Abstract
As the primary physical barrier between blood and tissue compartments within the body, blood vessel endothelial cells and integrity of the cell junctions connecting them must be carefully regulated to support leukocyte transendothelial migration only when necessary. Leukocytes utilize two independent routes across the endothelium: the paracellular route involves migration in-between adjacent endothelial cells and requires the transient disassembly of endothelial cell junctions, while the transcellular route occurs directly through an individual endothelial cell, likely requiring the formation of a channel or pore. In this review, I will first summarize the signaling events that are transduced by leukocyte engagement of endothelial cell-surface receptors like ICAM-1 and VCAM-1. Some of these signals include activation of GTPases, production of reactive oxygen species, and phosphorylation of target proteins. These signaling pathways converge to cause junctional disruption, cytoskeletal remodeling, and/or the membrane fusion events that are associated with leukocyte transendothelial migration. The review will conclude with a detailed discussion of the newly characterized transmigratory cup structure, and the recent advances made towards understanding the mechanisms of transcellular transendothelial migration.
Keywords: Endothelial cell, Transendothelial migration, Paracellular, Transcellular, Reactive oxygen species, GTPase, Cytoskeleton, Cell junctions, Transmigratory cup structures, Podosomes, Review
2. INTRODUCTION
Leukocyte transendothelial migration (TEM) is a vital physiological process that occurs during both the adaptive and innate immune response and during routine immune surveillance and homing. During TEM, leukocytes are stimulated to exit the bloodstream and enter lymphoid organs and infected tissues. Endothelial cells (ECs) lining the blood vessels are the primary barrier between the blood circulation and underlying tissue that leukocytes must navigate during TEM. Tight regulation of this process is critical, because if TEM occurs excessively or at inappropriate locations, it can cause inflammatory disorders such as atherosclerosis, chronic inflammation, multiple sclerosis, and rheumatoid arthritis. As such, research towards a better understanding of how this process is regulated will enable design of potential therapies for many of these pathological situations.
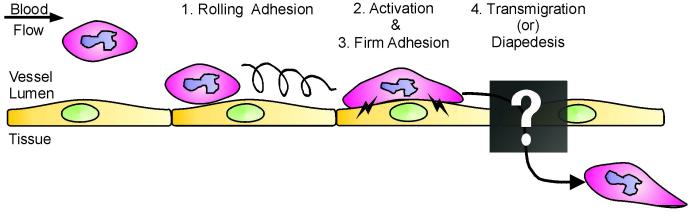
Leukocyte TEM has been described as a sequential set of events, each of which facilitates progression to the next stage. The original leukocyte adhesion multistep model comprised of three steps: 1. rolling adhesion, mediated by selectins, 2. activation, triggered by chemokine signals, and 3. firm adhesion/arrest, mediated by integrins (1, 2). TEM is added as the fourth and last step, concluding a complete inflammation cascade (Figure 1). During the initial rolling adhesion step, leukocytes become loosely tethered to the blood vessel wall due to transient weak interactions between selectins on activated ECs and leukocyte carbohydrate ligands. This step is thought to slow leukocyte passage through the blood vessel, allowing binding to ECs and exposure to a local environment that facilitates progression to the second step, activation. Local production of cytokines such as TNF-alpha by leukocytes serves a dual purpose: initiating a positive feedback loop resulting in further production of more cytokines, as well as causing upregulated expression of EC-leukocyte adhesion molecules such as ICAM and VCAM on the EC surface. Furthermore, production and display of specific chemokines (i.e. interleukin-8, MCP-1, SDF-1) by ECs attracts leukocytes and causes them to migrate towards the site of infection. Binding of these chemokines to leukocyte heterotrimeric G-protein coupled receptors results in rapid activation of beta-1 and beta-2 integrin activation. The third step in the adhesion cascade, firm adhesion, is promoted by this increased affinity of leukocyte integrins α4β1 (VLA-4), αLβ2 (LFA-2, CD11a/CD18), and αMβ2 (Mac-1, CD11b/CD18) for EC Ig-family adhesion molecules such as the previously mentioned VCAM and ICAM. Engagement and clustering of these EC cell adhesion molecules is required in turn to initiate signals within the EC which ultimately trigger diapedesis. It is now widely appreciated that ECs are active participants during these later processes (3, 4). The focus of this review will be on the transendothelial migration stage, and how signaling within the ECs influences this process.
Figure 1.
Schematic diagram of the leukocyte adhesion and transmigration cascade. Given an inflammatory stimulus, leukocytes initially loosely adhere on the vascular ECs, rolling along the blood vessel wall via transient selectin-mediated interactions (1). During the activation stage, both the EC and leukocyte begin to upregulate expression and/or activity of adhesion receptors on the cell surfaces (2), and this is required for initiating the firm adhesion stage (3). Finally, leukocytes exit the bloodstream, crossing the endothelium by the process known as transmigration or diapedesis (4). The mechanisms and pathways by which transmigration occurs are poorly understood and thus will be the focus of this review.
The multistage paradigm has undergone some additional refinements recently (5). For example, the TEM stage can be broken down into several more discrete steps occurring both before and during the actual diapedesis process. Additional steps prior to actual diapedesis include intraluminal crawling (6, 7), and podosome-mediated sensing by leukocytes interacting with the EC apical surface (8). Endothelial-dependent events include formation of transmigratory cup structures, followed by TEM, which can be further broken down into two distinct modes: paracellular and transcellular. “Paracellular” TEM requires transient junctional disruption as leukocytes migrate between adjacent cells. Migration thus occurs “alongside of” or “beside” adjacent ECs. Alternatively, some leukocyte subtypes such as neutrophils have been observed migrating through pre-existing gaps at cell borders where three ECs meet (tricellular junctions) (9). In contrast, “transcellular” TEM occurs when a leukocyte transmigrates directly “through” a single EC. Once across the EC barrier, the leukocyte must additionally traverse a second barrier which is the underlying perivascular basement membrane. In vivo, there is evidence for the existence of specific regions of low matrix protein deposition in the venular wall, and interestingly, leukocytes preferentially transmigrate at these sites (10).
This review will begin with an introduction of key EC junction and cell surface adhesion proteins, along with a description of key regulatory proteins involved in junction and cytoskeleton regulation (Section 3). Following this, Section 4 of the review will provide a broad overview of what is known about signaling events downstream from leukocyte engagement of EC adhesion proteins, touching on how this affects regulation of the actin cytoskeleton, EC junctions, and thus paracellular TEM. The last two sections of this review will provide a more detailed discussion of the newly characterized EC apical cup structure, incorporating the most recent advances made towards learning how its assembly is regulated (Section 5). Finally, Section 6 of the review will conclude with an in-depth discussion of transcellular TEM; its renewed recognition as a bona fide pathway, its regulation, and hypotheses about mechanisms for transcellular pore formation.
3. MOLECULAR PLAYERS
At the interface between blood and tissue, blood vessel ECs provide the primary physical barrier between these two distinct compartments within the body. The integrity of this barrier is maintained by junctional structures which seal the space between adjacent ECs (Figure 2). Junctions of ECs are morphologically heterogeneous (11), giving rise to different permeability and barrier characteristics between different vascular beds (12). At two extremes of the continuum are the relatively impermeable endothelium which comprises the blood brain barrier microvasculature, and the lymph node vessels known as high endothelial venules (HEVs) which support constitutive lymphocyte extravasation during homing and immune surveillance. It is important for EC junctions to be regulated appropriately in order to allow selective and specific passage of macromolecules and immune cells across this barrier only when necessary. Cell-cell junctions contain both transmembrane proteins with extracellular adhesive domains, and intracellular proteins which bind to the cytoplasmic domains of these transmembrane proteins. The cytoplasmic proteins often act as scaffolds, recruiting signaling molecules and providing an important link to the cell cytoskeleton which is critical for maintenance of barrier function. The transmembrane adhesion proteins contain extracellular adhesion domains to interact in a homo-or heterophilic manner to those on adjacent cells; they therefore constitute the actual physical barrier to a transmigrating leukocyte exiting the bloodstream via the paracellular route. ECs interact with neighboring cells using structures known as adherens junctions and tight junctions.
Figure 2.
Molecular components of EC and EC-leukocyte interactions. ECs contain both adherens junctions and tight junctions. Transmembrane proteins located along the paracellular cleft of two adjoining ECs interact, and thus provide the physical barrier to the transmigrating leukocyte. Cytoplasmic junctional proteins provide a link between the transmembrane proteins and the cell cytoskeleton. Leukocytes interact via integrins to EC adhesion molecules present on the apical surface. In endothelial cells, adherens junctions and tight junctions can be interspersed along the entire length of the lateral membrane.
3.1. Tight junction proteins
In contrast to the well-developed and apically restricted tight junctions of epithelial cells, EC tight junctions are less structured and are found along the entire length of the paracellular cleft (13, 14). An exception to this is found in the blood-brain barrier; here ECs contain relatively impermeable tight junctions similar to those found in epithelial cells (15), and this is thought to be primarily due to differences in molecular composition with respect to transmembrane proteins (16, 17).
Occludin was the first transmembrane protein of the tight junction to be identified in epithelia (18), and later in endothelia (16). Indications that occludin plays a functional role in leukocyte TEM come from many sources. During experimentally induced inflammation, areas of neutrophil accumulation were often found adjacent to brain microvessels that had a disrupted staining pattern and lower expression of the tight junction proteins occludin and ZO-1 (19). This loss of occludin from cell junctions during TEM can be caused by matrix metalloprotease-mediated degradation (20). Furthermore, in a mouse model of HIV-1 encephalitis, sites of neuro-inflammation were not only correlated with diminished staining of total occludin, but there was an increase in occludin phosphorylation at those same locations; intriguingly, the phosphorylation was directly mediated by Rho kinase (21). However, occludin as a barrier protein in the paracellular pathway may not be the entire story; one intriguing study has suggested that at least in epithelial cells, certain domains of occludin may be required for efficient neutrophil trans-epithelial migration (22). It has even been reported that activated T-cells express occludin; raising the possibility that homophilic binding of leukocyte to EC occludin might help preserve barrier function as leukocytes migrate through EC junctions (23).
Claudin-5 is a vascular EC-specific member of the claudin family of transmembrane tight junction proteins, and this isoform is particularly enriched in the brain (24). Claudin-5 knockout mice exhibit impaired blood-brain barrier permeability to small molecules (25), and increased monocyte TEM has been correlated with local loss of claudin-5 expression in a neuro-AIDS model (21).
The junctional adhesion molecule (JAM) family proteins are another type of transmembrane protein found at the tight junction (26). JAM proteins can engage in homophilic interactions between molecules on adjacent cells (27-29), and when transfected into epithelial cells, JAM expression increases paracellular barrier sealing (30, 31). However, JAM proteins are unique junctional proteins in that they are also receptors for leukocyte integrins (32-35). This raised the possibility that JAM proteins aid adhesion and guidance of leukocyte transmigration across vessels (36). This hypothesis is supported by observations that neutralizing antibodies to JAM-A inhibit spontaneous and chemokine-induced neutrophil TEM (30, 37). Furthermore, intravital microscopy experiments with JAM-A knockout mice and adoptive cell transfer demonstrated decreased leukocyte TEM during IL-1β-induced inflammation (38), and ischemia-reperfusion (39), although the two studies reported differences in whether EC-derived JAM-A (38) or neutrophil JAM-A (39) mediated the effect. In contrast, other groups report no inhibition of leukocyte TEM under flow using different JAM-A antibodies (40). The apparent differences have been attributed to the use of different inflammatory models, or the unique aspects of static versus flow-based assays. JAM-C involvement in positively mediating leukocyte TEM is less controversial; over-expression of JAM-C in ECs promoted lymphocyte TEM (41), while neutralizing antibodies against JAM-C partially blocked TEM during pulmonary inflammation (42), and in a mouse model of acute thioglycollate-induced peritonitis (43). Interestingly, this is not due to a decrease in TEM out of the blood vessel but instead represents an increase in “reverse” TEM from the interstitial tissues back into the blood circulation, indicating that JAM-C plays a key role in egressed leukocyte retention (44).
ESAM, or endothelial-specific adhesion molecule (45), is another tight junction transmembrane protein that contains extracellular immunoglobulin domains, similar to JAM proteins. ESAM knockout mice display inhibition of neutrophil but not lymphocyte TEM using in vivo inflammation models (46). Thus, both ESAM and JAM protein functions correlate positively with leukocyte TEM, reflecting their role as leukocyte counter-receptors. Additionally, knockdown of ESAM protein expression in cultured ECs reduced Rho activity (46), which as I will discuss later, is required for leukocyte TEM.
In addition to these transmembrane proteins, there are also a number of intracellular proteins which form a complex on the cytoplasmic face of the plasma membrane immediately underlying the tight junction. The predominant cytoplasmic TJ proteins in endothelia are the zonula occludens family proteins ZO-1 and ZO-2 (47, 48). These play a scaffolding role, linking the cytoplasmic domain of occludin to the actin cytoskeleton (49, 50), and also recruiting other signaling molecules that function during junctional regulation. Some of these proteins include AF-6, a putative GTPase effector, exchange factors for GTPases (GEF-H1), kinases such as aPKC, and phosphatases (RPTPβ, PP2A) (51).
3.2. Adherens junction proteins
Adherens junctions present along the paracellular cleft form the other major barrier to leukocyte TEM, and the predominant transmembrane protein forming contacts between adjacent cells is VE-cadherin. The extracellular domain interacts with other VE-cadherin molecules on adjacent ECs in a calcium-dependent manner (52). That VE-cadherin is relevant during leukocyte TEM is highlighted by the observation that injection of function-blocking antibodies cause increased leukocyte TEM in several mouse models of inflammation (53, 54), as well as in in vitro studies (55, 56). Real-time video microscopy has revealed that during TEM, VE-cadherin is physically displaced from adherens junctions during TEM and then relocalizes within minutes once the leukocyte has completed transmigration (57). Thus, regulation of VE-cadherin at the adherens junction is an important aspect of regulating leukocyte TEM. Further discussion of VE-cadherin-mediated signal transduction as it occurs during TEM will be elaborated in later sections of this review.
At the cytoplasmic face of the adherens junction, VE-cadherin forms a complex with alpha-, beta-, and p120-catenin. Through these interactions, VE-cadherin is connected to the actin cytoskeleton and this connection is required for maintenance of EC barrier function (55). The dogma has been that alpha-catenin acts as an intermediate binding protein linking F-actin with the beta-catenin/VE-cadherin complex (58). However, the idea of a static VE-cadherin/beta-catenin/alpha-catenin/F-actin complex has been challenged recently by reconstitution experiments showing that alpha-catenin does not interact simultaneously with the cadherin/beta-catenin complex and F-actin (59). Instead, the interaction between these proteins may be too weak or transient to be readily detected in vitro, or there may be unidentified components or physical properties such as mechanical tension present in a cellular context that are absent in vitro (60). Recent data show that the actin-binding protein EPLIN (epithelial protein lost in neoplasm) (61) may be one of these unidentified components linking the cadherin-catenin complex to F-actin (62). p120 catenin does not participate in connecting the cadherin complex to the cytoskeleton, but it does contribute to barrier function by regulating the stability of the complex (63). Junctional proteins like VE-cadherin and the catenins are often targeted for phosphorylation; high level of phosphorylation is an indicator of junctional immaturity, junctional disassembly, and increased TEM events (64-66).
3.3. PECAM and CD99
PECAM-1 (CD31) is a member of the Ig superfamily of transmembrane proteins and is expressed on ECs, platelets, and subsets of leukocytes (67). In numerous studies, PECAM-1 has been described as being present along the full length of EC lateral membranes, with particular concentration at cell-cell junctions (68, 69). PECAM-1 has also been found evenly distributed over the entire apical surface (70); this differential localization was attributed to accessibility of different antibodies used in these studies. Interestingly, localization of cell surface PECAM-1 is regulated by various angiogenic (angiopoietin-1) and inflammatory agents (TNF-alpha, IFN-gamma) (71, 72). The junctional pool of PECAM-1 plays a critical role in cell-cell adhesion, owing to its ability to engage in homotypic binding interactions with PECAM molecules on the adjacent endothelial cells (73, 74). In addition to its homophilic binding capabilities, the intracellular domain of PECAM-1 also acts as a molecular scaffold, recruiting and binding other junctional proteins like beta- and gamma-catenin (75), as well as a host of signaling proteins including phosphatases and kinases (reviewed in (76)). The importance of this scaffolding function is underscored by the recent observation that upon histamine treatment of ECs, phosphorylated PECAM-1 recruits both the phosphatase SHP-2 and phosphorylated beta-catenin to its cytoplasmic domain. This facilitates the subsequent dephosphorylation of beta-catenin, allowing it to bind to VE-cadherin and thus promotes adherens junction reassembly (77). A unique PECAM-containing membranous compartment underlying EC junctions has also been described (78). During TEM, PECAM-1 is specifically targeted to sites of monocyte migration, and blocking antibodies inhibit both this targeting (78). PECAM-1 has long been implicated in the process of leukocyte TEM. Early studies demonstrated that pre-treatment of either monocytes or ECs with PECAM antibodies blocks TEM (79), and this also holds true in models of acute inflammation in vivo (80-82). Furthermore, PECAM-1 knockout mice exhibit increased vascular permeability (83), and reduced leukocyte TEM in the majority of mouse strains (80). PECAM-1 engagement and subsequent upregulation of alpha-6/beta-1 integrin expression on extravasating neutrophils may also aid their migration through the basement membrane (84).
CD99 is a heavily glycosylated protein expressed by most leukocytes and ECs (reviewed in (85)). Addition of blocking antibodies inhibited in vitro TEM by >90% (86). Confocal microscopy has shown that CD99 acts at a later stage of diapedesis than PECAM-1; while PECAM antibody treatment arrests leukocytes on the apical surface of endothelium (79), CD99 antibody-blocked neutrophils are seen lodged part-way through adjacent ECs in the area of cell junctions (87). This, together with the observation that antibody blockade of both PECAM-1 and CD99 together have additive effects on leukocyte TEM (87), indicates a non-overlapping role for these two proteins. Recently a homolog called CD99L2 was identified, and it was shown to be required for monocyte and neutrophil, but not lymphocyte TEM in vivo (88).
3.4. EC-leukocyte adhesion molecules
Other EC adhesion proteins are involved in leukocyte TEM not as part of a junctional structure per se, but as receptors for leukocyte adhesion (Figure 2). When they are engaged and clustered by leukocyte binding, they transduce intracellular signals which we will see later on in this review, can lead to the weakening of cell-cell junctions, cytoskeleton remodeling, and/or membrane fusion events that all contribute to leukocyte TEM.
3.4.1. ICAM-1
Intercellular adhesion molecule 1 (ICAM-1) is an Ig-like adhesion receptor that binds the leukocyte integrin LFA-1 (89, 90). The importance of ICAM-1 for TEM is underscored by the fact that transfection of ICAM-1 alone is completely sufficient to reconstitute neutrophil transmigration across a non-endothelial cell type (CHO, epithelial) that lacks any other leukocyte adhesion receptors (91). The crucial nature of ICAM-1 and also its close relative ICAM-2, is further supported by the observation that knockout mice exhibit deficiencies in the immune response stimulated by chemically-induced peritonitis in vivo (92, 93). Antibody blockade of ICAM-1 also inhibits leukocyte TEM; despite being able to adhere properly, monocytes are not able to direct their lateral movement across the apical surface of the ECs to search out permissible sites for TEM (6). Using real-time imaging of neutrophils migrating across HUVEC monolayers, Shaw and colleagues found that neutrophil integrins rapidly redistributed to form a ring structure which co-clustered with endothelial ICAM-1 close to endothelial cell junctions, and this ring structure was the origin of pseudopods which extended from the leukocyte through EC junctions (94).
3.4.2. VCAM-1
VCAM-1 also mediates adhesion of leukocytes expressing VLA-4 to the endothelium; however it is probably not the principle player during the transmigration stage. This is because antibody blockade of VCAM-1 alone does not significantly reduce monocyte TEM, but blocking both ICAM-1 and VCAM-1 simultaneously has an additive effect (95). Recently, an unbiased screen for VCAM-interacting proteins identified SPARC (secreted protein acidic and rich in cysteine) as a non-integrin receptor for VCAM-1 that is necessary for efficient TEM (96). During leukocyte TEM, the major role of VCAM seems to be more as a signal transducer, whereby engagement of VCAM-1 on the EC surface generates intracellular signals resulting in junctional disruption (97). The signaling mechanisms downstream of both VCAM-1 and ICAM-1 engagement will be discussed in detail in Section 4.
3.5. GTPases and the cytoskeleton
Linking transmembrane junctional proteins to the actin cytoskeleton is critical for the dynamic regulation of EC junctions during permeability fluctuations and leukocyte TEM (98). Indeed, the process of TEM requires the ability of ECs to activate GTPases and rearrange the cytoskeleton (99).
3.5.1. Cytoskeleton
Cortical actin bundles in the cell periphery promote junctional stability and function (13). However, during junctional disruption, for example after acute exposure to shear flow, the distribution of F-actin changes from a peripheral localization to an organization exhibiting cytoplasmic stress fibers (100). Insertion of these stress fibers into junctional sites and the resulting generation of isometric tension are proposed to be major mechanisms of junctional breakdown and induction of permeability. Generation of actomyosin contractility can be mediated either by intracellular calcium signaling or activation of the Rho/Rho kinase pathway (reviewed in (101, 102)). In the first scenario, intracellular calcium leads to Ca2+/calmodulin-dependent myosin light chain kinase (MLCK)-mediated phosphorylation of myosin light chain (MLC). In the second case, Rho kinase activity also results in increased phosphorylation of MLC, both by inhibiting myosin light chain phosphatase (MLCP) (103, 104), and by phosphorylating MLC directly (105). In either situation, the resulting actin-myosin interactions allow contractile force generation (106) which promotes the cell shape changes and junctional breakdown necessary for paracellular TEM. Indeed, pre-treating ECs with specific MLCK inhibitors significantly reduced neutrophil transmigration (107, 108), and furthermore, the adhesion of neutrophils to ECs directly increases MLC phosphorylation (107, 109). Collectively these results confirm that tension-generation by the actomyosin system is used to facilitate the actual paracellular TEM of leukocytes across the endothelium.
3.5.2. Rho family GTPases
When discussing regulation of the cytoskeleton, low molecular weight GTPases are usually implicated as central players (110, 111). These include the familiar members of the Rho family, RhoA, Rac1, and Cdc42, but also include the less-studied RhoG, and GTPases like Rap1, which is actually a member of the Ras subfamily (112). RhoG and Rap1 will be discussed in detail in later sections.
Low molecular weight GTPases are proteins that act as molecular switches which cycle between an active (GTP-bound) and inactive (GDP-bound) form. GTP-binding and subsequent activation of Rho proteins is facilitated by guanine nucleotide exchange factors (GEFs), while inactivation of Rho proteins by hydrolysis of GTP to GDP is catalyzed by GTPase-activating proteins (GAPs) (Figure 3A). Extracellular stimuli are transduced by EC-leukocyte receptors, activating the GTPases, which now bind downstream effectors that lead to multiple downstream signaling cascades (Figure 3B). In ECs these signaling pathways in turn influence cell-cell junctions, actin cytoskeleton remodeling, and membrane dynamics, putting them in a central position to be important regulators of leukocyte TEM events (113).
Figure 3.

Low molecular weight GTPases, via downstream effectors, are key participants of EC signaling pathways involved in leukocyte TEM. (A) Signals transduced by EC adhesion molecules downstream of leukocyte engagement regulate activity of low molecular weight GTPases. GEFs activate the GTPase, enabling it to interact with downstream effectors. In turn, GAPs aid the hydrolysis of GTP to GDP, inactivating the GTPase and inhibiting downstream signaling events. (B) Effector signaling downstream from GTPase activation can control cell adhesion, cytoskeleton remodeling, and membrane dynamics. In turn, this influences barrier function, membrane fusion events, and the formation of cytoskeleton-enriched structures such as apical cups that are involved in leukocyte TEM.
In particular, RhoA and Rac1 were the first small GTPases implicated in modulating the cytoskeleton and cell junctions in ECs. Microinjection of a constitutively active RhoA mutant increased the amount of F-actin stress fibers in ECs, ultimately inducing loss of VE-cadherin from cell junctions and causing EC contraction and gap formation (114). These were qualitatively similar effects to that seen during neutrophil transmigration across a HUVEC monolayer (108), thrombin-induced permeability (115), and TNF-alpha stimulation (114). In agreement with the idea that EC Rho activity is required for TEM is the observation that inhibiting Rho/Rho kinase with C3 toxin or Y-27632 significantly inhibits MLC phosphorylation and the associated leukocyte TEM (116, 117).
On the other hand, Rac involvement in regulating EC junctions is much more controversial. Some experiments, involving over-expression of constitutively active Rac or using sphingosine 1-phosphate to activate Rac have indicated that Rac activity promotes junction formation and reduces EC permeability (118, 119). However, other experiments indicate that Rac activation disrupts EC junctions and increases permeability by a ROS-mediated mechanism (120). In the latter study, the authors showed that cells transduced with constitutively active Rac had a greater permeability increase than those expressing constitutively active Rho (120). A second mechanism by which Rac activation may induce junctional disruption is via its downstream effector, PAK (121). Stockton et al. have shown that EC fluid permeability is regulated by direct phosphorylation of MLC by PAK family kinase activity downstream from Rac (121, 122). PAK activation downstream of Rac can also induce VE-cadherin phosphorylation, subsequent β-arrestin-mediated internalization, and resulting junctional disassembly (123). These discrepancies highlight the need to strictly control GTPase activity for optimal junction integrity because either excessive, or deficient, activity can negatively impact the formation and maintenance of cell junctions (124).
3.5.3. Rap1 GTPase
Rap1 is another member of the small G-protein superfamily, but unlike Rho, Rac, and cdc42, it is a member of the Ras subfamily (125). Previously, Rap1 had been well-studied in the context of leukocyte integrin activation (126). With regard to leukocyte TEM, this is critical during the transition to firm adhesion onto ECs as well as aiding polarization during directed migration on the apical surface of ECs before the transmigration step (127). It is now appreciated that Rap1 has important integrin-independent functions within the EC. For example, Rap1 activation downstream of tissue inhibitors of metalloproteinase (TIMP)-signaling has been implicated as a mechanism regulating EC adhesion and migration during processes such as angiogenesis (128, 129). A number of groups have also documented a role for Rap1 and its cAMP-responsive GEF Epac-1, in promoting EC junctional integrity and barrier function (4, 130-132). Rap activation and subsequent EC junctional strengthening has also been implicated as a mechanism for inhibiting leukocyte TEM (133). Calcium switch experiments have shown that endogenous Rap1 is activated at early time points of junctional re-assembly (133), while biosensor experiments have confirmed that Rap1 is specifically localized to, and activated at, sites of junctional contact (134). The exact mechanism of how Rap1 promotes junctional stability is still elusive, although it likely depends on cytoskeletal rearrangements, in particular, the loss of actin stress fibers and increased cortical actin that is seen upon Rap1 activation (130-132). Besides the actin-based cytoskeleton, an intact microtubule cytoskeleton is also required for the dynamic regulation of EC junctions (135, 136). The Rap GEF Epac1 may perform another function that aids in junction stability independent of Rap1 activation, via its effects on these microtubules. Epac-1 co-localizes with microtubules, promoting their growth and stabilization (137). The authors propose that Epac-1 promotes barrier function in two ways: activating Rap which induces cortical actin remodeling, as well as a more direct effect on stabilizing the microtubule network; it is the coordinate regulation of both of these cytoskeletal structures that is critical for EC junction integrity (137).
Recently, the tight junction protein JAM-C was shown to be a key regulator of Rap1 activity in ECs, with JAM-C downregulating Rap1 activity and subsequently destabilizing adherens junctions (138). Intriguingly, this is opposite to the case for JAM-A in epithelial cells, in which JAM-A engagement improved junctional integrity along with Rap1 activation (139). An interesting possibility is that JAM protein regulation of Rap1 activity and thus VE-cadherin-based adhesion is in analogy to the role of nectins in epithelial cells, in which the stability of E-cadherin cell contacts is also mediated through effects on Rap1 (140). Thus Rap1 may be a central player linking regulation of both adherens and tight junctions in endothelia (141).
Progress is also being made in identification of downstream Rap effectors that may play a role in EC junctional regulation. One of these, KRIT-1 (protein mutated in cerebral cavernous malformation, a disease of vascular impairment) localizes to EC junctions in a Rap-GTP dependent manner, possibly by binding to beta-or p120-catenin (142). Using an siRNA approach, KRIT-1 was shown to be necessary for such Rap1-mediated junctional effects like decreased stress fibers, increased cortical actin, and decreased permeability. Another putative Rap effector at cell-cell junctions is AF-6 (afadin), which is a multidomain scaffolding protein that participates in linking transmembrane junctional components like cadherins, nectins, and JAM proteins to the actin cytoskeleton (143-145). AF-6 also selectively binds active Rap1-GTP, along with its GAPs Spa-1 and RapGAP-1, suggesting that AF-6 could be a central player in regulating EC junctions by controlling local Rap1 activation status through the recruitment of both Rap and its GAPs (146). However, besides negatively regulating local Rap1 activity, AF-6 might also be involved in the cytoskeleton remodeling effects seen upon Rap1 activation at cell-cell junctions, since it also binds profilin, which has been shown to promote cortical actin assembly (147). MAGI-1 (Membrane-Associated Guanylate kinase with Inverted orientation), yet another multi-domain scaffolding protein that localizes to cell-cell junctions by binding to ESAM and/or beta-catenin (134, 148), also binds a Rap GEF, PDZ-GEF1 (149). Homophilic engagement of VE-cadherin activates Rap1 in a MAGI-1-dependent manner, owing to the recruitment of PDZ-GEF1 by MAGI-1 (134). In conclusion, the existence of multiple junctional proteins that recruit both positive and negative regulators of Rap GTPase activity points toward a role for Rap1 in fine-tuning dynamic junction assembly/disassembly events such as those that occur during leukocyte TEM.
4. SIGNALING EVENTS DURING TEM
During the early stages of leukocyte TEM, firm adhesion of leukocytes induces clustering of EC adhesion molecules like ICAM-1 and VCAM-1 (150-152). This engagement triggers activation of many intracellular signaling pathways which induce EC actin cytoskeleton and cell junction remodeling. This sets the stage for weakening of EC junctions, allowing paracellular TEM to occur. Next, I will summarize the diverse signaling mechanisms downstream of leukocyte engagement of EC adhesion proteins and how this influences TEM events.
4.1. Signaling downstream of ICAM
ICAM-1 is perhaps the most prominent upstream adhesion molecule responsible for transducing outside-in signals that aid leukocyte TEM. The observation that deletion of the intracellular region of ICAM-1 completely abolished neutrophil TEM provided a convincing demonstration that ICAM-1-mediated signals generated by the EC were required for TEM, and that ECs were active participants in the leukocyte TEM process (91). ICAM-1 involvement in some of the key pathways is described in the following sections and is illustrated schematically in Figure 4.
Figure 4.
Schematic diagram of signaling events initiated downstream of ICAM-1 engagement. Leukocyte binding to ICAM-1 triggers diverse signaling pathways within the EC (highlighted in red). Phosphorylation of target proteins (Section 4.1.3.), particularly the VE-cadherin complex (Section 4.1.4.), production of ROS (Section 4.1.5.), activation of Rho family GTPases (Section 4.1.1.), and calcium signaling (Section 4.1.2.) are centrally involved. These pathways all contribute to the junctional disruption and/or actin remodeling that is permissive for leukocyte TEM to occur. (see text for details)
4.1.1. Rho GTPase signaling
Subsequently, it was discovered through the use of bacterial toxins that inhibit Rho activity, that Rho GTPase signaling and its effects on the actin cytoskeleton were a critical aspect of this ICAM-1-mediated signal (153, 154). Artificially simulating leukocyte adhesion to ECs by cross-linking ICAM-1 with antibodies revealed that RhoA activation was an early event occurring downstream of ICAM-1, but not ICAM-2 receptor clustering (155). Rho activation correlated with stress fiber formation as shown previously (99, 155), and furthermore, this cytoskeletal remodeling occurred in conjunction with recruitment of ERM proteins to sites of ICAM-1 clustering (150, 155). The ERM (ezrin/radixin/moesin) family of proteins functions to link cytoskeletal elements to transmembrane proteins and therefore they play an important role during cytoskeletal dynamics and regulation of signaling pathways downstream from proteins like ICAM-1 (156). The recruitment and activation of ERM proteins is a potential mechanism for connecting Rho activation to localized cytoskeleton rearrangements such as formation of stress fibers (157) and actin-rich cell surface membrane structures such as microvilli and transmigratory cups (158) (discussed in Section 5.) Upon ICAM-induced Rho activation, ERM proteins participate to further increase Rho activity in two ways: by binding to and recruiting the Rho GEF Dbl (159), and by binding to RhoGDI which releases its inhibition of Rho (160).
4.1.2. Calcium signaling
Another signaling event downstream from ICAM-1 engagement is increased intracellular calcium levels. Calcium-dependent activation of PKC activates Src and mediates tyrosine phosphorylation of actin-associated proteins like FAK and paxillin (161). Furthermore, the same group showed that chelating intracellular calcium or treating ECs with PKC inhibitors significantly decreased T-cell TEM, highlighting the importance of calcium-mediated signaling. Another outcome of calcium release that impacts TEM events is calcium/calmodulin-driven MLCK activation which as mentioned previously, generates actomyosin-based cell contractility and junctional disruption (101).
4.1.3. Phosphorylation
In addition to activating RhoA, ICAM-1 crosslinking also induces the tyrosine phosphorylation of many EC proteins. Some of these are cytoskeleton-associated proteins, like FAK, paxillin, p130Cas, ezrin, and cortactin (99, 151,162-164). In addition, kinases like Src and p38MAPK have been identified as being phosphorylated and/or activated after ICAM-1 crosslinking (165, 166). Activation of the tyrosine kinase Src is a key step downstream of ICAM-1 engagement, responsible for many of these phosphorylation events. In particular, ICAM-1-mediated phosphorylation of cortactin by Src is clearly involved in leukocyte TEM. Cortactin is a protein scaffold that is well-studied for its role in Arp2/3-mediated actin remodeling events especially in the cell cortex (167). Thus cortactin is an ideal candidate for linking ICAM-1 engagement during leukocyte adhesion to the downstream clustering of adhesion molecules required during TEM. Src-mediated phosphorylation of cortactin was required for both E-selectin and ICAM-1 clustering on the EC surface (151). Furthermore, Src phosphorylation of Y421, Y466, and Y482 of cortactin was necessary for its dynamic redistribution to junctional regions where neutrophils were actively transmigrating, and inhibition of Src or knockdown of cortactin inhibited neutrophil TEM (164). Collectively, these results imply a role for cortactin in TEM, not only for its association with the actin polymerization machinery at the cell cortex, but also for stabilizing the association of actin with adhesion molecules such as ICAM-1.
4.1.4. VE-cadherin
Most recently, the adherens junction protein VE-cadherin has been identified as target for Src and/or pyk2-mediated tyrosine phosphorylation downstream of ICAM-1 engagement (168). As mentioned previously, VE-cadherin is one of the main transmembrane proteins that act as a barrier to leukocyte TEM via the paracellular route, and tyrosine phosphorylation of both cadherins and catenins has been implicated as a mechanism for inducing junctional disassembly (64, 65, 169, 170). Furthermore, increased tyrosine phosphorylation of the VE-cadherin-catenin complex is seen upon treatment of ECs with permeability-inducing compounds such as histamine and VEGF (171, 172). Previous experiments indicated that VE-cadherin was a target for Src kinase and that tyrosine phosphorylation of residues Y658 and Y731 inhibited the binding of beta-catenin and p120 catenin, thus negatively regulating adherens junction function and stability (173). Allingham et al. (168) showed that this phosphorylation of VE-cadherin occurred downstream of ICAM-1 cross-linking, and that both Src and pyk2 tyrosine kinases are specifically recruited to sites of ICAM-1 engagement. Moreover, inhibition of either kinase or expression of nonphosphorylatable VE-cadherin mutants significantly attenuated neutrophil TEM (168). These findings are corroborated by a recent report where ICAM-mediated VE-cadherin phosphorylation in brain microvascular cells also increased paracellular TEM of lymphocytes (174).
4.1.5. Reactive oxygen species
Another EC-generated signal downstream of ICAM-1 engagement is production of intracellular reactive oxygen species (ROS). In one study, neutrophil adherence to ECs triggered production of xanthine oxidase-generated ROS and this could be inhibited with anti-ICAM-1 antibodies (175). The production of ROS is now known to be downstream of other EC-leukocyte receptors such as ICAM-3 and VCAM-1, and ROS has been implicated during conditions of increased permeability and leukocyte TEM (97, 176). Subsequent work placed ICAM-1-induced ROS production upstream of both Src and p38MAPK activation, and showed that this entire pathway was required for ICAM-1-induced cytoskeletal remodeling such as the generation of actin stress fibers (165, 166). EC-derived ROS may mediate leukocyte TEM in several ways, one of which is by promoting the necessary EC actin cytoskeleton rearrangement to facilitate TEM. Indeed, one of the proteins that is activated upon ROS production in ECs is hsp27 (177). Hsp27 is a substrate of p38MAPK, and is thought to be an important effector protein regulating actin polymerization (178); whether this has relevance for mediating leukocyte TEM remains to be tested. ROS-induced cytoskeleton rearrangements might be necessary for efficient leukocyte migration towards EC junctions as hypothesized by Wang et al. (179), or alternatively, the ROS-mediated generation of actin-based contractile elements within the EC may have a more direct effect on junction instability. A second way EC-derived ROS may mediate leukocyte TEM is via effects on tyrosine phosphatases. ROS can inhibit phosphatase activity by reversible oxidation of the catalytic cysteine residue (180), and the resulting increase in tyrosine phosphorylated junctional proteins might cause junctional disruption and concomitantly increased TEM.
4.2. Signaling downstream of VCAM
VCAM-1, the other major EC leukocyte-adhesion molecule, also acts as a signal transducer during leukocyte TEM. Engagement of VCAM-1 by adhering leukocytes or artificial stimulation with VCAM-1 antibody-coated beads generates two major intracellular signals: activation of the GTPase Rac1 and production of ROS, these pathways are shown in Figure 5.
Figure 5.
Schematic diagram of signaling pathways initiated downstream of VCAM-1 engagement. Leukocyte adhesion to VCAM-1 mainly signals via Rac1-mediated ROS generation. ROS inhibition of phosphatases and activation of redox-sensitive kinases serve to increase phosphorylation of junctional proteins, and together with production of MMPs, leads to junctional disruption. The Rac effector PAK has also been implicated in actin remodeling via MLC-generated tension and contractility. (see text for details)
4.2.1. Rac activation and ROS generation
It is now appreciated that non-phagocytic cells also produce ROS, which have been implicated as novel second messengers that help regulate cellular events like angiogenesis and inflammation (181). Interestingly, Rac1 is a component of the vascular NADPH oxidase complex, and active Rac promotes the assembly and activity of the complex (182). Several key observations linked ROS production and Rac activity to increased EC permeability and leukocyte TEM. For example, introduction of constitutively active Rac caused loss of VE-cadherin-mediated adhesion, and blocking ROS production with free radical scavengers protected against this loss of cell-cell adhesion (120). Treating ECs with inhibitors of NADPH oxidase and ROS scavengers was also found to block lymphocyte TEM (183). Subsequently, it was discovered that cross-linking of VCAM leads to a similar increase in permeability, while preventing engagement of VCAM with blocking antibodies inhibited T-cell TEM; these effects also required the production of EC-derived ROS (56). Together, this leads to a pathway whereby VCAM-1 cross-linking results in Rac activation, followed by the production of ROS via Rac-dependent activation of the NADPH oxidase, and Rho-dependent induction of stress fibers, all of which result in the gap formation or at least junctional weakening, that facilitates paracellular TEM (97). ROS may also in turn influence the activity of Rho and Rac, providing a feedback loop of cytoskeleton regulation.
4.2.2. Phosphorylation
Rac-mediated ROS production downstream of VCAM also influences other signaling pathways that are implicated in regulation of leukocyte TEM. As mentioned earlier, phosphorylation of junctional proteins is a predominant mechanism for regulating their stability and localization at the junctional complex (180). In this regard, ROS production has been correlated with increased tyrosine phosphorylation of alpha-catenin (120). In addition, ROS can activate a redox-sensitive kinase pyk2, which phosphorylates beta-catenin, leading to its dissociation from VE-cadherin and the dissolution of the cadherin/catenin complex (170). PKC has also been shown to be activated downstream of VCAM-1 by direct oxidation, and this transient activation is required for efficient TEM (184). Activation of PKC-alpha in this manner can also phosphorylate PTP1b, a phosphatase (185). While it was shown that PTP1b activity is required for TEM, the exact mechanism of how its activity promotes lymphocyte TEM remains to be determined. Another potential signaling target of Rac-induced ROS produced downstream of VCAM engagement is the activation of cell-associated proteases. Experiments by Deem et al. showed that lymphocyte binding to VCAM rapidly activates matrix metalloproteases (MMPs); this activation was accompanied by production of ROS, and scavenging that ROS prevented VCAM-1-dependent lymphocyte TEM (186).
5. EC APICAL CUP STRUCTURES
As discussed in the previous section, it is evident that ECs play an active role during the process of leukocyte TEM. This can occur either via activation of signaling events which alter the EC cytoskeleton, or through opening of intercellular junctions. Recently however, another dramatic example of EC participation in TEM was also described. Upon leukocyte adhesion, a novel endothelial “docking structure” was induced that anchored and partially embraced the adherent leukocyte (158). In fact, earlier in vivo ultrastructural studies also described ECs “embracing” migrating leukocytes (187-189). Barreiro et al. were the first group to describe the structure in terms of its protein components as actin-rich, containing α-actinin, vinculin, VASP, the ERM proteins ezrin and moesin, and the EC adhesion molecules ICAM-1 and VCAM-1 (158). In their model, leukocyte attachment induced clustering of VCAM-1 and ICAM-1 on the EC surface, activating bound ERM proteins and causing local actin remodeling and formation of the membrane protrusions around the attached leukocyte (Figure 6). It was hypothesized that the formation of these docking structures was necessary for efficient capture of leukocytes by the EC, especially under conditions of shear flow, and that disassembly of these structures must presumably occur before diapedesis. Expanding on these findings, Carman et al. determined that leukocyte LFA-1 binding to ICAM-1 was necessary for formation of these “microvilli-like” endothelial membrane projections (190). Further characterization revealed that “cup” structure formation around adherent leukocytes required an intact actin and microtubule cytoskeleton as indicated by the inhibitory effect of cytochalasin D and colchicine treatment. In contrast to Barreiro et al. (158), formation of cup structures did not significantly increase adhesion under flow. Thus their hypothesis was that the function was not adhesion-strengthening, but instead could be required for the actual TEM process. This idea was validated by further data that ICAM-1-containing projections are highly associated with leukocytes that are actively transmigrating (191). Cumulatively, these findings provided more evidence for “transmigratory cup structures” as directional guides for leukocytes to traverse the endothelium.
Figure 6.
Molecular architecture of EC transmigratory cup structures. EC participation in TEM is dramatically illustrated by the formation of apical cup structures. These EC-derived actin and intermediate filament-rich membrane protrusions partially surround and “embrace” the leukocyte. Assembly of apical cup structures requires clustering of EC adhesion proteins like ICAM-1 and VCAM-1, coupled to cortactin and/or ERM protein-mediated local and directed actin polymerization.
5.1. Mechanisms of assembly
These seminal papers described the morphology and molecular components of cup structures, and began to characterize their putative function in TEM. However, much work remains to be done to describe the signaling mechanism(s) required for assembly. One recent report implicated cortactin as a possible mechanistic link between adhesion molecule engagement/redistribution and the subsequent association with the underlying actin cytoskeleton that ultimately leads to cup formation and enhanced TEM (152). A scheme has been envisioned whereby leukocyte engagement of ICAM-1 leads to recruitment of Src and its phosphorylated substrate cortactin, which then leads to temporal and spatial actin remodeling. Further clustering and adhesive strengthening between the leukocyte integrins and EC adhesion molecules such as ICAM-1 may feedback to amplify signals leading to more efficient leukocyte TEM (152). Whether the ring-like docking structures observed in these studies are transitionary, leading to transmigratory cup formation was left unclear. However, the role for cortactin in assembly of either of these structures leading to TEM was certainly established.
Apical cup formation has also been reported to require localized polymerization of actin filaments (158, 190), an intact microtubule cytoskeleton (190), and also vimentin intermediate filament networks (192). Rho family GTPases are well-known regulators of such cytoskeletal dynamics (193). One of the most recent studies to delve into the molecular mechanisms of cup formation and the signaling pathways that regulate their assembly focused on one of these small GTPases, RhoG, and its upstream regulator SGEF (SH3-containing GEF). SGEF is a RhoG-specific GEF that when expressed in fibroblasts, induces the formation of dorsal membrane ruffles and enhances macropinocytosis (194). Since dorsal ruffles induced by SGEF superficially resemble endothelial cup structures induced by leukocyte adhesion, the rationale was that they may be regulated by similar mechanisms. Van Buul et al. (195) discovered that engagement of ICAM-1 rapidly activates RhoG, and both RhoG and SGEF become colocalized with clustered ICAM-1 in the apical membrane protrusions surrounding the adherent leukocyte. SGEF/RhoG involvement in apical cup formation was confirmed by siRNA-mediated knockdown of both proteins, and while leukocyte adhesion was not affected, cup formation and subsequent leukocyte TEM was inhibited. The exact mechanism of SGEF-mediated RhoG activation downstream of ICAM engagement remains unclear. The SGEF SH3 domain binds to the ICAM-1 cytoplasmic region; however, this interaction is independent of SGEF activation state since binding occurs even when SGEF is mutationally inactivated. Presumably ICAM engagement by leukocyte adhesion must trigger activation of the already-bound SGEF, possibly through phosphorylation by a kinase such as Src (195), which is known to target multiple substrates downstream of ICAM such as cortactin (99, 151, 163, 164, 166) and VE-cadherin (168). Interestingly, SGEF and RhoG have also been associated with engulfment of Salmonella by phagocytic cup structures on the apical surface of epithelial cells (196). While apical cups have been discussed so far in the context of paracellular TEM, it is important to note that these structures have also been observed during leukocyte transcellular migration, independently of EC junctions (188, 189, 191). This alternate route of TEM is discussed in the next section.
6. TRANSCELLULAR TEM
Thus far, leukocyte TEM has mostly been described in terms of crossing the endothelial barrier by breaching the intercellular junctions that connect adjacent ECs, i.e. the “paracellular” route (Figure 7). However, as mentioned in the introduction, this is not the only route, or possibly even the most prevalent means by which leukocytes extravasate. In the early 1960s, detailed morphological studies of leukocyte TEM revealed that a significant amount of leukocytes seemed to transmigrate without disrupting the EC junctions (197). Instead of using the paracellular course, they transmigrated directly “across” or “through” an individual EC cytoplasm, using this so-called “transcellular” route (198) (Figure 7). One reason why the notion of transcellular TEM fell out of favor is likely due to the advances made in identifying the molecular constituents of cell-cell junctions. The ability to inhibit or activate known protein components gave researchers new experimental tools that were ideally suited to study paracellular (junctional) TEM. A second reason why the idea of transcellular TEM was slow to gain widespread acceptance had to do with the technical challenges of capturing it in an experimental setting. Advances in electron microscopy techniques and computer generated three-dimensional reconstructions ultimately permitted visualization of neutrophils migrating through a transcellular pore that passed cleanly through the cytoplasm of the EC very close to, but decidedly distinct from EC junctions (189). This also raised the caveat that many studies might be underestimating the amount of transcellular TEM if migration occurs so close to the EC junctions that they are mistaken for paracellular events. Other in vivo studies have since confirmed the existence of a transcellular route (199, 200). Besides the inherent difficulty in visualizing transcellular TEM, the last major obstacle to the acceptance of this phenomenon was probably conceptual. It is rather more difficult to envision a leukocyte penetrating and passing straight through the cytoplasm of another cell, than it is to imagine the leukocyte squeezing in between adjacent cells in a zipper-like model of junction disassembly. The recent resurgence in the recognition of the transcellular mode of TEM may in part be due to more in-depth descriptions of membrane structures which could aid transcellular pore formation. The subsequent section will describe these structures, putting them in context with the current experimental evidence for transcellular TEM.
Figure 7.
Modes of leukocyte TEM: paracellular versus transcellular. Leukocytes can transmigrate across the endothelium via two independent routes. Use of the “paracellular” route requires transient junctional disruption as leukocytes migrate between adjacent ECs. Paracellular TEM may involve a series of PECAM-enriched EC surface-connected membrane compartments that are located adjacent to the junctional region. Conversely, “transcellular” TEM involves leukocyte passage directly through an individual EC, bypassing the need to disassemble EC junctions. Local fusion of caveolae or vesiculo-vacuolar organelles may be a potential mechanism of transcellular pore formation. Recent data suggest that transcellular TEM is strongly dependent on the formation of “invasive podosomes” that are extended by the leukocyte, while EC apical cup structures may aid in both types of TEM.
6.1. EC membranous structures in relation to transcellular TEM
ECs contain subcellular membranous structures such as caveolae, fenestrae, and vesiculo-vacuolar organelles (VVOs) which function in regulating microvascular permeability and inflammation. These specialized membrane microdomains have been implicated as the sites of transendothelial exchange of fluid and plasma proteins in and out of the bloodstream (201), but may also directly or indirectly impact leukocyte TEM. Fenestrae are specialized plasma membrane microdomains, where the membrane “thins out” into circular openings usually spanned by a non-membranous diaphragm. Fenestrated endothelium is normally present only in specialized vascular beds that are functionally required to have high permeability e.g. in endocrine organs where secretion of hormones into the bloodstream occurs (11). Interestingly, tumor neovasculature is also highly fenestrated (202), and VEGF application in vivo rapidly (<10 min) induced the formation of fenestrae in skeletal muscle and dermal endothelium which are both normally non-fenestrated (continuous) (203). In addition to inducing fenestrations, VEGF-treatment in vitro was also correlated with increased appearance of fused, clustered, caveolae-like vesicles separated by diaphragms (204). Such aggregated vesicles have been referred to as vesiculo-vacuolar organelles (VVOs) (205). An important unresolved question is whether caveolae fuse and give rise to VVOs and/or fenestrations. Caveolae, but not the VVO or fenestrations contain caveolin-1 (204), which suggests that caveolin-1 must be specifically excluded if there is a transition from caveolae to fused clustered vesicles (VVO’s) and fenestrae. The hypothesis that membrane fusion events are required to form a transcellular channel permissive for leukocyte passage is bolstered by the observation that lymphocytes transmigrate at caveolin-1 enriched areas, and that reduction of caveolin-1 expression by siRNA specifically decreases transcellular TEM (206). Local fusion of caveolae that are concentrated around the transmigrating leukocyte might therefore be one way to form a transcellular pore linking the apical with the basal EC membrane (Figure 7). Caveolin-independent mechanisms may exist as well, since the site of leukocyte transcellular TEM is often associated with an abundance of vesicles or VVO-like structures in the adjacent EC cytoplasm (8). In these cases, membrane fusion events leading to transcellular pore formation was dependent on the SNARE-containing membrane fusion complex. Membrane fusion events need not be restricted to transcellular TEM. Another related membrane structure, apparently distinct from VVOs, concentrates adjacent to lateral EC junctions (78). This novel “EC surface-connected compartment” is comprised of PECAM-containing membrane vesicles that continually recycle in and out of this network under steady state, but upon leukocyte adhesion, membrane recycling becomes specifically targeted to junctional leukocyte adhesion sites in a kinesin and microtubule-dependent process (136). The disgorgement of membrane would increase the surface area of PECAM-containing membrane in contact with the leukocyte, assisting in maintaining the tight seal between EC and leukocyte during paracellular TEM (207).
6.2. Factors regulating transcellular migration
Mechanistic study of transcellular TEM requires the ability to observe it in an experimentally malleable setting. Further bolstered by the in vivo studies, several groups began to redouble their efforts to observe transcellular TEM in vitro. Ultimately, high resolution confocal microscopy provided in vitro evidence that leukocyte TEM can occur by both a paracellular and transcellular route (191). The physiological factors that promote transcellular TEM are currently an area of intense research. Are specific vascular beds more prone to using one route over the other, and/or do certain leukocyte subtypes utilize different modes preferentially? Are there other signaling factors upstream of cup formation such as inflammatory stimulus? The remainder of this section will address these intriguing issues.
By and large, transcellular TEM has been more readily observed in vivo; while in vitro it is the less preferred route. Furthermore, microvascular ECs support transcellular TEM more readily than macrovascular ECs, although the reason for this phenomenon is not known (8, 206). In one experimental system, transcellular movement of lymphocytes accounted for ∼30% of the total TEM across microvascular cells, but less than 10% across macrovascular cells such as HUVECs (8). With respect to leukocyte type, one study reported that T-lymphocytes migrated exclusively paracellularly (208), yet other studies observe a detectable level of T-cell transcellular migration across the same endothelial cell type (8, 192). Another group observed that neutrophil intraluminal crawling was an important mechanism for determining which route was taken i.e. Mac-1 deficient leukocytes, which adhere but do not crawl on the EC apical surface, mainly use the transcellular pathway (7). This group further made the observation that ECs form “domes” that encapsulate neutrophils undergoing transcellular TEM, and formation of these structures help preserve barrier integrity while TEM is occurring (200). A final hypothesis is that exposure to shear forces might affect transmigration route preference (209). Although shear flow can globally increase TEM, in one study it did not promote one route over the other (208). Thus, whether any of these factors affect transcellular TEM is still under investigation. Another factor that can influence TEM route preference has to do with endothelial ICAM-1 levels. Yang et al. (208) demonstrated that transcellular TEM events were positively correlated with increased expression of ICAM-1, for example after 24 hour treatment with TNF-alpha. They surmised that ICAM-1 levels influence the route of TEM due to higher occupancy of the cognate receptor on the leukocyte (LFA-1) promoting rapid, non-junctional arrest and thus transcellular TEM. This phenotype could be reconstituted by artificially raising ICAM-1 levels by overexpression of full-length ICAM-1, but not ICAM-1 lacking the cytoplasmic domain. In fact, this cytoplasmic domain deletion of ICAM-1 not only decreased overall TEM as previously described (91, 153, 154), but this was specifically due to a decrease in the transcellular fraction (208).
Another reason for differences in route choice may depend on strength of intercellular junctions within the monolayer in question, which in turn depends on locally regulated cytokine exposure. This idea was tested using IL-1β treatment which increases the amount of stress fibers, and disrupts EC junctions (210). Most importantly, this treatment caused a 40% decrease in the number of monocytes migrating transcellularly. A similar explanation was proposed for the observation that TEM of neutrophils was exclusively paracellular on HUVECs stimulated for only 4 hours with TNF-alpha, but after 24 hours of stimulation a significant (10%) number of TEM events now occurred transcellularly (208). Such short-term acute application of cytokines such as TNF-alpha has been shown to cause junctional disruption (114, 211, 212). Therefore, in this scenario, neutrophils could sense and utilize the path of least resistance; if junctions were weakened, paracellular migration was facilitated at the expense of transcellular TEM. It remains to be seen whether inhibition of paracellular migration by strengthening junctions results in a compensatory increase in transcellular TEM. This may be a relevant factor in vivo because historically it was transcellular, not paracellular TEM that was invariably observed across vessels of the brain microvasculature which have very elaborate and impermeable cell junctions (198). Lastly, in other studies where transcellular migration was inhibited through caveolin-1 knockdown, overall TEM was partially compensated for by enhanced paracellular migration (206). In summary, it is now acknowledged that leukocytes can in fact use the transcellular route of TEM, and the differential signaling involved in route selection will be an exciting area of intense research.
6.3. Integrating EC membrane fusion events with leukocyte invasive podosomes
Much attention has been given to EC-derived structures that are actively involved in the progression of leukocyte transcellular TEM. However, the leukocyte also plays an active role in this process. Lymphocytes have been observed extending podosome-like protrusions which probe the EC apical surface, and then initiate transcellular invasion at a permissive location of the cytoplasm (8) (Figure 7). In response to palpation by leukocyte podosomes, EC surface indentations termed “podoprints” form. . Recent work by Carman et al. (8) demonstrated that transcellular TEM, but not paracellular TEM was highly dependent on these “invasive” podosomes, and that their formation required lymphocyte Src and N-WASP activity. The authors propose that this dynamic probing is a mechanism for leukocytes to sense areas of low endothelial resistance, and once a permissive area is identified, leukocyte protrusions can progress to become true invasive podosomes which ultimately breach the endothelium. Leukocyte invasive podosomes may act in concert with endothelial membrane protrusions (cups), which might provide a vertical traction substrate for the podosomes against the EC surface (8). Intriguingly, there was often an abundance of vesicles or VVO-like structures in the EC cytoplasm adjacent to these invasive podosomes, which also lends credence to the idea that podosomes elicit an EC response to trigger membrane fusion events that would facilitate formation of a transcellular pore.
7. SUMMARY AND PERSPECTIVE
Exciting progress is being made in elucidating the complex mechanisms that orchestrate leukocyte TEM, and a current theme is that ECs are active participants in this process. Leukocyte engagement of EC adhesion receptors triggers signaling events impacting actin cytoskeleton and EC junction remodeling, both of which contribute to the TEM response. The renewed recognition of the transcellular mode of TEM is an exciting example of how the field is constantly progressing. Perhaps the most important unresolved issue in this area of research is the classification of stimuli and signaling that contribute to the relative utilization of the paracellular versus transcellular route of TEM. Interestingly, endothelial apical cup structures may participate in both modes of TEM by synergizing with leukocyte podosome formation to help stabilize and guide TEM. Continued research into the process of leukocyte TEM will be vital to the understanding and rational design of potential therapies for clinical conditions where an inappropriate inflammatory response leads to disease.
8. ACKNOWLEDGMENTS
This work was supported by Grants HL-080166 and HL-45100 from the National Institutes of Health. The author thanks Keith Burridge and members of the Burridge laboratory for helpful comments on the manuscript.
Abbreviations
- EC
endothelial cell
- ESAM
endothelial-specific adhesion molecule
- GAP
GTPase activating protein
- GEF
guanine nucleotide exchange factor
- HUVEC(s)
human umbilical vein endothelial cell(s)
- ICAM
intercellular adhesion molecule
- IL-1-beta
interleukin-1-beta
- JAM
junctional adhesion molecule
- MAGI-1
membrane-associated guanylate kinase-inverted
- MLC
myosin light chain
- MLCK
myosin light chain kinase
- MMPs
matrix metalloproteinases
- PECAM
platelet endothelial cell adhesion molecule
- ROS
reactive oxygen species
- SGEF
SH3-containing GEF
- TEM
transendothelial migration
- TNF-alpha
tumor necrosis factor alpha
- VCAM
vascular cell adhesion molecule
- VE-cadherin
vascular endothelial cadherin
- VEGF
vascular endothelial growth factor
- VVO
vesiculo-vacuolar organelle
- ZO
zonula occludens
Footnotes
Publisher's Disclaimer: “This is an un-copyedited, author manuscript that has been accepted for publication in the Frontiers in Bioscience. Cite this article as appearing in the Journal of Frontiers in Bioscience. Full citation can be found by searching the Frontiers in Bioscience (http://bioscience.org/search/authors/htm/search.htm) following publication and at PubMed (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?CMD=search&DB=pubmed) following indexing. This article may not be duplicated or reproduced, other than for personal use or within the rule of “Fair Use of Copyrighted Materials” (section 107, Title 17, U.S. Code) without permission of the copyright holder, the Frontiers in Bioscience. From the time of acceptance following peer review, the full final copy edited article of this manuscript will be made available at http://www.bioscience.org/. The Frontiers in Bioscience disclaims any responsibility or liability for errors or omissions in this version of the un-copyedited manuscript or in any version derived from it by the National Institutes of Health or other parties”
9. REFERENCES
- 1.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–6. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 2.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 3.Cook-Mills JM, Deem TL. Active participation of endothelial cells in inflammation. J Leukoc Biol. 2005;77:487–95. doi: 10.1189/jlb.0904554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wittchen ES, van Buul JD, Burridge K, Worthylake RA. Trading spaces: Rap, Rac, and Rho as architects of transendothelial migration. Curr Opin Hematol. 2005;12:14–21. doi: 10.1097/01.moh.0000147892.83713.a7. [DOI] [PubMed] [Google Scholar]
- 5.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 6.Schenkel AR, Mamdouh Z, Muller WA. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat Immunol. 2004;5:393–400. doi: 10.1038/ni1051. [DOI] [PubMed] [Google Scholar]
- 7.Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006;203:2569–75. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carman CV, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, Ochs HD, Dvorak HF, Dvorak AM, Springer TA. Transcellular diapedesis is initiated by invasive podosomes. Immunity. 2007;26:784–97. doi: 10.1016/j.immuni.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns AR, Walker DC, Brown ES, Thurmon LT, Bowden RA, Keese CR, Simon SI, Entman ML, Smith CW. Neutrophil transendothelial migration is independent of tight junctions and occurs preferentially at tricellular corners. J Immunol. 1997;159:2893–903. [PubMed] [Google Scholar]
- 10.Wang S, Voisin MB, Larbi KY, Dangerfield J, Scheiermann C, Tran M, Maxwell PH, Sorokin L, Nourshargh S. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med. 2006;203:1519–32. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–73. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 12.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100:174–90. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 13.Schnittler HJ. Structural and functional aspects of intercellular junctions in vascular endothelium. Basic Res Cardiol. 1998;93(Suppl 3):30–9. doi: 10.1007/s003950050205. [DOI] [PubMed] [Google Scholar]
- 14.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–70. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 15.Rubin LL, Staddon JM. The cell biology of the blood-brain barrier. Annu Rev Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- 16.Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Fujimoto K, Tsukita S, Rubin LL. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci. 1997;110(Pt 14):1603–13. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- 17.Aurrand-Lions M, Johnson-Leger C, Wong C, Du Pasquier L, Imhof BA. Heterogeneity of endothelial junctions is reflected by differential expression and specific subcellular localization of the three JAM family members. Blood. 2001;98:3699–707. doi: 10.1182/blood.v98.13.3699. [DOI] [PubMed] [Google Scholar]
- 18.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–88. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolton SJ, Anthony DC, Perry VH. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood-brain barrier breakdown in vivo. Neuroscience. 1998;86:1245–57. doi: 10.1016/s0306-4522(98)00058-x. [DOI] [PubMed] [Google Scholar]
- 20.Reijerkerk A, Kooij G, van der Pol SM, Khazen S, Dijkstra CD, de Vries HE. Diapedesis of monocytes is associated with MMP-mediated occludin disappearance in brain endothelial cells. Faseb J. 2006;20:2550–2. doi: 10.1096/fj.06-6099fje. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto M, Ramirez SH, Sato S, Kiyota T, Cerny RL, Kaibuchi K, Persidsky Y, Ikezu T. Phosphorylation of claudin-5 and occludin by rho kinase in brain endothelial cells. Am J Pathol. 2008;172:521–33. doi: 10.2353/ajpath.2008.070076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber D, Balda MS, Matter K. Occludin modulates transepithelial migration of neutrophils. J Biol Chem. 2000;275:5773–8. doi: 10.1074/jbc.275.8.5773. [DOI] [PubMed] [Google Scholar]
- 23.Alexander JS, Dayton T, Davis C, Hill S, Jackson TH, Blaschuk O, Symonds M, Okayama N, Kevil CG, Laroux FS, Berney SM, Kimpel D. Activated T-lymphocytes express occludin, a component of tight junctions. Inflammation. 1998;22:573–82. doi: 10.1023/a:1022310429868. [DOI] [PubMed] [Google Scholar]
- 24.Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147:185–94. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–60. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bazzoni G. The JAM family of junctional adhesion molecules. Curr Opin Cell Biol. 2003;15:525–30. doi: 10.1016/s0955-0674(03)00104-2. [DOI] [PubMed] [Google Scholar]
- 27.Bazzoni G, Martinez-Estrada OM, Mueller F, Nelboeck P, Schmid G, Bartfai T, Dejana E, Brockhaus M. Homophilic interaction of junctional adhesion molecule. J Biol Chem. 2000;275:30970–6. doi: 10.1074/jbc.M003946200. [DOI] [PubMed] [Google Scholar]
- 28.Kostrewa D, Brockhaus M, D’Arcy A, Dale GE, Nelboeck P, Schmid G, Mueller F, Bazzoni G, Dejana E, Bartfai T, Winkler FK, Hennig M. X-ray structure of junctional adhesion molecule: structural basis for homophilic adhesion via a novel dimerization motif. Embo J. 2001;20:4391–8. doi: 10.1093/emboj/20.16.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santoso S, Orlova VV, Song K, Sachs UJ, Andrei-Selmer CL, Chavakis T. The homophilic binding of junctional adhesion molecule-C mediates tumor cell-endothelial cell interactions. J Biol Chem. 2005;280:36326–33. doi: 10.1074/jbc.M505059200. [DOI] [PubMed] [Google Scholar]
- 30.Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D, Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–27. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aurrand-Lions M, Duncan L, Ballestrem C, Imhof BA. JAM-2, a novel immunoglobulin superfamily molecule, expressed by endothelial and lymphatic cells. J Biol Chem. 2001;276:2733–41. doi: 10.1074/jbc.M005458200. [DOI] [PubMed] [Google Scholar]
- 32.Ostermann G, Weber KS, Zernecke A, Schroder A, Weber C. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol. 2002;3:151–8. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- 33.Cunningham SA, Rodriguez JM, Arrate MP, Tran TM, Brock TA. JAM2 interacts with alpha4beta1. Facilitation by JAM3. J Biol Chem. 2002;277:27589–92. doi: 10.1074/jbc.C200331200. [DOI] [PubMed] [Google Scholar]
- 34.Santoso S, Sachs UJ, Kroll H, Linder M, Ruf A, Preissner KT, Chavakis T. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J Exp Med. 2002;196:679–91. doi: 10.1084/jem.20020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zen K, Babbin BA, Liu Y, Whelan JB, Nusrat A, Parkos CA. JAM-C is a component of desmosomes and a ligand for CD11b/CD18-mediated neutrophil transepithelial migration. Mol Biol Cell. 2004;15:3926–37. doi: 10.1091/mbc.E04-04-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber C, Fraemohs L, Dejana E. The role of junctional adhesion molecules in vascular inflammation. Nat Rev Immunol. 2007;7:467–77. doi: 10.1038/nri2096. [DOI] [PubMed] [Google Scholar]
- 37.Del Maschio A, De Luigi A, Martin-Padura I, Brockhaus M, Bartfai T, Fruscella P, Adorini L, Martino G, Furlan R, De Simoni MG, Dejana E. Leukocyte recruitment in the cerebrospinal fluid of mice with experimental meningitis is inhibited by an antibody to junctional adhesion molecule (JAM) J Exp Med. 1999;190:1351–6. doi: 10.1084/jem.190.9.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woodfin A, Reichel CA, Khandoga A, Corada M, Voisin MB, Scheiermann C, Haskard DO, Dejana E, Krombach F, Nourshargh S. JAM-A mediates neutrophil transmigration in a stimulus-specific manner in vivo: evidence for sequential roles for JAM-A and PECAM-1 in neutrophil transmigration. Blood. 2007;110:1848–56. doi: 10.1182/blood-2006-09-047431. [DOI] [PubMed] [Google Scholar]
- 39.Corada M, Chimenti S, Cera MR, Vinci M, Salio M, Fiordaliso F, De Angelis N, Villa A, Bossi M, Staszewsky LI, Vecchi A, Parazzoli D, Motoike T, Latini R, Dejana E. Junctional adhesion molecule-A-deficient polymorphonuclear cells show reduced diapedesis in peritonitis and heart ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2005;102:10634–9. doi: 10.1073/pnas.0500147102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw SK, Perkins BN, Lim YC, Liu Y, Nusrat A, Schnell FJ, Parkos CA, Luscinskas FW. Reduced expression of junctional adhesion molecule and platelet/endothelial cell adhesion molecule-1 (CD31) at human vascular endothelial junctions by cytokines tumor necrosis factor-alpha plus interferon-gamma Does not reduce leukocyte transmigration under flow. Am J Pathol. 2001;159:2281–91. doi: 10.1016/s0002-9440(10)63078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson-Leger CA, Aurrand-Lions M, Beltraminelli N, Fasel N, Imhof BA. Junctional adhesion molecule-2 (JAM-2) promotes lymphocyte transendothelial migration. Blood. 2002;100:2479–86. doi: 10.1182/blood-2001-11-0098. [DOI] [PubMed] [Google Scholar]
- 42.Aurrand-Lions M, Lamagna C, Dangerfield JP, Wang S, Herrera P, Nourshargh S, Imhof BA. Junctional adhesion molecule-C regulates the early influx of leukocytes into tissues during inflammation. J Immunol. 2005;174:6406–15. doi: 10.4049/jimmunol.174.10.6406. [DOI] [PubMed] [Google Scholar]
- 43.Chavakis T, Keiper T, Matz-Westphal R, Hersemeyer K, Sachs UJ, Nawroth PP, Preissner KT, Santoso S. The junctional adhesion molecule-C promotes neutrophil transendothelial migration in vitro and in vivo. J Biol Chem. 2004;279:55602–8. doi: 10.1074/jbc.M404676200. [DOI] [PubMed] [Google Scholar]
- 44.Bradfield PF, Scheiermann C, Nourshargh S, Ody C, Luscinskas FW, Rainger GE, Nash GB, Miljkovic-Licina M, Aurrand-Lions M, Imhof BA. JAM-C regulates unidirectional monocyte transendothelial migration in inflammation. Blood. 2007;110:2545–55. doi: 10.1182/blood-2007-03-078733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nasdala I, Wolburg-Buchholz K, Wolburg H, Kuhn A, Ebnet K, Brachtendorf G, Samulowitz U, Kuster B, Engelhardt B, Vestweber D, Butz S. A transmembrane tight junction protein selectively expressed on endothelial cells and platelets. J Biol Chem. 2002;277:16294–303. doi: 10.1074/jbc.M111999200. [DOI] [PubMed] [Google Scholar]
- 46.Wegmann F, Petri B, Khandoga AG, Moser C, Khandoga A, Volkery S, Li H, Nasdala I, Brandau O, Fassler R, Butz S, Krombach F, Vestweber D. ESAM supports neutrophil extravasation, activation of Rho, and VEGF-induced vascular permeability. J Exp Med. 2006;203:1671–7. doi: 10.1084/jem.20060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–66. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jesaitis LA, Goodenough DA. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J Cell Biol. 1994;124:949–61. doi: 10.1083/jcb.124.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wittchen ES, Haskins J, Stevenson BR. Protein interactions at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3. J Biol Chem. 1999;274:35179–85. doi: 10.1074/jbc.274.49.35179. [DOI] [PubMed] [Google Scholar]
- 50.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–53. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 51.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–28. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 52.Kemler R, Ozawa M, Ringwald M. Calcium-dependent cell adhesion molecules. Curr Opin Cell Biol. 1989;1:892–7. doi: 10.1016/0955-0674(89)90055-0. [DOI] [PubMed] [Google Scholar]
- 53.Gotsch U, Borges E, Bosse R, Boggemeyer E, Simon M, Mossmann H, Vestweber D. VE-cadherin antibody accelerates neutrophil recruitment in vivo. J Cell Sci. 1997;110(Pt 5):583–8. doi: 10.1242/jcs.110.5.583. [DOI] [PubMed] [Google Scholar]
- 54.Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, McDonald DM, Ward PA, Dejana E. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci U S A. 1999;96:9815–20. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hordijk PL, Anthony E, Mul FP, Rientsma R, Oomen LC, Roos D. Vascular-endothelial-cadherin modulates endothelial monolayer permeability. J Cell Sci. 1999;112(Pt 12):1915–23. doi: 10.1242/jcs.112.12.1915. [DOI] [PubMed] [Google Scholar]
- 56.van Buul JD, Voermans C, van den Berg V, Anthony EC, Mul FP, van Wetering S, van der Schoot CE, Hordijk PL. Migration of human hematopoietic progenitor cells across bone marrow endothelium is regulated by vascular endothelial cadherin. J Immunol. 2002;168:588–96. doi: 10.4049/jimmunol.168.2.588. [DOI] [PubMed] [Google Scholar]
- 57.Shaw SK, Bamba PS, Perkins BN, Luscinskas FW. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J Immunol. 2001;167:2323–30. doi: 10.4049/jimmunol.167.4.2323. [DOI] [PubMed] [Google Scholar]
- 58.Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci U S A. 1995;92:8813–7. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burridge K. Cell biology: a break in the chain? Nature. 2006;440:38–9. doi: 10.1038/440038b. [DOI] [PubMed] [Google Scholar]
- 61.Maul RS, Song Y, Amann KJ, Gerbin SC, Pollard TD, Chang DD. EPLIN regulates actin dynamics by cross-linking and stabilizing filaments. J Cell Biol. 2003;160:399–407. doi: 10.1083/jcb.200212057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abe K, Takeichi M. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci U S A. 2008;105:13–9. doi: 10.1073/pnas.0710504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, Kowalczyk AP. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J Cell Biol. 2003;163:535–45. doi: 10.1083/jcb.200306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lampugnani MG, Corada M, Andriopoulou P, Esser S, Risau W, Dejana E. Cell confluence regulates tyrosine phosphorylation of adherens junction components in endothelial cells. J Cell Sci. 1997;110(Pt 17):2065–77. doi: 10.1242/jcs.110.17.2065. [DOI] [PubMed] [Google Scholar]
- 65.Garcia JG, Schaphorst KL, Verin AD, Vepa S, Patterson CE, Natarajan V. Diperoxovanadate alters endothelial cell focal contacts and barrier function: role of tyrosine phosphorylation. J Appl Physiol. 2000;89:2333–43. doi: 10.1152/jappl.2000.89.6.2333. [DOI] [PubMed] [Google Scholar]
- 66.Young BA, Sui X, Kiser TD, Hyun SW, Wang P, Sakarya S, Angelini DJ, Schaphorst KL, Hasday JD, Cross AS, Romer LH, Passaniti A, Goldblum SE. Protein tyrosine phosphatase activity regulates endothelial cell-cell interactions, the paracellular pathway, and capillary tube stability. Am J Physiol Lung Cell Mol Physiol. 2003;285:L63–75. doi: 10.1152/ajplung.00423.2002. [DOI] [PubMed] [Google Scholar]
- 67.Newman PJ, Berndt MC, Gorski J, White GC, 2nd, Lyman S, Paddock C, Muller WA. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247:1219–22. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- 68.Muller WA, Ratti CM, McDonnell SL, Cohn ZA. A human endothelial cell-restricted, externally disposed plasmalemmal protein enriched in intercellular junctions. J Exp Med. 1989;170:399–414. doi: 10.1084/jem.170.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ayalon O, Sabanai H, Lampugnani MG, Dejana E, Geiger B. Spatial and temporal relationships between cadherins and PECAM-1 in cell-cell junctions of human endothelial cells. J Cell Biol. 1994;126:247–58. doi: 10.1083/jcb.126.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feng D, Nagy JA, Pyne K, Dvorak HF, Dvorak AM. Ultrastructural localization of platelet endothelial cell adhesion molecule (PECAM-1, CD31) in vascular endothelium. J Histochem Cytochem. 2004;52:87–101. doi: 10.1177/002215540405200109. [DOI] [PubMed] [Google Scholar]
- 71.Romer LH, McLean NV, Yan HC, Daise M, Sun J, DeLisser HM. IFN-gamma and TNF-alpha induce redistribution of PECAM-1 (CD31) on human endothelial cells. J Immunol. 1995;154:6582–92. [PubMed] [Google Scholar]
- 72.Gamble JR, Drew J, Trezise L, Underwood A, Parsons M, Kasminkas L, Rudge J, Yancopoulos G, Vadas MA. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ Res. 2000;87:603–7. doi: 10.1161/01.res.87.7.603. [DOI] [PubMed] [Google Scholar]
- 73.Fawcett J, Buckley C, Holness CL, Bird IN, Spragg JH, Saunders J, Harris A, Simmons DL. Mapping the homotypic binding sites in CD31 and the role of CD31 adhesion in the formation of interendothelial cell contacts. J Cell Biol. 1995;128:1229–41. doi: 10.1083/jcb.128.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun QH, DeLisser HM, Zukowski MM, Paddock C, Albelda SM, Newman PJ. Individually distinct Ig homology domains in PECAM-1 regulate homophilic binding and modulate receptor affinity. J Biol Chem. 1996;271:11090–8. doi: 10.1074/jbc.271.19.11090. [DOI] [PubMed] [Google Scholar]
- 75.Biswas P, Zhang J, Schoenfeld JD, Schoenfeld D, Gratzinger D, Canosa S, Madri JA. Identification of the regions of PECAM-1 involved in beta- and gamma-catenin associations. Biochem Biophys Res Commun. 2005;329:1225–33. doi: 10.1016/j.bbrc.2005.02.095. [DOI] [PubMed] [Google Scholar]
- 76.Ilan N, Madri JA. PECAM-1: old friend, new partners. Curr Opin Cell Biol. 2003;15:515–24. doi: 10.1016/s0955-0674(03)00100-5. [DOI] [PubMed] [Google Scholar]
- 77.Biswas P, Canosa S, Schoenfeld D, Schoenfeld J, Li P, Cheas LC, Zhang J, Cordova A, Sumpio B, Madri JA. PECAM-1 affects GSK-3beta-mediated beta-catenin phosphorylation and degradation. Am J Pathol. 2006;169:314–24. doi: 10.2353/ajpath.2006.051112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mamdouh Z, Chen X, Pierini LM, Maxfield FR, Muller WA. Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature. 2003;421:748–53. doi: 10.1038/nature01300. [DOI] [PubMed] [Google Scholar]
- 79.Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–60. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schenkel AR, Chew TW, Muller WA. Platelet endothelial cell adhesion molecule deficiency or blockade significantly reduces leukocyte emigration in a majority of mouse strains. J Immunol. 2004;173:6403–8. doi: 10.4049/jimmunol.173.10.6403. [DOI] [PubMed] [Google Scholar]
- 81.Bogen S, Pak J, Garifallou M, Deng X, Muller WA. Monoclonal antibody to murine PECAM-1 (CD31) blocks acute inflammation in vivo. J Exp Med. 1994;179:1059–64. doi: 10.1084/jem.179.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vaporciyan AA, DeLisser HM, Yan HC, Mendiguren, Thom SR, Jones ML, Ward PA, Albelda SM. Involvement of platelet-endothelial cell adhesion molecule-1 in neutrophil recruitment in vivo. Science. 1993;262:1580–2. doi: 10.1126/science.8248808. [DOI] [PubMed] [Google Scholar]
- 83.Graesser D, Solowiej A, Bruckner M, Osterweil E, Juedes A, Davis S, Ruddle NH, Engelhardt B, Madri JA. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J Clin Invest. 2002;109:383–92. doi: 10.1172/JCI13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dangerfield J, Larbi KY, Huang MT, Dewar A, Nourshargh S. PECAM-1 (CD31) homophilic interaction up-regulates alpha6beta1 on transmigrated neutrophils in vivo and plays a functional role in the ability of alpha6 integrins to mediate leukocyte migration through the perivascular basement membrane. J Exp Med. 2002;196:1201–11. doi: 10.1084/jem.20020324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Petri B, Bixel MG. Molecular events during leukocyte diapedesis. Febs J. 2006;273:4399–407. doi: 10.1111/j.1742-4658.2006.05439.x. [DOI] [PubMed] [Google Scholar]
- 86.Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol. 2002;3:143–50. doi: 10.1038/ni749. [DOI] [PubMed] [Google Scholar]
- 87.Lou O, Alcaide P, Luscinskas FW, Muller WA. CD99 is a key mediator of the transendothelial migration of neutrophils. J Immunol. 2007;178:1136–43. doi: 10.4049/jimmunol.178.2.1136. [DOI] [PubMed] [Google Scholar]
- 88.Bixel MG, Petri B, Khandoga AG, Khandoga A, Wolburg-Buchholz K, Wolburg H, Marz S, Krombach F, Vestweber D. A CD99-related antigen on endothelial cells mediates neutrophil but not lymphocyte extravasation in vivo. Blood. 2007;109:5327–36. doi: 10.1182/blood-2006-08-043109. [DOI] [PubMed] [Google Scholar]
- 89.Marlin SD, Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1) Cell. 1987;51:813–9. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- 90.Alon R, Kassner PD, Carr MW, Finger EB, Hemler ME, Springer TA. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J Cell Biol. 1995;128:1243–53. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sans E, Delachanal E, Duperray A. Analysis of the roles of ICAM-1 in neutrophil transmigration using a reconstituted mammalian cell expression model: implication of ICAM-1 cytoplasmic domain and Rho-dependent signaling pathway. J Immunol. 2001;166:544–51. doi: 10.4049/jimmunol.166.1.544. [DOI] [PubMed] [Google Scholar]
- 92.Sligh JE, Jr., Ballantyne CM, Rich SS, Hawkins HK, Smith CW, Bradley A, Beaudet AL. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1993;90:8529–33. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang MT, Larbi KY, Scheiermann C, Woodfin A, Gerwin N, Haskard DO, Nourshargh S. ICAM-2 mediates neutrophil transmigration in vivo: evidence for stimulus specificity and a role in PECAM-1-independent transmigration. Blood. 2006;107:4721–7. doi: 10.1182/blood-2005-11-4683. [DOI] [PubMed] [Google Scholar]
- 94.Shaw SK, Ma S, Kim MB, Rao RM, Hartman CU, Froio RM, Yang L, Jones T, Liu Y, Nusrat A, Parkos CA, Luscinskas FW. Coordinated redistribution of leukocyte LFA-1 and endothelial cell ICAM-1 accompany neutrophil transmigration. J Exp Med. 2004;200:1571–80. doi: 10.1084/jem.20040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ronald JA, Ionescu CV, Rogers KA, Sandig M. Differential regulation of transendothelial migration of THP-1 cells by ICAM-1/LFA-1 and VCAM-1/VLA-4. J Leukoc Biol. 2001;70:601–9. [PubMed] [Google Scholar]
- 96.Kelly KA, Allport JR, Yu AM, Sinh S, Sage EH, Gerszten RE, Weissleder R. SPARC is a VCAM-1 counter-ligand that mediates leukocyte transmigration. J Leukoc Biol. 2007;81:748–56. doi: 10.1189/jlb.1105664. [DOI] [PubMed] [Google Scholar]
- 97.van Wetering S, van den Berk N, van Buul JD, Mul FP, Lommerse I, Mous R, ten Klooster JP, Zwaginga JJ, Hordijk PL. VCAM-1-mediated Rac signaling controls endothelial cell-cell contacts and leukocyte transmigration. Am J Physiol Cell Physiol. 2003;285:C343–52. doi: 10.1152/ajpcell.00048.2003. [DOI] [PubMed] [Google Scholar]
- 98.Lai CH, Kuo KH, Leo JM. Critical role of actin in modulating BBB permeability. Brain Res Brain Res Rev. 2005;50:7–13. doi: 10.1016/j.brainresrev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 99.Adamson P, Etienne S, Couraud PO, Calder V, Greenwood J. Lymphocyte migration through brain endothelial cell monolayers involves signaling through endothelial ICAM-1 via a rho-dependent pathway. J Immunol. 1999;162:2964–73. [PubMed] [Google Scholar]
- 100.Noria S, Cowan DB, Gotlieb AI, Langille BL. Transient and steady-state effects of shear stress on endothelial cell adherens junctions. Circ Res. 1999;85:504–14. doi: 10.1161/01.res.85.6.504. [DOI] [PubMed] [Google Scholar]
- 101.Tiruppathi C, Minshall RD, Paria BC, Vogel SM, Malik AB. Role of Ca2+ signaling in the regulation of endothelial permeability. Vascul Pharmacol. 2002;39:173–85. doi: 10.1016/s1537-1891(03)00007-7. [DOI] [PubMed] [Google Scholar]
- 102.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 103.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–8. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 104.Essler M, Amano M, Kruse HJ, Kaibuchi K, Weber PC, Aepfelbacher M. Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. J Biol Chem. 1998;273:21867–74. doi: 10.1074/jbc.273.34.21867. [DOI] [PubMed] [Google Scholar]
- 105.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–9. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 106.van Hinsbergh VW, van Nieuw Amerongen GP. Intracellular signalling involved in modulating human endothelial barrier function. J Anat. 2002;200:549–60. doi: 10.1046/j.1469-7580.2002.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garcia JG, Verin AD, Herenyiova M, English D. Adherent neutrophils activate endothelial myosin light chain kinase: role in transendothelial migration. J Appl Physiol. 1998;84:1817–21. doi: 10.1152/jappl.1998.84.5.1817. [DOI] [PubMed] [Google Scholar]
- 108.Saito H, Minamiya Y, Kitamura M, Saito S, Enomoto K, Terada K, Ogawa J. Endothelial myosin light chain kinase regulates neutrophil migration across human umbilical vein endothelial cell monolayer. J Immunol. 1998;161:1533–40. [PubMed] [Google Scholar]
- 109.Hixenbaugh EA, Goeckeler ZM, Papaiya NN, Wysolmerski RB, Silverstein SC, Huang AJ. Stimulated neutrophils induce myosin light chain phosphorylation and isometric tension in endothelial cells. Am J Physiol. 1997;273:H981–8. doi: 10.1152/ajpheart.1997.273.2.H981. [DOI] [PubMed] [Google Scholar]
- 110.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–79. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 111.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 112.Zwartkruis FJ, Bos JL. Ras and Rap1: two highly related small GTPases with distinct function. Exp Cell Res. 1999:157–65. doi: 10.1006/excr.1999.4695. [DOI] [PubMed] [Google Scholar]
- 113.Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vascul Pharmacol. 2002;39:187–99. doi: 10.1016/s1537-1891(03)00008-9. [DOI] [PubMed] [Google Scholar]
- 114.Wojciak-Stothard B, Entwistle A, Garg R, Ridley AJ. Regulation of TNF-alpha-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. J Cell Physiol. 1998;176:150–65. doi: 10.1002/(SICI)1097-4652(199807)176:1<150::AID-JCP17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 115.Moy AB, Blackwell K, Wang N, Haxhinasto K, Kasiske MK, Bodmer J, Reyes G, English A. Phorbol ester-mediated pulmonary artery endothelial barrier dysfunction through regulation of actin cytoskeletal mechanics. Am J Physiol Lung Cell Mol Physiol. 2004;287:L153–67. doi: 10.1152/ajplung.00292.2003. [DOI] [PubMed] [Google Scholar]
- 116.Strey A, Janning A, Barth H, Gerke V. Endothelial Rho signaling is required for monocyte transendothelial migration. FEBS Lett. 2002;517:261–6. doi: 10.1016/s0014-5793(02)02643-1. [DOI] [PubMed] [Google Scholar]
- 117.Saito H, Minamiya Y, Saito S, Ogawa J. Endothelial Rho and Rho kinase regulate neutrophil migration via endothelial myosin light chain phosphorylation. J Leukoc Biol. 2002;72:829–36. [PubMed] [Google Scholar]
- 118.Wojciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci. 2001;114:1343–55. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- 119.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.van Wetering S, van Buul JD, Quik S, Mul FP, Anthony EC, ten Klooster JP, Collard JG, Hordijk PL. Reactive oxygen species mediate Rac-induced loss of cell-cell adhesion in primary human endothelial cells. J Cell Sci. 2002;115:1837–46. doi: 10.1242/jcs.115.9.1837. [DOI] [PubMed] [Google Scholar]
- 121.Stockton RA, Schaefer E, Schwartz MA. p21-activated kinase regulates endothelial permeability through modulation of contractility. J Biol Chem. 2004;279:46621–30. doi: 10.1074/jbc.M408877200. [DOI] [PubMed] [Google Scholar]
- 122.Stockton R, Reutershan J, Scott D, Sanders J, Ley K, Schwartz MA. Induction of vascular permeability: beta PIX and GIT1 scaffold the activation of extracellular signal-regulated kinase by PAK. Mol Biol Cell. 2007;18:2346–55. doi: 10.1091/mbc.E06-07-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–34. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 124.Braga VM, Del Maschio A, Machesky L, Dejana E. Regulation of cadherin function by Rho and Rac: modulation by junction maturation and cellular context. Mol Biol Cell. 1999;10:9–22. doi: 10.1091/mbc.10.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bos JL. All in the family? New insights and questions regarding interconnectivity of Ras, Rap1 and Ral. Embo J. 1998:6776–82. doi: 10.1093/emboj/17.23.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bos JL, de Bruyn K, Enserink J, Kuiperij B, Rangarajan S, Rehmann H, Riedl J, de Rooij J, van Mansfeld F, Zwartkruis F. The role of Rap1 in integrin-mediated cell adhesion. Biochem Soc Trans. 2003:83–6. doi: 10.1042/bst0310083. [DOI] [PubMed] [Google Scholar]
- 127.Lorenowicz MJ, Fernandez-Borja M, Hordijk PL. cAMP signaling in leukocyte transendothelial migration. Arterioscler Thromb Vasc Biol. 2007;27:1014–22. doi: 10.1161/ATVBAHA.106.132282. [DOI] [PubMed] [Google Scholar]
- 128.Oh J, Seo DW, Diaz T, Wei B, Ward Y, Ray JM, Morioka Y, Shi S, Kitayama H, Takahashi C, Noda M, Stetler-Stevenson WG. Tissue inhibitors of metalloproteinase 2 inhibits endothelial cell migration through increased expression of RECK. Cancer Res. 2004;64:9062–9. doi: 10.1158/0008-5472.CAN-04-1981. [DOI] [PubMed] [Google Scholar]
- 129.Chang H, Lee J, Poo H, Noda M, Diaz T, Wei B, Stetler-Stevenson WG, Oh J. TIMP-2 promotes cell spreading and adhesion via upregulation of Rap1 signaling. Biochem Biophys Res Commun. 2006;345:1201–6. doi: 10.1016/j.bbrc.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 130.Gakidis MA, Cullere X, Olson T, Wilsbacher JL, Zhang B, Moores SL, Ley K, Swat W, Mayadas T, Brugge JS. Vav GEFs are required for beta2 integrin-dependent functions of neutrophils. J Cell Biol. 2004;166:273–82. doi: 10.1083/jcb.200404166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 2005;25:136–46. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 2005;579:4966–72. doi: 10.1016/j.febslet.2005.07.080. [DOI] [PubMed] [Google Scholar]
- 133.Wittchen ES, Worthylake RA, Kelly P, Casey PJ, Quilliam LA, Burridge K. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J Biol Chem. 2005;280:11675–82. doi: 10.1074/jbc.M412595200. [DOI] [PubMed] [Google Scholar]
- 134.Sakurai A, Fukuhara S, Yamagishi A, Sako K, Kamioka Y, Masuda M, Nakaoka Y, Mochizuki N. MAGI-1 is required for Rap1 activation upon cell-cell contact and for enhancement of vascular endothelial cadherin-mediated cell adhesion. Mol Biol Cell. 2006;17:966–76. doi: 10.1091/mbc.E05-07-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Birukova AA, Birukov KG, Smurova K, Adyshev D, Kaibuchi K, Alieva I, Garcia JG, Verin AD. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. Faseb J. 2004;18:1879–90. doi: 10.1096/fj.04-2328com. [DOI] [PubMed] [Google Scholar]
- 136.Mamdouh Z, Kreitzer GE, Muller WA. Leukocyte transmigration requires kinesin-mediated microtubule-dependent membrane trafficking from the lateral border recycling compartment. J Exp Med. 2008;205:951–66. doi: 10.1084/jem.20072328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sehrawat S, Cullere X, Patel S, Italiano J, Jr., Mayadas TN. Role of epac1, an exchange factor for rap GTPases, in endothelial microtubule dynamics and barrier function. Mol Biol Cell. 2008;19:1261–70. doi: 10.1091/mbc.E06-10-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Orlova VV, Economopoulou M, Lupu F, Santoso S, Chavakis T. Junctional adhesion molecule-C regulates vascular endothelial permeability by modulating VE-cadherin-mediated cell-cell contacts. J Exp Med. 2006;203:2703–14. doi: 10.1084/jem.20051730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mandell KJ, Babbin BA, Nusrat A, Parkos CA. Junctional adhesion molecule 1 regulates epithelial cell morphology through effects on beta1 integrins and Rap1 activity. J Biol Chem. 2005;280:11665–74. doi: 10.1074/jbc.M412650200. [DOI] [PubMed] [Google Scholar]
- 140.Hoshino T, Sakisaka T, Baba T, Yamada T, Kimura T, Takai Y. Regulation of E-cadherin endocytosis by nectin through afadin, Rap1, and p120ctn. J Biol Chem. 2005;280:24095–103. doi: 10.1074/jbc.M414447200. [DOI] [PubMed] [Google Scholar]
- 141.Orlova VV, Chavakis T. Regulation of vascular endothelial permeability by junctional adhesion molecules (JAM) Thromb Haemost. 2007;98:327–32. [PubMed] [Google Scholar]
- 142.Glading A, Han J, Stockton RA, Ginsberg MH. KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell cell junctions. J Cell Biol. 2007;179:247–54. doi: 10.1083/jcb.200705175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, Itoh M, Mizoguchi A, Aoki T, Fujimoto T, Matsuda Y, Tsukita S, Takai Y. Afadin: A novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol. 1997;139:517–28. doi: 10.1083/jcb.139.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tachibana K, Nakanishi H, Mandai K, Ozaki K, Ikeda W, Yamamoto Y, Nagafuchi A, Tsukita S, Takai Y. Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J Cell Biol. 2000;150:1161–76. doi: 10.1083/jcb.150.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ebnet K, Schulz CU, Meyer Zu Brickwedde MK, Pendl GG, Vestweber D. Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J Biol Chem. 2000;275:27979–88. doi: 10.1074/jbc.M002363200. [DOI] [PubMed] [Google Scholar]
- 146.Su L, Hattori M, Moriyama M, Murata N, Harazaki M, Kaibuchi K, Minato N. AF-6 controls integrin-mediated cell adhesion by regulating Rap1 activation through the specific recruitment of Rap1GTP and SPA-1. J Biol Chem. 2003;278:15232–8. doi: 10.1074/jbc.M211888200. [DOI] [PubMed] [Google Scholar]
- 147.Boettner B, Govek EE, Cross J, Van Aelst L. The junctional multidomain protein AF-6 is a binding partner of the Rap1A GTPase and associates with the actin cytoskeletal regulator profilin. Proc Natl Acad Sci U S A. 2000;97:9064–9. doi: 10.1073/pnas.97.16.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wegmann F, Ebnet K, Du Pasquier L, Vestweber D, Butz S. Endothelial adhesion molecule ESAM binds directly to the multidomain adaptor MAGI-1 and recruits it to cell contacts. Exp Cell Res. 2004;300:121–33. doi: 10.1016/j.yexcr.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 149.Mino A, Ohtsuka T, Inoue E, Takai Y. Membrane-associated guanylate kinase with inverted orientation (MAGI)-1/brain angiogenesis inhibitor 1-associated protein (BAP1) as a scaffolding molecule for Rap small G protein GDP/GTP exchange protein at tight junctions. Genes Cells. 2000;5:1009–16. doi: 10.1046/j.1365-2443.2000.00385.x. [DOI] [PubMed] [Google Scholar]
- 150.Wojciak-Stothard B, Williams L, Ridley AJ. Monocyte adhesion and spreading on human endothelial cells is dependent on Rho-regulated receptor clustering. J Cell Biol. 1999;145:1293–307. doi: 10.1083/jcb.145.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tilghman RW, Hoover RL. The Src-cortactin pathway is required for clustering of E-selectin and ICAM-1 in endothelial cells. Faseb J. 2002;16:1257–9. doi: 10.1096/fj.01-0969fje. [DOI] [PubMed] [Google Scholar]
- 152.Yang L, Kowalski JR, Yacono P, Bajmoczi M, Shaw SK, Froio RM, Golan DE, Thomas SM, Luscinskas FW. Endothelial cell cortactin coordinates intercellular adhesion molecule-1 clustering and actin cytoskeleton remodeling during polymorphonuclear leukocyte adhesion and transmigration. J Immunol. 2006;177:6440–9. doi: 10.4049/jimmunol.177.9.6440. [DOI] [PubMed] [Google Scholar]
- 153.Greenwood J, Amos CL, Walters CE, Couraud PO, Lyck R, Engelhardt B, Adamson P. Intracellular domain of brain endothelial intercellular adhesion molecule-1 is essential for T lymphocyte-mediated signaling and migration. J Immunol. 2003;171:2099–108. doi: 10.4049/jimmunol.171.4.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lyck R, Reiss Y, Gerwin N, Greenwood J, Adamson P, Engelhardt B. T-cell interaction with ICAM-1/ICAM-2 double-deficient brain endothelium in vitro: the cytoplasmic tail of endothelial ICAM-1 is necessary for transendothelial migration of T cells. Blood. 2003;102:3675–83. doi: 10.1182/blood-2003-02-0358. [DOI] [PubMed] [Google Scholar]
- 155.Thompson PW, Randi AM, Ridley AJ. Intercellular adhesion molecule (ICAM)-1, but not ICAM-2, activates RhoA and stimulates c-fos and rhoA transcription in endothelial cells. J Immunol. 2002;169:1007–13. doi: 10.4049/jimmunol.169.2.1007. [DOI] [PubMed] [Google Scholar]
- 156.Louvet-Vallee S. ERM proteins: from cellular architecture to cell signaling. Biol Cell. 2000;92:305–16. doi: 10.1016/s0248-4900(00)01078-9. [DOI] [PubMed] [Google Scholar]
- 157.Mackay DJ, Esch F, Furthmayr H, Hall A. Rho- and rac-dependent assembly of focal adhesion complexes and actin filaments in permeabilized fibroblasts: an essential role for ezrin/radixin/moesin proteins. J Cell Biol. 1997;138:927–38. doi: 10.1083/jcb.138.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol. 2002;157:1233–45. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Takahashi K, Sasaki T, Mammoto A, Hotta I, Takaishi K, Imamura H, Nakano K, Kodama A, Takai Y. Interaction of radixin with Rho small G protein GDP/GTP exchange protein Dbl. Oncogene. 1998;16:3279–84. doi: 10.1038/sj.onc.1201874. [DOI] [PubMed] [Google Scholar]
- 160.Takahashi K, Sasaki T, Mammoto A, Takaishi K, Kameyama T, Tsukita S, Takai Y. Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J Biol Chem. 1997;272:23371–5. doi: 10.1074/jbc.272.37.23371. [DOI] [PubMed] [Google Scholar]
- 161.Etienne-Manneville S, Manneville JB, Adamson P, Wilbourn B, Greenwood J, Couraud PO. ICAM-1-coupled cytoskeletal rearrangements and transendothelial lymphocyte migration involve intracellular calcium signaling in brain endothelial cell lines. J Immunol. 2000;165:3375–83. doi: 10.4049/jimmunol.165.6.3375. [DOI] [PubMed] [Google Scholar]
- 162.Etienne S, Adamson P, Greenwood J, Strosberg AD, Cazaubon S, Couraud PO. ICAM-1 signaling pathways associated with Rho activation in microvascular brain endothelial cells. J Immunol. 1998;161:5755–61. [PubMed] [Google Scholar]
- 163.Durieu-Trautmann O, Chaverot N, Cazaubon S, Strosberg AD, Couraud PO. Intercellular adhesion molecule 1 activation induces tyrosine phosphorylation of the cytoskeleton-associated protein cortactin in brain microvessel endothelial cells. J Biol Chem. 1994;269:12536–40. [PubMed] [Google Scholar]
- 164.Yang L, Kowalski JR, Zhan X, Thomas SM, Luscinskas FW. Endothelial cell cortactin phosphorylation by Src contributes to polymorphonuclear leukocyte transmigration in vitro. Circ Res. 2006;98:394–402. doi: 10.1161/01.RES.0000201958.59020.1a. [DOI] [PubMed] [Google Scholar]
- 165.Wang Q, Doerschuk CM. The p38 mitogen-activated protein kinase mediates cytoskeletal remodeling in pulmonary microvascular endothelial cells upon intracellular adhesion molecule-1 ligation. J Immunol. 2001;166:6877–84. doi: 10.4049/jimmunol.166.11.6877. [DOI] [PubMed] [Google Scholar]
- 166.Wang Q, Pfeiffer GR, 2nd, Gaarde WA. Activation of SRC tyrosine kinases in response to ICAM-1 ligation in pulmonary microvascular endothelial cells. J Biol Chem. 2003;278:47731–43. doi: 10.1074/jbc.M308466200. [DOI] [PubMed] [Google Scholar]
- 167.Weaver AM, Young ME, Lee WL, Cooper JA. Integration of signals to the Arp2/3 complex. Curr Opin Cell Biol. 2003;15:23–30. doi: 10.1016/s0955-0674(02)00015-7. [DOI] [PubMed] [Google Scholar]
- 168.Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol. 2007;179:4053–64. doi: 10.4049/jimmunol.179.6.4053. [DOI] [PubMed] [Google Scholar]
- 169.Kinch MS, Clark GJ, Der CJ, Burridge K. Tyrosine phosphorylation regulates the adhesions of ras-transformed breast epithelia. J Cell Biol. 1995;130:461–71. doi: 10.1083/jcb.130.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.van Buul JD, Anthony EC, Fernandez-Borja M, Burridge K, Hordijk PL. Proline-rich tyrosine kinase 2 (Pyk2) mediates vascular endothelial-cadherin-based cell-cell adhesion by regulating beta-catenin tyrosine phosphorylation. J Biol Chem. 2005;280:21129–36. doi: 10.1074/jbc.M500898200. [DOI] [PubMed] [Google Scholar]
- 171.Andriopoulou P, Navarro P, Zanetti A, Lampugnani MG, Dejana E. Histamine induces tyrosine phosphorylation of endothelial cell-to-cell adherens junctions. Arterioscler Thromb Vasc Biol. 1999;19:2286–97. doi: 10.1161/01.atv.19.10.2286. [DOI] [PubMed] [Google Scholar]
- 172.Esser S, Lampugnani MG, Corada M, Dejana E, Risau W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci. 1998;111(Pt 13):1853–65. doi: 10.1242/jcs.111.13.1853. [DOI] [PubMed] [Google Scholar]
- 173.Potter MD, Barbero S, Cheresh DA. Tyrosine phosphorylation of VE-cadherin prevents binding of p120- and beta-catenin and maintains the cellular mesenchymal state. J Biol Chem. 2005;280:31906–12. doi: 10.1074/jbc.M505568200. [DOI] [PubMed] [Google Scholar]
- 174.Turowski P, Martinelli R, Crawford R, Wateridge D, Papageorgiou AP, Lampugnani MG, Gamp AC, Vestweber D, Adamson P, Dejana E, Greenwood J. Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J Cell Sci. 2008;121:29–3. doi: 10.1242/jcs.022681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang Q, Doerschuk CM. Neutrophil-induced changes in the biomechanical properties of endothelial cells: roles of ICAM-1 and reactive oxygen species. J Immunol. 2000;164:6487–94. doi: 10.4049/jimmunol.164.12.6487. [DOI] [PubMed] [Google Scholar]
- 176.van Buul JD, Mul FP, van der Schoot CE, Hordijk PL. ICAM-3 activation modulates cell-cell contacts of human bone marrow endothelial cells. J Vasc Res. 2004;41:28–37. doi: 10.1159/000076126. [DOI] [PubMed] [Google Scholar]
- 177.Nguyen A, Chen P, Cai H. Role of CaMKII in hydrogen peroxide activation of ERK1/2, p38 MAPK, HSP27 and actin reorganization in endothelial cells. FEBS Lett. 2004;572:307–13. doi: 10.1016/j.febslet.2004.06.061. [DOI] [PubMed] [Google Scholar]
- 178.Gerthoffer WT, Gunst SJ. Invited review: focal adhesion and small heat shock proteins in the regulation of actin remodeling and contractility in smooth muscle. J Appl Physiol. 2001;91:963–72. doi: 10.1152/jappl.2001.91.2.963. [DOI] [PubMed] [Google Scholar]
- 179.Wang Q, Yerukhimovich M, Gaarde WA, Popoff IJ, Doerschuk CM. MKK3 and -6-dependent activation of p38alpha MAP kinase is required for cytoskeletal changes in pulmonary microvascular endothelial cells induced by ICAM-1 ligation. Am J Physiol Lung Cell Mol Physiol. 2005;288:L359–69. doi: 10.1152/ajplung.00292.2004. [DOI] [PubMed] [Google Scholar]
- 180.Sallee JL, Wittchen ES, Burridge K. Regulation of cell adhesion by protein-tyrosine phosphatases: II. Cell-cell adhesion. J Biol Chem. 2006;281:16189–92. doi: 10.1074/jbc.R600003200. [DOI] [PubMed] [Google Scholar]
- 181.Hordijk PL. Endothelial signalling events during leukocyte transmigration. Febs J. 2006;273:4408–15. doi: 10.1111/j.1742-4658.2006.05440.x. [DOI] [PubMed] [Google Scholar]
- 182.Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, Quinn MT, Pagano PJ, Johnson C, Alexander RW. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160–7. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- 183.Matheny HE, Deem TL, Cook-Mills JM. Lymphocyte migration through monolayers of endothelial cell lines involves VCAM-1 signaling via endothelial cell NADPH oxidase. J Immunol. 2000;164:6550–9. doi: 10.4049/jimmunol.164.12.6550. [DOI] [PubMed] [Google Scholar]
- 184.Abdala-Valencia H, Cook-Mills JM. VCAM-1 signals activate endothelial cell protein kinase Calpha via oxidation. J Immunol. 2006;177:6379–87. doi: 10.4049/jimmunol.177.9.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Deem TL, Abdala-Valencia H, Cook-Mills JM. VCAM-1 activation of endothelial cell protein tyrosine phosphatase 1B. J Immunol. 2007;178:3865–73. doi: 10.4049/jimmunol.178.6.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Deem TL, Cook-Mills JM. Vascular cell adhesion molecule 1 (VCAM-1) activation of endothelial cell matrix metalloproteinases: role of reactive oxygen species. Blood. 2004;104:2385–93. doi: 10.1182/blood-2004-02-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Faustmann PM, Dermietzel R. Extravasation of polymorphonuclear leukocytes from the cerebral microvasculature. Inflammatory response induced by alpha-bungarotoxin. Cell Tissue Res. 1985;242:399–407. doi: 10.1007/BF00214554. [DOI] [PubMed] [Google Scholar]
- 188.Fujita S, Puri RK, Yu ZX, Travis WD, Ferrans VJ. An ultrastructural study of in vivo interactions between lymphocytes and endothelial cells in the pathogenesis of the vascular leak syndrome induced by interleukin-2. Cancer. 1991;68:2169–74. doi: 10.1002/1097-0142(19911115)68:10<2169::aid-cncr2820681014>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 189.Feng D, Nagy JA, Pyne K, Dvorak HF, Dvorak AM. Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J Exp Med. 1998;187:903–15. doi: 10.1084/jem.187.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Carman CV, Jun CD, Salas A, Springer TA. Endothelial cells proactively form microvilli-like membrane projections upon intercellular adhesion molecule 1 engagement of leukocyte LFA-1. J Immunol. 2003;171:6135–44. doi: 10.4049/jimmunol.171.11.6135. [DOI] [PubMed] [Google Scholar]
- 191.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167:377–88. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nieminen M, Henttinen T, Merinen M, Marttila-Ichihara F, Eriksson JE, Jalkanen S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol. 2006;8:156–62. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- 193.Nobes C, Hall A. Regulation and function of the Rho subfamily of small GTPases. Curr Opin Genet Dev. 1994;4:77–81. doi: 10.1016/0959-437x(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 194.Ellerbroek SM, Wennerberg K, Arthur WT, Dunty JM, Bowman DR, DeMali KA, Der C, Burridge K. SGEF, a RhoG guanine nucleotide exchange factor that stimulates macropinocytosis. Mol Biol Cell. 2004;15:3309–19. doi: 10.1091/mbc.E04-02-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.van Buul JD, Allingham MJ, Samson T, Meller J, Boulter E, Garcia-Mata R, Burridge K. RhoG regulates endothelial apical cup assembly downstream from ICAM1 engagement and is involved in leukocyte trans-endothelial migration. J Cell Biol. 2007;178:1279–93. doi: 10.1083/jcb.200612053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Patel JC, Galan JE. Differential activation and function of Rho GTPases during Salmonella-host cell interactions. J Cell Biol. 2006;175:453–63. doi: 10.1083/jcb.200605144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Marchesi VT, Gowans JL. The Migration of Lymphocytes through the Endothelium of Venules in Lymph Nodes: An Electron Microscope Study. Proc R Soc Lond B Biol Sci. 1964;159:283–90. doi: 10.1098/rspb.1964.0002. [DOI] [PubMed] [Google Scholar]
- 198.Engelhardt B, Wolburg H. Mini-review: Transendothelial migration of leukocytes: through the front door or around the side of the house? Eur J Immunol. 2004;34:2955–63. doi: 10.1002/eji.200425327. [DOI] [PubMed] [Google Scholar]
- 199.Wolburg H, Wolburg-Buchholz K, Engelhardt B. Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact. Acta Neuropathol. 2005;109:181–90. doi: 10.1007/s00401-004-0928-x. [DOI] [PubMed] [Google Scholar]
- 200.Phillipson M, Kaur J, Colarusso P, Ballantyne CM, Kubes P. Endothelial domes encapsulate adherent neutrophils and minimize increases in vascular permeability in paracellular and transcellular emigration. PLoS ONE. 2008;3:e1649. doi: 10.1371/journal.pone.0001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Stan RV. Endothelial stomatal and fenestral diaphragms in normal vessels and angiogenesis. J Cell Mol Med. 2007;11:621–43. doi: 10.1111/j.1582-4934.2007.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Roberts WG, Palade GE. Neovasculature induced by vascular endothelial growth factor is fenestrated. Cancer Res. 1997;57:765–72. [PubMed] [Google Scholar]
- 203.Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108(Pt 6):2369–79. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- 204.Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol. 1998;140:947–59. doi: 10.1083/jcb.140.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dvorak AM, Feng D. The vesiculo-vacuolar organelle (VVO). A new endothelial cell permeability organelle. J Histochem Cytochem. 2001;49:419–32. doi: 10.1177/002215540104900401. [DOI] [PubMed] [Google Scholar]
- 206.Millan J, Hewlett L, Glyn M, Toomre D, Clark P, Ridley AJ. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol. 2006;8:113–23. doi: 10.1038/ncb1356. [DOI] [PubMed] [Google Scholar]
- 207.Ager A. Inflammation: Border crossings. Nature. 2003;421:703–5. doi: 10.1038/421703a. [DOI] [PubMed] [Google Scholar]
- 208.Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005;106:584–92. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cinamon G, Shinder V, Shamri R, Alon R. Chemoattractant signals and beta 2 integrin occupancy at apical endothelial contacts combine with shear stress signals to promote transendothelial neutrophil migration. J Immunol. 2004;173:7282–91. doi: 10.4049/jimmunol.173.12.7282. [DOI] [PubMed] [Google Scholar]
- 210.Ferreira AM, McNeil CJ, Stallaert KM, Rogers KA, Sandig M. Interleukin-1beta reduces transcellular monocyte diapedesis and compromises endothelial adherens junction integrity. Microcirculation. 2005;12:563–79. doi: 10.1080/10739680500253493. [DOI] [PubMed] [Google Scholar]
- 211.Camussi G, Turello E, Bussolino F, Baglioni C. Tumor necrosis factor alters cytoskeletal organization and barrier function of endothelial cells. Int Arch Allergy Appl Immunol. 1991;96:84–91. doi: 10.1159/000235539. [DOI] [PubMed] [Google Scholar]
- 212.Nwariaku FE, Liu Z, Zhu X, Nahari D, Ingle C, Wu RF, Gu Y, Sarosi G, Terada LS. NADPH oxidase mediates vascular endothelial cadherin phosphorylation and endothelial dysfunction. Blood. 2004;104:3214–20. doi: 10.1182/blood-2004-05-1868. [DOI] [PubMed] [Google Scholar]