Abstract
Visual disappearance illusions, such as motion-induced blindness, are commonly used to study the neural correlates of visual perception. In such illusions a salient visual target becomes perceptually invisible. Previous studies are inconsistent regarding the role of early visual areas in these illusions. Here we provide physiological and psychophysical evidence suggesting a role for early visual areas in generating motion-induced blindness, and we provide a conceptual model by which different brain areas might contribute to the perceptual disappearance in this illusion.
Keywords: motion-induced blindness, perception, V1, physiology, illusion, bistable
Introduction
Some of the most striking visual illusions fall into the category of multistable phenomena. These are situations in which an unchanging stimulus generates alternating perceptual states; for example: Necker cube reversals, binocular rivalry, ambiguous structure from motion and motion-induced blindness (MIB) (Bonneh, Cooperman, & Sagi, 2001).
Motion-induced blindness is a striking phenomenon of visual disappearance in which a salient target becomes intermittently invisible when surrounded by a field of rotating distractors (Bonneh et al., 2001). In the first, perceptual, series of experiments we examined the necessity for a large, surface-inducing mask for generating target disappearances in MIB. The results of these experiments led us to do a second series of experiments to look physiologically at the responses of V1 cells in alert monkeys to the MIB stimulus. The observations in the physiological studies in turn led to testable perceptual predictions, which we explored in a third experiment.
Experiment series 1: Psychophysical evidence for a role for early visual areas in MIB
Several explanations have been proposed for the MIB illusion: a slowdown of the attentional switch (Bonneh et al., 2001), interhemispheric competition (Funk & Pettigrew, 2003), depth ordering, surface completion (Graf, Adams, & Lages, 2002) and perceptual filling-in (Hsu, Yeh, & Kramer, 2006), among others. All these explanations would require interactions at quite high levels in the visual system, where receptive fields are large and span the vertical midline. However we first wanted to see if changes in activity in early visual areas, where receptive fields are small, could account for at least some of the suppressive effects of the MIB mask. We therefore designed experiments to test whether local masks could also induce MIB.
Methods
Six naive observers participated in Experiment 1A and three naive observers and two authors (CL and TS) participated in Experiment 1B. All of them had normal or corrected-to-normal vision.
For stimulus presentation and data collection we used Matlab OpenGL psychtoolbox (Brainard, 1997; Pelli, 1997). Stimuli for Experiment 1A are shown in Figures 1A, 1B, and 1C. For the stimulus shown in Figure 1A the mask consisted of an array of 81 (9 × 9) blue crosses with a luminance of 19.4 cd/m2; the crosses formed a square 10° × 10°. We will refer to this mask as ‘full-field’. The mask rotated around the fixation spot at a speed of 0.25 revolutions per second (except for the human control experiment shown in Figure 3 where the mask rotated at 0.12 revolutions per second). The target was a yellow circle 0.1° in radius with a luminance of 111 cd/m2. The crosses were absent within a protection zone 0.4° in radius around the target. The stimulus shown in Figure 1B was the same as that in Figure 1A except that the rotating mask was present only within an annulus with an inner radius of 0.4° and an outer radius of 0.8°. The stimulus shown in Figure 1C consisted of the same target as in Figure 1A but the rotating mask was replaced by flashing bars 1° in length, 0.5° from the target. Two horizontal bars alternated with two vertical bars at 5 Hz.
Figure 1.
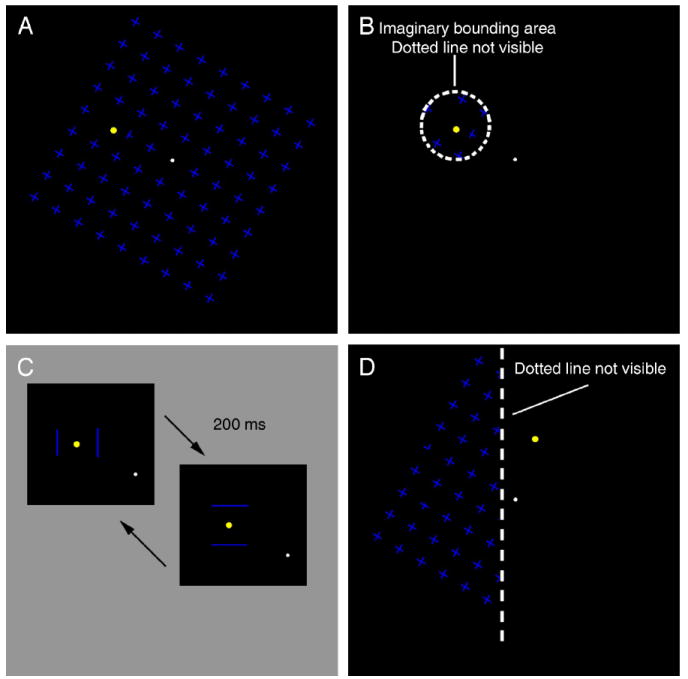
Visual stimuli used in Experiments 1A and 1B. (A) Full-field mask, a 9 × 9 field composed of 81 equally spaced blue crosses rotated around a fixation spot at 0.25 revolutions/s. A yellow target was located 2° from fixation in the top-left quadrant, as shown. (B) Local mask. This was the same stimulus as the full-field mask (A) except that the mask was restricted to an annulus with an outer radius of 0.8° centered on the yellow target. (C) Flashing bars. The target was the same as in A, but the mask was replaced by two pairs of sequentially flashing bars, flanking the target alternately horizontally or vertically. The two pairs alternated at 5 Hz. (D) Stimulus used in Experiment 1B, midline condition. Here the mask was present only to the left of an imaginary line 1° to the left of the target; in comparison conditions the fixation spot was moved by 1° or 2° to the right or left of this imaginary line (for detailed descriptions see Methods).
Figure 3.
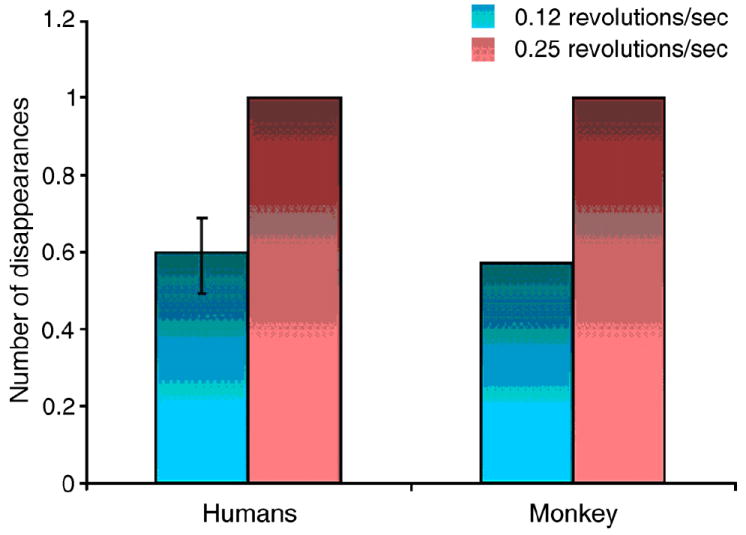
Effect of mask speed on the rates of disappearance for three human subjects and a trained monkey during MIB. All values are normalized to the high-speed (0.25 revolutions/sec) condition for each subject. Error bar in humans is the standard error (for the monkey there are no error bars because we had only one subject).
For Experiment 1B the stimulus shown in Figure 1D was the same as that shown in Figure 1A, except that the mask was visible only to the left side of a vertical border 1° to the left of the target in each display (Figure 1D). Five different conditions were tested by changing the position of the fixation spot with respect to the mask and target. For the midline condition (shown in Figure 1D) the fixation spot was located on a vertical line halfway between the mask and the target, so the mask was in the left visual field and the target in the right. For Left1 and Left2 conditions the fixation spot was moved to the right by 1° and 2° respectively (so that both mask and target were in the left visual field), and for Right1 and Right2 conditions the fixation spot was moved to the left by 1°and 2° respectively (so both mask and target were in the right visual field). To control for effects of eccentricity, we also tested a full-field mask control condition with the same five target and fixation locations.
Each subject was positioned 60 cm from the display and reported with different key presses when the yellow target appeared and disappeared. Each experiment consisted of five 1-minute trials for each subject per condition. In Experiments 1A and 1B data were pooled for each subject over each condition. To account for intersubject variability, we normalized the values of each subject by their average over all conditions within each experiment. Paired two-tailed t-tests were carried out for all pairs of conditions.
Results and discussion
In order to explore the possibility that at least some MIB masking could arise at early stages in the visual system, we tested whether small, local masks could be as effective as large surface-inducing masks in causing MIB. This experiment is based on the fact that receptive fields in early visual areas tend to be smaller than receptive fields in higher visual areas. We compared the effects on the rate of target disappearance of a full mask, a mask that only just surrounded the target, and flashing bars around the target (Kawabe & Miura, 2007; Wallis & Arnold, 2008) (Figures 1A, 1B, and 1C, demos of stimuli used are available in “Supporting Information”). For different subjects, responses ranged from the target being invisible 9% to 39% of the time, with an average of 25.8%, 24.4% and 25.4% for full mask, local mask, and bars respectively. The number of disappearances per minute ranged from 7 to 19, with an average of 14.4, 13.1, and 13.5 for full mask, local mask and bars respectively. In Figure 2A the average normalized number of disappearances and the average normalized percent time invisible are presented for each condition. There was no significant difference between the rates of disappearance or the time the target remained invisible between any pair of these conditions (Figure 2A, paired t-test, 2-tailed, df = 4; p > 0.05). This result suggests that local masking is more important than surface-induction for the generation of MIB.
Figure 2.
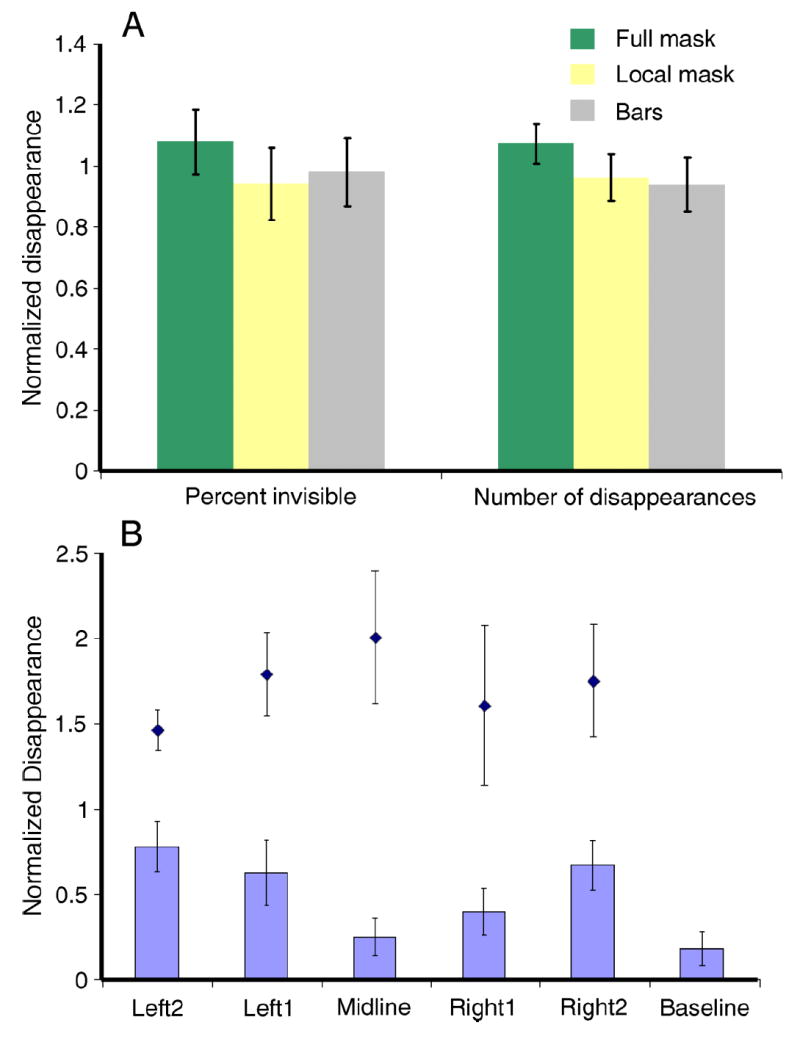
Results of Experiment 1. (A) Average of normalized (to average under all 3 conditions) time of invisibility (left) and number of disappearances (right) under Full Mask (green), Local Mask (yellow) and Flashing Bars (gray). (B) Reduced masking when target and mask are in different hemifields. Midline stimulus is shown in Figure 1D. Black diamonds show the average normalized disappearances for the full-mask control condition when the target was at the same locations as used to test the same vs. different hemifield conditions. Data are normalized to the average of the 11 conditions (5 with partial mask, 5 with full mask and one without a mask). Error bars represent the standard error.
In order to further explore the possibility that at least some MIB masking could arise at early stages in the visual system, we designed an experiment to test whether MIB masking crosses the vertical midline. This experiment is based on the fact that receptive fields (including the inhibitory surround) in early visual areas do not cross the vertical midline, but receptive fields in higher visual areas do (Hubel & Wiesel, 1967; Van Essen, Newsome, & Bixby, 1982). We arranged the target and mask as shown in Figure 1D, with the mask present only 1 degree to the left of the horizontal position of the target. Then we varied the fixation location so that the mask and target were both on the same side of the vertical meridian, or on opposite sides. We found that the target disappeared less often when it was on the opposite side of the vertical midline from the mask, compared to the same-side conditions (Figure 2B). Significant differences were found between the opposite hemifield condition (Midline) and the two more extreme same hemifield conditions Left2 (2-tailed t-test, t(4) = 3.19, p = 0.033) and Right2 (t(4) = 2.89, p = 0.045). The difference was not significant between Left1 or Right1 and Midline (df = 4; P > 0.05).
In Experiment 1B we observed more disappearances when the target and mask were on the same side of the vertical midline than when they were on opposite sides. In order to maintain the same distance between target and mask, yet change whether the target and mask were on the same or different sides of the midline, we had to change the eccentricity of the target. To investigate the possibility that our result accurately reflects the influence of having the target and mask in the same vs. the opposite hemisphere, rather than reflecting asymmetric rates of disappearance between different retinotopic locations, we ran a control experiment to test for the effects of retinotopy on the rate of disappearances. In this control experiment we measured the rates of disappearance in the standard MIB condition (with a full mask, as in Figure 1A) at the same five target and fixation locations. No significant effects of retinotopy across the range in question were observed.
Our result that the suppressive interactions causing MIB are stronger in the same hemifield than across the midline further supports the idea that MIB can be generated by suppressive interactions arising in early visual areas. Thus, Experiments 1A and 1B suggest that local processing, possibly originating in early visual areas, can generate sufficient suppressive interactions to cause target disappearances during MIB.
Experiment series 2: Physiological support for a role of early visual areas in MIB
Since the perceptual studies in Experiment 1 indicated a role for early visual areas in generating MIB, we looked at the firing patterns of individual V1 neurons in two alert fixating macaques while they viewed the MIB stimulus.
Methods
Single units were recorded in primary visual cortex of two alert fixating adult male macaque monkeys while they viewed the MIB stimulus shown in Figure 1A and maintained fixation on the fixation point. The target was centered on the receptive field of each cell recorded. A protection zone surrounding the target prevented the mask from entering the activating zone of the V1 cells; the size of the protection zone was tailored for each cell, so that it was large enough that the mask when presented alone produced no change in activity.
For Experiment 2A, one of the monkeys was trained to maintain fixation while reporting the appearance or disappearance of a target using a lever with 3 positions. The resting position (middle) was the default state of the lever. The monkey was trained to pull the lever leftward when the target disappeared and to pull the lever rightward when the target reappeared. The monkey was required to fixate a central spot throughout the task. Each trial lasted 15 seconds, at the end of which a juice reward was given if the target appearance and disappearance transitions had been correctly reported within 1 second of each transition throughout the trial. This reward schedule was used to discourage the monkey from signaling changes in the presence or absence of the target when none were experienced, since more reports did not result in more rewards, and only accuracy was rewarded (Leopold, Maier, & Logothetis, 2003). Trials were aborted, with a 5 second time-out period, if the animal broke fixation, failed to report a target change, or reported the wrong change. During training the animal was presented with real target transitions at a random rate every 1.5–4.5 seconds in the absence of a mask. Once the animal performed well at this task (>95% correct) the mask was slowly faded in, until he was again performing at more than 95% correct with the high contrast mask. Having the target turned ON and OFF every 1.5 to 4.5 seconds almost entirely prevents the illusory disappearance of the target for humans (Leopold et al., 2003). When the animal’s performance was above 95% in the presence of the MIB mask, we presented him with a prolonged period of target ON (8–9 secs) in 25% of the trials. In humans this condition induces perceptual disappearances; i.e. MIB. During this period the monkey was required to maintain fixation, but was not punished or rewarded for reporting changes in the target. The monkey had no incentive to pull the lever during this period, since reward was given only at the end of the 15 second trial. All trials ended with the target disappearing and reappearing to ensure that he was still faithfully reporting his perceptual state up until the end of each trial.
For the MIB-reporting task, V1 cells were first tested for receptive field location and, for each cell, the target stimulus was centered on the receptive field, where it would evoke the maximal response. While the animal performed the task, activity was recorded with a tungsten microelectrode (9 ± 1 MΩ initial impedance, Frederick Haer and Co). Electrical signals were amplified, band-pass filtered and recorded at 20 kHz. Spike trains for each trial were averaged over a period of 200 ms around each time point and averaged together with other trials for the same cell. Cells were categorized as giving an ON or an OFF response (or both) by whether the average firing rate 50 ms around the peak exceeded the mean firing rate by more than 3 standard deviations. Firing patterns for all cells giving an ON or OFF response were then averaged together (Figure 4A). To test the statistical significance of the peaks we carried out a randomization test, in which the lever press time was randomly assigned during the periods when the target stayed ON (1000 repetitions).
Figure 4.
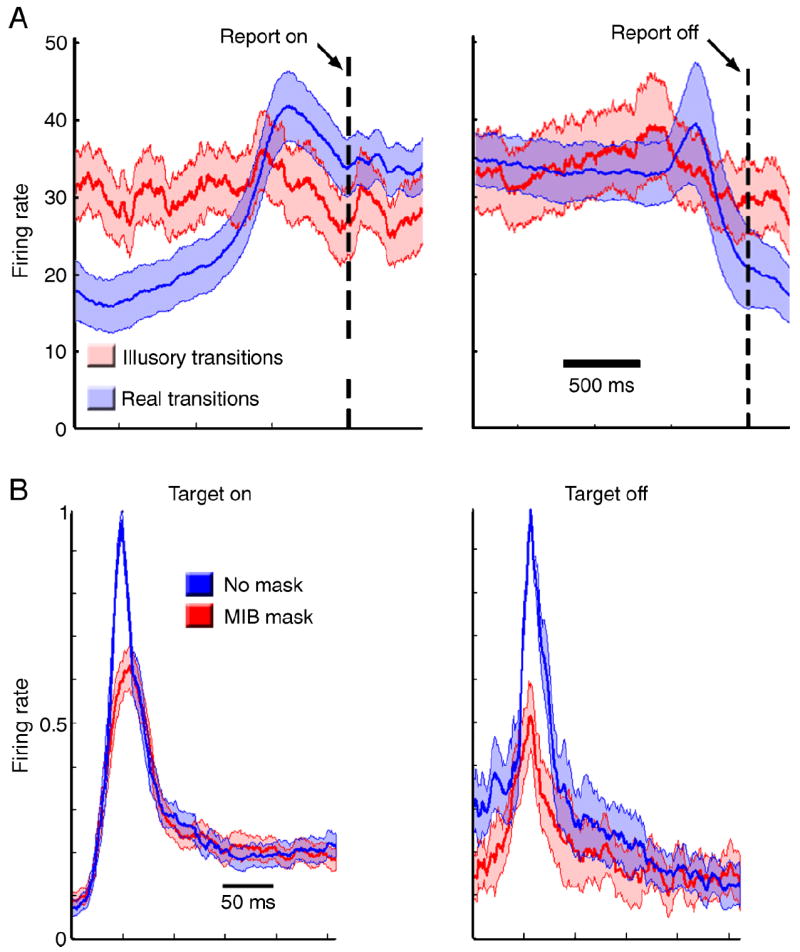
Results of Experiment 2. (A) Average responses of V1 cells when the monkey reported target appearance (left) and target disappearance (right) for illusory (red) or real (blue) transitions. Activity is aligned with the time of lever press, indicated by the dotted line. The shaded area denotes standard error. (B) Population average of V1 cells during passive fixation (mean ± standard error) to target ON (left) and target OFF (right) from cells with ON and OFF responses respectively. The blue line represents the average firing rate when no mask was present and red the average firing rate when the MIB mask was present. Responses were aligned by time to peak and normalized by the maximum firing rate for each cell.
For Experiment 2B, activity was recorded from 21 single units in two monkeys during passive fixation. The MIB mask condition was as shown in Figure 1A, except the size of the protection zone was tailored for each cell as described above, and the No-Mask condition was the same except that no mask was present. In both conditions the yellow target flickered ON and OFF every 500 ms in the receptive field of the cell. Spike trains for each cell were averaged over a period of 10 ms around each time point and averaged together with other cells. A paired t-test was carried out comparing the average firing rate ±50 ms around the peak activity for each condition, as well as the average activity from 300 to 500 ms after target onset. There was a significant difference between the peaks (t(17) = 6.66, p < 0.001 for target ON and t(6) = 2.57, p = 0.043 for target OFF) but no difference in the 300–500 ms period (p > 0.05). In Figure 4B the average activity for all 21 cells is plotted. Responses from different cells were aligned by their time-to-peak in the No-Mask condition to reduce the variability introduced by different response latencies.
The monkeys were prepared for chronic recording as previously described (Livingstone, 1998). Monkeys fixated for a juice reward; data collected when the monkey’s eye position was more than 0.5° from fixation were discarded. Eye position was monitored using a scleral search coil in a magnetic field (Judge, Richmond, & Chu, 1980). All procedures were in accordance with NIH guidelines and were approved by the Harvard Medical Area Standing committee on Animals.
Results and discussion
One of our monkeys was trained to report the visibility of a peripheral yellow target in the presence of an MIB mask while maintaining fixation on a small spot. Each trial started with the target present in the cell’s receptive field, and the monkey was trained to move a lever rightward when he saw the target disappear, and to move it leftward when the target re-appeared. In some trials the target actually disappeared and reappeared, and in some trials it remained present throughout the trial. The monkey was rewarded at the end of the trial. Great care was taken to ensure that the lever pulls reflected perceptual states (see Methods).
The speed of the mask rotation, which is known to affect the rate of target disappearance in humans (Bonneh et al., 2001), also affected the rate of illusory target disappearance reports by the monkey (Figure 3). When we repeated this experiment on human subjects, they reported that the (continuously present) target on average disappeared in 61% of the 8 second-long trials when the mask rotated at 0.25 revolutions per second, and in 36% of the trials when the mask rotated at 0.125 revolutions per second; the monkey reported that the (continuously present) mask disappeared in 42% of the 8 second-long trials when the mask rotated at 0.25 revolutions per second, and in 24% of the trials when the mask rotated at 0.125 revolutions per second. This similar mask-speed dependence suggests that macaques, like humans, perceive disappearances of the salient target in the presence of a moving field of dark blue crosses. We will therefore refer to occasions when the monkey reported that the target disappeared, even though it was continuously present, as ‘illusory disappearances’.
We recorded from single units in V1 while the monkey viewed the MIB stimulus shown in Figure 1A and reported target disappearances and reappearances using the lever press; the target was centered on the receptive field of each cell recorded. A protection zone surrounding the target prevented the mask from entering the activating zone of the V1 cells (the size of the protection zone was tailored for each cell, and was made large enough that the mask when presented alone produced no change in activity). We compared V1 neural activity preceding lever presses in trials when the target actually disappeared and re-appeared (unambiguous trials) to the activity in trials when the monkey moved the lever even though the target was continuously present throughout the trial (ambiguous trials resulting in illusory appearances and disappearances). Only 12 cells were held long enough to map their receptive fields and collect responses from both ambiguous and unambiguous correct behavioral trials. We observed, as expected, an increase in neural activity around 500 ms before lever presses in response to actual target appearances or disappearances, but we also observed similar, but smaller, average increases in activity before lever presses indicating illusory transitions. However the increases in neural activity preceding illusory transitions were smaller than the peaks of activity preceding real target transitions and did not reach statistical significance at the p < 0.05 level (Figure 4A). We therefore cannot explain the illusory disappearances simply by parallel changes in the activity of V1 cells.
However, we noticed during these MIB-reporting trials that in the presence of the MIB mask the responses of the V1 cells to actual appearances and disappearances of the target were attenuated compared to the mask-absent condition, leading us to ask whether the mask might weaken or interfere with neural responses to the target in V1. To answer this question we measured the responses of 21 single cells in V1 to the presentation of the same target with and without the MIB mask in two monkeys during passive fixation. On average, the neurons gave smaller responses to both appearances and disappearances of the target in the presence of a surrounding mask compared to the no-mask control condition (Figure 4B). On average, there was a significant decrease in the initial peak response to both target ON and target OFF in the presence of the MIB mask (paired t-test, p < 0.05), and no significant difference in the sustained responses (300–500 ms after target transition, paired t-test, p > 0.05).
These results suggest that even though the activity in V1 does not correlate directly with the perceptual state of the animal, early visual areas might nevertheless be involved in the disappearance of the target by conveying a weaker signal in the presence of a mask. Furthermore, this signal decrease may affect the detection of transitions of the target (from On to Off and Off to On) yet may not affect ongoing signals reflecting the current state of the target. Further physiological data are needed in order to test this idea, however, our results raise the possibility that the sustained level of activity in ON or OFF cells is not key in signaling whether the target is present or absent, but instead the crucial influence of the mask may be to reduce transient signals whose role is to indicate whether some change has taken place.
Experiment 3: Psychophysical evidence for transient-induced disappearances
Our physiological recordings from V1 in one monkey trained to report MIB disappearances did not show a significant correlation between V1 firing rates and target visibility. Nevertheless, our physiological recordings from V1 in two monkeys passively fixating the standard MIB stimulus showed that the transient phases of V1 responses to target appearance and disappearance were significantly reduced in the presence of the MIB mask, even though the sustained phase of the responses to the target were unaffected by the MIB mask. Our failure to observe any effect of the mask on the sustained part of the responses to the target suggests that the perceptual disappearances might not be attributable to the reduction of activity directly correlated with the stable presence of the target, but rather to suppression of activity signaling target transitions—appearances or disappearances. That is, our physiological results raise the question of whether the mask actually renders the target “less visible” or whether it makes the target “more likely to disappear.”
Therefore in a third, perceptual, experiment we explored the question of whether the MIB mask renders the target ‘less visible’ or ‘more likely to disappear’. To do this we presented a stimulus in which the target luminance was sinusoidally modulated around two values (a high and a low luminance value), in the presence and absence of the MIB mask. If the MIB mask simply renders the target ‘less visible’ then we expect the target to disappear more frequently during the dimmest periods of the luminance cycle; if the MIB disappearances are caused by changes in the likelihood of ‘disappearances’ then we expect the target to disappear more frequently during the decreasing brightness phases of the brightness cycle.
Methods
Three naive observers and two authors (CL and TS) participated in Experiment 3. All of them had normal or corrected-to-normal vision.
Stimulus and data collection were the same as in Experiment 1. The stimulus was the same as shown in Figure 1A, except that the target fluctuated in luminance sinusoidally over time at 0.3 Hz between 8.8 cd/m2 and 39.7 cd/m2 or between 39.7 cd/m2 and 111.0 cd/m2 in the low and high luminance conditions respectively. Each experiment consisted of three 1-minute trials for each subject per condition. We also measured the reaction times for reporting target appearance and disappearance for each subject in intermixed trials, where real target transitions occurred in the absence of a mask.
To account for intersubject variability we normalized the instantaneous disappearance rate at each point in the cycle for the high or the low luminance condition to the average rate of disappearances and reappearances for the high and low contrast condition averaged together and averaged over the entire cycle.
Results and discussion
The average instantaneous normalized disappearance and reappearance rates as a function of target luminance are shown in Figure 5. Overall, the disappearance and reappearance rates were similar for the high and the low luminance conditions, consistent with a previous study that found no effect of target luminance on disappearance rate (Bonneh et al., 2001). In addition, we found that subjects reported target disappearances most often right after the target started dimming in the presence of the MIB mask, for both high and low luminance levels; in the absence of the mask the target simply appeared to dim, not disappear. Figure 5A shows that for both luminance conditions the disappearance rate was highest during the dimming phase of the target luminance cycle (when the luminance cycle is delayed by the average reaction time—gray areas in Figure 5). That is, the disappearance rate correlated with the dimming of the target, not with its maximum dimness, though the disappearances do peak at the dimmest phase of the cycle. Target reappearances also increased when the target began to brighten, and not at the peak target brightness; there was also a peak in target reappearances during the dimming phase of the target, probably simply because the target can only reappear if it has first disappeared.
Figure 5.
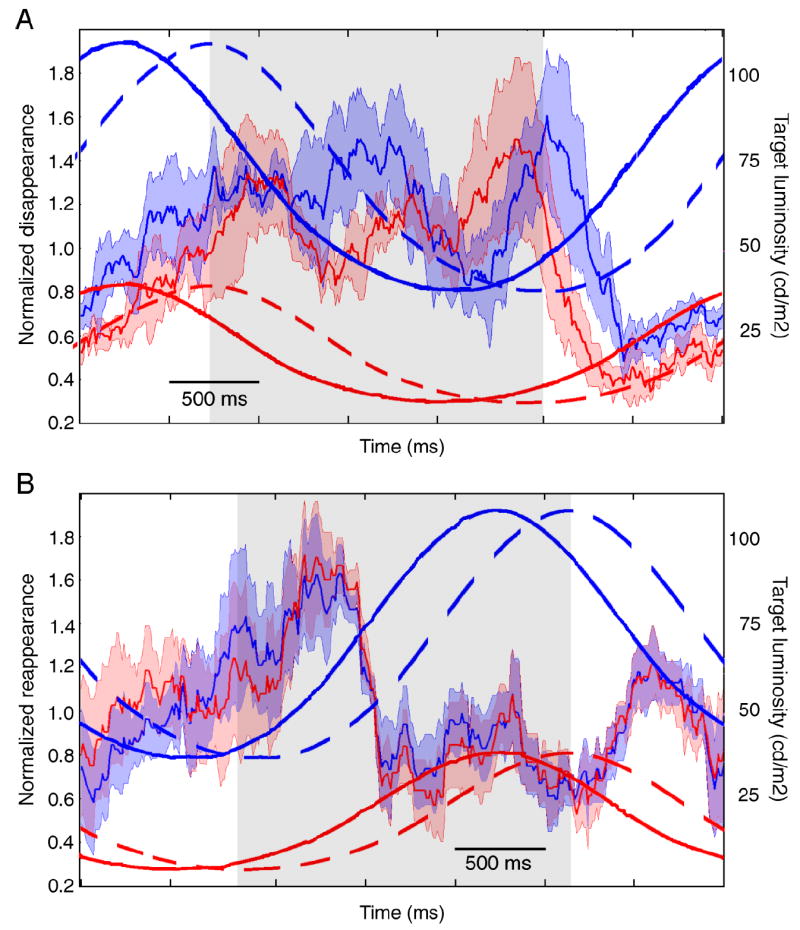
Results of Experiment 3. Normalized time of disappearance (A) and reappearance (B) as a function of time in the luminance cycle. The normalized time of disappearance was calculated as the percent of times the target disappeared in a 250 ms window around each time point, normalized to the average disappearance rate for each subject over the entire experiment. The irregular red and blue traces show the average normalized disappearance rate for the low (red) and high (blue) luminance cycles. The lighter red and blue shading denotes 1 standard deviation around the mean. The overlaid solid sinusoidal traces show the luminosity of the target during the cycle for the low (red) and high (blue) luminance cycles. The overlaid dotted sinusoidal traces show the luminosity of the target shifted in time by the average reaction time for all subjects (315 ms for appearances and 380 ms for disappearances) for the low (red) and high (blue) luminance conditions. Gray shaded areas show the time during which the target was decreasing in luminance (A) and increasing in luminance (B) once reaction times are accounted for.
Overall, the results of this experiment are consistent with the idea that, regardless of the absolute value of luminance of the target, the MIB mask renders the target not ‘less visible” but rather “more likely to disappear,” and small transients signal the disappearance or reappearance of the target, regardless of whether the transients are caused by a change in the target or within the brain itself.
General discussion
In summary, our physiological and perceptual results were both consistent with the hypothesis that MIB can originate in early visual areas. We established that macaque monkeys, like humans, perceive the MIB illusion, and, even though the level of activity of V1 cells did not correlate directly with the perceptual presence or absence of the target, the transient phases of responses in V1 to target transitions were diminished by the MIB mask. Furthermore, we found that in humans transient decreases in target luminosity, regardless of absolute luminosity level, induced perceptual disappearances of the target. Such decreases in target luminosity should cause transient increases of activity in a subpopulation of V1 cells (OFF cells). Since perceptual disappearances tended to occur while the target was decreasing in luminance, we assume that these disappearances were caused by OFF transient responses, rather than by the sustained levels of activity in either the ON or the OFF population. Because we also found that under MIB conditions the initial transient responses of V1 cells were reduced (thus bringing the peak response closer to the noise level), we suggest that the spontaneous perceptual transitions during MIB are caused by the ‘chance’ event that a sufficiently large population of OFF cells in visual cortex fired. Since during MIB real transitions evoke smaller transient responses, the read-out of V1 activity to decide that the target appeared or disappeared must be subject to a lower criterion during MIB. In other words, the exact same pattern of activity that under the no-MIB condition would be interpreted by higher visual areas as no-change in the target, under MIB conditions could be interpreted as a target transition. This would also explain why we found a weak (but not significant) correlation between V1 activity and perceptual state during the monkey MIB-reporting study.
If this interpretation is correct, then the percept that an object in the world has disappeared must depend on the transient activity of OFF cells, not on the termination of sustained activity in ON cells. This idea is consistent with a large body of evidence supporting the idea that activity in the visual system does not represent the world isomorphically, or isotemporally, but rather that it carries information about spatial and temporal deviations from the status quo. Indeed previous results from our laboratory have implicated the transient phases of V1 cells’ responses in visibility and invisibility during masking (Macknik & Livingstone, 1998). Furthermore, our results and interpretations allow us to predict a correlation between parameters known to affect the rate of disappearance in humans (such as mask speed, target size, protection zone size, etc) and the degree of suppression of transient responses in V1. Future research should address these issues.
We found that the transient phases of the responses of V1 cells to target appearance or disappearance were reduced in the presence of an MIB mask. So, even though the mask fell well beyond V1 classical receptive fields due to the protection-zone, it still produced a modulatory influence on the V1 responses. This modulatory influence could originate in V1 via lateral interactions, or could be the product of feedback from other brain areas, or both. We observed suppressive interactions in V1 responses up to distances of several degrees of visual angle, so the observation in Bonneh et al., 2001 that in the presence of a large protection zone MIB still occurs does not rule out a role for V1 in MIB. Furthermore, primary visual cortex is likely not the only area signaling the disappearances, as contextual surround suppression could arise at any cortical level. But the perceptual and physiological results presented here suggest that suppressive effects of the mask on activity in early visual areas can contribute to the phenomenon.
The involvement of early visual areas in MIB has been overlooked because several lines of evidence point away from early topographic visual areas as an important locus for MIB. Aftereffects and adaptations, which are assumed to arise in V1, are not affected by MIB disappearances (Hofstoetter, Koch, & Kiper, 2004; Montaser-Kouhsari, Moradi, Zandvakili, & Esteky, 2004; Rajimehr, 2004). Furthermore, factors assumed to be important for MIB, such as attention, object selectivity (Bonneh et al., 2001), surface completion, depth ordering (Funk & Pettigrew, 2003) and interhemispheric switching (Funk & Pettigrew, 2003) are thought to arise at levels higher than V1. It has also been shown that V1 activity does not correlate with perceptual state for other visual disappearance illusions (Leopold & Logothetis, 1996). On the other hand, Kawabe and Miura (2007) and Wilke, Logothetis, and Leopold (2003) provided evidence that low-level signals can be involved in visual disappearance phenomena. Because our results implicate the transient phase of visual responses, we can now argue that adaptations and aftereffects not being affected by MIB is not inconsistent with a role for early visual areas, since adaptations and aftereffects result from prolonged sensory stimulation and are not dependent on response transients (Maffei, Fiorentini, & Bisti, 1973).
Even if high-level effects such as object competition or attentional modulation are the read-out stages responsible for perceptual target visibility, our results and interpretations suggest that mask-induced suppression of target responses as early as V1 can also play an important role. That is, when the signals from lower levels are noisier, the detection processes in higher-level cells will also be noisier. In this view, we would expect activity in the population of V1 cells that respond to the target to correlate to some degree with the perceptual report, although this correlation need not be as strong as during real transitions, since errors could be initiated anywhere along the pathway, not just at the first stage.
Acknowledgments
This research was supported by NIH grant EY16187. CL was supported by NIH training grant T32 EY07110. We would like to thank Arash Yazdanbakhsh, Michael-John Tavantzis, Krishna Srihasam, Bevil Conway, John Assad, and an anonymous reviewer for their helpful input.
Footnotes
Citation: Libedinsky, C., Savage, T., & Livingstone, M. (2009). Perceptual and physiological evidence for a role for early visual areas in motion-induced blindness.
Commercial relationships: none.
Contributor Information
Camilo Libedinsky, Email: camilo_libedinsky@hms.harvard.edu.
Tristram Savage, Email: savage.tr@gmail.com.
Margaret Livingstone, Email: margaret_livingstone@hms.harvard.edu.
References
- Bonneh YS, Cooperman A, Sagi D. Motion-induced blindness in normal observers. Nature. 2001;411:798–801. doi: 10.1038/35081073. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Funk AP, Pettigrew JD. Does interhemispheric competition mediate motion-induced blindness? A transcranial magnetic stimulation study. Perception. 2003;32:1325–1338. doi: 10.1068/p5088. [DOI] [PubMed] [Google Scholar]
- Graf EW, Adams WJ, Lages M. Modulating motion-induced blindness with depth ordering and surface completion. Vision Research. 2002;42:2731–2735. doi: 10.1016/s0042-6989(02)00390-5. [DOI] [PubMed] [Google Scholar]
- Hofstoetter C, Koch C, Kiper DC. Motion-induced blindness does not affect the formation of negative afterimages. Conscious Cognitive. 2004;13:691–708. doi: 10.1016/j.concog.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Hsu LC, Yeh SL, Kramer P. A common mechanism for perceptual filling-in and motion-induced blindness. Vision Research. 2006;46:1973–1981. doi: 10.1016/j.visres.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Cortical and callosal connections concerned with the vertical meridian of visual fields in the cat. Journal of Neurophysiology. 1967;30:1561–1573. doi: 10.1152/jn.1967.30.6.1561. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: An improved method. Vision Research. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Kawabe T, Miura K. Subjective disappearance of a target by flickering flankers. Vision Research. 2007;47:913–918. doi: 10.1016/j.visres.2006.10.026. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Logothetis NK. Activity changes in early visual cortex reflect monkeys’ percepts during binocular rivalry. Nature. 1996;379:549–553. doi: 10.1038/379549a0. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Maier A, Logothetis NK. Measuring subjective visual perception in the nonhuman primate. Journal of Conscience Studies. 2003;10:115–130. [Google Scholar]
- Livingstone MS. Mechanisms of direction selectivity in macaque V1. Neuron. 1998;20:509–526. doi: 10.1016/s0896-6273(00)80991-5. [DOI] [PubMed] [Google Scholar]
- Macknik SL, Livingstone MS. Neuronal correlates of visibility and invisibility in the primate visual system. Nature Neuroscience. 1998;1:144–149. doi: 10.1038/393. [DOI] [PubMed] [Google Scholar]
- Maffei L, Fiorentini A, Bisti S. Neural correlate of perceptual adaptation to gratings. Science. 1973;182:1036–1038. doi: 10.1126/science.182.4116.1036. [DOI] [PubMed] [Google Scholar]
- Montaser-Kouhsari L, Moradi F, Zandvakili A, Esteky H. Orientation-selective adaptation during motion-induced blindness. Perception. 2004;33:249–254. doi: 10.1068/p5174. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Rajimehr R. Unconscious orientation processing. Neuron. 2004;41:663–673. doi: 10.1016/s0896-6273(04)00041-8. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Newsome WT, Bixby JL. The pattern of interhemispheric connections and its relationship to extrastriate visual areas in the macaque monkey. Journal of Neuroscience. 1982;2:265–283. doi: 10.1523/JNEUROSCI.02-03-00265.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis TS, Arnold DH. Motion-induced blindness is not tuned to retinal speed. Journal of Vision. 2008;8(2):11, 1–7. doi: 10.1167/8.2.11. http://journalofvision.org/8/2/11/ [DOI] [PubMed]
- Wilke M, Logothetis NK, Leopold DA. Generalized flash suppression of salient visual targets. Neuron. 2003;39:1043–1052. doi: 10.1016/j.neuron.2003.08.003. [DOI] [PubMed] [Google Scholar]


