Summary
Stress granules aid cell survival in response to environmental stressors by acting as sites of translational repression. We report an unanticipated link between stress granules and the Ser/Thr kinase, RSK2. In stressed breast cells endogenous RSK2 co-localizes in granules with TIA-1 and poly-(A) binding protein 1, and the sequestration of RSK2 and TIA-1 exhibits co-dependency. The RSK2 N-terminal kinase domain controls the direct interaction with the prion-related domain of TIA-1. Silencing RSK2 decreases cell survival in response to stress. Mitogen releases RSK2 from the stress granules and permits its nuclear import via a nucleo-cytoplasmic shuttling sequence in the C-terminal domain. Nuclear accumulation is dependent on TIA-1. Surprisingly, nuclear localization of RSK2 is sufficient to enhance proliferation through induction of cyclin D1, in the absence of other active signaling pathways. Hence, RSK2 is a pivotal factor linking the stress response to survival and proliferation.
Introduction
The p90 ribosomal S6 kinase (RSK) family of Ser/Thr kinases is proposed to control the activity of a plethora of downstream effectors (Roux and Blenis, 2004). However, many of these substrates were identified by over-expression or in vitro kinase assays and the physiological significance of their phosphorylation by RSK is not clear. For example, it is now widely accepted that the cre element binding protein (CREB) and histone H3, proteins previously reported as RSK2 substrates, are in fact physiological substrates for mitogen- and stress-activated kinase (MSK) (Sapkota et al., 2007; Soloaga et al., 2003; Wiggin et al., 2002). Moreover, for most proposed RSK substrates it is not known whether the substrate is phosphorylated by a particular isoform or by multiple family members. The RSKs are frequently expressed in the same cell type but each of the isoforms likely has a unique substrate profile, because they have distinct in vivo functions (Bignone et al., 2007; Dufresne et al., 2001; Poirier et al., 2007; Yang et al., 2004; Yntema et al., 1999). RSK substrates are presumably not confined to a particular subcellular compartment as, in addition to their cytoplasmic distribution, RSK1 has been reported to localize to the plasma membrane (Richards et al., 2001) and RSK1, RSK2 and RSK3 to the nucleus (Chen et al., 1992; Cude et al., 2007; Dunham-Ems et al., 2006; Sananbenesi et al., 2002; Willard and Crouch, 2001; Zhao et al., 1995). Some studies have suggested that the phosphorylation status of RSK is important in nuclear translocation (Chaturvedi et al., 2006; Watson and Fan, 2005) but it is not clear from these studies which isoform was studied because the phosphospecific antibodies used were not isoform-specific. Moreover, the regulation of RSK nuclear translocation is not understood, because RSK does not contain a canonical nuclear localization signal (NLS) and is too large to diffuse into the nucleus. Other studies have suggested that various binding partners regulate RSK subcellular distribution. However, these observations are based on over-expression systems and their physiological relevance is not clear (Cavet et al., 2003; Vaidyanathan and Ramos, 2003). Thus, our knowledge of the various functions of RSK and the regulation of these functions in somatic cells is strikingly incomplete.
Cells have evolved multiple strategies to cope with the varied and inevitable stresses of existence. One recently discovered mechanism involves the formation of stress granules. Stress granules form under conditions where translation initiation has been reduced or inhibited (Kedersha et al., 2002). These granules recruit selected mRNAs and associated proteins from polyribosomes, for storage, or for triage through processing bodies (P-bodies) (Anderson and Kedersha, 2006). Stress granules and P-bodies have many of the same components. For example, both types contain TIA-1, a RNA-binding protein that acts as a translational inhibitor (Lopez de Silanes et al., 2005). Stress granules differ from P-bodies in that they uniquely contain poly-(A) binding protein 1 (PABP1), RasGAP SH3-domain binding protein-1, elongation initiation factor 2 (eIF2), eIF3 and 40S ribosomal components (Kedersha et al., 2002; Kedersha et al., 2005; Kedersha et al., 1999; Tourriere et al., 2003). Stress granules are thought to aid cell survival by acting as sites of translational repression and to facilitate post-stress recovery by acting as reservoirs of poly (A)+ RNA.
We have discovered an unanticipated link between stress granules and RSK2. We found that in breast cells subjected to stress, endogenous RSK2 associates with and co-localizes with TIA-1 and PABP1. Unexpectedly, RSK2 controls TIA-1 recruitment into stress granules and this regulation is physiologically important, because loss of RSK2 decreases cell survival in response to stress. Addition of mitogen triggers the dissolution of stress granules, and the released RSK2 accumulates in the nucleus, where it induces cyclin D1 expression, driving entry into the cell cycle. RSK2 has not previously been implicated as a regulatory component in the stress response.
Results
RSK2 is a Nucleocytoplasmic Shuttling Protein
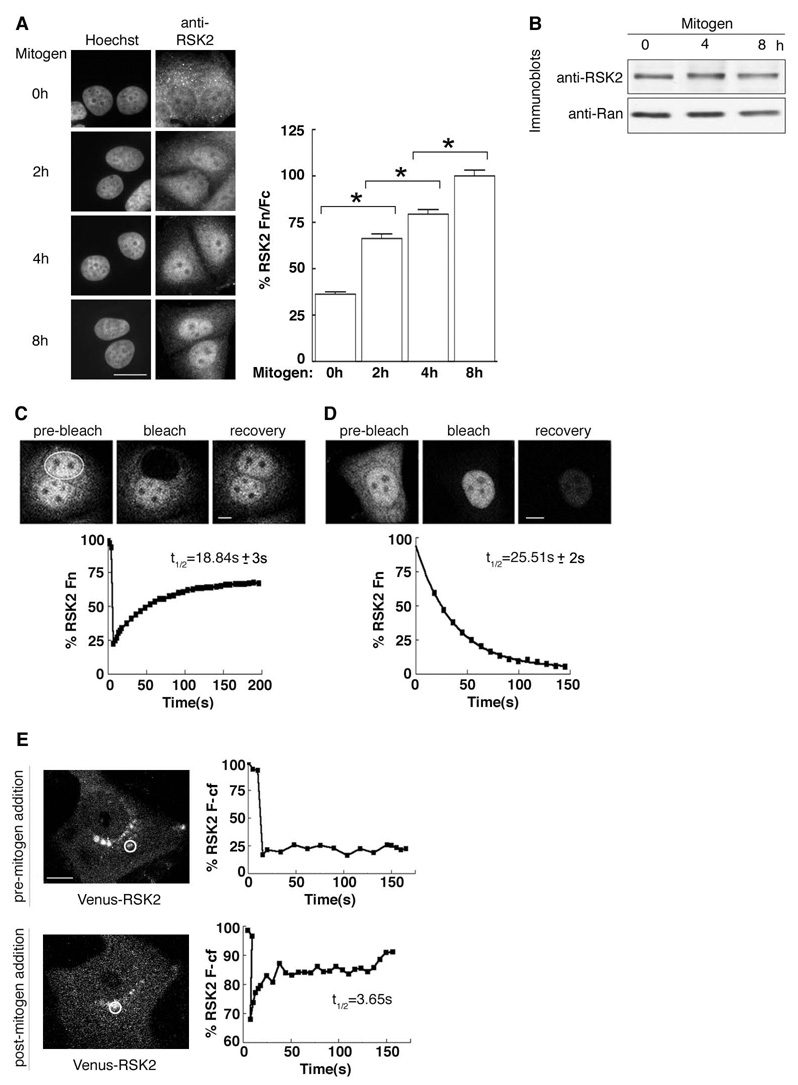
To define the molecular mechanisms that regulate RSK function we initially analyzed the mitogen-induced nuclear accumulation of RSK2. In serum-starved MCF-7 cells, RSK2 was predominantly cytoplasmic and concentrated in distinct granules (Fig. 1A). In response to mitogen treatment, RSK2 accumulated in the nucleus over a 4 – 8 h period, with an ~3X increase in the ratio of nuclear to cytoplasmic staining. No change in RSK2 protein levels was observed over the 8 h mitogen treatment (Fig. 1B). These results suggest that RSK2 accumulates in the nucleus through enhanced import rather than increased synthesis.
Figure 1. Regulation of RSK2 subcellular localization by mitogen.
(A) MCF-7 cells treated with mitogen for various times and stained with goat anti-RSK2; DNA stained with Hoechst. Columns, mean (n=2, ≥ 15 cells/time point); bars, SEM, p<0.01. Fn=nuclear fluorescence; Fc=cytoplasmic fluorescence (B) MCF-7 cells treated as in (A). (C) Rate of Venus-RSK2 nuclear import as determined by FRAP in mitogen-treated (8 h) MCF-7 cells. (D) Rate of Venus-RSK2 nuclear export as determined by FLIP in cells treated as in (C). (E) Mobility of Venus-RSK2 in cytoplasmic foci as determined by FRAP without and with mitogen (2 h). F-cf= cytoplasmic foci fluorescence
To investigate whether RSK2 shuttles in and out of the nucleus we used MCF-7 cells that stably express a Venus-RSK2 fusion. Venus-RSK2 is expressed at ~3X higher levels than endogenous RSK2 and only the full length Venus-RSK2 was detected using an anti-Venus antibody (Fig. S1A). We determined the rate of RSK2 nuclear import by measuring fluorescence recovery after targeted bleaching (FRAP) of the nuclei. In serum-starved conditions RSK2 is absent from the nuclei and we could not measure nuclear import. Therefore, we treated cells with mitogen for 8 h then measured the rate of import after bleaching the nucleus. Nuclear fluorescence quickly recovered with t1/2= ~19 s (95% confidence interval (CI); 17 s to 21 s, n=3; ≥3 cells) (Fig. 1C). To measure nuclear export we used fluorescence loss in photobleaching (FLIP), in which the cytoplasm was reiteratively bleached and the loss of fluorescence in the nuclei was measured. Nuclear fluorescence disappeared with t1/2= ~ 26 s (95% CI; 24 s to 27 s, n=3; ≥3 cells) (Fig 1D). These results show that in the presence of mitogen RSK2 shuttles rapidly in and out of the nucleus.
To understand why RSK2 is absent from the nuclei in serum-starved cells we examined the dynamic properties of the cytoplasmic RSK2 pool. Venus-RSK2, like the endogenous RSK2, was reproducibly localized to cytoplasmic granules in serum-starved cells (Figs. 1A, 1E). Fluorescence recovery was measured after targeted bleaching of Venus-RSK2-containing granules. Surprisingly, fluorescence did not recover (Fig. 1E) even after 10 min (data not shown). To ensure that the lack of recovery was not a consequence of damage caused by photobleaching we added mitogen and imaged the same cell every 20 min over a 120 min period. During this time we observed that the individual granules slowly dissipated in response to mitogen. After 120 min we performed FRAP on Venus-RSK2 still present in the dissipating granules and observed that a portion of the fluorescence rapidly recovered with an t1/2= ~3.7s (95% CI; 3.3 s to 4.2 s, n=4). These results support the idea that RSK2 accumulation into nuclei is prevented by its stable sequestration into cytoplasmic foci. Mitogen-induced release of RSK2 from granules then allows RSK2 to cycle rapidly between the nuclear and cytoplasmic compartments, which permit nuclear accumulation.
RSK2 is Sequestered in Stress Granules
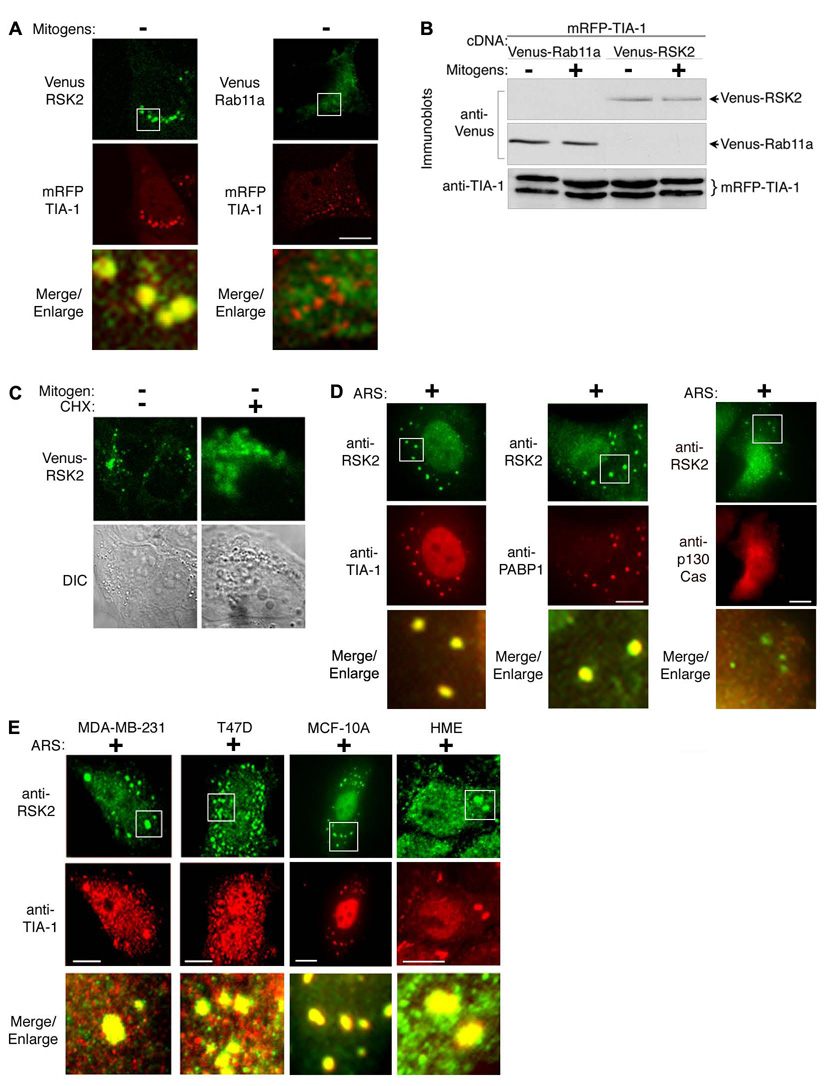
To understand the sequestration mechanism we used mass spectrometry to identify proteins that associate with RSK2 in serum-starved MCF-7 cells. For these experiments we used a cell line that constitutively expresses HA-RSK2 at similar levels to endogenous RSK2 (Fig. S1B). Unexpectedly, a number of proteins co-precipitated with the HA-RSK2 isolated from serum-starved cells that are present in stress granules, including TIA-1 (STable 1). TIA-1 regulates the assembly of stress granules (Gilks et al., 2004) and we asked, therefore, whether TIA-1 and RSK2 co-localize in serum-starved cells. The granules formed in response to serum-starvation are quite small, so to increase the sensitivity of our detection we ectopically expressed Venus-RSK2 and mRFP-TIA-1 in MCF-7 cells (Fig. 2A). The RSK2 and TIA-1 fusion proteins co-localized in serum-starved cells, consistent with the mass spectrometry data. This co-localization is specific, as RSK2 did not co-localize with Rab11a, even though Rab11a and RSK2 are expressed at similar levels (Fig. 2B). To further characterize the cytoplasmic foci we treated serum-starved cells with the protein synthesis inhibitor cycloheximide. Cycloheximide stabilizes polysomes and thereby inhibits stress granule formation (Kedersha et al., 2000). We found that RSK2 became dispersed throughout the cytoplasm by cycloheximide treatment (Fig. 2C). Together, these results suggest that RSK2 is localized in stress granules.
Figure 2. In stressed breast cells RSK2 is localized into stress granules.
(A) Serum-starved MCF-7 cells co-expressing mRFP-TIA-1 and Venus-RSK2 or Venus-Rab11a. (B) MCF-7 cells treated as in (A) or with mitogen. (C) Serum-starved MCF-7 clone stably expressing Venus-RSK2 treated with vehicle or cycloheximide (50 µM; 2.5 h). (D) ARS-treated (500 µM; 1 h) MCF-7 cells co-stained with mouse monoclonal anti-RSK2 and goat anti-TIA-1 antibodies, rabbit anti-PABP1 antibodies or rabbit anti-p130Cas. (E) ARS-treated breast cells.
Stress granules also form in response to oxidative stress, which can be induced by arsenite (Kedersha et al., 2000). The arsenite-induced granules were substantially larger than those that formed in response to serum starvation, and strong co-localization of endogenous RSK2 with endogenous TIA-1 was apparent (Fig. 2D). Importantly, we also observed that RSK2 co-localized with PABP1 (Fig. 2D), which is found exclusively in stress granules (Kedersha et al., 2005). This co-localization is consistent with our mass spectrometry results (STable 1). The co-localization of RSK2 with TIA-1 and PABP1 is specific because we did not observe co-localization with the cytosolic protein, p130Cas (Fig. 2D). To confirm that RSK2 sequestration is not peculiar to MCF-7 cells we examined other transformed breast cancer cell lines, MD-MB-231 and T47D, the untransformed breast line, MCF-10A and primary human epithelial (HME) cells grown in 3D culture. In each cell type RSK2 co-localized with TIA-1 in cytoplasmic granules of arsenite-treated cells (Fig. 2E). Therefore, we conclude that RSK2 localizes to stress granules in response to stress in breast cells.
TIA-1 Controls RSK2 Localization
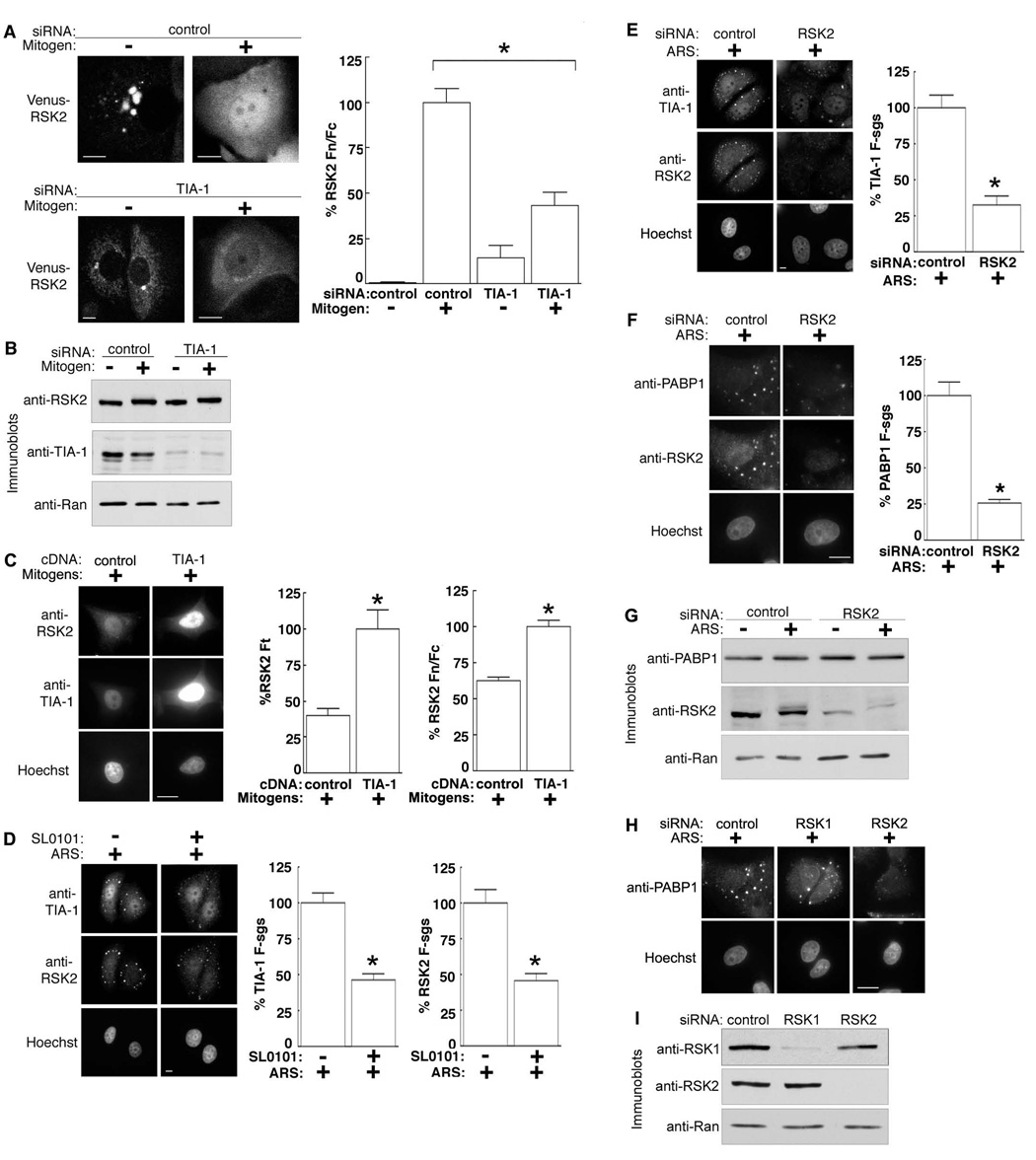
To understand the significance of the co-localization of RSK2 and TIA-1 we specifically silenced TIA-1 in the MCF-7 line stably expressing Venus-RSK2. Strikingly, the loss of TIA-1 caused a diffuse redistribution of RSK2 throughout the cytoplasm of serum-starved cells (Fig. 3A). These results are consistent with previous observations that TIA-1 regulates stress granule formation (Gilks et al., 2004) but also further confirm that RSK2 is targeted to stress granules. Remarkably, however, depletion of TIA-1 also inhibited the nuclear accumulation of RSK2 by ~ 60% in response to mitogen, suggesting that TIA-1 is necessary for efficient nuclear retention or import of RSK2 (Fig. 3A). Silencing TIA-1 did not alter RSK2 expression or prevent its electrophoretic mobility upshift, caused by activating phosphorylations (Dalby et al., 1998) in response to mitogen (Fig. 3B). Therefore, even though RSK2 is activated, it is not efficiently translocated into the nucleus. Ectopic addition of TIA-1 raised total levels of RSK2 but also clearly increased the nuclear accumulation of RSK2 by ~ 2X (Fig. 3C). Conversely, forcing constitutive nuclear accumulation of RSK2 using an NLS had no effect on the nuclear levels of TIA-1 (Fig. S2A). Consistent with this observation, silencing RSK2 did not alter the levels of nuclear TIA-1 (Fig. S2B). Thus, the majority of TIA-1 is imported into the nucleus independently of RSK2. Importantly, our data demonstrate that TIA-1 regulates RSK2 localization both into stress granules and into the nucleus.
Figure 3. Co-dependence of TIA-1 and RSK2 localization.
(A) Stably expressing Venus-RSK2 MCF-7 clone transfected with TIA-1-specific or control siRNA. Transfected cells were serum-starved or mitogen (8 h) treated. Columns, mean (n=2, ≥ 8 cells/condition); bars, SEM, p<0.0001. (B) MCF-7 cells treated as in (A). (C) Mitogen-treated MCF-7 cells transfected with TIA-1 or control vector. Columns, mean (n=3, ≥ 11 cells/condition); bars, SEM, p<0.0005. Ft= total cellular fluorescence (D) ARS-treated MCF-7 cells pre-treated with SL0101 (100 µM; 4 h). Columns, mean (n=2, ≥72 granules in ≥ 7 cells); bars, SEM, p<0.0001. F-sgs; stress granule fluorescence. (E) ARS-treated MCF-7 cells transfected with RSK2-specific or control siRNA. Columns, mean (n=2, ≥60 granules in ≥ 6 cells); bars, SEM, p<0.0001. (F) Cells treated as in (E). Columns, mean (n=2, 37 ≥ granules in ≥ 30 cells); bars, SEM, p<0.0001. (G) MCF-7 cells treated as in (F). (H) ARS-treated MCF-7 cells transfected with control, RSK1- or RSK2-specific or control siRNA. (I) MCF-7 cells treated as in (H).
Because of the association of RSK2 with TIA-1 we investigated whether RSK2 influences stress granule formation. First, we used a specific, small molecule inhibitor of RSK activity, SL0101 (Smith et al., 2005). This inhibitor reduced both the amount of RSK2 and TIA-1 in the granules by ~50% compared to the vehicle control (Fig. 3D). In agreement with these observations, silencing RSK2 by RNA interference resulted in a ~3X reduction in the amount of TIA-1 present in stress granules (Fig. 3E). Moreover, knockdown of RSK2 decreased the amount of PABP1 associated with stress granules by ~4X compared to the control (Fig. 3F). Silencing RSK2 did not alter PABP1 expression (Fig. 3G). RSK2 regulation of stress granule formation is specific because silencing RSK1 did not alter the localization of PABP1 to stress granules (Fig. 3H, 3I). We conclude that RSK2 and its kinase activity are important for stress granule formation because they control both TIA-1 and PABP1 sequestration.
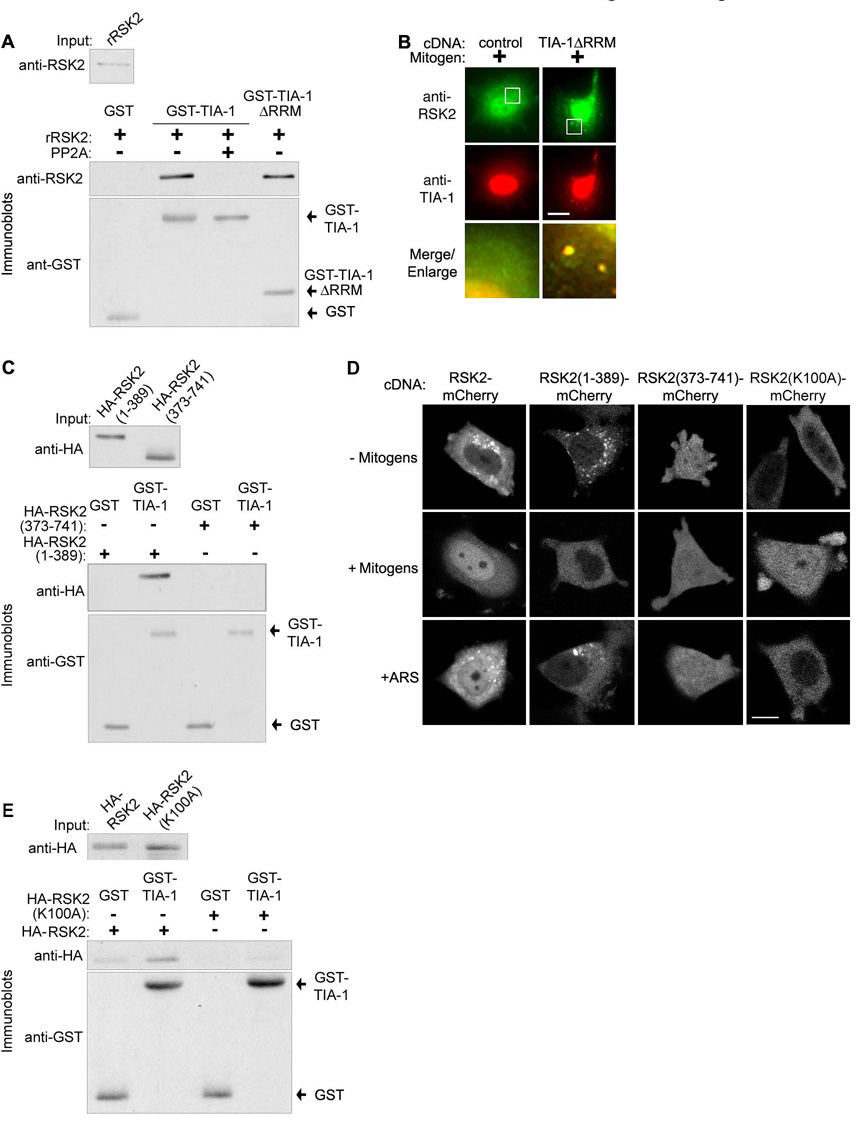
To determine the mechanism by which RSK2 localization is regulated we asked whether RSK2 and TIA-1 interact directly, using an in vitro binding assay with purified recombinant proteins. His6-RSK2 was specifically precipitated with GST-TIA-1 bound to glutathione-beads (Fig. 4A).
Figure 4. NTKD controls the direct interaction of RSK2 with the TIA-1 prion-related domain.
(A) GST-pulldown assay using recombinant proteins incubated with or without protein phosphatase 2A (PP2A). Input is 1/10th of the total added. (B) MCF-7 cells transfected with TIA-1ΔRRM or control vector. (C) GST-pulldown assay using recombinant GST-TIA-1 with lysates of MCF-7 cells transfected with RSK2 deletion mutants. (D) MCF-7 cells transfected with various C-terminal mcherry-tagged RSK2 mutants. (E) As in (C) except MCF-7 cells were transfected with wild type or kinase dead RSK2.
To identify the domain of TIA-1 important for the interaction with RSK2 we expressed the trans-dominant mutant TIA-1ΔRRM. This N-terminal truncation lacks almost all the RNA recognition motifs, but does contain an intact prion-related domain (PRD) (Kedersha et al., 1999). TIA-1ΔRRM constitutively sequesters endogenous TIA-1 and associated stress granule proteins in the absence of upstream signaling events. However, the granules formed by TIA-1ΔRRM are much smaller than those formed in response to stress and do not contain poly(A)+ mRNA. In agreement with the literature, we found that expression of TIA-1ΔRRM induced the formation of small TIA-1 granules and, importantly, that RSK2 was co-localized with TIA-1 in those granules (Fig. 4B). An in vitro binding assay demonstrated that RSK2 and the prion-related domain of TIA-1 interact directly (Fig. 4A). We conclude that RSK2 is able to bind directly to TIA-1, independent of RNA, through interaction with the C-terminal region of TIA-1 from amino acids 219 to 386.
To identify the domains of RSK2 responsible for its localization we created the deletion mutants RSK2(1–389) and RSK2(373–741). RSK2 is a dual domain kinase (Hauge and Frodin, 2006). RSK2(1– 389) contains the N-terminal kinase domain (NTKD) plus the linker region. RSK2(373–741) contains a portion of the linker domain, the C-terminal kinase domain (CTKD) and the extracellular signal-regulated kinase (ERK)1/2 docking site (Smith et al., 1999). Both deletion mutants are known to be functional (Chrestensen and Sturgill, 2002; Richards et al., 1999). We prepared lysates from MCF-7 cells transfected with the deletion mutants. In the GST-pulldown assay RSK2(1–389) preferentially bound to TIA-1 compared to RSK2(373–741) (Fig. 4C). To further study localization of the mutants we generated C-terminal tagged mCherry fusion proteins, which are too large to diffuse into the nucleus. In agreement with the binding studies, the C-terminal domain was not targeted to stress granules in response to stress (Fig. 4D). In contrast, RSK2(1–389) localized to stress granules like wild type RSK2 (Fig. 4D). We conclude that the region of RSK2 from amino acids 1 to 389 is responsible for targeting RSK2 to stress granules.
NTKD activity is important in targeting to stress granules because SL0101, which inhibits NTKD activity, decreased the amount of RSK2 present in stress granules (Fig. 3D). To understand how NTKD activity regulates RSK2 sequestration we tested whether kinase dead, recombinant RSK2 was able to interact with TIA-1 in an in vitro binding assay. Deactivation of recombinant RSK2 by addition of protein phosphatase 2A (Sturgill et al., 1988) eliminated the interaction between RSK2 and TIA-1 (Fig. 4A). Additionally, we prepared lysates from MCF-7 cells transfected with wild type RSK2 or a RSK2 mutant, which has the essential Lys in the catalytic domain of the NTKD altered to Ala (RSK2(K100A)). The interaction of RSK2(K100A) with recombinant TIA-1 was substantially lower than that for the wild type (Fig. 4E). Consistent with these observations, RSK2(K100A)-mCherry fusion did not localize to stress granules (Fig. 4D). As a further control we determined that stress granule formation was not disrupted by RSK2(K100A) and RSK2(373–741) (Fig. S2C), and therefore, these mutants do not act as dominant negatives. We conclude that the NTKD catalytic activity regulates the interaction of RSK2 with TIA-1.
Although the NTKD is important for targeting RSK2 to stress granules, RSK2(1–389)-mCherry did not accumulate in the nucleus in response to mitogen, even though it was released from stress granules (Fig. 4D). Strikingly, however the CTKD-mCherry fusion was dispersed throughout the nucleus and cytoplasm, even in stressed cells. Because the fusion protein is too large to diffuse passively through the nuclear pore complex, we conclude that the region of RSK2 from amino acids 373 to 741 contains a sequence essential for RSK2 nuclear-cytoplasmic shuttling. NTKD kinase activity is not important for shuttling, as RSK2(K100A) distributed between the cytoplasm and nucleus in the presence of mitogen, though it did not accumulate in the nucleus. Together, these data reveal previously unknown functions for both the N- and C-terminal domains of RSK2, and provide a mechanism by which RSK2 localization is controlled.
RSK2 Regulates Cyclin D1 mRNA Levels
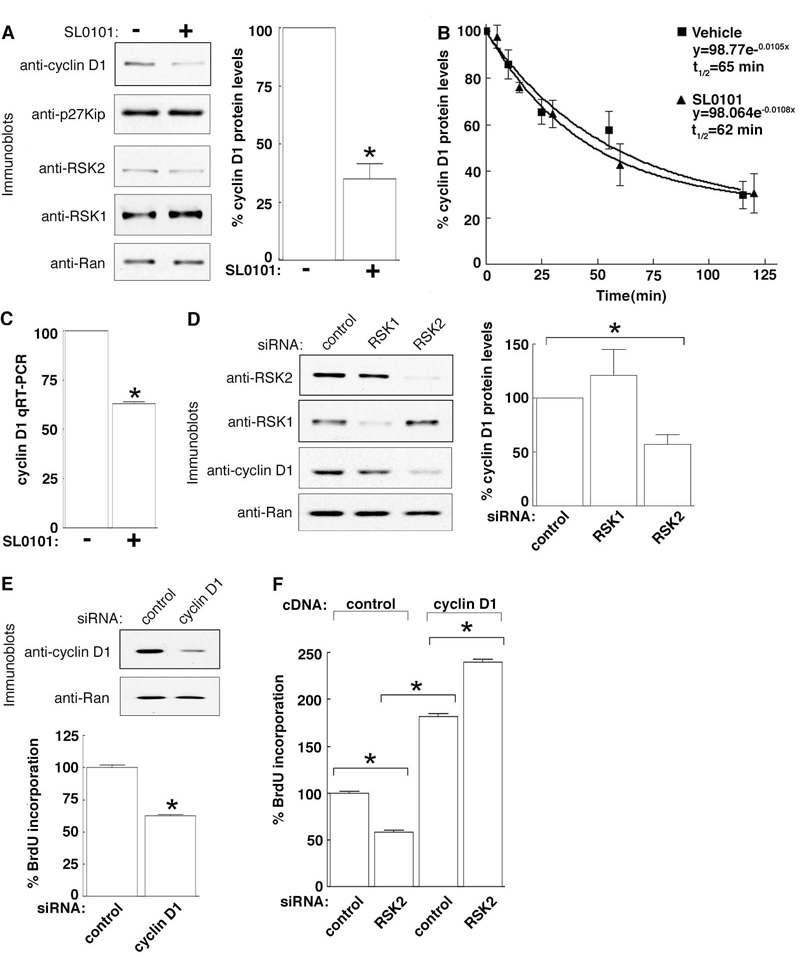
Previously, we identified that treatment of MCF-7 cells with SL0101 produced a cell cycle block in G1 (Smith et al., 2005). We investigated whether there is a connection between the control of proliferation by RSK2 and its association with stress granules. To identify how RSK2 regulates proliferation we analyzed the expression levels of the G1 cell cycle regulators, cyclin D1 and p27Kip. We found that SL0101 reduced cyclin D1 expression in MCF-7 cells by ~ 70%, but did not significantly alter the level of p27Kip (Fig. 5A). RSK does not regulate cyclin D1 protein stability, because the t1/2 for cyclin D1 degradation in MCF-7 cells was ~65 mins in the presence or absence of SL0101 (Fig. 5B). However, SL0101 did decrease cyclin D1 mRNA levels by ~40% compared to the vehicle, as determined using quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) (Fig. 5C) or by RNase protection assay (Fig. S3A). SL0101 inhibits the kinase activity of RSK1 and RSK2 in in vitro kinase assays (Smith et al., 2005), so to determine if one of these isoforms preferentially regulates cyclin D1 expression we silenced RSK1 and RSK2. Knockdown of RSK2 reduced cyclin D1 levels by ~ 50% (Fig. 5D). Conversely, ectopically expressed RSK2 increased cyclin D1 ~ 2.5X (Fig. S3B). However, silencing RSK1 expression did not significantly alter cyclin D1 levels. Together, these results demonstrate that the RSK2 isoform is primarily responsible for the maintenance of cyclin D1 mRNA levels in MCF-7 cells. Silencing cyclin D1 in MCF-7 cells, the S-phase population decreased by ~40% (Fig. 5E), suggesting that control of cyclin D1 levels might be important to the mechanism by which RSK2 regulates proliferation.
Figure 5. RSK2 regulates cyclin D1 mRNA levels.
(A) MCF-7 cells treated with or without SL0101 (4 h). Columns, mean (n=3); bars, SEM, p< 0.005. (B) Rate of cyclin D1 protein degradation in MCF-7 cells with cycloheximide and pretreated with vehicle or SL0101 (4 h). Symbols, mean (n=2, triplicate), SEM. (C) Cells treated as in (A), RNA extracted and a qRT-PCR assay performed. Columns, mean (n=3); bars, SEM, p<0.0001. (D) MCF-7 cells transfected with control, RSK1- or RSK2-specific siRNA. Columns, mean (n=3); bars, SEM, p<0.05. (E) MCF-7 cells transfected with control or cyclin D1-specific siRNA. Columns, mean (n=2, 16 replicates); bars, SEM, p< 0.0001. (F) MCF-7 cells transfected with control or RSK2-specific siRNA followed by a second transfection with control or cyclin D1 vector. Columns, mean (n=2, 16 replicates); bars, SEM, p <0.0001.
To test whether cyclin D1 is an important target for control of proliferation by RSK2, we asked whether the ectopic expression of cyclin D1 would reverse the drop in proliferation caused by reduced RSK2 levels. MCF-7 cells were transfected with RSK2-specific or control siRNA, then transfected a second time with a vector encoding cyclin D1 or a vector control. In the absence of ectopically expressed cyclin D1, silencing RSK2 decreased the S-phase population to ~50% of the control (Fig. 5F). As expected, over-expression of cyclin D1 increased the number of control cells in S-phase by ~ 1.7X (Grillo et al., 2006). Importantly, the forced expression of cyclin D1 after RSK2 knockdown not only prevented the decrease in S-phase but even increased the number of cells in S-phase. We conclude, therefore, that cyclin D1 is an important target for the control of proliferation by RSK2.
Spatial Regulation Controls RSK2 Function
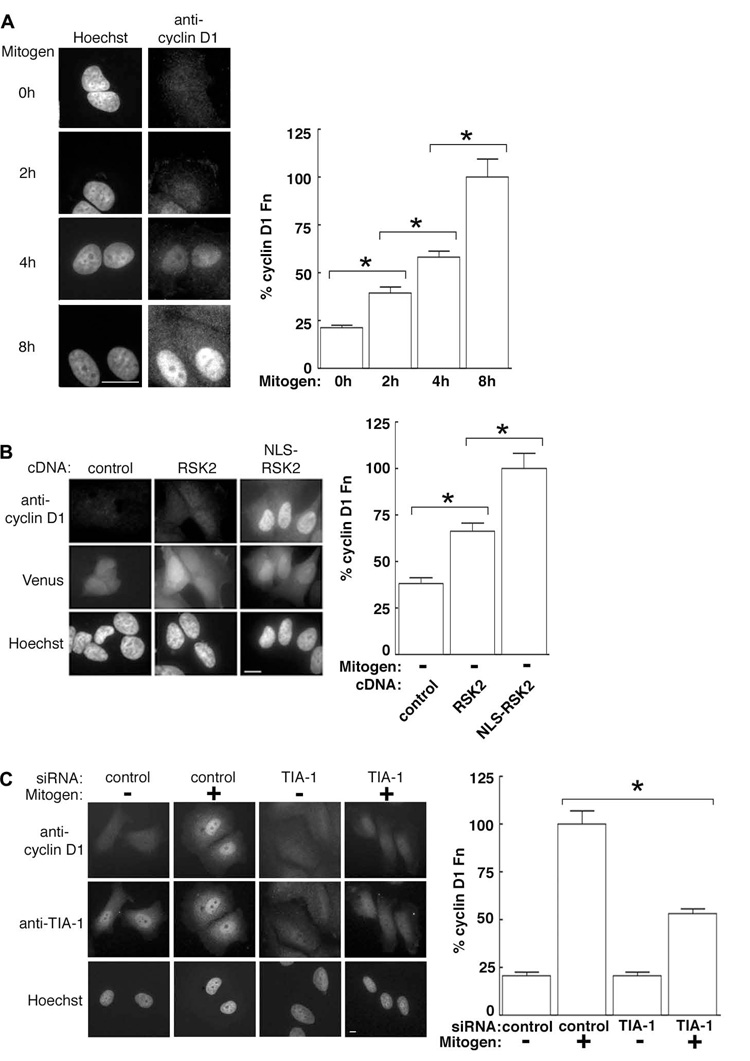
Nuclear accumulation of RSK2 occurred over a 4–8 h period, and this increase in nuclear RSK2 paralleled an elevation in cyclin D1 levels (Fig. 6A). Moreover, ectopic expression of wild type RSK2, which elevates RSK2 in both the cytoplasm and nucleus, raised cyclin D1 levels in serum-starved MCF-7 cells by ~ 75% as compared to the control. However, the expression of NLS-RSK2 enhanced the amount of cyclin D1 by ~ 3X (Fig. 6B). The amounts of expressed RSK2 and NLS-RSK2 were similar (Fig. S3C). Remarkably, therefore, nuclear translocation of RSK2, in the absence of activation of any other signaling pathway, can induce cyclin D1 expression in MCF-7 cells. Based on these results we predicted that silencing TIA-1 would decrease cyclin D1 levels, because loss of TIA-1 reduces RSK2 nuclear translocation. Consistent with this hypothesis we found that TIA-1 depletion caused a significant drop in the level of cyclin D1 (Fig. 6C). Together, these data demonstrate that TIA-1 regulates RSK2 function by controlling its localization.
Figure 6. Nuclear RSK2 controls proliferation by regulating cyclin D1.
(A) Serum-starved MCF-7 cells treated with mitogen for various times and stained with a mouse monoclonal anti-cyclin D1 antibody. Columns, mean (n=2, ≥ 13 cells/condition); bars, SEM, p< 0.0001. (B) Serum-starved MCF-7 cells co-transfected with Venus and RSK2, NLS-RSK2 or control. Venus was used as a marker of transfection. Columns, mean (n=2, ≥12 cells/condition); bars, SEM, p< 0.0001. (C) MCF-7 cells transfected with control or TIA-1-specific siRNA. Columns, mean (n=2, ≥21 cells/condition); bars, SEM, p<0.0001.
RSK2 Enhances Cell Survival in Response to Oxidative Stress
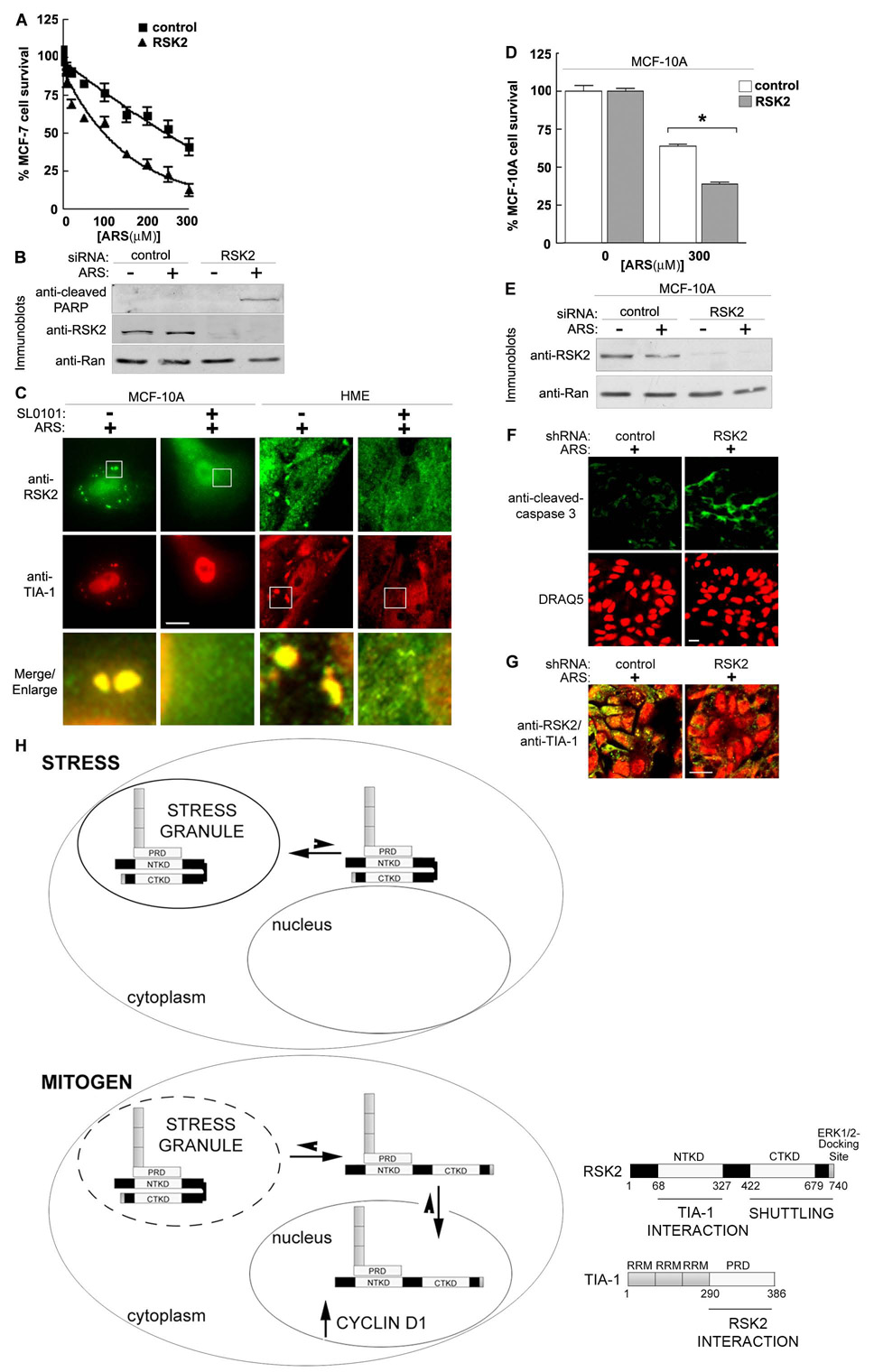
Because we observed that silencing RSK2 reduces stress granule formation we investigated whether RSK2 is important for cell survival in response to stress. To test this hypothesis we silenced RSK2 and treated MCF-7 cells with varying doses of arsenite, then measured cell viability and an apoptotic marker. The inhibitory arsenite concentration (IC50) for wild type cells was ~250 µM, while for cells depleted of RSK2 the IC50 was ~100 µM (Fig. 7A). Thus, with increasing oxidative stress, MCF-7 cell survival is decreased by ~ 2.5X by silencing RSK2 expression. This decrease in cell viability is due to an increase in apoptosis, as shown by the increased levels of cleaved poly ADP ribose polymerase (PARP) in arsenite-treated RSK2 knockdown cells (Fig. 7B). In addition, consistent with the observations in MCF-7 cells, treatment of MCF-10A cells or primary HME cells with SL0101 inhibited stress granule formation (Fig. 7C). Silencing RSK2 in MCF-10A cells also decreased cell survival in response to oxidative stress (Figs. 7D, 7E). Furthermore, knockdown of RSK2 in stressed HME cells grown in 3D culture led to an increase in apoptosis, as shown by the increased levels of cleaved-caspase-3 (Figs. 7F, 7G). Taken together, these results argue that RSK2 plays an integral role in the stress response.
Figure 7. RSK2 regulates cell survival in response to stress.
(A) MCF-7 cells were transfected with control or RSK2-specific siRNA. The transfected cells were treated with various doses of ARS (48 h) and cell viability measured. The data was normalized to 100% in the absence of ARS. Symbols, mean (n=2 in quadruplicate); bars, SEM. (B) MCF-7 cells treated as in (A) using 20 µM ARS. (C) ARS-treated MCF-10A and primary HME cells treated with or without SL0101 (4 h). (D) MCF-10A cells transfected and analyzed as in (A) with or without 300 µM ARS. Columns, mean (n=2, quadruplicate); bars, SEM., p<0.0001. E) MCF-10A cells treated as in (D). (F) HME cells were transduced with control or RSK2 lentiviral-based shRNA, treated with ARS (500 µM; 1h) and stained with anti-cleaved caspase-3. DNA stained with DRAQ5. (G) HME cells treated as in (F) showing the merge of anti-RSK2 and anti-TIA-1 staining. (H) Schematic showing the regulation of RSK2 subcellular localization. See text for details.
Discussion
Stress granule formation is a fundamental component of the organismal response to environmental stress (e.g., oxidative conditions, heat, UV irradiation, hypoxia). In response to these stresses, translation of selective mRNAs is halted by their retention into stress granules, to facilitate cell survival (Anderson and Kedersha, 2006). We propose that RSK2 facilitates stress granule assembly to repress translation and to enhance cell survival (Fig. 7H). This model is based on our observation that RSK2 regulates sequestration of TIA-1, which is critical for stress granule formation (Gilks et al., 2004). In addition RSK2 expression is required for the organization of PABP1, a stress granule marker, into the granules. Dispersal of RSK2 throughout the cytoplasm by cycloheximide is also consistent with RSK2 association with stress granules. Moreover, RSK2 has been found to be associated with polyribosomes (Angenstein et al., 1998), and components of polyribosomes and stress granules have been proposed to exist in equilibrium with each other (Kedersha et al., 2005). RSK2 regulation of stress granule formation is physiologically important because silencing RSK2 decreased cell survival through the apoptotic pathway in response to stress. Therefore, RSK2 sequestration into stress granules is an integral component for cell survival and not simply a mechanism to control RSK2 activity.
The NTKD of RSK2 is responsible for direct interaction with the PRD of TIA-1. This conclusion is based on the observations that RSK2 directly interacts with the TIA-1 PRD in an in vitro binding assay using recombinant proteins, and that an inactivating point mutation in the RSK2 NTKD inhibits its interaction with TIA-1. Furthermore, the isolated RSK2 N-terminal domain binds to TIA-1 in an in vitro binding assay and is sequestered into stress granules.
RSK2 may serve as a scaffold for stress granule assembly, because TIA-1 association with granules is blocked more efficiently by silencing RSK2 than by just inhibiting its kinase activity. Thus, the amount of RSK2, as well as its kinase activity, is important in stress granule assembly. Consistent with a scaffolding function for RSK2 is the observation that RSK2 is stably sequestered in stress granules. Moreover, a kinase-dead RSK2 mutant does not interact with TIA-1 and does not go to stress granules, suggesting that sequestration of RSK2 is regulated by NTKD catalytic activity. However, it is unlikely that the interaction between RSK2 and TIA-1 is regulated by direct RSK2 phosphorylation of TIA-1, as it does not contain RSK2 consensus phosphorylation sites. It is more probable that the interaction with TIA-1 is controlled by conformational differences between the active and inactive forms of the NTKD.
In response to mitogen treatment RSK2 is slowly released from stress granules and upon release is able to shuttle rapidly in and out of the nucleus. RSK2 does not contain a classical polybasic NLS and the mechanism of RSK2 translocation has not been previously examined. We have discovered that the sequence responsible for RSK2 nuclear-cytoplasmic shuttling is within the C-terminal domain. This domain contains the CTKD and the ERK1/2 docking site. It is unlikely that ERK1/2 is involved in RSK2 nuclear translocation because activated ERK1/2 is released from RSK (Roux et al., 2003). Curiously, RSK2 nuclear accumulation is dependent on TIA-1, but it is the N-terminal domain that interacts with TIA-1, and the isolated N-terminal domain is not able to shuttle into the nucleus. These data are consistent with the idea that the C-terminal domain associates with a nucleo-cytoplasmic shuttling protein but that TIA-1 enhances RSK2 nuclear accumulation, by interacting with a nuclear binding partner. Evidence for this hypothesis is provided by the fact that the kinase-dead RSK2 translocates in response to mitogen but neither accumulates in the nucleus nor binds TIA-1. TIA-1 does not contain a classical NLS (Zhang et al., 2005) and, therefore, it is likely that both RSK2 and TIA-1 piggyback into the nucleus in association with an NLS-containing protein. However, TIA-1 import can also occur independently of RSK2 because altering the levels of RSK2 does not alter TIA-1 nuclear accumulation. We also propose that the NTKD regulates shuttling of the C-terminal domain by controlling its interaction with an NLS-containing protein. This suggestion is supported by the observations that the kinase-dead RSK2 mutant does not translocate in stressed cells even though it is dispersed in the cytoplasm; whereas, the isolated C-terminal domain is able to shuttle. In summary, we conclude that the RSK2 C-terminal domain contains a nuclear-cytoplasmic shuttling sequence but that the NTKD controls translocation by regulating the binding of an NLS-containing protein and also determines nuclear accumulation via its interaction with TIA-1.
In the nuclei of MCF7 cells, cyclin D1 is a critical RSK2 target for regulation of proliferation. This reasoning is based on the following data: (1) silencing or inhibition of RSK2 reduces cyclin D1 levels and proliferation; (2) ectopic expression of cyclin D1 prevents the inhibition of proliferation resulting from knockdown of RSK2; and (3) forced nuclear localization of RSK2 increases cyclin D1 levels in the absence of activating any other signaling pathway. The CCND1 (cyclin D1 gene) promoter is regulated by the transcription factor, CREB, and RSK was earlier proposed to activate CREB. However, CREB does not appear to be a physiological substrate for RSK (Sapkota et al., 2007; Wiggin et al., 2002). In agreement with the literature, inhibition of RSK did not alter basal or the mitogen-induced increase in CREB phosphorylation (Fig. S3D). Furthermore, NLS-RSK2 did not increase phosphorylation of CREB (Fig. S3E). The transcription factor, c-fos, a RSK substrate is known to regulate the CCND1 promoter. However, although RSK phosphorylation does contribute to the stabilization of the c-fos protein, ERK1/2 phosphorylation is required for further stabilization and for c-fos activation (Murphy et al., 2002). Importantly, NLS-RSK2, in the absence of active ERK1/2, is able to stimulate cyclin D1 levels. Therefore, it is unlikely that the RSK2-induced increase in cyclin D1 is mediated by c-fos. Thus, our data are the first to establish a specific function for RSK2 in connection with cyclin D1 regulation. We conclude that RSK2 is a pivotal regulatory factor linking the stress response to survival and ultimately to proliferation through its association with TIA-1.
We have found that RSK2 is important for cell survival in response to stress. We hypothesize that RSK2 protects cells through its control of stress granule formation. This conclusion is supported by our observations that silencing RSK2 decreases cell survival and stress granule formation. Stress granules represent an ancient mechanism in eukaryotes for the post-translational regulation of mRNA. For example, stress granule-like mRNA granules have been shown to form in the unicellular eukaryote, trypanosome, when in the intestinal tract of the insect vector or in starvation conditions in culture (Cassola et al., 2007). A number of human viral pathogens inhibit or induce stress granule formation (McInerney et al., 2005; White et al., 2007; Smith et al., 2006; Emara and Brinton, 2007). The difference between how these viruses alter the host’s stress response is related to their particular replication requirements. Stress granules have also been shown to form in vivo in hypoxic areas within tumors and are thought to contribute to the radioresistance of the tumor vasculature (Moeller et al., 2004). Additionally, the persistence of stress granule formation in the hippocampal cornu ammonis 1 neurons is thought to prevent their recovery in in vivo stroke models (DeGracia et al., 2007). Stress granules have also been found in muscle biopsies of patients with sporadic inclusion body myositis, an inflammatory muscle disease, and not in the controls; but the causal relationship of these stress granules to the disease is unknown (Nakano et al., 2005). There is also in vivo evidence demonstrating that inhibition of stress granule formation decreases organism survival in response to stress (McEwen et al., 2005). Thus there is substantial evidence for the physiological significance of stress granules and we provide the first evidence that RSK2 may play a fundamental role in regulating the response of an organism to stress.
Experimental Procedures
Cell culture
Information on culturing cell lines and primary HME cells is in the Supplementary Materials.
Immunodetection
Cells were lysed (Joel et al., 1998). Primary antibodies used were monoclonal anti-RSK2 (C-21), rabbit anti-RSK1 (C-21), goat anti-TIA-1 (C-20) (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-GFP (Venus) (Invitrogen, Carlsbad, CA), monoclonal anti-Ran (BD Biosciences, San Jose, CA), monoclonal anti-cyclin D1, rabbit anti-cleaved PARP and rabbit anti-p27Kip (Cell Signaling Technology, Danvers, MA). Immunoblots were analyzed and quantitated by densitometry (Joel et al., 1998). p values determined using Student’s t-test.
Quantitative RT-PCR
Total RNA was isolated using RNeasy (Qiagen Inc, Valencia, CA). Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) was done on an ABI PRISM 7900 HT sequence detection system (Applied Biosystems, Foster City, CA) using SYBR® Green detection. Cyclin D1 mRNA levels were normalized to the geometric mean of GAPDH, β-Actin, and α-Tubulin mRNA levels. p values determined using Student’s t-test.
Transient and stable transfections
MCF-7 cells were transfected as described (Smith et al., 2005). siGENOME SMARTpool short interfering RNA and the siCONTROL nontargeting siRNA1 (Dharmacon Research, Inc., Lafayette, CO) were used for gene silencing. MCF-7 lines stably expressing Venus-HA-RSK2 (Venus is super-enhanced yellow fluorescent protein containing the following mutations: F46L, M153T, V163A, S175G) or HA-RSK2 were generated by G418 selection (600 ng/ µL). HME cells were transduced with lentivirus expressing RSK2 or control shRNA.
Plasmids
The plasmids, pKH3-RSK2 and pKH3-RSK2(K100A) have been described (Smith et al., 1999; Clark et al., 2001). The constructs, pMT2-HA-TIA-1 and pMT2-HA-TIA-1ΔRRM, were generously provided by Paul Anderson (Brigham and Women’s Hospital). Additional constructs are described in Supplementary Materials.
BrdU (Bromodeoxyuridine) incorporation assay
BrdU incorporation was performed with Cell Proliferation Biotrak ELISA System, v2 (Amersham Biosciences, Piscataway, NJ) on an Elx 800 Automated Microplate Reader (Bio-Tek Winooski, VT).
Immunostaining
Primary antibodies used were monoclonal anti-RSK2 (C-21), goat anti-RSK2 (C-19) and goat anti-TIA-1 (C-20) (Santa Cruz Biotechnology, Santa Cruz, CA), monoclonal anti-cyclin D1, rabbit anti-PABP1 and rabbit anti-cleaved caspase-3 (Asp 175) (Cell Signaling Technology, Danvers, MA). AlexaFluor fluorescent secondary antibodies (Invitrogen, Carlsbad, CA) and a Cy3-labeled donkey anti-goat antibody (Jackson ImmunoResearch, West Grove, PA) were also used. DNA was stained using Hoechst dye (Sigma-Aldrich, St. Louis, MO) or DRAQ5 (Axxora, San Diego, CA). Cells were examined using an upright Eclipse E800 microscope (Nikon, Tokyo, Japan) equipped with a 40× (NA 1.3) or 60× (NA 1.4) oil immersion lens and an Orca C4742-95 charge-coupled device camera (Hamamatsu Corporation, Bridgewater, NJ) using Openlab 3.1.4 software (Improvision Inc, Lexington MA). For confocal images a 510/Meta/FCS laser-scanning microscope (Carl Zeiss, Thornwood, NY) and a 100× oil immersion lens (1.3 NA) were used. Images were manipulated in Adobe Photoshop version 7.0 (Adobe systems, Mountain View, CA). Images were quantitated using Openlab 3.1.4 software. Scale bars represent 10 µm. p values determined using Student’s t-test.
Live cell imaging
Images were captured with a 488-nm laser line at 75% power, and 0.1% transmission. For FRAP, regions of interest were selected and bleached with the 488-nm laser line, at 75% power, 100% transmission for 7 iterations. Recovery was monitored for at least 150 seconds. FLIP and FRAP imaging and bleaching settings were identical. To measure loss of nuclear fluorescence the entire cytoplasm was reiteratively bleached. Half-life analysis was performed with GraphPad Prism software (GraphPad Software, Inc. San Diego, CA). Images were processed with Adobe Photoshop version 7.0. Scale bars represent 10 µm.
In vitro binding assay
GST, GST-TIA-1, or GST-TIA-1ΔRRM protein (1 µg) in 10 mM HEPES, pH 7.4, 1 mM EDTA, and 10 mM KCl were incubated (1 h; 4 °C) with 25 ng purified, recombinant RSK2. Alternatively, lysates from ~ 1×104 MCF-7 cells were used in the binding assay. Recombinant (His)6-tagged RSK2 was purified as described (Smith et al., 2005). Magnetic glutathione beads (100 µl) (Bioclone Inc., San Diego, CA) were washed with 1% BSA in phosphate-buffered saline and added to the incubation (1 h, 4 °C). The washed beads and supernatant were processed for Western analysis.
Protein turnover measurements
Cells were pretreated with 100 µM SL0101 or DMSO for 4h, followed by 50 µM cycloheximide for 20 min. Immunoblots of cell lysates were quantitated by densitometry. Cyclin D1 protein half-life was determined using GraphPad Prism software.
Supplementary Material
Acknowledgements
This work was supported by GM084386 (D.A.L.), GM37537 (D.F.H.), GM50526 (I.G.M.), T32CA009109-30 (T.S.K.E.), Patients and Friends of the UVa Cancer Center (D.A.L.), Swing Fore the Cure (D.A.L.), Virginia Kincaid Cancer Research Fund (D.A.L.) and the James and Rebecca Craig Foundation (I.G.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson P, Kedersha N. RNA granules. J Cell Biol. 2006;172:803–808. doi: 10.1083/jcb.200512082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angenstein F, Greenough WT, Weiler IJ. Metabotropic glutamate receptor-initiated translocation of protein kinase p90rsk to polyribosomes: a possible factor regulating synaptic protein synthesis. Proc Natl Acad Sci U S A. 1998;95:15078–15083. doi: 10.1073/pnas.95.25.15078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignone PA, Lee KY, Liu Y, Emilion G, Finch J, Soosay AE, Charnock FM, Beck S, Dunham I, Mungall AJ, Ganesan TS. RPS6KA2, a putative tumour suppressor gene at 6q27 in sporadic epithelial ovarian cancer. Oncogene. 2007;26:683–700. doi: 10.1038/sj.onc.1209827. [DOI] [PubMed] [Google Scholar]
- Cassola A, De Gaudenzi JG, Frasch AC. Recruitment of mRNAs to cytoplasmic ribonucleoprotein granules in trypanosomes. Mol Microbiol. 2007;65:655–670. doi: 10.1111/j.1365-2958.2007.05833.x. [DOI] [PubMed] [Google Scholar]
- Cavet ME, Lehoux S, Berk BC. 14-3-3beta is a p90 ribosomal S6 kinase (RSK) isoform 1-binding protein that negatively regulates RSK kinase activity. J Biol Chem. 2003;278:18376–18383. doi: 10.1074/jbc.M208475200. [DOI] [PubMed] [Google Scholar]
- Chaturvedi D, Poppleton HM, Stringfield T, Barbier A, Patel TB. Subcellular localization and biological actions of activated RSK1 are determined by its interactions with subunits of cyclic AMP-dependent protein kinase. Mol Cell Biol. 2006;26:4586–4600. doi: 10.1128/MCB.01422-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RH, Sarnecki C, Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrestensen CA, Sturgill TW. Characterization of the RSK2 carboxy-terminal domain as a protein kinase. J Biol Chem. 2002;16:16. doi: 10.1074/jbc.M202663200. [DOI] [PubMed] [Google Scholar]
- Clark DE, Poteet-Smith CE, Smith JA, Lannigan DA. Rsk2 allosterically activates estrogen receptor alpha by docking to the hormone-binding domain. Embo J. 2001;20:3484–3494. doi: 10.1093/emboj/20.13.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cude K, Wang Y, Choi HJ, Hsuan SL, Zhang H, Wang CY, Xia Z. Regulation of the G2-M cell cycle progression by the ERK5-NFkappaB signaling pathway. J Cell Biol. 2007;177:253–264. doi: 10.1083/jcb.200609166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby KN, Morrice N, Caudwell FB, Avruch J, Cohen P. Identification of regulatory phosphorylation sites in mitogen-activated protein kinase (MAPK)-activated protein kinase-1a/p90rsk that are inducible by MAPK. J Biol Chem. 1998;273:1496–1505. doi: 10.1074/jbc.273.3.1496. [DOI] [PubMed] [Google Scholar]
- DeGracia DJ, Rudolph J, Roberts GG, Rafols JA, Wang J. Convergence of stress granules and protein aggregates in hippocampal cornu ammonis 1 at later reperfusion following global brain ischemia. Neuroscience. 2007;146:562–572. doi: 10.1016/j.neuroscience.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufresne SD, Bjorbaek C, El-Haschimi K, Zhao Y, Aschenbach WG, Moller DE, Goodyear LJ. Altered extracellular signal-regulated kinase signaling and glycogen metabolism in skeletal muscle from p90 ribosomal S6 kinase 2 knockout mice. Mol Cell Biol. 2001;21:81–87. doi: 10.1128/MCB.21.1.81-87.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham-Ems SM, Pudavar HE, Myers JM, Maher PA, Prasad PN, Stachowiak MK. Factors controlling fibroblast growth factor receptor-1's cytoplasmic trafficking and its regulation as revealed by FRAP analysis. Mol Biol Cell. 2006;17:2223–2235. doi: 10.1091/mbc.E05-08-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emara MM, Brinton MA. Interaction of TIA-1/TIAR with West Nile and dengue virus products in infected cells interferes with stress granule formation and processing body assembly. Proc Natl Acad Sci U S A. 2007;104:9041–9046. doi: 10.1073/pnas.0703348104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, Anderson P. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell. 2004;15:5383–5398. doi: 10.1091/mbc.E04-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo M, Bott MJ, Khandke N, McGinnis JP, Miranda M, Meyyappan M, Rosfjord EC, Rabindran SK. Validation of cyclin D1/CDK4 as an anticancer drug target in MCF-7 breast cancer cells: Effect of regulated overexpression of cyclin D1 and siRNA-mediated inhibition of endogenous cyclin D1 and CDK4 expression. Breast Cancer Res Treat. 2006;95:185–194. doi: 10.1007/s10549-005-9066-y. [DOI] [PubMed] [Google Scholar]
- Hauge C, Frodin M. RSK and MSK in MAP kinase signalling. J Cell Sci. 2006;119:3021–3023. doi: 10.1242/jcs.02950. [DOI] [PubMed] [Google Scholar]
- Joel PB, Traish AM, Lannigan DA. Estradiol-Induced Phosphorylation of serine 118 In the estrogen receptor is independent of p42/p44 mitogen-activated protein kinase. J Biol Chem. 1998;273:13317–13323. doi: 10.1074/jbc.273.21.13317. [DOI] [PubMed] [Google Scholar]
- Kedersha N, Chen S, Gilks N, Li W, Miller IJ, Stahl J, Anderson P. Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol Biol Cell. 2002;13:195–210. doi: 10.1091/mbc.01-05-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Cho MR, Li W, Yacono PW, Chen S, Gilks N, Golan DE, Anderson P. Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J Cell Biol. 2000;151:1257–1268. doi: 10.1083/jcb.151.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fitzler MJ, Scheuner D, Kaufman RJ, Golan DE, Anderson P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Biol. 2005;169:871–884. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha NL, Gupta M, Li W, Miller I, Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol. 1999;147:1431–1442. doi: 10.1083/jcb.147.7.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez de Silanes I, Galban S, Martindale JL, Yang X, Mazan-Mamczarz K, Indig FE, Falco G, Zhan M, Gorospe M. Identification and functional outcome of mRNAs associated with RNA-binding protein TIA-1. Mol Cell Biol. 2005;25:9520–9531. doi: 10.1128/MCB.25.21.9520-9531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen E, Kedersha N, Song B, Scheuner D, Gilks N, Han A, Chen JJ, Anderson P, Kaufman RJ. Heme-regulated inhibitor kinase-mediated phosphorylation of eukaryotic translation initiation factor 2 inhibits translation, induces stress granule formation, and mediates survival upon arsenite exposure. J Biol Chem. 2005;280:16925–16933. doi: 10.1074/jbc.M412882200. [DOI] [PubMed] [Google Scholar]
- McInerney GM, Kedersha NL, Kaufman RJ, Anderson P, Liljestrom P. Importance of eIF2alpha phosphorylation and stress granule assembly in alphavirus translation regulation. Mol Biol Cell. 2005;16:3753–3763. doi: 10.1091/mbc.E05-02-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Murphy LO, Smith S, Chen RH, Fingar DC, Blenis J. Molecular interpretation of ERK signal duration by immediate early gene products. Nat Cell Biol. 2002;4:556–564. doi: 10.1038/ncb822. [DOI] [PubMed] [Google Scholar]
- Nakano S, Shinde A, Ito H, Kusaka H. Messenger RNA degradation may be inhibited in sporadic inclusion body myositis. Neurology. 2005;65:420–425. doi: 10.1212/01.wnl.0000171341.76482.15. [DOI] [PubMed] [Google Scholar]
- Poirier R, Jacquot S, Vaillend C, Soutthiphong AA, Libbey M, Davis S, Laroche S, Hanauer A, Welzl H, Lipp HP, Wolfer DP. Deletion of the Coffin-Lowry syndrome gene Rsk2 in mice is associated with impaired spatial learning and reduced control of exploratory behavior. Behav Genet. 2007;37:31–50. doi: 10.1007/s10519-006-9116-1. [DOI] [PubMed] [Google Scholar]
- Richards SA, Dreisbach VC, Murphy LO, Blenis J. Characterization of regulatory events associated with membrane targeting of p90 ribosomal S6 kinase 1. Mol Cell Biol. 2001;21:7470–7480. doi: 10.1128/MCB.21.21.7470-7480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards SA, Fu J, Romanelli A, Shimamura A, Blenis J. Ribosomal S6 kinase 1 (RSK1)activation requires signals dependent on and independent of the MAP kinase ERK. Curr Biol. 1999;9:810–820. doi: 10.1016/s0960-9822(99)80364-9. [DOI] [PubMed] [Google Scholar]
- Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, Richards SA, Blenis J. Phosphorylation of p90 ribosomal S6 kinase (RSK) regulates extracellular signal-regulated kinase docking and RSK activity. Mol Cell Biol. 2003;23:4796–4804. doi: 10.1128/MCB.23.14.4796-4804.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sananbenesi F, Fischer A, Schrick C, Spiess J, Radulovic J. Phosphorylation of hippocampal Erk-1/2, Elk-1, and p90-Rsk-1 during contextual fear conditioning: interactions between Erk-1/2 and Elk-1. Mol Cell Neurosci. 2002;21:463–476. doi: 10.1006/mcne.2002.1188. [DOI] [PubMed] [Google Scholar]
- Sapkota GP, Cummings L, Newell FS, Armstrong C, Bain J, Frodin M, Grauert M, Hoffmann M, Schnapp G, Steegmaier M, et al. BI-D1870 is a specific inhibitor of the p90 RSK (ribosomal S6 kinase) isoforms in vitro and in vivo. Biochem J. 2007;401:29–38. doi: 10.1042/BJ20061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Poteet-Smith CE, Malarkey K, Sturgill TW. Identification of an extracellular signal-regulated kinase (ERK) docking site in ribosomal S6 kinase, a sequence critical for activation by ERK in vivo. J Biol Chem. 1999;274:2893–2898. doi: 10.1074/jbc.274.5.2893. [DOI] [PubMed] [Google Scholar]
- Smith JA, Poteet-Smith CE, Xu Y, Errington TM, Hecht SM, Lannigan DA. Identification of the first specific inhibitor of p90 Ribosomal S6 Kinase (RSK) reveals an unexpected role for RSK in cancer cell proliferation. Cancer Res. 2005;65:1027–1034. [PubMed] [Google Scholar]
- Smith JA, Schmechel SC, Raghavan A, Abelson M, Reilly C, Katze MG, Kaufman RJ, Bohjanen PR, Schiff LA. Reovirus induces and benefits from an integrated cellular stress response. J Virol. 2006;80:2019–2033. doi: 10.1128/JVI.80.4.2019-2033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloaga A, Thomson S, Wiggin GR, Rampersaud N, Dyson MH, Hazzalin CA, Mahadevan LC, Arthur JS. MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14. Embo J. 2003;22:2788–2797. doi: 10.1093/emboj/cdg273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill TW, Ray LB, Erikson E, Maller JL. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988;334:715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- Tourriere H, Chebli K, Zekri L, Courselaud B, Blanchard JM, Bertrand E, Tazi J. The RasGAP-associated endoribonuclease G3BP assembles stress granules. J Cell Biol. 2003;160:823–831. doi: 10.1083/jcb.200212128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Vaidyanathan H, Ramos JW. RSK2 activity is regulated by its interaction with PEA-15. J Biol Chem. 2003;278:32367–32372. doi: 10.1074/jbc.M303988200. [DOI] [PubMed] [Google Scholar]
- Watson K, Fan GH. Macrophage inflammatory protein 2 inhibits beta-amyloid peptide (1–42)-mediated hippocampal neuronal apoptosis through activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase signaling pathways. Mol Pharmacol. 2005;67:757–765. doi: 10.1124/mol.104.004812. [DOI] [PubMed] [Google Scholar]
- White JP, Cardenas AM, Marissen WE, Lloyd RE. Inhibition of cytoplasmic mRNA stress granule formation by a viral proteinase. Cell Host Microbe. 2007;2:295–305. doi: 10.1016/j.chom.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Wiggin GR, Soloaga A, Foster JM, Murray-Tait V, Cohen P, Arthur JS. MSK1 and MSK2 are required for the mitogen- and stress-induced phosphorylation of CREB and ATF1 in fibroblasts. Mol Cell Biol. 2002;22:2871–2881. doi: 10.1128/MCB.22.8.2871-2881.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard FS, Crouch MF. MEK, ERK, and p90RSK are present on mitotic tubulin in Swiss 3T3 cells: a role for the MAP kinase pathway in regulating mitotic exit. Cell Signal. 2001;13:653–664. doi: 10.1016/s0898-6568(01)00185-1. [DOI] [PubMed] [Google Scholar]
- Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, Li L, Brancorsini S, Sassone-Corsi P, Townes TM, et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell. 2004;117:387–398. doi: 10.1016/s0092-8674(04)00344-7. [DOI] [PubMed] [Google Scholar]
- Yntema HG, van den Helm B, Kissing J, van Duijnhoven G, Poppelaars F, Chelly J, Moraine C, Fryns JP, Hamel BC, Heilbronner H, et al. A novel ribosomal S6-kinase (RSK4;RPS6KA6) is commonly deleted in patients with complex X-linked mental retardation. Genomics. 1999;62:332–343. doi: 10.1006/geno.1999.6004. [DOI] [PubMed] [Google Scholar]
- Zhang T, Delestienne N, Huez G, Kruys v, Gueydan C. Identification of the sequence determinants mediating the nucleo-cytoplasmic shuttling of TIAR and TIA-1 RNA-binding proteins. J Cell Sci. 2005;118:5453–5463. doi: 10.1242/jcs.02669. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Bjorbaek C, Weremowicz S, Morton CC, Moller DE. RSK3 encodes a novel pp90rsk isoform with a unique N-terminal sequence: growth factor-stimulated kinase function and nuclear translocation. Mol Cell Biol. 1995;15:4353–4363. doi: 10.1128/mcb.15.8.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.