepatitis C virus infections among injection drug users. Medicine (Baltimore) 1995;4:212–220. doi: 10.1097/00005792-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 23.United Nations AIDS (UNAIDS) Report on the global epidemic of AIDS: Executive Summary; UNAIDS/06-20E, English. Joint United Nations Programme on HIV/AIDS/WHO; 2006. ISBN 929-1735-116. [Google Scholar]
- 24.Dore GJ, Law M, MacDonald M, Kaldor JM. Epidemiology of hepatitis C virus infection in Australia. J Clin Virol. 2003;26(2):171–84. doi: 10.1016/s1386-6532(02)00116-6. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan LE, Fiellin DA. Hepatitis C and HIV infections: implications for clinical care in injection drug users. Am J Addict. 2004;13(1):1–20. doi: 10.1080/10550490490265271. [DOI] [PubMed] [Google Scholar]
- 26.Howard AA, Klein RS, Schoenbaum EE. Association of hepatitis C infection and antiretroviral use with diabetes mellitus in drug users. Clin Infect Dis. 2003;36(10):1318–23. doi: 10.1086/374838. [DOI] [PubMed] [Google Scholar]
- 27.Balasubramanian A, Koziel M, Groopman JE, Ganju RK. Molecular mechanisms of hepatic injury in co-infection with hepatitis C and human immunodeficiency virus. Clin Infect Dis. 2005;41(suppl 1):s32–s37. doi: 10.1086/429493. [DOI] [PubMed] [Google Scholar]
- 28.Baum MK. Role of micronutrients in HIV-infected intravenous drug users. J Acquir Immune Defic Syndr. 2000;25(Suppl 1):S49–52. doi: 10.1097/00042560-200010001-00008. [DOI] [PubMed] [Google Scholar]
- 29.Hurwitz BE, Klaus JR, Llabre MM, Gonzalez A, Lawrence PJ, Maher KJ, Greeson JM, Baum MK, Shor-Posner G, Skyler JS, Schneiderman N. Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: a randomized controlled trial. Arch Intern Med. 2007;167(2):148–54. doi: 10.1001/archinte.167.2.148. [DOI] [PubMed] [Google Scholar]
- 30.National Institutes of Health Consensus Development Conference Statement . Management of hepatitis C: 2002. National Institutes of Health; Bethesda, Md: Jun 10-12, 2002. [Google Scholar]
- 31.Rosenberg S, Goodman L, Osher F, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31–7. doi: 10.2105/ajph.91.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angelino AF, Treisman GJ. Evidence-informed assessment and treatment of depression in HCV and interferon-treated patients. Int Rev Psychiatry. 2005;17(6):471–6. doi: 10.1080/02646830500381567. [DOI] [PubMed] [Google Scholar]
- 33.el-Serag HB, Kunik M, Richardson P, et al. Psychiatric disorders among veterans with hepatitis C infection. Gastroenterology. 2002;123(2):476–82. doi: 10.1053/gast.2002.34750. [DOI] [PubMed] [Google Scholar]
- 34.Loftis JM, Matthews AM, Hauser P. Psychiatric and substance use disorders in individuals with hepatitis C: epidemiology and management. Drugs. 2006;66(2):155–74. doi: 10.2165/00003495-200666020-00003. [DOI] [PubMed] [Google Scholar]
- 35.Patrick DM, Buxton JA, Bigham M, et al. Public health and hepatitis C. Can J Public Health. 2000;91(Suppl 1):S18–21. S19–23. [PubMed] [Google Scholar]
- 36.Salloum IM, Thase ME. Impact of substance abuse on the course and treatment of bipolar disorder. Bipolar Disord. 2000 Sep;2(3 Pt 2):269–80. doi: 10.1034/j.1399-5618.2000.20308.x. [DOI] [PubMed] [Google Scholar]
- 37.Safdar K, Schiff ER. Alcohol and hepatitis C. Semin Liver Dis. 2004;24(3):305–15. doi: 10.1055/s-2004-832942. [DOI] [PubMed] [Google Scholar]
- 38.Huckans MS, Loftis JM, Blackwell AD, Linke A, Hauser P. Interferon alpha therapy for hepatitis C: treatment completion and response rates among patients with substance use disorders. Subst Abuse Treat Prev Policy. 2007;12:2–4. doi: 10.1186/1747-597X-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sylvestre DL. Treating hepatitis C virus infection in substance users. Clin Infect Dis. 2005;40(Suppl 5):S321–4. doi: 10.1086/427447. [DOI] [PubMed] [Google Scholar]
- 40.Himelhock S, Moore RD, Treisman GJ, Gebo KA. Does the Presence of a Current Psychiatric Disorder in AIDS Patients Affect the Initiation of Antiretroviral Treatment and Duration of Therapy? J Acquir Immune Defic Syndr. 2004;37(4):1457–63. doi: 10.1097/01.qai.0000136739.01219.6d. [DOI] [PubMed] [Google Scholar]
- 41.Treisman GJ, Angelino AF, Hutton HH. Psychiatric Issues in the Management of Patients with HIV Infection. JAMA. 2001;286(22):2857–2864. doi: 10.1001/jama.286.22.2857. [DOI] [PubMed] [Google Scholar]
- 42.Angelino AF, Treisman GJ. Evidence-informed assessment and treatment of depression in HCV and interferon-treated patients. Int Rev Psychiatry. 2005;17(6):471–6. doi: 10.1080/02646830500381567. [DOI] [PubMed] [Google Scholar]
- 43.Himelhoch S, Medoff DR. Efficacy of antidepressant medication among HIV-positive individuals with depression: a systematic review and meta-analysis. AIDS Patient Care STDS. 2005;19(12):813–22. doi: 10.1089/apc.2005.19.813. [DOI] [PubMed] [Google Scholar]
- 44.Puzantian T. Central nervous system adverse effects with efavirenz: case report and review. Pharmacotherapy. 2002;22(7):930–3. doi: 10.1592/phco.22.11.930.33624. [DOI] [PubMed] [Google Scholar]
- 45.Treisman GJ, Kaplin AI. Neurologic and Psychiatric Complications of Antiretroviral Agents. AIDS. 2002;16(9):1201–15. doi: 10.1097/00002030-200206140-00002. [DOI] [PubMed] [Google Scholar]
- 46.Center for Disease Control [Accessed Dec 3, 2006];HIV and AIDS in the United States: a picture of today’s epidemic. http://www.cdc.gov/hiv/resources.
- 47.Celentano DD, Galai N, Sethi AK, Shah NG, Strathdee SA, Vlahov D, Gallant JE. Time to initiating highly active antiretroviral therapy among HIV-infected drug users. AIDS. 2001;15:1707–1715. doi: 10.1097/00002030-200109070-00015. [DOI] [PubMed] [Google Scholar]
- 48.Strathdee SA, Palepu A, Cornelisse PG, Yip B, O’Shaughnessy MV, Montaner JS, Schecter MT, Hogg RS. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 1998;280:547–549. doi: 10.1001/jama.280.6.547. [DOI] [PubMed] [Google Scholar]
- 49.Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV infections. J Acquir Immune Defic Syndr. 2001;27:251–259. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- 50.Gifford AL, Bormann JE, Shively MJ, Wright BC, Richman DD, Bozette SA. Predictors of self-reported adherence and plasma HIV concentrations in patients on multidrug antiretroviral regimens. J Acquir Immune Defic Syndr. 2000;23:386–395. doi: 10.1097/00126334-200004150-00005. [DOI] [PubMed] [Google Scholar]
- 51.McCance-Katz EF. Treatment of opioid dependence and HIV/HCV co-infection in opioid dependent patients: the importance of drug interactions between opioids and antiretroviral medications. Clin Inf Dis. 2005;41:S89–S95. doi: 10.1086/429503. [DOI] [PubMed] [Google Scholar]
- 52.Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, Schoenbaum EE. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17:377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCance-Katz EF, Jatlow P, Rainey P, Friedland G. Methadone effects on zidovudine (AZT) disposition (ACTG 262) J Acquir Immune Defic Syn Hum Retrovirol. 1998;18:435–443. doi: 10.1097/00042560-199808150-00004. [DOI] [PubMed] [Google Scholar]
- 54.McCance-Katz EF, Rainey PM, Friedland G, Kosten TR, Jatlow P. Effect of opioid dependence pharmacotherapies on zidovudine disposition. Am J Addictions. 2001;10:296–307. [PubMed] [Google Scholar]
- 55.McCance-Katz EF, Moody DE, Morse G, Pade P, Friedland G, Baker J, Alvanzo A, Smith P, Abayomi O, Jatlow P, Rainey PM. Interactions Between Buprenorphine and antiretrovirals I: Non-Nucleoside Reverse Transcriptase Inhibitors I: Efavirenz and Delavirdine. Clinical Infectious Diseases. 2006;43(Suppl 4):S224–34. doi: 10.1086/508187. [DOI] [PubMed] [Google Scholar]
- 56.McCance-Katz EF, Moody DE, Morse G, Pade P, Friedland G, Baker J, Alvanzo A, Smith P, Jatlow P, Rainey PM. Interactions between buprenorphine and antiretrovirals II: Protease inhibitors, nelfinavir, lopinavir/ritonavir, or ritonavir. Clin Inf Dis. 2006;43(Suppl 4):S235–46. doi: 10.1086/508188. [DOI] [PubMed] [Google Scholar]
- 57.McCance-Katz EF, Rainey P, Friedland G, Jatlow P. The protease inhibitor lopinavir/ritonavir may produce opiate withdrawal in methadone-maintained patients. Clinical Infectious Diseases. 2003;37:476–482. doi: 10.1086/376907. [DOI] [PubMed] [Google Scholar]
- 58.McCance-Katz EF, Rainey P, Smith P, Morse G, Friedland G, Gourevitch M, Jatlow P. Drug interactions between opioid and antiretroviral medications: Interaction between methadone, LAAM, and nelfinavir. Am J Addictions. 2004;13:163–180. doi: 10.1080/10550490490436037. [DOI] [PubMed] [Google Scholar]
- 59.McCance-Katz EF, Gourevitch MN, Arnsten J, Sarlo J, Rainey P, Jatlow P. Modified Directly Observed Therapy (MDOT) For Injection Drug Users With HIV Disease. Am J Addict. 2002;11:271–278. doi: 10.1080/10550490290088072. [DOI] [PubMed] [Google Scholar]
- 60.McCance-Katz EF, Rainey P, Smith P, Morse GD, Friedland G, Boyarsky B, Gourevitch M, Jatlow P. Drug interactions between opioids and antiretroviral medications: interaction between methadone, LAAM, and delavirdine. Am J Addict. 2006;15:23–34. doi: 10.1080/10550490500419029. [DOI] [PubMed] [Google Scholar]
- 61.McCance-Katz EF, Pade P, Morse GD, Moody DE, Rainey PM. Interaction of atazanavir and atazanavir/ritonavir with buprenorphine. College on Problems of Drug Dependence; Scottsdale, AZ: Jun, 2006. [Google Scholar]
- 62.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomized trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 63.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 64.Chung RT, Andersen J, Volberding P, et al. Peginterferon alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-co-infected persons. N Engl J Med. 2004;351:451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carrat F, Bani-Sadr F, Pol S, et al. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA. 2004;292:2839–2848. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
- 66.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 67.von Wagner M, Huber M, Berg T, Hinrichsen H, Rasenack J, Heintges T, Bergk A, Bernsmeier C, Haussinger D, Herrmann E, Zeuzem S. Peginterferon-alpha-2a (40KD) and ribavirin for 16 or 24 weeks in patients with genotype 2 or 3 chronic hepatitis C. Gastroenterology. 2005 Aug;129(2):522–7. doi: 10.1016/j.gastro.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 68.Conjeevaram HS, Fried MW, Jeffers LJ, Terrault NA, Wiley-Lucas TE, Afdhal N, Brown RS, Belle SH, Hoofnagle JH, Kleiner DE, Howell CD. Virahep-C Study Group Peginterferon and ribavirin treatment in African American and Caucasian American patients with hepatitis C genotype 1. Gastroenterology. 2006 Aug;131(2):470–7. doi: 10.1053/j.gastro.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 69.Chung RT. Assessment of efficacy of treatment in hepatitis C: Infection and disease. J Hepatol. 2006;44(1 suppl):S56–S59. doi: 10.1016/j.jhep.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 70.Mehta SH, Lucas GM, Mirel LB, Torbenson M, Higgins Y, Moore RD, Thomas DL, Sulkowski MS. Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV Clinic. AIDS. 2006 Nov 28;20(18):2361–9. doi: 10.1097/QAD.0b013e32801086da. [DOI] [PubMed] [Google Scholar]
- 71.Braitstein P, Li K, Kerr T, Montaner JS, Hogg RS, Wood E. Differences in access to care among injection drug users infected either with HIV and hepatitis C or hepatitis C alone. AIDS Care. 2006 Oct;18(7):690–3. doi: 10.1080/09540120500359330. [DOI] [PubMed] [Google Scholar]
- 72.AU Lohmann V, Korner F, Koch J, Herian U, Theilmann L, Bartenschlager R SO. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999 Jul 2;285(5424):110–3. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 73.Lindenbach BD, Evans MJ, Syder AJ, Wolk B, Tellinghuisen TL, Liu CC, Maruyama T, Hynes RO, Burton DR, McKeating JA, Rice CM. Complete Replication of Hepatitis C Virus in Cell Culture. Science. 2005 July 22;309(5734):623–626. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 74.Lázaro CA, Chang M, Tang W, Campbell J, Sullivan DG, Gretch DR, Corey L, Coombs RW, Fausto N. Hepatitis C virus replication in transfected and serum-infected cultured human fetal hepatocytes. Am J Pathol. 2007;170:478–489. doi: 10.2353/ajpath.2007.060789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pawlotsky JM, Gish RG. Future therapies for hepatitis C. Antivir Ther. 2006;11(4):397–408. [PubMed] [Google Scholar]
- 76.Nelson D, Rustgi V, Balan V, et al. A phase 2 study of albuferon in combination with ribavirin in non-responders to prior interferon therapy for chronic hepatitis C; Program and abstracts of the 56th Annual Meeting of the American Association for the Study of Liver Diseases; San Francisco, California. November 11-15, 2005; Abstract 204. [Google Scholar]; Bacon B, Regev A, Ghalib R, et al. Use of daily interferon alfacon-1 (Infergen) plus ribavirin in patients infected with hepatitis C (HCV) who are nonresponders to previous pegylated interferon plus ribavirin therapy: 24-week and end of treatment data from the DIRECT trial; Program and abstracts of the 57th Annual Meeting of the American Association for the Study of Liver Diseases; Boston, Massachusetts. October 27-31, 2006; Abstract LB18. [Google Scholar]