Abstract
Purpose
Despite their risk for serious late sequelae, childhood cancer survivors do not adhere to recommended medical screenings. We identified treatment, survivor, physician, and contextual factors that may influence survivors' adherence to recommended echocardiography and bone densitometry screening.
Methods
Structural equation modeling of data from the Childhood Cancer Survivor Study (CCSS); participants (N=838) were diagnosed and treated for pediatric malignancies between 1970 and 1986 .
Results
Survivors ( Mean age = 31 years; Mean age @ diagnosis = 10 years; Mean time since diagnosis = 21 years) at risk of cardiac sequelae (N=316) who reported more cancer-related visits (P = 0.01), having discussed heart disease with a physician (P ≤ 0.001), a sedentary lifestyle (P = 0.05), and less frequent health fears (P=0.05) were most likely to follow the recommended echocardiogram schedule (R2 = 23%). Survivors (Mean age=30 years; Mean age @ diagnosis = 9 years; Mean time since diagnosis = 21 years) at risk for osteoporosis (N=324) who reported more cancer-related visits (P = 0.05), were followed up at an oncology clinic (P = 0.01), had discussed osteoporosis with a physician (P ≤ 0.001), and had a lower BMI (P = 0.05) were most likely to adhere to the recommended bone density screening guidelines (R2 = 26%). Symptoms and motivation influenced screening frequency in both models.
Conclusions
Multiple factors influence survivors' adherence to screening recommendations. It is likely that tailored interventions would be more successful in encouraging recommended screening among childhood cancer survivors than will traditional health education approaches.
Introduction
Improvement in the rates of childhood cancer survival has prompted greater awareness of late treatment-related morbidity. Among the potential sequelae of therapy are osteoporosis, cardiomyopathy, and secondary neoplasms [1-4]. The Children's Oncology Group (COG) has compiled risk-based, exposure-related clinical practice guidelines for screening and management of late effects resulting from treatment for pediatric malignancies [5]. A baseline echocardiography screening is recommended for survivors at entry into long-term follow-up and then periodically based on age at treatment, radiation dose, and cumulative anthracycline dose (Table 1). Survivors who are at highest risk and therefore should undergo more frequent screening are those who were less than five years old at treatment, and who had any anthracycline or radiation exposure. Baseline DEXA screening for bone densitometry is recommended at entry into long-term follow-up and is repeated as clinically indicated (Table 1). While exposure based guidelines for screening the late effects of pediatric cancer treatment have been established, survivors' medical screening practices are sub-optimal [6-8] .
Table 1.
Treatment Exposure and Screening Guidelinesa
| Treatment Exposure | Recommended Screening |
Frequency |
|---|---|---|
| Abbreviations: tx, treatment; ECHO, echocardiogram; DEXA: dual-energy X-ray absorptiometry | ||
| Anthracyclines | ECHO | Baseline at entry to follow-up, then periodically based on age at tx, history of chest radiation, and cumulative anthracycline |
| <1 yr old at tx + chest radiation + any dose anthracycline | ECHO | Every year |
| <1 yr old at tx + no radiation + <200 mg/m2 anthracycline | ECHO | Every 2 years |
| <1 yr old at tx + no radiation + ≥200 mg/m2 anthracycline | ECHO | Every year |
| 1-4 yrs old at tx+ chest radiation + any anthracycline | ECHO | Every year |
| 1-4 yrs old at tx + no radiation + <100 mg/m2 anthracycline | ECHO | Every 5 years |
| 1-4 yrs old at tx + no radiation + ≥100 to <300mg/m2 anthracycline | ECHO | Every 2 years |
| 1-4 yrs old at tx + no radiation + ≥300 mg/m2 anthracycline | ECHO | Every year |
| ≥5 yrs old at tx + chest radiation + <300 mg/m2 anthracycline | ECHO | Every 2 years |
| ≥5 yrs old at tx + chest radiation + ≥300 mg/m2 anthracycline | ECHO | Every year |
| ≥5 yrs old at tx + no radiation + <200 mg/m2 anthracycline | ECHO | Every 5 years |
| ≥5 yrs old at tx + no radiation + ≥200 to <300mg/m2 anthracycline | ECHO | Every 2 years |
| ≥5 yrs old at tx + no radiation + ≥300 mg/m2 anthracycline | ECHO | Every year |
| Any age with decrease in serial function | ECHO | Every year |
| Radiation (Mantle, Spine, Abdomen) | ECHO | Baseline at entry to follow-up, then periodically based on age at tx, radiation dose, and cumulative anthracycline dose |
| <5 yr old at tx + no anthracycline + any dose radiation | ECHO | Every 2 years |
| <5 yr old at tx + any anthracycline + any dose radiation | ECHO | Every year |
| ≥5 yr old at tx + no anthracycline + <30 Gy radiation | ECHO | Every 5 years |
| ≥5 yr old at tx + no anthracycline + ≥30 Gy radiation | ECHO | Every 2 years |
| ≥5 yr old at tx + <300 mg/m2 anthracycline + any radiation | ECHO | Every 2 years |
| ≥5 yr old at tx + ≥300 mg/m2 anthracycline + any radiation | ECHO | Every year |
| Any age with serial decrease in function | ECHO | Every year |
| Corticosteroids | DEXA | Baseline at entry into long-term follow-up. Repeat clinically as indicated. |
Table created from information obtained from the Children's Oncology Group Survivorship Guidelines available at http://www.survivorshipguidelines.org/pdf/HR/LTFUGuidelines_HR.pdf
The medical screening literature limited to childhood cancer survivors is confined to breast and cervical cancer, [8, 9] and cardiovascular disease [9]. Females in the CCSS (78.2%) reported undergoing a Papanicolaou smear within the previous 3 years, 62.4% underwent a clinical breast examination within the last year, and 20.9% had gotten a mammogram at least once in their lifetime [8]. Childhood cancer survivors who received chest radiation are at an increased risk for developing breast cancer before the age of 40 [10-12]. A prospective study of Hodgkin Disease survivors found that only 47% (41 of 87) reported having had a mammogram in the previous 24 months; only 417 (49%) of 852 female survivors at increased risk of breast cancer underwent mammography within the previous 24 months [9]. Treatment of childhood cancer with anthracyclines and/or radiation increases risk of late cardiotoxicity [4, 13-15]. However, only 503 (28%) of 1798 childhood cancer survivors at increased risk of cardiac disease received the recommended cardiac screening in the previous 24 months [9].
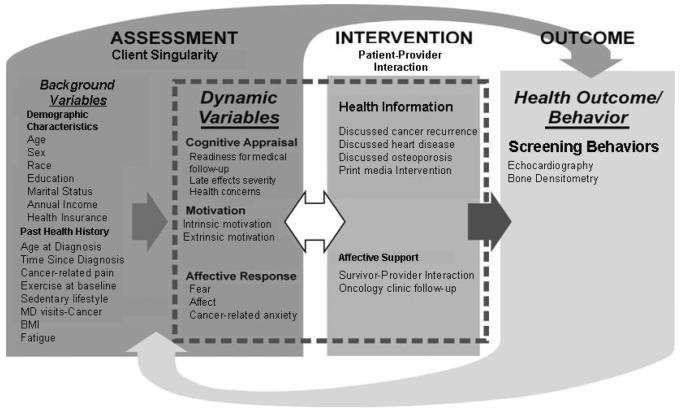
In addition to disease and treatment factors, personal and contextual factors influence health behavior choices [16-21]. To describe the multiple influences on survivors' screening behaviors, we selected the Interaction Model of Client Health Behavior (IMCHB) [22, 23] which incorporates both intrapersonal and contextual variables and has been adapted to the study of childhood cancer survivors (Fig. 1). Structural equation modeling (SEM), which combines factor and path analyses into a comprehensive methodology [24], allowed us to test the model's hypotheses simultaneously rather than sequentially. Our goal was to identify treatment, survivor, physician, and contextual factors that could be targeted with behavioral interventions to support recommended screening.
Fig. 1.
Correspondence of the Interaction Model of Client Health Behavior (IMCHB) with Study Variables
Methods
Data Source
The CCSS is a multi-institutional retrospective cohort study initiated in 1994 to examine the late effects of pediatric cancer diagnosed and treated between 1970 and 1986. Survivors completed a baseline questionnaire at study entry and respond to follow-up questionnaires sent at regular intervals. Questionnaires and sampling methods are detailed by Robison, et al. [25] and are available for review at http://www.stjude.org/ccss. The study was approved by the institutional review board of St. Jude Children's Research Hospital.
Sample
Originally 20,346 survivors were contacted to participate in CCSS. Eligible participants were those who had survived 5 or more years after being treated for a malignant disease diagnosed (before the age of 21 years) between 1970 and 1986 (approximately 12,423 are alive to date). An ancillary study, the Health Care Needs Survey (HCNS; initiated by KO), randomly sampled 1600 of the survivors. Of the 978 (61%) participants who completed and returned the survey, 838 (86%) returned the Follow-up 2 survey of the CCSS within the same data collection period. Non-respondents to the HCNS were typically male (59%), minorities (37%), or had less than a high school education (56%). Survivors who completed the HCNS but not the Follow-up 2 survey were younger at diagnosis (P=0.019) and diagnosed more recently (P=<0.001). No survivor reported here was younger than 18 years at the time of data collection; data were self-reported (Table 2).
Table 2.
Descriptive Summary of the Total Sample (N = 838) and Sample Completing Barriers Survey Only (N =121)a
| Variables | Total Sample [N (%)] |
Bone Density Risk Group [N (%)] |
Cardiac Risk Group [N (%)] |
P-Value Between Total & Risk Groups |
||||
|---|---|---|---|---|---|---|---|---|
| (X2) | ||||||||
| Sex | 0.156 | |||||||
| Male | 385 (45.9) | 280 (47.4) | 197 (41.6) | |||||
| Female | 453 (54.1) | 311 (52.6) | 276 (58.2) | |||||
| Race | 0.863 | |||||||
| White | 599 (71.5) | 437 (73.9) | 359 (75.7) | |||||
| Black | 64 (7.6 ) | 42 (7.1) | 39 (8.2) | |||||
| Hispanic | 96 (11.5) | 70 (11.8) | 52 (11) | |||||
| Other | 46 (5.5 ) | 41 (6.9) | 24(5.1)_ | |||||
| Personal Annual Income ($US) | 0.901 | |||||||
| None | 94 (11.5) | 64 (11.7) | 51 (11.5) | |||||
| <19,999 - 39,999 | 490 (60.3) | 340 (62.2) | 265 (59.5) | |||||
| 40,000 - 59,999 | 123 (15.1) | 80 (14.6) | 76 (17.1) | |||||
| ≥ 60,000 | 107 (13.1) | 63 (11.5) | 53 (11.9) | |||||
| Marital Status | 0.183 | |||||||
| Ever Married | 329 (39.4) | 209 (35.5) | 192 (40.7) | |||||
| Never Married | 506 (60.6) | 379 (64.5) | 280 (59.3) | |||||
| Health Insurance | 0.438 | |||||||
| Yes | 732 (88.1) | 513 (86.8) | 428 (90.3) | |||||
| No | 99 (11.9) | 73 (12.5) | 44 (9.3) | |||||
| Education | 0.002 | |||||||
| 1-12 Years | 20 (2.4) | 41 (6.1) | 19 (4.0) | |||||
| Completed High School/GED | 106 (12.8) | 82 (14.1) | 55 (11.7) | |||||
| Post High School Training/Some College | 305 (36.9) | 204 (35.2) | 165 (35.2) | |||||
| College Graduate/Post-graduate work | 397 (48.0) | 253 (43.6) | 230 (49.1) | |||||
| Seen at Oncology Clinic Within Past 2 Yrs | 0.008 | |||||||
| Yes | 76 (9.9%) | 76 (12.9) | 70 (14.8) | |||||
| No | 690 (90.1%) | 474 (80.2) | 376 (79.3) | |||||
| Mean | SD | Mean | SD | Mean | SD |
P-Value Between Risk Groups (F-ratio) |
Post-hoc P- Value (Bonferroni Adj) |
|
| Age (years) | 30.98 | 7.50 | 30.20 | 7.09 | 31.01 | 7.40 | 0.094 | |
| Age at Diagnosis (years) | 9.25 | 5.87 | 9.01 | 5.51 | 9.88 | 5.88 | 0.036 | BD/Cb=0.012 |
| Time Since Diagnosis (years) | 21.74 | 4.54 | 21.20 | 4.27 | 21.14 | 4.37 | 0.021 | T/BD=0.023 |
| T/C=0.018 | ||||||||
N varies because of missing data.
T=Total Group;BD=Bone Density Risk Group; C=Cardiac Risk Group
CCSS respondents self-identified their racial category based on structured response categories used in the baseline questionnaire (white, black, American Indian or Alaskan Native, Asian or Pacific Islander, Hispanic, and other). The analyses in this article were restricted to survivors classified as white, black, Hispanic, and other because there were too few respondents in the other categories to permit meaningful analyses.
We selected and modeled two at-risk subsamples based on treatment exposures (Table 1) who had responded to the baseline, HCNS and Follow-up 2 surveys: A cardiac risk group (anthracycline, radiation exposure, or both) and a bone density risk group (cranial radiotherapy, glucocorticoids, methotrexate, and prolonged corticosteroid exposure).
Outcome Measures
Single items addressed the recency of the last echocardiogram or bone densitometry evaluation (1 = Never; 2 = 5 or more years ago; 3 = More than 2 years but less than 5 years; 4 = 1-2 years ago; 5 = Less than 1 year ago) (Table 3). Survivors who answered “don't know” for any of the screening exams were excluded from the analysis.
Table 3.
Descriptive Summary of Study Measures
| Medical Screening Behaviors | Total Sample [N (%)] |
Bone Density Risk Group [N (%)] |
Cardiac Risk Group [N (%)] |
P-Value Between Total & Risk Groups |
P-Value Between Risk Groups |
|||
|---|---|---|---|---|---|---|---|---|
| (X2) | (X2) | |||||||
| Recency of Echocardiogram (N = 836)a | <0.001 | 0.003 | ||||||
| Never | 354 (36.2) | 194 (38.9) | 105 (25.4) | |||||
| 5 or more years ago | 134 (13.7) | 54 (10.8) | 69 (16.7) | |||||
| More than 2 years but less than 5 years | 87 (8.9) | 55 (11.0) | 53 (12.8) | |||||
| 1-2 years ago | 82 (8.4) | 55 (11.0) | 57 (13.8) | |||||
| Less than a year ago | 95 (9.7) | 85 (17.0) | 89 (21.5) | |||||
| Don't know | 84 (8.6) | 56 (11.2) | 40 (9.7) | |||||
| Recency of DEXA Scan (Bone Density) (N + 839) | 0.531 | 0.932 | ||||||
| Never | 589 (60.2) | 343 (68.5) | 271 (65.3) | |||||
| 5 or more years ago | 61 (6.2) | 29 (5.8) | 26 (6.3) | |||||
| More than 2 years but less than 5 years | 31 (3.2) | 20 (4.0) | 21 (5.1) | |||||
| 1-2 years ago | 34 (3.5) | 20 (4.0) | 17 (4.1) | |||||
| Less than a year ago | 44 (4.5) | 39 (7.8) | 34 (8.2) | |||||
| Don't know |
80 (8.2) |
50 (10.0) |
46 (11.1) |
|||||
| INDEPENDENT VARIABLES |
P-Value Between Total Sample & Risk Groups |
P-Value Between Risk Groups |
||||||
| (X2) | (X2) | |||||||
| Physician Discussed Risk of Developing Cancer | 0.128 | 0.064 | ||||||
| Yes | 183 (23.9) | 106 (23.0) | 111 (28.6) | |||||
| No | 583 (76.1) | 354 (77.0) | 277 (71.4) | |||||
| Physician Discussed Risk of Developing Osteoporosis | 0.328 | 0.317 | ||||||
| Yes | 75 (7.7) | 48 (10.4) | 49 (12.6) | |||||
| No | 692 (70.8) | 412 (89.6) | 339 (87.4) | |||||
| Physician Discussed Risk of Developing Heart Disease | 0.017 | 0.012 | ||||||
| Yes | 131 (13.4) | 75 (16.3) | 90 (23.2) | |||||
| No | 636 (65.0) | 385 (83.7) | 298 (76.8) | |||||
| Survivors' Severity of Late Effects | 0.487 | 0.350 | ||||||
| Moderate, Severe, Life-threatening | 164 (19.7) | 117 (20.1) | 105 (22.2) | |||||
| Mild or No Chronic Problems | 667 (80.3) | 466 (79.9) | 363 (76.6) | |||||
| Print Media Intervention | <0.001 | 0.005 | ||||||
| Yes | 6166 (73.5) | 408 (69.0) | 288 (60.8) | |||||
| No | 222 (26.5) | 183 (31.0) | 186 (39.2) | |||||
| Age 40 Years or More | 0.027 | 0.011 | ||||||
| Yes | 65 (7.8) | 37 (6.3) | 50 (10.5) | |||||
| No | 773 (92.2) | 554 (93.7) | 424 (89.5) | |||||
| Likelihood of Cancer-Related Follow-up | 0.488 | 0.777 | ||||||
| Precontemplation | 419 (51.7) | 276 (48.8) | 213 (46.6) | |||||
| Contemplation | 242 (29.9) | 177 (31.3) | 147 (32.2) | |||||
| Action | 149 (18.4) | 113 (20.0) | 97 (21.2) | |||||
| Mean | SD | Mean | SD | Mean | SD |
P-Value Between Total Sample and Risk Groups (ANOVA) |
||
| Cancer Pain | 1.36 | 0.749 | 1.36 | 0.764 | 1.40 | 0.786 | 0.620 | |
| Cancer Anxiety | 1.56 | 0.855 | 1.59 | 0.891 | 1.60 | 0.860 | 0.686 | |
| Fatigue | 3.86 | 1.253 | 3.88 | 1.223 | 3.89 | 1.247 | 0.922 | |
| Intrinsic Motivation | 18.19 | 3.636 | 18.10 | 3.625 | 18.06 | 3.528 | 0.802 | |
| Extrinsic Motivation | 7.59 | 3.291 | 8.05 | 3.429 | 7.79 | 3.231 | 0.451 | |
| Health Concerns | 3.92 | 0.943 | 3.89 | 0.956 | 3.87 | 0.948 | 0.638 | |
| Fear/Worry | 5.52 | 0.835 | 5.51 | 0.856 | 5.48 | 0.866 | 0.711 | |
| Patient-Physician Relationship | 13.82 | 3.696 | 13.96 | 3.625 | 14.01 | 3.540 | 0.624 | |
| No. of Cancer-related Physician Visits | 1.84 | 1.416 | 1.88 | 1.401 | 1.97 | 1.497 | 0.302 | |
| Exercise Frequency at Baseline | 2.27 | 2.185 | 2.30 | 2.150 | 2.20 | 2.147 | 0.750 | |
N varies because of missing data.
Independent Measures
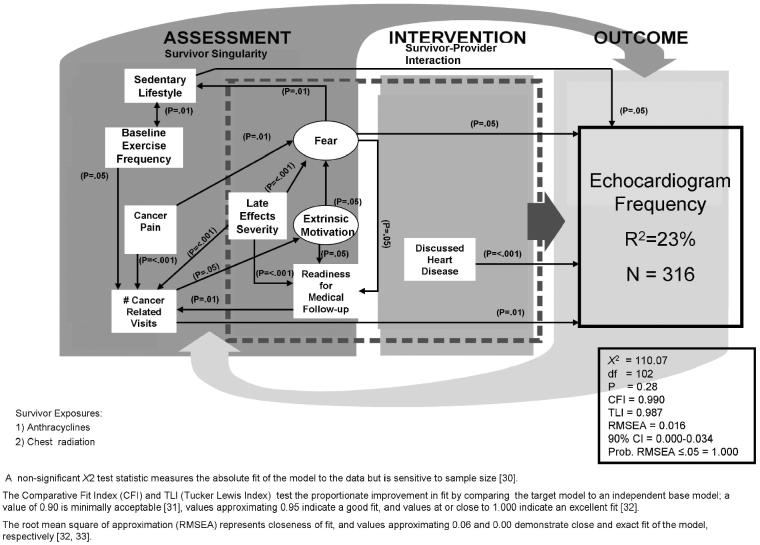
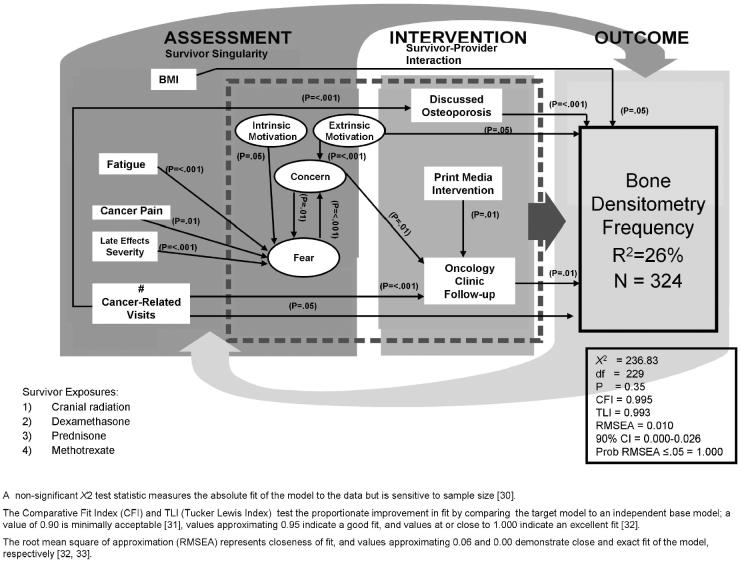
Two types of variables are modeled in SEM: Observed and latent. In contrast to observed variables that can be directly measured (e.g., test scores), latent variables (e.g., depression) are measured indirectly by a set of observed variables [26]. Our final models have 10 directly observed measures (represented in Figs. 2-3 as rectangles) and 4 latent measures (represented in Figs. 2-3 as ovals) that contributed directly, indirectly, or both to the explained variance in frequency of echocardiography or bone densitometry.
Fig. 2.
Predictors of Echocardiograpic Screening
Fig. 3.
Predictors of Bone Densitometry Screening
Directly observed independent variables
Although all variables corresponding to the conceptual model were examined as potential covariates, the following directly observed independent variables were statistically significant in the final models: (1) survivors' pain resulting from cancer or its treatment (1 = No pain; 5 = Excruciating pain); (2) number of cancer-related visits last 2 years (1 = None; 7 = More than 20); (3) survivors' perceptions of the severity of their late effects (1 = moderate, severe, life-threatening; 2 = mild or no chronic problems); (4) physician/survivor discussion of osteoporosis (1=Yes; 2 = No); (5) physician/survivor discussion of heart disease (1 = Yes ; 2 = No); (6) follow-up at an oncology clinic in the past 2 years (1 = Yes; 2 = No); (7) receipt of a print media intervention detailing exposure risks and recommended follow-up for cardiac or bone density sequelae (1 = Yes; 0 = No); (8) baseline aerobic exercise frequency (sweat or breathe hard for 20 min) (0-7 days); (9) physically active leisure-time lifestyle during the past month (1=Yes; 2=No); (10) level of readiness for medical follow-up (1=Precontemplation; 2=Contemplation; 3=Action).
Latent independent variables
The following latent measures were significant in the final models.
Fear/Worry: Three observed variables: Survivors' worry about their future health, the recurrence of their cancer, and their fear that a problem would be discovered in a check-up (1 = Moderate, quite a bit, or extremely concerned; 2 = Not at all or a little concerned) (α = 0.76)
Health Concerns: Three observed variables: Survivors' general concerns about their health, their concerns about chances of getting sick, and their perceptions about the importance of a check-up (1 = Moderate, quite a bit, or extremely concerned; 2 = Not at all or a little concerned) (α = 0.79).
Intrinsic Motivation: Five observed items from the Multidimensional Health Locus of Control Scale (MHLC) [27] (“I am in control of my health”) (1 = Strongly disagree; 6 = Strongly agree) (α = 0.79).
Extrinsic Motivation: Five MHLC [27] items (e.g., “Health professionals control my health”) (1= Strongly disagree; 6 = Strongly agree) (α = 0.80).
Statistical Analyses
SEM has two components: (1) the measurement model evaluates whether observed measures (scales, self-reports, etc.) adequately represent the latent variables and (2) model hypotheses (see Fig. 1) are then tested with respect to the interrelation of the latent variables and covariates [28]. SEM was performed with Mplus 4.2 [26]. The models are based on subjects with complete data; sample sizes for each model were more than adequate [29].
Multiple indicators assess how the SEM fit the data [30-33] (see Fig. 2-3). Factor loading values for the latent variables were less than or equal to P = 0.01 across both models and factor score determinacy values were ≥ 0.80, suggesting that measures of the latent constructs were strong. The final models have significant parameter estimates (Appendix 1) corresponding to the hypothesized relationships, meet the established SEM fit criteria (See Figs. 2-3), and offer the highest percentage of explained variance for the outcome.
Results
The typical respondent was a white, unmarried female college graduate with a personal income of $19,999-39,999; she had health insurance and had not been seen at an oncology clinic in the past 2 years (Table 2). Participants in the risk groups were more recently diagnosed (not significant with Bonferroni adjustment for multiple comparisons), had slightly more education, and were more likely to have been followed recently in an oncology clinic compared to the total sample. The cardiac risk group was slightly older than the bone density risk group at diagnosis (Table 2). In the cardiac risk group, 42.1% of participants had never or not within the last 5 years had an echocardiogram; however, those at cardiac risk were more likely to have had an echocardiogram more recently (2-4 years) than either the total sample or the bone density risk group. Nearly 75% of those in the bone density group had never or not within the last 5 years had a bone densitometry evaluation, and they were no more likely than the cardiac risk group to have had bone densitometry at the recommended intervals (Table 3).
Survivors at cardiac risk were more likely than the total sample and those in the bone density risk group to have discussed heart disease and to be at least 40 years of age. Predictably, survivors in the total sample were more likely to have received a print media intervention detailing their treatment risks; however, a greater proportion of those in the bone density risk group received the intervention than did those in the cardiac risk group (Table 3).
Echocardiogram Frequency
A strong model (N = 316; X2 = 110. 07, df = 102, P = 0.28; CFI = 0.990, TLI = 0.987; RMSEA = 0.016; 90% CI = 0.00-0.034; Probability RMSEA ≤ 0.05 = 1.000 explained 23% of the variance in echocardiogram recency in survivors most at risk for cardiac sequelae (Figure 2a). Survivors who were most likely to follow a more frequent echocardiogram schedule reported more cancer-related visits, discussion of heart disease with a physician, a sedentary lifestyle, and less frequent health fears. The number of cancer-related visits was predicted by reports of increased pain, lower levels of aerobic exercise at baseline, increased readiness for medical follow-up, and perceptions of more severe late effects. Less frequent health fears predicted an active lifestyle. More cancer pain, higher levels of extrinsic motivation, and perceptions of more severe late effects predicted more frequent health fears. Increased readiness to seek medical follow-up was predicted by increased extrinsic motivation, frequent health fears, and more severe late effects. Significant positive indirect effects on echocardiogram recency included cancer-related pain (P = 0.01) and an increased readiness for medical follow-up (P = 0.05) through cancer-related visits.
Bone Densitometry Frequency
A well-fitting bone densitometry model (N = 324; X2 = 236.83, df = 229, P = 0.35; CFI = 0.995, TLI = 0.993; RMSEA = 0.010; 90% CI = 0.00-0.026; Probability RMSEA ≤ .05 = 1.000), described participants who were adherent to the bone density screening guidelines (R2 = 26%) as having made more cancer-related visits, received follow-up at an oncology clinic, were more extrinsically motivated, had discussed osteoporosis with a physician, and had a lower BMI. More health concerns, more cancer-related visits, and having received a print media intervention detailing the individualized risk of sequelae predicted recent oncology clinic follow-up (Fig. 2). Health concerns were predicted by more frequent health fears and reported higher extrinsic motivation. Greater health concerns, decreased intrinsic motivation, more cancer-related pain, perceptions of more severe late effects, and more frequent fatigue predicted increased fear about future health. More cancer-related visits predicted having discussed osteoporosis with the physician. More cancer-related visits and increased concerns about health indirectly predicted bone densitometry frequency through follow-up at an oncology clinic.
Discussion
High-risk adult survivors of childhood cancer frequently do not adhere to recommended medical screening guidelines. Most survivors reported having never discussed heart disease or osteoporosis with their physician. Survivors were most likely to adhere to recommended echocardiogram and bone densitometry screening schedules if they reported more frequent cancer-related visits or were followed up at an oncology clinic, or both. The extent to which our findings reflect the increase in sequelae of treatment, increase in confidence in the knowledge of the specialty provider, familiarity with the facility and its staff in case the treatment was more recent, or more targeted delivery of care, as compared with that available in a non-specialty facility needs further study. Only 7% of the study sample were followed by a cancer specialist; only 4% were followed at a cancer center. Since most survivors are not followed in specialty clinics, this finding is particularly relevant for primary care providers who often lack knowledge about the unique health risks inherent in the treatment for childhood cancer [7, 34-37]. Chronic health conditions in childhood cancer survivors become more prevalent with increasing intervals from cancer treatment and are exacerbated by comorbid illnesses associated with aging and maladaptive health behaviors [38]. Since specific treatment and survivor factors are linked to adverse health outcomes in childhood cancer survivors, informed provider intervention based on risk-stratified medical surveillance represents an important opportunity to reduce cancer-related morbidity.
Pain, fatigue, and perceptions of severity of late effects were strong exogenous variables (unaffected by other variables) in both models. They were antecedent to increased health concerns, more frequent health fears, and a negative affect, which in turn directly and/or indirectly impacted screening frequency. Pain is a frequently reported late effect [39, 40]; 22.3% of 9034 childhood cancer survivors reported having moderate to very severe pain and 14.3% reported pain sufficient to interfere with daily activities [41]. Nineteen percent of 2645 [42], and 30% of 161 adult childhood cancer survivors reported fatigue [43]. Fatigue and pain negatively impact quality of life [43] and health behaviors that have the potential to modify late effects [44, 45].
More frequent health fears were a deterrent to obtaining echocardiograms; however, more frequent fear also increased health concerns, which predicted more recent follow-up at an oncology clinic. Fear, worry, and anxiety exert both positive and negative influence on health-related behaviors [18, 46]. Even though early detection through medical screening may positively modify a disease course, the prospect of learning that one has a serious health condition can be profoundly frightening [47, 48]. Survivors may resort to avoidance behavior [46] (e.g., not going for routine screens) to reduce fear, anxiety, and a negative affect, or, in contrast, use screening as a means (e.g., negative screening exam) to reduce the discomfort of fear and anxiety [30].
Lack of specific information on risk factors and misconceptions can exacerbate fear or contribute to the denial of the existence of significant health problems [49-51]. Discussing late effects (heart disease, osteoporosis) with physicians predicted more recent screening in both models. In the general population, specific physician recommendation is associated with a higher rate of screening for cervical [52], breast [53, 54], prostate [54], colorectal [55-57], and skin cancers [58]. More recent oncology clinic follow-up was predicted by survivors' receipt of an individualized print media intervention that detailed treatment exposure risks for bone density-related late effects and recommendations for follow-up. The impact of the print media intervention on the bone density risk group may reflect the fact that a larger proportion of this group received the intervention; additionally this group may have had greater sensitivity or receptivity because of discernible symptoms (e.g, pain, physical dysfunction).
Motivation played a prominent role in all the models. Extrinsically motivated individuals are more worried and fearful about their health, think they are less able to exert control over health matters, and are more likely to rely on health professionals for direction [17, 23, 59]; intrinsically motivated individuals are more self-reliant and self-directed instead of being physician-directed [17, 60] in their health care choices. Because they may not have accurate health and risk information and have infrequent contact with a physician, intrinsically motivated survivors may be at greater risk for not adhering to screening guidelines. The complex interactions among fear, the patient-physician relationship, affect, and intrinsic motivation should be further explored.
The unique contributions of baseline exercise frequency and sedentary lifestyle to the echocardiogram model may reflect survivors who have early symptoms of treatment-related cardiac sequelae [45]; similarly, survivors with a low BMI were more likely to adhere to bone densitometry recommendations.
Limitations
The study sample reflects a subset of the overall CCSS population - those who responded to the Health Care Needs and CCSS Follow-up 2 Surveys; therefore, survivors included in the current analysis may not be fully representative of the population from which they were derived. The information utilized to classify the health screening outcomes, as well as the independent measures, was based upon self-reported data. Lastly, while the CCSS population represents a large and heterogeneous cohort of five year survivors, results may not be generalizable to all childhood cancer survivors. As a group, CCSS participants may be more informed regarding risks and health promotion because of newsletters received as part of participation in the study.
Clinical Implications
Primary care physicians are encouraged to specifically inquire about treatment-related symptoms, particularly pain, fatigue, and anxiety [1, 43, 61]. These symptoms may share common biological mechanisms [62-64] and, until addressed, obstruct positive health behaviors. Physicians should elicit survivors' concerns and address any misconceptions that may contribute to survivors' lack of understanding about the significance of their late effects risks. Therapeutically increasing or decreasing fear arousal [65, 66] by providing personalized information on late effects risks and the benefits of medical screening may enhance screening behavior. Focused interactions with survivors are important to reduce anxiety, support motivation, and contribute to a more positive affect, which in turn support adherence to screening.
Conclusions
Multiple factors can influence survivors' adherence to screening recommendations, including already established sequelae (e.g., pain, fatigue, functional decline). Early interventions (before completion of therapy, early post-therapy follow-up) that consider the multiple influences on survivors' medical screening behaviors may be instrumental in modifying sequelae and supporting earlier screening. Providing the childhood cancer survivor with written summaries of pediatric cancer therapy together with recommendations for screening and follow-up that can be shared with the primary care physician may be a useful adjunct for targeting increased medical screening.
Acknowledgements
The authors acknowledge the contributions of Sharon Naron and Vani Shanker (for editorial assistance) and Kelly Shempert (for illustrations).
This work was supported by grants RO3 NR009203 and U24 CA55727 from the U.S. Public Health Service, a grant from the Robert Wood Johnson Foundation, and support to St. Jude Children's Research Hospital from the American Lebanese Syrian Associated Charities (ALSAC). This work is original and has not previously been published. An abstract of the work has been accepted for podium presentation at the American Public Health Association in October, 2008.
References
- 1.Hudson MM, Mertens AC, Yasui Y, Hobbie W, Chen H, Gurney JG, Yeazel M, Recklitis CJ, Marina N, Robison LL, Oeffinger KC. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA. 2003;290:1583–1592. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- 2.Mattano LA, Sather HN, Trigg ME, et al. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: a report from the children's cancer group. Clinical Oncology. 2000;18:3262–3272. doi: 10.1200/JCO.2000.18.18.3262. [DOI] [PubMed] [Google Scholar]
- 3.Neglia JP, Friedman DL, Yasui Y, Mertens AC, Hammond S, Stovall M, Donaldson SS, Meadows AT, Robison LL. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst. 2001;93:618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 4.Simbre VC, Duffy SA, Dadlani GH, Miller TL, Lipshultz SE. Cardiotoxicity of cancer chemotherapy: implications for children. Paediatric Drugs. 2005;7:187–202. doi: 10.2165/00148581-200507030-00005. [DOI] [PubMed] [Google Scholar]
- 5.Children's Oncology Group The Children's Oncology Group Long-Term Follow-up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. 2006 [cited October 22, 2007]; Available from: http://www.survivorshipguidelines.org.
- 6.Bellizzi KM, Rowland JH, Jeffery DD, McNeel T. Health behaviors of cancer survivors: Examining opportunities for cancer control intervention. Journal of Clinical Oncology. 2005;23:8884–8893. doi: 10.1200/JCO.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- 7.Diller L, Medeiros Nancarrow C, Shaffer K, Matulonis U, Mauch P, Neuberg D, et al. Breast cancer screening in women previously treated for Hodgkin's Disease: A prospective cohort study. Journal of Clinical Oncology. 2002;20:2085–2091. doi: 10.1200/JCO.2002.08.031. [DOI] [PubMed] [Google Scholar]
- 8.Yeazel MW, Oeffinger KC, Gurney JG, Mertens AC, Hudson MM, Emmons KM, Chen H, Robison LL. The cancer screening practices of adult survivors of childhood cancer. Cancer. 2004;100:631–640. doi: 10.1002/cncr.20008. [DOI] [PubMed] [Google Scholar]
- 9.Nathan PC, Greenberg ML, Ness KK, Mahone MC, Gurney JG, Hudson MM, et al. Risk-based care in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study (CCSS) Journal of Clinical Oncology. 2007;25(18S):6502. [Google Scholar]
- 10.Bhatia S, Yasui Y, Robison L, Birch J, Bogue M, Diller L, et al. High Risk of Subsequent Neoplasms Continues With Extended Follow-Up of Childhood Hodgkin's Disease: Report From the Late Effects Study Group. Journal of Clinical Oncology. 2003;21(23):4386–4394. doi: 10.1200/JCO.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 11.Kenney LB, Yasui Y, Inskip PD, Hammond S, Neglia JP, Mertens AC, et al. Breast cancer after childhood cancer: a report from the Childhood Cancer Survivor Study. Annals of Internal Medicine. 2004;141(8):590–597. doi: 10.7326/0003-4819-141-8-200410190-00006. [DOI] [PubMed] [Google Scholar]
- 12.Taylor AJ, Winter DL, Stiller CA, Murphy M, Hawkins MM. Risk of breast cancer in female survivors of childhood Hodgkin's disease in Britain: A population-based study. International Journal of Cancer. 2006;120:384–391. doi: 10.1002/ijc.22261. [DOI] [PubMed] [Google Scholar]
- 13.Adams MJ, et al. Radiation-associated cardiovascular disease: manifestations and management. Semin Radiat Oncol. 2003;13(3):346–56. doi: 10.1016/S1053-4296(03)00026-2. [DOI] [PubMed] [Google Scholar]
- 14.Berry GJ, Jorden M. Pathology of radiation and anthracycline cardiotoxicity. Pediatr Blood Cancer. 2005;44(7):630–7. doi: 10.1002/pbc.20346. [DOI] [PubMed] [Google Scholar]
- 15.Lipshultz SE, Alvarez JA, Scully RE. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart. 2008;94(4):525–33. doi: 10.1136/hrt.2007.136093. [DOI] [PubMed] [Google Scholar]
- 16.Breslow L, Lloyd D, Shumaker SA. Disease Prevention Research at NIH: An agenda for all. Workshop B: Health behaviors-predictors, mediators, and endpoints. Prev Med. 1994;23:552–553. doi: 10.1006/pmed.1994.1079. [DOI] [PubMed] [Google Scholar]
- 17.Cox CL, McLaughlin RA, Rai SN, Steen BD, Hudson MM. Adolescent survivors: a secondary analysis of a clinical trial targeting behavior change. Pediatric Blood and Cancer. 2005;45:144–154. doi: 10.1002/pbc.20389. [DOI] [PubMed] [Google Scholar]
- 18.Cox CL, McLaughlin RA, Steen BD, Hudson MM. Predicting and modifying substance use in childhood cancer survivors: application of a conceptual model. Oncology Nursing Forum. 2006;33:51–60. doi: 10.1188/06.ONF.51-60. [DOI] [PubMed] [Google Scholar]
- 19.Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59:877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 20.Prochaska J. Health behavior change research: A consortium approach to collaborative science. Ann Behav Med. 2005;29:4–6. doi: 10.1207/s15324796abm2902s_2. [DOI] [PubMed] [Google Scholar]
- 21.Rejeski WJ, Brawley LR, McAuley E, Rapp S. An examination of theory and behavior change in randomized clinical trials. Control Clin Trials. 2000;21:164S–70S. doi: 10.1016/s0197-2456(00)00074-x. [DOI] [PubMed] [Google Scholar]
- 22.Cox C. An interaction model of client health behavior: Theoretical prescription for research. Adv Nurs Sci. 1982;5:41–56. doi: 10.1097/00012272-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Cox CL. Online exclusive: a model of health behavior to guide studies of childhood cancer survivors. Oncol Nurs Forum. 2003;30:E92–9. doi: 10.1188/03.ONF.E92-E99. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan D. Structural Equation Modeling: Foundations and Extensions. Sage Publications; Newbury Park, CA: 2000. [Google Scholar]
- 25.Robison LL, Mertens AC, Boice JD, Breslow NE, Donaldson SS, Green DM, Li FP, Meadows AT, Mulvihill JJ, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: A multi-institutional collaborative project. Medical and Pediatric Oncology. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 26.Muthen K, Muthen BO. Mplus User's Guide. 4th ed. Muthen & Muthen; Los Angeles, CA: 19982007. [Google Scholar]
- 27.Wallston KA, Wallston BS, DeVellis R. Development of the Multidimensional Health Locus of Control (MHLC) Scales. Health Educ Monogr. 1978;6:160–170. doi: 10.1177/109019817800600107. [DOI] [PubMed] [Google Scholar]
- 28.Raykov T, Marcoulides GA. A first course in structural equation modeling. 1st ed. Lawrence Erlbaum Associates; Mahway, NJ: 2000. [Google Scholar]
- 29.Kline RB. Principles and Practice of Structural Equation Modeling (Methodology in the Social Sciences) 2nd ed. The Guilford Press; New York, NY: 2005. [Google Scholar]
- 30.Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 31.Bollen K. Overall fit in covariance structure models: two types of sample size effects. Psychol Bull. 1990;107:256–259. [Google Scholar]
- 32.Browne M, Cudeck R. In: Alternative ways of assessing model fit, in Testing structural equation models. Bollen KA, Long JA, editors. Sage; Newbury Park, CA: 1993. [Google Scholar]
- 33.Hu L, Bentler P. Cutoff criteria for fit indices in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- 34.Aziz NM, Oeffinger KC, Brooks S, Turoff AJ. Comprehensive long-term follow-up programs for pediatric cancer survivors. Cancer. 2006;107:841–848. doi: 10.1002/cncr.22096. [DOI] [PubMed] [Google Scholar]
- 35.Landier W, Wallace WH, Hudson MM. Long-term follow-up of pediatric cancer survivors: education, surveillance, and screening. Pediatr Blood Cancer. 2006;46:149–158. doi: 10.1002/pbc.20612. [DOI] [PubMed] [Google Scholar]
- 36.Oeffinger KC, Mertens AC, Hudson MM, Gurney JG, Casillas J, Chen H, et al. Health care of young adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Fam Med. 2004;2(1):61–70. doi: 10.1370/afm.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol. 2006;24:5117–5124. doi: 10.1200/JCO.2006.07.0474. [DOI] [PubMed] [Google Scholar]
- 38.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. New England Journal of Medicine. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 39.Children's Oncology Group Chronic Pain Version 2.0. Health Link: Health living after treatment for childhood cancer. 2006 [cited January 28, 2008]; Available from: http://www.survivorshipguidelines.org/pdf/ChronicPain.pdf.
- 40.Lu Q. Identifying risk factors of pain among childhood cancer survivors. n.d. [cited January 28, 2008]; Available from: http://www.mattel.ucla.edu/pedspain/bio.php?n=QianLu&p=5.
- 41.Lu Q, Tsao J, Leisenring W, Robison L, Zeltzer L. Pain among long-term survivors of childhood cancer: A preliminary report from the Childhood Cancer Survivor Study (CCSS) The Journal of Pain. 2007;8(Suppl S):S76. [Google Scholar]
- 42.Mulrooney DA, Ness KK, Neglia JP, Whitton JA, Green DM, Zeltzer LK, et al. Fatigue and sleep disturbance in adult survivors of childhood cancer: a report from the childhood cancer survivor study (CCSS) Sleep. 2008;31:271–281. doi: 10.1093/sleep/31.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meeske KA, Siegel SE, Globe DR, Mack WJ, Bernstein L. Prevalence and correlates of fatigue in long-term survivors of childhood leukemia. J Clin Oncol. 2005;23:5501–5510. doi: 10.1200/JCO.2005.03.210. [DOI] [PubMed] [Google Scholar]
- 44.Cox CL, Montgomery M, Oeffinger KC, Leisenring W, Zeltzer L, Whitton JA, et al. Promoting physical activity in childhood cancer survivors: Targets for intervention. Cancer. 2008 doi: 10.1002/cncr.24043. In Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cox CL, Rai SN, Rosenthal D, et al. Subclinical late cardiac toxicity in childhood cancer survivors: impact on self-reported health. Cancer. 2008;112:1835–1844. doi: 10.1002/cncr.23378. [DOI] [PubMed] [Google Scholar]
- 46.Mullens AB, McCaul KD, Erickson SC, Sandgren AK. Coping after cancer: Risk perceptions, worry, and health behaviors among colorectal cancer survivors. Psycho-Oncology. 2004;13:367–376. doi: 10.1002/pon.751. [DOI] [PubMed] [Google Scholar]
- 47.Cameron LD. In: Screening for cancer: Illness perceptions and illness worry, in Perceptions of health and illness: Current research and applications. Petrie KJ, Weinman JA, editors. Hardwood Academic; Amsterdam: 1997. [Google Scholar]
- 48.Millar MG, Millar K. Negative affective consequences of thinking about disease detection behaviors. Health Psychology. 1995;14:141–146. doi: 10.1037//0278-6133.14.2.141. [DOI] [PubMed] [Google Scholar]
- 49.Hopwood P. Breast cancer risk perception: what do we know and understand? Breast Cancer Res. 2000;2:387–391. doi: 10.1186/bcr83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahdy NH, Fatohy IM, Mounir GM, El-Deghedi BM. Assessment of students' knowledge, attitude and practice concerning cancer and its prevention. Part I. J Egypt Public Health Assoc. 1998;73:399–431. [PubMed] [Google Scholar]
- 51.Pohls UG, Renner SP, Fasching PA, Lux MP, Kreis H, Ackermann S, Bender H-G, Beckmann MW. Awareness of breast cancer incidence and risk factors among healthy women. European Journal of Cancer Prevention. 2004;13:249–256. doi: 10.1097/01.cej.0000136718.03089.a5. [DOI] [PubMed] [Google Scholar]
- 52.Coughlin SS, Breslau ES, Thompson T, Bernard VB. Physician recommendation for papanicolaou testing among U.S. women, 2000. Cancer Epidemiol Biomarkers Prev. 2005;14:1143–1148. doi: 10.1158/1055-9965.EPI-04-0559. [DOI] [PubMed] [Google Scholar]
- 53.Garbers S, Chiasson MA. Breast cancer screening and health behaviors among African American and Caribbean women in New York City. J Health Care Poor Underserved. 2006;17:37–46. doi: 10.1353/hpu.2006.0024. [DOI] [PubMed] [Google Scholar]
- 54.Mayer DK, Terrin NC, Menon U, Kreps GL, McCance K, Parsons SK, Mooney KH. Screening practices in cancer survivors. Journal of Cancer Survivorship. 2007;1:17–26. doi: 10.1007/s11764-007-0007-0. [DOI] [PubMed] [Google Scholar]
- 55.Katz ML, James AS, Pignone MP, Hudson MA, Jackson E, Oates V, Campbell MK. Colorectal cancer screening among African American church members: a qualitative and quantitative study of patient-provider communication. BMC Public Health. 2004;4:62–69. doi: 10.1186/1471-2458-4-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ling BS, Klein WM, Dang Q. Relationship of communication and information measures to colorectal cancer screening utilization: results from HINTS. J Health Commun. 2006;11:181–190. doi: 10.1080/10810730600639190. [DOI] [PubMed] [Google Scholar]
- 57.Matthews BA, Nattinger AB, Venkatesan T, Shaker R. Colorectal cancer screening among midwestern community-based residents: indicators of success. J Community Health. 2007;32:103–120. doi: 10.1007/s10900-006-9038-0. [DOI] [PubMed] [Google Scholar]
- 58.Manne S, Lessin S. Prevalence and correlates of sun protection and skin self-examination practices among cutaneous malignant melanoma survivors. Journal of Behavioral Medicine. 2006;29:419–434. doi: 10.1007/s10865-006-9064-5. [DOI] [PubMed] [Google Scholar]
- 59.Deci EL, Ryan RM. Handbook of Self-Determination Research. University of Rochester Press; Rochester, NY: 2002. [Google Scholar]
- 60.Cox CL, Montgomery M, Rai SN, McLaughlin R, Steen B, Hudson MM. Supporting breast self-examination in female childhood cancer survivors: A secondary analysis of a behavioral intervention. Oncol Nurs Forum. 2008 doi: 10.1188/08.ONF.423-430. In Press. [DOI] [PubMed] [Google Scholar]
- 61.Langeveld NE, Ubbink M, Smets E. ‘I don't have any energy’: The experience of fatigue in young adult survivors of childhood cancer. Eur J Oncol Nurs. 2000;4:20–28. doi: 10.1054/ejon.1999.0063. [DOI] [PubMed] [Google Scholar]
- 62.Cleeland CS, Bennett GJ, Dantzer R, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? Cancer. 2003;97:2919–2925. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 63.Lee BN, Dantzer R, Langley KE, et al. A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation. 2004;11:279–292. doi: 10.1159/000079408. [DOI] [PubMed] [Google Scholar]
- 64.Miaskowski C, Aouizerat BE. Is there a biological basis for the clustering of symptoms? Semin Oncol Nurs. 2007;23:99–105. doi: 10.1016/j.soncn.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 65.Dijker AJ, Koomen W, Kok G. Interpersonal determinants of fear of people with AIDS: the moderating role of predictable behavior. Basic and Applied Social Psychology. 1997;19:61–79. [Google Scholar]
- 66.Geller ES. Designing an effective fear appeal. n.d. [cited August 20, 2007]; Available from: http://www.safetyperformance.com/pdf/Articles/2001/DesigninganEffectiveFearAppeal.pdf.