Abstract
Trichuris trichiura is a common intestinal nematode parasite of captive baboons. We evaluated the efficacy of fenbendazole formulated in a commercial primate diet (FBZ-PD) for treating specific pathogen-free (SPF) baboons (Papio cynocephalus anubis) naturally infected with Trichuris trichiura. Twenty-nine baboons, housed indoors in 3 separate rooms, were fed FBZ-PD for 5 d, whereas 4 baboons housed in another isolated area served as untreated controls. The efficacy of FBZ-PD was measured as reduction in the number of T. trichiura eggs in host feces after treatment as determined by quantitative fecal flotation examination. All baboons that received FBZ-PD stopped shedding T. trichiura eggs by 7 d after initiation of treatment, and remained negative until at least 119 d after treatment. However, eggs of T. trichiura were present in the feces of 3 (10.3%) experimental baboons at 154 d after treatment. Untreated control baboons shed T. trichiura eggs throughout the entire study. Our results indicate that FBZ-PD was efficacious for treating SPF baboons infected with T. trichiura.
Abbreviation: EPG, eggs per gram; FBZ-PD, fenbendazole formulated in a primate diet; FEC, fecal egg count; SPF, specific pathogen-free
Trichuris trichiura infects the large intestine and cecum of captive and wild baboons (Papio spp.);4,5,9-12 this parasite also infects humans.13,16 T. trichiura is also known as a ‘whipworm’ because its gross morphology resembles that of a bullwhip. The anterior portion of adult T. trichiura is embedded within the intestinal mucosa while their posterior end extends into the gut lumen. Consequently, pathogenesis commonly involves anaphylactoid-type reactions and ischemic necrosis, both limited to tissue where adult worms reside in situ and direct injury occurs. Clinical trichuriasis in nonhuman primates is similar to that seen in humans, and signs range from subclincal to profuse watery diarrhea, lethargy, abdominal pain, weight loss, inappetance, and dehydration.6,10,13
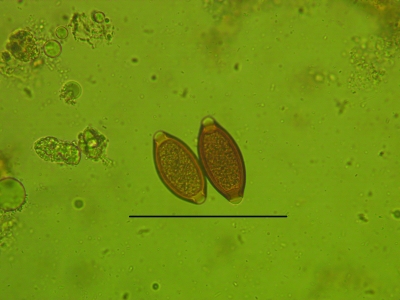
Transmission of T. trichiura from 1 host to the next is direct and occurs through the ingestion of embryonated eggs. Eggs require approximately 30 d (at 25 °C) in the external environment to embryonate and become infective to the next host.2 Eggs of T. trichiura (Figure 1) are resistant in the external environment, and their persistence is paramount in the transmission of the parasites from 1 host to the next.2 Because of its ability to cause disease, direct fecal–oral route of transmission, and persistence in the external environment, treatment and control of T. trichiura is requisite to maintaining healthy baboon colonies, providing quality animals for research, and reducing the threat of zoonotic transmission.
Figure 1.
Eggs of Trichuris trichiura. Whipworm eggs have thick, brown shells that have a plug on both ends. The shell wall surface is smooth, and the eggs are symmetrical about the bipolar plugs. Magnification, ×400; scale bar, 100 μm
Granulated fenbendazole fed at 50 mg/kg for 3 consecutive days was effective for treating singly housed baboons infected with T. trichiura.15 Although effective, this method15 involved hiding the fenbendazole in food given individually to the baboons and would be impractical for treating group-housed primates. The purpose of the present study was to determine the efficacy of fenbendazole that had been formulated in a commercial primate diet (FBZ-PD) for treating specific pathogen-free (SPF) baboons that were group-housed. FBZ-PD was effective: all treated SPF baboons stopped shedding T. trichiura eggs within 7 d of treatment, and fecal egg counts remained negative until 119 d after treatment.
Materials and Methods
Experimental design.
Thirty-three SPF olive baboons (Papio cynocephalus anubis), naturally infected with T. trichiura and without signs of clinical trichuriasis, were used for the present study. Baboons were group-housed in 4 rooms that were separate and isolated from each other (Table 1). One room of 4 baboons infected with T. trichiura served as positive controls and did not receive anthelmintic medication. The remaining 29 baboons, housed in 3 separate rooms, were fed FBZ-PD. Efficacy of the deworming was determined by the reduction of T. trichiura eggs in host feces after treatment.
Table 1.
Demographic data of SPF baboons
| Room | No. of SPF baboons (male, female) | Age (y) | Weight (kg; mean ± SE) |
| ARA 154 | 4 (0,4) | 2.11–2.49 | 8.1 ± 0.4 |
| ARA A | 8 (3,5) | 2.51–2.59 | 9.9 ± 0.3 |
| ARA B | 7 (5,2) | 3.09–3.32 | 12.5 ± 1.1 |
| ARA D | 14 (4,10) | 3.34–4.32 | 15.1 ± 1.0 |
Establishment of SPF baboons.
All baboons were housed and cared for according to the standards detailed in the Guide for the Care and Use of Laboratory Animals.7 Protocols for maintenance of the baboon colonies were approved by the University of Oklahoma Health Sciences Center Institutional Animal Care and Use Committee. Both male and female SPF baboons were used in the present study and ranged from 2.11 to 4.32 y of age and averaged in weight from 8.1 to 15.1 kg (Table 1).
Baboons in the SPF program were derived from the conventional colony at the University of Oklahoma Health Sciences Center. The conventional colony is housed in large indoor–outdoor corrals. Neonates were delivered naturally, typically during the hours between dusk and dawn, and removed from their dams within 24 h after birth. Dams were separated from the colony into a squeeze cage and anesthetized with ketamine (10 mg/kg; Fort Dodge, Fort Dodge, IA), during which time their infants were collected. Neonates were then transported to the SPF nursery to be hand-raised, and the dam was returned to the conventional colony.
Each acquired neonate received a warm soapy bath to remove all apparent soil contamination and then was washed quickly with chlorhexidine, after which the neonate was allowed to air-dry. Baboons were cared for by dedicated technicians, who used strict barrier principles to prevent disease transmission from conventional baboons housed in the same building. Blood was collected by sterile venipuncture monthly through 4 mo of age and then at then at 6, 9, 12, 18, and 24 mo and tested for the select viruses (Table 2). After 2 y of age, SPF baboons were tested for select viruses annually. Any baboon that tested positive for a select virus was immediately removed from the SPF program.
Table 2.
Infectious agents eliminated from SPF baboons
| Herpesvirus | Papovavirus | Retrovirus | Other agents |
| Baboon cytomegalovirus | Simian virus 40 | Simian foamy virus | Tuberculosis (Mycobacterium spp.) |
| Herpesvirus papio 1 | Simian immunodeficiency virus | Measles virus | |
| Herpesvirus papio 2 | Simian retrovirus D | ||
| Human herpesvirus 6 | Simian T-lymphotropic virus | ||
| Rhadinovirus | |||
| Simian varicella virus |
Housing of SPF baboons.
For the first 2 wk after birth, each SPF baboon was housed individually at 29 °C in stainless steel cages (60.96 cm × 60.96 cm × 40.64 cm). After 2 weeks, they began ever-increasing periods of time in a gang cage (182.88 cm × 121.92 cm × 195.58 cm ) until they were living communally full-time at 3 mo of age. At about 18 mo of age, groups were combined and moved into large aluminum-and-concrete indoor gang cages, where they were housed throughout the present study.
Cages and rooms that had housed conventional baboons were sanitized thoroughly before being used to house SPF baboons. Conventional baboons were removed for a minimum of 4 wk prior to use by SPF baboons. During that 4-wk period, all caging and each room were sprayed with a 10% bleach solution, followed by chlorhexidine solution and a quaternary ammonium solution (mixed to label directions) on alternating days. In addition, each room was meticulously cleaned with a high-pressure sprayer once weekly. Finally, twice during the 4-wk period, each room was completely wet down with a glutaraldehyde solution, which stayed on for 30 min before being rinsed off. The effect of these chemical and mechanical decontamination procedures are unknown on the survivability of T. trichiura eggs in contaminated environments.
SPF baboons were maintained on a standard 25% protein primate diet (Harlan Primate Diet 2055, Harlan Teklad, Madison, WI) that was supplemented 3 times weekly with fresh fruit and vegetables; animals also received enrichment grains such as trail mix and dry cereal. Potable water was available ad libitum. None of the baboons used in the present study had ever been treated with an anthelmintic.
Sample collection and evaluation.
Baboons infected with T. trichiura were identified by observation of eggs characteristic of whipworms during microscopic examination of feces (Figure 1). Eggs of T. trichiura measure 49.5 to 65.0 µm in length and 22.5 to 30.0 µm in width and are distinctive barrel- or lemon-shaped ellipses with polar plugs at each end.18 Quantitative fecal eggs counts (FEC) were accomplished by using double-centrifugation with sugar flotation.19 Briefly, 5 g of fecal material was mixed thoroughly with 30 ml water, passed through a tea strainer, divided into two 15-ml conical tubes, and centrifuged in a swinging-bucket rotor at 176 × g for 10 min. Tubes were decanted without disturbing the sediment and refilled with sugar solution (specific gravity, 1.27). To release the eggs from the plug of debris in the tube bottom, the sediment and sugar solution were mixed thoroughly with an applicator stick. The tubes again were placed in a swinging-bucket rotor, and sugar solution was added dropwise to each tube until a positive meniscus formed. A 22-mm square coverslip was placed over each tube, and the tubes were centrifuged at 176 × g for 10 min. Each coverslip was removed and placed on a microscope slide; each 5-g sample yielded 2 coverslips. The number of Trichuris eggs shed per gram of feces (EPG) was estimated by counting the whipworm eggs visible under each coverslip at ×100 magnification and dividing that sum by the number of grams of feces used. Fecal samples were collected and analyzed from treatment days –14, –6, and 0 to establish a baseline T trichiura EPG for each baboon in the present study. Posttreatment fecal samples from individual baboons were collected on treatment days 7, 14, 21, 28, 63, 92, 119, and 154.
Anthelmintic treatments.
SPF baboons housed in rooms ARA A, ARA B, and ARA D (Table 1) were fed FBZ-PD for 5 consecutive days. The FBZ-PD was a 20% protein primate diet formulated with fenbendazole at 600 mg/kg (TD06561, Harlan Teklad, Madison, WI). SPF baboons in ARA 154 were untreated controls and received primate diet that was not formulated with FBZ. No other food, including fruits and treats, were fed during the 5-d treatment period.
Determination of anthelmintic efficacy.
As outlined by the World Association for the Advancement of Veterinary Parasitology regarding methods for the detection of anthelmintic resistance in nematodes of veterinary importance,3 descriptive statistics as well as the percentage FEC reduction of T. trichiura eggs are reported. The percentage of FEC reduction of T. trichiura was calculated as ([pretreatment EPG – posttreatment EPG/pretreatment EPG] × 100%).3 Data on T. trichiura EPG from baboons housed in the same room were combined for statistical comparisons.
Pretreatment T. trichiura EPG from baboons in different rooms were compared by using Kruskal–Wallis 1-way ANOVA on ranks.17 Mann–Whitney rank sum tests were used to compare pretreatment with posttreatment T. trichiura EPG from baboons within rooms.17 The Dunn method was used for multiple comparisons, and the criterion for statistical significance was a P value of 0.05.17 Analyses were performed with SigmaStat 3.1 statistical software (SyStat Software, Point Richmond, CA).
Results
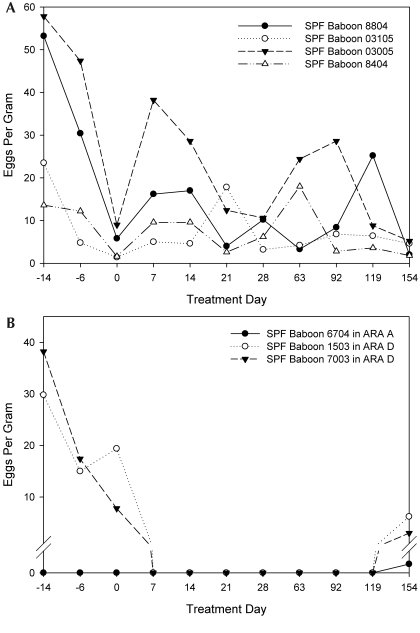
Prior to treatment, T. trichiura FEC varied considerably across individual baboons (Figure 2; not all data shown), ranging from 0 to 57.8 EPG (Table 3) and differing significantly (H = 23.0, P < 0.001) among rooms. Baboons in ARA 154 shed significantly more (Q = 3.139, P < 0.05; Q = 4.327, P < 0.05) T. trichiura eggs than did baboons in ARA A and ARA B, respectively. Similarly, baboons in ARA D shed significantly (Q = 3.433, P < 0.05) more T. trichiura eggs than did baboons in ARA B.
Figure 2.
The number of Trichuris trichiura eggs shed from control baboons (A) and from 3 baboons (B) that were fed fenbendazole formulated in a primate diet. Treatment was initiated on day 0 and continued for 5 consecutive days. The 3 baboons in panel B were the only FBZ-PD animals shedding T. trichiura eggs at 154 d after treatment.
Table 3.
Descriptive statistics and percentage reduction of Trichuris trichiuraegg counts from baboons
| Room |
||||
| ARA 154 (Controls) | ARA A | ARA B | ARA D | |
| Pretreatment | ||||
| Sample size | 12 | 24 | 21 | 42 |
| Mean ± SE | 21.8 ± 6.0 | 3.7 ± 0.7 | 1.8 ± 0.7 | 10.8 ± 1.8 |
| 95% Confidence interval | 8.7–34.9 | 2.2–5.2 | 0.4–3.2 | 7.1–14.5 |
| Median | 12.9 | 3.4 | 0.2 | 5.4 |
| Range | 1.4–57.8 | 0.0–12.2 | 0.0–13.0 | 0.0–38.2 |
| Posttreatment | ||||
| Sample size | 36 | 64 | 56 | 112 |
| Mean ± SE | 11.1 ± 1.6 | 0.003 ± 0.003 | 0.0 ± 0.0 | 0.8 ± 0.6 |
| 95% Confidence interval | 7.9–14.2 | 0.003–0.009 | 0.0–0.0 | (−)0.02–0.18 |
| Median | 7.6 | 0.0 | 0.0 | 0.0 |
| Range | 1.8–38.2 | 0.0–0.2 | 0.0–0.0 | 0.0–6.2 |
| % Reduction | 49.1 | 99.9 | 100.0 | 92.6 |
A single 5-d course of FBZ-PD significantly reduced the number of T. trichiura eggs shed from baboons in ARA A (T = 1640.5, P < 0.001), ARA B (T = 1155.0, P < 0.001), and ARA D (T = 5185.0, P < 0.001) compared with pretreatment values. The percentage reduction in T. trichiura eggs shed by each of the 3 groups of baboons was 99.9%, 100.0%, and 92.6%, respectively (Table 3). All baboons that received FBZ-PD stopped shedding T. trichiura eggs within 7 d initiation of treatment, and FEC remained negative for at least 119 d. T. trichiura eggs were detected in the feces of 3 of 29 (10.3%) experimental baboons at 154 d after treatment (Figure 2).
Discussion
Reductions in fecal egg counts provide an estimation of anthelmintic efficacy by enabling comparison of animals before and after treatment. Although the FEC reduction test initially was developed for detecting anthelmintic resistance in domestic animals,3,8 we used this assay to determine the efficacy of FBZ-PD in the treatment of SPF baboons infected with T. trichiura. As demonstrated by the significant reduction of T. trichiura eggs from infected SPF baboons, FBZ-PD was effective for treating baboons naturally infected with T. trichiura.
FBZ is a benzimidazole agent that selectively binds to nematode tubulin, disrupting mitosis and mitochondrial function.1 Mebendazole, in the same drug family as FBZ, is indicated for treatment of T. trichiura in humans. FBZ, effective in eradicating T. trichiura eggs from the feces of baboons in the present study, is a broad-spectrum anthelmintic and is effective in whipworm treatment of other animals, as well as nematode, trematode, and cestode parasites of domestic and wild animals.1,14 We chose to determine the efficacy of FBZ-PD because granulated FBZ was effective for treating baboons infected with T. trichiura15 and because the FBZ-PD formulation was available commercially.
Infections of intestinal nematodes typically are treated with at least 2 anthelmintic doses, the first given to eradicate adult worms and the second to eliminate immature forms that survived the primary dose. Timing of the second dose should coincide with the interval between infection and egg production, typically 14 to 21 d. However, adjustments must be made in scheduling the second dose when treating whipworms because Trichuris has a longer prepatent period than most intestinal nematodes.1 In the present study, 29 SPF baboons each were fed a single 5-d course of FBZ-PD, which eliminated T. trichiura eggs from feces at least until 119 d after initiation of treatment. However, T. trichiura eggs were present in the feces of 3 (10.3%) SPF baboons at 154 d after treatment. The prepatent period of T. trichiura in baboons is unknown currently but is estimated to be approximately 60 d in humans.2 If T. trichiura has a similar prepatent period in baboons as in humans, the 3 baboons likely became reinfected during the course of the present study, given that FEC for these 3 baboons were negative from 7 d until at least 119 d after treatment. Alternatively, perhaps the FBZ-PD treatments did not completely eliminate all T. trichiura; some immature larva may have survived and remained undetected until they matured and began to shed eggs.
One goal of our SPF program is to provide quality animals that are free of select infectious agents (Table 2) and can therefore be used for research with less likelihood of confounding variables. In the present study, SPF baboons had been housed under strict quarantine conditions that prevented the transmission of particular viruses from other baboons in the colony. However, these precautions did not prevent SPF baboons from becoming infected with T. trichiura. The rooms that had housed the SPF baboons since 24 h of age had been meticulously decontaminated with chemical and mechanical means before introduction of animals. Therefore, we surmise that the neonates most likely were infected with T. trichiura within the first 24 h of life. We also speculate that our decontamination procedures were not totally effective for rooms that held conventional baboons prior to the SPF animals in the present study, because 3 SPF baboons apparently became reinfected and started to shed T. trichiura eggs 154 d after treatment. Another possibility is that SPF animals became infected with T. trichiura through ingestion of eggs adhered to the hair coat of other gang-housed baboons.
Using primate diets that have been formulated with medication reduces labor costs as compared with the costs of dosing baboons individually. Because T. trichiura eggs require 30 d to become infective and because embryonated eggs can maintain viability in the external environment for several months, repeating 5-d treatments every 4 to 6 mo should be considered for routine control of whipworms when baboons are housed in contaminated environments. In addition to yielding quality animals for research, deworming baboons infected with T. trichiura will improve animal health and decrease the risk of transmission to caretakers, handlers, and researcher staff.
Acknowledgments
FBZ-PD was provided by Harlan Teklad (Madison, WI). The present project was funded through a Howard Hughes Medical Institute scholarship (to LCC) and National Institutes of Health grants 5R24RR016556-07 and 2P40RR012317-011 (to Gary L White).
References
- 1.Bowman DD, Carl RC, Eberhard ML. 2003. Georgis’ parasitology for veterinarians, 8th ed. St Louis (MO): Elsevier Science [Google Scholar]
- 2.Bundy DAP, Cooper ES. 1989. Trichuris and trichuriasis in humans. Adv Parasitol 28:107–173 [DOI] [PubMed] [Google Scholar]
- 3.Coles GC, Bauer C, Borgsteede FHM, Geerts S, Klei TR, Taylor MA, Waller PJ. 1992. World Association for the Advancement of Veterinary Parasitology (WAAVP) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol 44:35–44 [DOI] [PubMed] [Google Scholar]
- 4.Flynn RJ. 1973. Parasites of laboratory animals. Ames (IA): Iowa State University Press [Google Scholar]
- 5.Hahn NE, Proulx D, Muruthi PM, Alberts S, Altmann J. 2003. Gastrointestinal parasites in free-ranging Kenyan baboons (Papio cynocephalus and P. anubis). Int J Primatol 24:271–279 [Google Scholar]
- 6.Hennessy A, Phippard AF, Harewood WJ, Horam CJ, Horvath JS. 1994. Helminthic infestation complicated by intussusception in baboons (Papio hamadryas). Lab Anim 28:270–273 [DOI] [PubMed] [Google Scholar]
- 7.Institute of Laboratory Animal Resources. N. R. C 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C [Google Scholar]
- 8.Kaplan RM, Klei TR, Lyons ET, Lester G, Courtney CH, French DD, Tolliver SC, Vidyashankar AN, Zhao Y. 2004. Prevalence of anthelmintic-resistant cyathostomes on horse farms. J Am Vet Med Assoc 225:903–910 [DOI] [PubMed] [Google Scholar]
- 9.Kuntz RE, Meyers BJ. 1967. Parasites of the Kenya baboon: arthropods, blood protozoa, and helminths (Kenya, 1966). Primates 8:75–82 [Google Scholar]
- 10.Munene E, Otsyula M, Mbaabu DAN, Mutahi WT, Muriuki SMK, Muchemi GM. 1998. Helminth and protozoan gastrointestinal tract parasites in captive and wild-trapped African nonhuman primates. Vet Parasitol 78:195–201 [DOI] [PubMed] [Google Scholar]
- 11.Murray S, Stem C, Boudreau B, Goodall J. 2000. Intestinal parasites of baboons (Papio cynocephalus anubis) and chimpanzees (Pan troglodytes) in Gombe National Park. J Zoo Wildl Med 31:176–178 [DOI] [PubMed] [Google Scholar]
- 12.Myers BJ, Kuntz RE. 1965. A checklist of parasites reported for the baboon. Primates 6:137–194 [DOI] [PubMed] [Google Scholar]
- 13.Ooi HK, Tenora F, Itoh K, Kamiya M. 1993. Comparative study of Trichuris trichiura from nonhuman primates and from man and their difference with T. suis. J Vet Med Sci 55:363–366 [DOI] [PubMed] [Google Scholar]
- 14.Plumb DC. 2005. Plumb's veterinary drug handbook, 7th ed. Ames (IA): Blackwell Publishing [Google Scholar]
- 15.Reichard MV, Wolf RF, Carey DW, Garrett JJ, Briscoe HA. 2007. Efficacy of fenbendazole and milbemycin oxime for treating baboons (Papio cynocephalus anubis) infected with Trichuris trichiura. J Am Assoc Lab Anim Sci 46:42–45 [PubMed] [Google Scholar]
- 16.Roberts LS, Janovy J., Jr 2005. Gerald D Schmidt and Larry S Roberts’ Foundations of parasitology, 7th ed. New York: McGraw Hill [Google Scholar]
- 17.Sokal RR, Rohlf FJ. 1997. Biometry, 3rd ed. San Francisco (CA): WH Freeman and Company [Google Scholar]
- 18.Soulsby EJL. 1982. Helminths, arthropods, and protozoa of domesticated animals, 7th ed. Philadelphia: Lea and Febiger [Google Scholar]
- 19.Zajac AM, Conboy GA. 2006. Veterinary clinical parasitology, 7th ed. Ames (IA): Blackwell Publishing [Google Scholar]