Introduction
Polymerase chain reaction (PCR) is a technique involving enzymatic amplification of nucleic acid sequences in repeated cycles of denaturation, oligonucleotide annealing, and DNA polymerase extension.1,2 The PCR uses in vitro enzymatic synthesis to amplify specific DNA sequences within a few hours. Since its inception in 1985, PCR has revolutionized research in the biologic sciences and medicine and has influenced criminology and law.3 The inventor of PCR, Kary Mullis, was awarded the Nobel Prize in Chemistry in 1993 in recognition of the extraordinary impact of PCR technology on scientific research.4,5
Polymerase chain reaction consists of repetitive cycles of specific DNA synthesis, defined by short stretches of preselected DNA. With each cycle, there is a doubling of the final, desired DNA product such that a million-fold amplification is possible.6 This powerful technique has numerous applications in diagnostic pathology, especially in the fields of microbiology, genetics, and oncology. Polymerase chain reaction has been used to diagnose uveitis, including viral uveitis, mycobacterial intraocular infections, infectious endophthalmitis, and protozoa eye diseases.7 However, the extremely high sensitivity of PCR can produce false-positive results, whereas its high specificity may produce false-negative results. These pitfalls can be minimized by techniques such as the use of both positive and negative controls, real-time PCR, and the performance of tests in an experienced laboratory. In any case, to ensure an accurate diagnosis, one must consider clinical data in the interpretation of a PCR result. We present diagnostic applications and examples of utilization of PCR in infectious and noninfectious uveitis, as well as in masquerade syndromes and other common ocular diseases with inflammatory components.
Infectious Uveitis
Polymerase chain reaction has had a major impact on our ability to detect infectious agents. Since the first identification of Toxoplasma gondii DNA in ocular tissue with the use of PCR in 1990,8 PCR has been applied to the detection and diagnosis of infectious uveitis caused by various viruses, bacteria, and fungi in ocular tissues and/or fluids.9–15 Polymerase chain reaction has frequently become a useful diagnostic aid.16 Although PCR detects the DNA of a pathogen, it does not confirm an active infection. Thus, positive culture from the tested ocular sample provides extremely useful complementary data to a positive PCR result. Elevated antibody against the specific pathogen is also helpful.17
In 1993, Aouizerate et al performed PCR on the aqueous of 59 eyes with suspected or confirmed infection with Toxoplasma gondii; the parasite was demonstrated in 20 cases (33.8%).18 The combination of PCR with serologic analysis of Witmer-Desmonts coefficient (the ratio of aqueous and serum antibodies) increased the probability to 60% to 72.7% of making a biologic diagnosis for ocular toxoplasmosis. Ten years later, Villard et al also reported the high yield of a combination of serologic analysis (enzyme-linked immunosorbent assay [ELISA] and immunoblotting) and PCR for ocular toxoplasmosis in 83% (15 of 18) patients.19 The relative specificities of the 3 techniques were 89% for ELISA and immuno-blotting and 100% for PCR.
Knox et al reviewed their experience using PCR to examine vitreous biopsies in the diagnosis of viral retinitis in 37 patients (39 eyes).20 Ten patients (11 eyes) were positive for cytomegalovirus (CMV), 8 for varicella zoster (VZV), and 6 for herpes simplex (HSV). The data closely correlated with clinical outcome of these patients, thus supporting the use of PCR in the diagnostic evaluation of infectious retinitis. Human T-cell lymphotro-pic virus type-1 was detected in uveitic eyes only by PCR.21,22 Multiple viral infections can be identified in immunocompromised patients with acquired immune deficiency syndrome (AIDS) or malignancies.23–25 Newer PCR techniques have further improved the ability to diagnose viral retinitis. Multiplex PCR shows adequate sensitivity to simultaneously screen for a substantial number of different diagnoses for posterior uveitis in a single reaction, without loss of specificity.26 A combination of micro-dissection and PCR offers the advantages of better handling of small quantities of ocular specimens and identification of potentiating viral coinfections.23,27
Although direct microscopy is the easiest and most rapid method to detect bacterial etiologies of endophthalmitis, its sensitivity is very low, with positive results varying from 4.2% to 46.5% for vitreous samples, which decreases further in aqueous fluid.28,29 More sensitive than microscopy, culture is considered “the gold standard.” However, there have been no significant improvements in the yield of culture methods.30 Reasons attributed to the lack of sensitivity include prior antibiotic therapy, small numbers of micro-organisms in small volumes of the ocular samples, and the fastidious growth requirements of some pathogens, that is, Propionibacterium acnes, Staphylococcus epidermidis, Tropheryma whippellii, and Mycobacterium tuberculosis.31–34 In cases where conventional techniques have low sensitivity, PCR, characterized by its high sensitivity and specificity, would be an ideal technique to detect bacterial pathogens in the eye. However, PCR can be laborious and challenging. It generally involves determining the bacterium responsible for the positive 16S PCR product.35,36 Thus, we recommend applying PCR only to cases of suspected clinical bacterial endophthalmitis but negative or no bacteriologic studies.37 Sometimes nested PCR or PCR-restriction fragment length polymorphism (RFLP) is required to improve the sensitivity and specificity for identification of bacteria.38
Fungi in the eye can be difficult to isolate and detect. Molecular methods will become important diagnostic tools in this clinical scenario.39 Polymerase chain reaction has successfully identified several fungal endo-phthalmitides, such as Fusarium,15 Candida,40 and Aspergillus.41 In a prospective case-control study in India, Anand et al reported that among 43 intraocular specimens from 30 patients with fungal endophthalmitis, 24 were positive by conventional mycologic methods and 32 were positive by PCR.42 Polymerase chain reaction increased the sensitivity of detection by 18.6%, which was statistically significant (P = 0.039). Using microdissection and nested PCR, we were able to detect Histoplasma capsulatum DNA in chronic choroidal lesions of an eye with earlier infection by this fungus and provide molecular biologic evidence linking the etiology of chronic ocular histoplasmosis syndrome.43
Case Example
A 37-year-old male with a history of toxoplasmosis as a child was diagnosed with acute retinal necrosis in his right eye. The patient was treated with acyclovir without improvement for 3 months. He was then referred to the National Eye Institute. A diagnosis of panuveitis in the right eye was made. The patient was treated prophylactically with antitoxoplasma medication but achieved no response. A year later, his right eye became phthisical and had no light perception; his left eye also developed uveitis with features of acute retinal necrosis. A diagnostic enucleation of the right eye was performed.
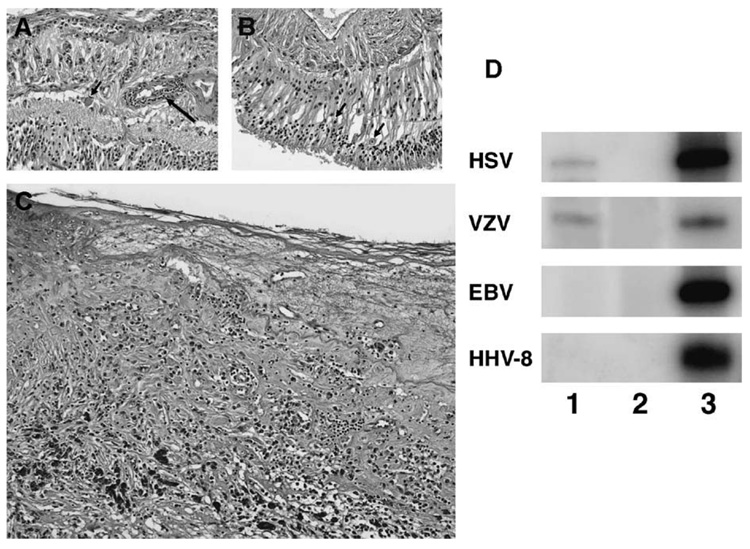
Microscopic examination disclosed a phthisis bulbi with focal retinal necrosis, retinal detachment, chorioretinal scars, chronic inflammation (T- > B-lymphocytes, CD8 > CD4 cells), cyclitic membrane, and vitreous neovascularization (Fig. 1, right). Polymerase chain reaction was conducted using primers HSV-P1 (5′-GTG GTG GAC TTT GCC AGC CTG TAC CC-3′) and HSV-P2 (5′-TAA ACATGG AGT CCG TGT CGC CGTAGA TGA-3′) for HSV, primers VZV-P1 (5′-GTC GTG TTT GAT TTT CAA AGT TTA TAT CC-3′) and VZV-P2 (5′-ATA AAC ACA CAA TCC GTA TCA CCA TAA ATA ACC T-3′) for VZV, S1 (5′-ACA GGT ACC TGC AGA TCT AGA-3′) and S2 (5′-TAC GAG CTC GCG AAT TCA TGA-3′) for HHV-8, L1 (5′-GTT AGA TCT TAC CAA GTA AGC A-3′) and L2 (5′-TTA TGA GTG ACT GGA CTG GAG GA-3′) for EBV, respectively. Each PCR was performed in a total volume of 10 µL containing 1 µL of microdissected DNA, 4.0 pmol each of 32P-labeled sense and antisense primers, 4.0 nmol each of dNTP, 1X GeneAmp buffer, 1.0 unit of AmpliTaq Gold Polymerase (Perkin Elmer, Hayward, CA), and a final concentration of 2.0 mM MgCl2.
Figure 1.
Photomicrograph showing (A) retinal vasculitis (arrow) and a large cell with cytoplasmic inclusions (arrowhead); (B) several retinal cells containing nuclear inclusion (arrowheads); (C) retinal necrosis; and (D) PCR demonstrating positive HSV and VZV but negative HHV-8 and EBV (lane 1, the case; lane 2, negative control; lane 3, positive control).
The cycling parameter with the HSV primer pair was an initial incubation at 94°C for 9 minutes; 3 cycles consisting of 94°C for 1 minute, 60°C for 1 minute and 72°C for 1 minute; 37 cycles consisting of 94°C for 45 seconds, 55°C for 45 seconds and 72°C for 1 minute; and a final incubation at 70°C for 10 minutes. For the VZV primer pair, the cycling parameter was an initial incubation at 94°C for 9 minutes; 40 cycles consisting of 94°C for 45 seconds, 47°C for 1 minute and 72°C for 1 minute; and a final incubation at 70°C for 10 minutes. For EBV and HHV-8 primer pairs, the cycling parameter was an initial incubation at 94°C for 9 minutes; 40 cycles consisting of 94°C for 1 minute, 59°C for 1 minute and 72°C for 1 minute; and a final incubation at 70°C for 10 minutes. Polyacrylamide gel electrophoresis and autoradiography were employed to visualize the PCR products. Polymerase chain reaction was positive for HSV and VZV but negative for HHV-8, CMV, and EBV (Fig. 1, left). The patient was again treated with high doses of antiviral medication. He responded to treatment this time.
Noninfectious Uveitis
Polymerase chain reaction technology has also been implicated in studies of noninfectious uveitis. The most common application is HLA typing. Saiki et al used PCR to enzymatically amplify a specific segment of the beta-globin or HLA-DQ alpha gene in human genomic DNA before hybridization with an allele-specific oligonucleotide for the detection of allelic polymorphisms.44 Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) methodology is applied to HLA-DR, -DQ, and -DW typing at the nucleotide level, eliminating the need for radioisotopes as well as allele specific oligonucleotide probes.45 Using this technique, Shino et al reported complete association of the HLA-DRB1*04 and -DQB1*04 alleles with Vogt-Koyanagi-Harada disease (VKH).46 Polymerase chain reaction-sequencing-based typing (PCR-SBT) is used for HLA-B*51 alleles. A significant association of HLA-B*5101 with Behçet disease was also found in both Japanese and Greek patients.47–49 HLA-B27 typing is associated with anterior uveitis.
Evaluation of intraocular cytokines, chemokines, and other inflammatory mediators and markers provides important information, particularly in identifying cellular immune processes underlying ocular inflammation in noninfectious uveitis.50 In general, the majority of noninfectious uveitides represent an autoimmune etiology.50 These patients with uveitis demonstrate a T-cell–mediated inflammatory response, predominately involving a TH1-like cytokine profile with expression of interleukin-2 (IL-2) and interferon alpha (IFN-α).51–53 Cytokine and inflammatory-related transcripts are usually detected via reverse transcription-PCR (RT-PCR).50,54,55 The results from RT-PCR are complementary to data from Western blotting and/or immunohistochemistry.
Imai et al reported that significant amounts of interferon gamma (IFN-γ) mRNA are found in the cerebrospinal fluid (CSF) of 8 out of 9 patients with VKH, although there was no measurable IFN-γ present.56 The discrepancies between the 2 methods, RT-PCR and ELISA, might be caused by the different sensitivities of these 2 techniques. In addition, transcripts and genes are not always expressed simultaneously and/or require different stimulations. As predominant TH1 cytokines and related proteins are thought to induce and activate noninfectious autoimmune uveitis, expression of their genes underlines the pathogenic mechanism.54
Case Examples
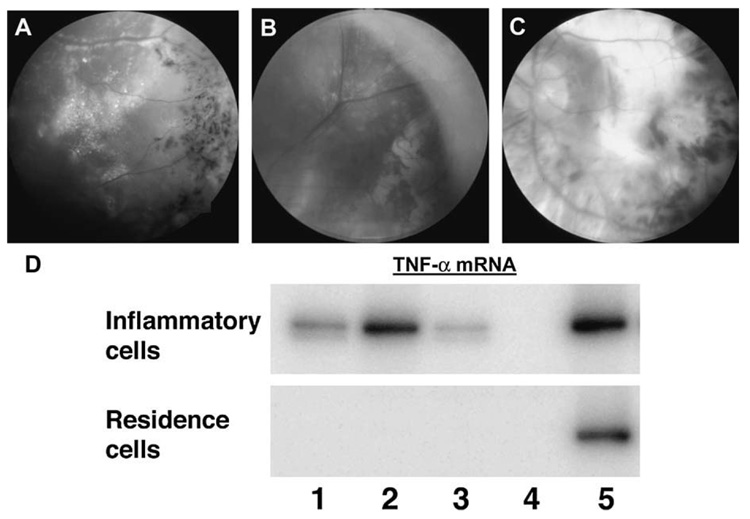
Inflammatory cells and ocular resident cells were collected via manual microdissection from 3 uveitic eyes, which we have reported previously.57–59 The first eye was enucleated from a 29-year-old female with a 9-year history of bilateral uveitis complicated by secondary glaucoma who presented with clinical features of Coats disease in the left eye, including exudative retinal detachment, extensive retinal exudates, and tortuous vascular anomalies with sheathing (Fig. 2A). The eye became blind and painful. The pathologic diagnosis was inflammatory Coats disease with predominant CD4 positive T-cell infiltration.57
Figure 2.
Funduscopic photographs showing (A) an active inflammatory Coats disease with crystalline-lipid exudates, retinal vascular sheathing, and pigmentary deposits; (B) an active sympathetic ophthalmia with many small Dalen-Fuchs’ nodules and retinal detachment; and (C) an end-stage VKH with large chorioretinal scars. D, Reverse transcription-PCR amplification showing expression of TNF-α messengers in ocular inflammatory cells of the 3 cases (lane 1–3, case A–C, respectively; lane 4, negative control; lane 5, positive control).
The second eye was enucleated from a 41-year-old woman with high myopia who developed a recurrent retinal detachment in her right eye.58 After extensive retinal repair surgery, the eye became phthisical and later developed sympathetic ophthalmia (Fig. 2B). This eye presented with diffuse infiltration of mainly CD4-positive T-cells and granulomas in the choroid, as well as Dalen-Fuchs’ nodules.58
The third eye was enucleated from a 72-year-old woman with a 30-year history of bilateral intermittent panuveitis, exudative retinal detachments, and disc swelling (Fig. 2C). She was diagnosed with VKH. In spite of immunosuppressive therapy, her right eye did not respond well and developed neovascular glaucoma, exotropia, and became blind and painful. The pathology of this eye demonstrated an end-stage VKH with extensive chorioretinal scars, retinal gliosis, and focal T-cellular and B-cellular infiltration.59
RNA was isolated from the microdissected cells and examined for tumor necrosis factor alpha (TNF-α) mRNA. The PicoPure RNA isolation kit (Arcturus, Mountain View, CA) was used for RNA isolation and the Superscript II Rnase H- reverse transcription system (Invitrogen, Carlsbad, CA) and random primers (Promega, Madison, WI) were employed for cDNA synthesis. Polymerase chain reaction amplification was performed using the Human TNF-α PCR kit (Maxim Biotech, San Francisco, CA) following the manufacturer’s manual. Briefly, the PCR mixture contained 1 µL of microdissected DNA, 4.0 pmol each of 32P-labeled sense and antisense primers, 4.0 nmol each of dNTP, 1X GeneAmp buffer, 1.0 unit of AmpliTaq Gold Polymerase (Perkin Elmer, Hayward, CA), and a final concentration of 2.0 mM MgCl2 in a total volume of 10 µL. The cycling parameter consisted of an initial incubation at 94°C for 9 minutes; 40 cycles of 94°C for 45 seconds, 58°C for 1 minute and 72°C for 2 minutes; and a final incubation at 70°C for 10 minutes. Polyacrylamide gel electrophoresis and autoradiography were employed to visualize the PCR products. The inflammatory cells in the 3 eyes expressed TNF-α transcript (Fig. 2), although the ocular resident cells did not. Higher levels of TNF-α message were noted in the eyes with intense active inflammation (cases 1 and 2).
Masquerade Syndrome
The masquerade syndrome is comprised of a group of disorders that occur with intraocular inflammation and are often misdiagnosed as a chronic idiopathic uveitis. The most common masquerade syndrome is malignancy; others include degeneration and foreign body in the eye. Polymerase chain reaction can be useful for the diagnosis of masquerade syndrome. Early diagnosis and prompt treatment are critical to improve disease outcome, particularly in ocular malignancies.
Primary intraocular lymphoma is a subtype of central nervous system (CNS) lymphoma involving the eye. Most are diffuse, large B-cell lymphomas. It can often mimic chronic uveitis, especially in elderly patients. The lymphoma cells in the vitreous and subretinal space may present as “chronic vitreitis” and “subretinal infiltrations.” Although cytology, immunohistochemistry, and/or flow cytometry demonstrating the malignant lymphoid cells in the eye remain the gold standard, these diagnostic evaluations are often hampered by the paucity and rapid degeneration of lymphoma cells in ocular fluid specimens. However, molecular analysis to demonstrate B-cell monoclonality of immunoglobulin heavy chain (IgH) gene rearrangements is highly sensitive and helpful. This technique combines microdissection of at least 15 suspicious lymphoid cells and PCR.60,61 Utilization of PCR has become a practical tool for the detection of IgHgene rearrangements and provides a helpful adjunct for the diagnosis of B-cell lymphoma in the eye.62
A number of nonmalignant conditions can masquerade as idiopathic uveitis. An intraocular foreign body may elicit a uveitis. Retinal detachment can sometimes produce enough intraocular inflammation to be misdiagnosed as a uveitis, and retinal degenerations may be associated with inflammatory components. Polymerase chain reaction techniques can demonstrate the involvement of inflammation in retinal degeneration including age-related macular degeneration (AMD).63–65 Johnson et al used end-point PCR to assess and find amyloid precursor protein isoform expression in a human retinal pigment epithelium (RPE) cell line.66 The authors believed that RPE has the capacity to synthesize significant amounts of amyloid precursor protein, which can act as a candidate activator of the complement cascade in the context of drusen formation. These abnormal pathologic deposits can be accompanied by chronic localized inflammation and accentuate the effects of primary pathologic stimuli of AMD. Using PCR-RFLP, we demonstrated associations between AMD and single nucleotide polymorphisms (SNPs) of CX3CR1, a CX3 chemokine receptor.65 Using RT-PCR, we also showed low expression of CX3CR1 transcript in eyes with the CX3CR1 SNP. Our data suggest that a decrease, caused by sequence variation and/or lower CX3CR1 expression, in CX3CR1-induced cellular activities could contribute to AMD development. The underlying mechanisms of AMD development may involve a decrease in the chemoattractant efficiency of inflammatory cells to the macula of those exposed to AMD risk factors.
Case Examples
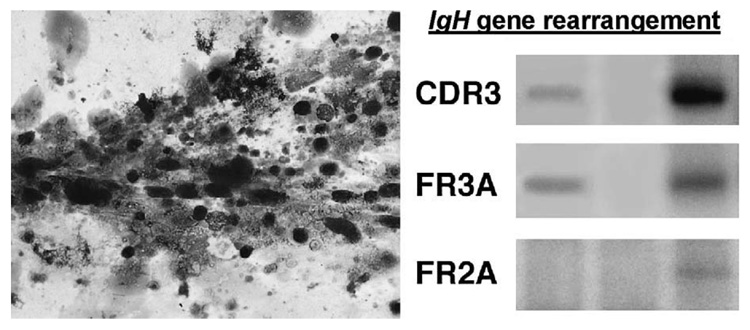
A 74-year-old female with a history of chronic bilateral uveitis was treated with systemic corticosteroids. In spite of medication, she continued to present with 3+ vitreous cells and subretinal infiltration. A diagnostic vitrectomy was performed. Cytology disclosed clumps of mononuclear cells with poor morphology (Fig. 3). Some cells were necrotic, and few had large nuclei and scanty basophilic cytoplasm. These atypical cells were positive for CD20 and kappa light chain. The atypical cells were microdissected and subjected to PCR. The PCR primer pairs were as follows: CDR3 sense (5′-CCG GRA ARR GTC TGG AGT GG-3′) and antisense (5′-ATC CTG AGG AGA CGG TGA CC-3′); FR3A sense (′-ACA CGG CYS TGT ATT ACT GT-3′) and antisense (5′-GGA TGG TAC CAA GCT TTG AGG AGA CGG TGA CCA-3′); and FR2A sense (5′-TGG RTC CGM CAG CAG SCV YCN GG-3′) and antisense (5′-ACC TGA GGA GAC GGT GAC C-3′). Polymerase chain reaction was performed in a total volume of 10 µL containing 1 µL of microdissected DNA, 4.0 pmol each of 32P-labeled sense primer and unlabeled antisense primer, 4.0 nmol each of dNTP, 1X GeneAmp buffer, 1.0 unit of AmpliTaq Gold Polymerase (Perkin Elmer, Hayward, CA), and a final concentration of 2.0 mM MgCl2. The cycling parameter with the CDR3 primer pair included an initial incubation at 94°C for 9 minutes; 40 cycles of 94°C for 45 seconds, 56°C for 45 seconds and 72°C for 1 minute; and a final incubation at 70°C for 10 minutes. For the FR3A and FR2A primer pairs, the cycling parameter included an initial incubation at 94°C for 9 minutes; 40 cycles consisting of 94°C for 1 minute, 59°C for 1 minute and 72°C for 1 minute; and a final incubation at 70°C for 10 minutes. Polyacrylamide gel electrophoresis and radio autograph were employed to visualize the PCR products. The PCR product showed IgH gene rearrangements at the CDR3 site using FR3A and CDR3 primers (Fig. 3). A diagnosis of primary intraocular lymphoma was made. The patient received systemic chemotherapy.
Figure 3.
Photomicrograph showing vitreous cytology of poor defined, large lymphoid cells, small lymphocytes, necrotic cells, and vitreous strands (left; Giemsa, original magnification, × 200); PCR amplification showing IgH gene rearrangement with FR3A and CDR3 primers in those large lymphoid cells (right).
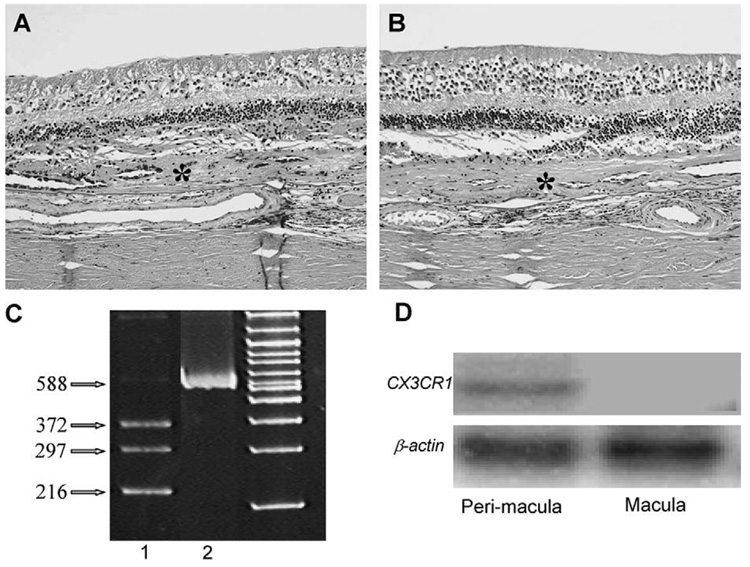
Autopsy eyes of an 89-year-old woman with a long-standing history of bilateral neovascular of related macular degeneration and cataract extractions revealed a disciform scar with subretinal component that contained hyperplastic RPE and small vascular lumens (Fig. 4A, B). There was total loss of photoreceptors. Mild amounts of chronic inflammatory cells were noted in the choroid. The nonretinal cells were microdissected and analyzed for CX3CR1 SNP typing; the retinal cells in the macular and perimacular were also microdissected for determining CX3CR1 mRNA by RT-PCR.
Figure 4.
Photomicrograph showing typical AMD with large disciform scars (asterisks) between the neuronal retina and Bruch membrane, loss of photoreceptors, and normal RPE cells (A, right eye; B, left eye; hematoxylin & eosin, original magnification, ×100). C, The gel image of the RFLP pattern showing heterozygous CX3CR-280 (T/M) with 216, 297, and 372 bp (lane 1), uncut in lane 2. D, Reverse transcription-PCR of CX3CR1 mRNA in the macular and perimacular regions showing no CX3CR1 in the macula but equal expressions of β-actin mRNA on both regions (small amplicons of 100 bp spanning from exon 1 and exon 2 of CX3CR1, and 103 bp spanning from exon 5 to exon 6 of β-actin were amplified using 32P-labeled primers. Polymerase chain reaction products were separated by gel electrophoresis. Images were captured by autoradiography.
Single nucleotide polymorphism typing of CX3CR1 T280M was performed by PCR amplification of a 588 base-pair CX3CR1 DNA fragment. The amplified DNA fragment containing the polymorphic sites was flanked by the following primers: forward: 5′-CCG AGG TCC TTC AGG AAA TCT-3′, and reverse: 3′-GAG TTC CTG AAC CTG ATG CTGA-5′. The PCR mixture included 1XJumpStart ReadyMix REDTaq (Sigma), 100 ng DNA, and 70 pmol of primers. The cycling parameter included an initial incubation at 94°C for 2 minutes and 34 cycles of 94°C for 30 seconds, 50°C for 40 seconds, and 72°C for 55 seconds. Restriction fragment length polymorphism analysis for the T280M SNP was conducted by incubating 3 µL of PCR product with 1.5 µL of BsMB1 buffer, 10 µL of water, and 0.5 µL of enzyme BsmBI at 55°C for at least 4 hours. Fragments were separated on 15% TBE polyacrylamide gels and visualized after ethidium bromide staining.
About 500 neuroretinal and RPE cells from the maculae were microdissected for total RNA extraction and purification using the PicoPure™ RNA isolation kit (Arcturus, Mountain View, CA). cDNA was synthesized with reverse transcriptase (Superscript II; Life Technologies, Grand Island, NY) and random hexamers (Promega, Madison, WI). Short amplicon PCR was designed for CX3CR1 and β-actin amplification. The primers for amplifying the 100 bp segment spanning from exon 1 and exon 2 of CX3CR1 were 5′-CAG ATC CAG AGG TTC CCT TG-3′ and 5′-TAA CAG GCC TCA GCC AAA TC-3′. The primers for amplifying the 103 bp segment spanning from exon 5 to exon 6 of β-actin were 5′-CCC AGC ACA ATG AAG ATC AA-3′ and 5′-ACA TCT GCT GGA AGG TGG AC-3′. Three picomoles of the 32P end-labeled sense primer and the unlabeled antisense oligonucleotides were used as appropriate. The 10 µL PCR amplification of 2 µL single-strand cDNA was performed with 0.5 U gold polymerase (AmpliTaq Gold; Perkin-Elmer Corp., Hayward, CA) using a cycling parameter with 34 cycles of 94°C for 30 seconds, 60°C for 30 seconds, 72°C for 40 seconds, and a final incubation at 72°C for 7 minutes. Polymerase chain reaction products were size fractionated using 15% polyacrylamide TBE gels (Bio-Rad, Richmond, CA). Images were then captured by autoradiography. CX3CR1 M280 SNP and low expression of CX3CR1 mRNA at the macula but not the perimacula were found in this case (Fig. 4C, D).
Conclusion
Polymerase chain reaction is a powerful molecular technique for evaluation of very small amounts of DNA and RNA. Polymerase chain reaction can be a simple, rapid, sensitive, and specific tool for the diagnosis of infection, autoimmunity, and masquerade syndromes in the eye. Polymerase chain reaction products amplified directly from intraocular specimens are able to provide useful information with important therapeutic implications. Although PCR may not be able to replace microscopy, culture, and immunologic tests in the immediate future, it will definitely be helpful as an adjunct in the diagnosis of cases that have failed to be identified by conventional methods in laboratories having access to good research facilities.
Acknowledgments
The authors thank Ms. Dana J. Wallace for editing assistance.
References
- 1.Erlich HA, Gelfand D, Sninsky JJ. Recent advances in the polymerase chain reaction. Science. 1991;252:1643–1651. doi: 10.1126/science.2047872. [DOI] [PubMed] [Google Scholar]
- 2.Gibbs RA. DNA amplification by the polymerase chain reaction. Anal Chem. 1990;62:1202–1214. doi: 10.1021/ac00212a004. [DOI] [PubMed] [Google Scholar]
- 3.Templeton NS. The polymerase chain reaction. History, methods, and applications. Diagn Mol Pathol. 1992;1:58–72. doi: 10.1097/00019606-199203000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Mullis K, Faloona F, Scharf S, et al. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb Symp Quant Biol. 1986;51:263–273. doi: 10.1101/sqb.1986.051.01.032. [DOI] [PubMed] [Google Scholar]
- 5.Saiki RK, Scharf S, Faloona F, et al. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985;230:1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- 6.Remick DG, Kunkel SL, Holbrook EA, et al. Theory and applications of the polymerase chain reaction. Am J Clin Pathol. 1990;93 Suppl 1:S49–S54. [PubMed] [Google Scholar]
- 7.Van Gelder RN. Applications of the polymerase chain reaction to diagnosis of ophthalmic disease. Surv Ophthalmol. 2001;46:248–258. doi: 10.1016/s0039-6257(01)00274-0. [DOI] [PubMed] [Google Scholar]
- 8.Brezin AP, Egwuagu CE, Burnier M, Jr, et al. Identification of toxoplasma gondii in paraffin-embedded sections by the polymerase chain reaction. Am J Ophthalmol. 1990;110:599–604. doi: 10.1016/s0002-9394(14)77055-2. [DOI] [PubMed] [Google Scholar]
- 9.Gerling J, Neumann-Haefelin D, Seuffert HM, et al. Diagnosis and management of the acute retinal necrosis syndrome. Ger J Ophthalmol. 1992;1:388–393. [PubMed] [Google Scholar]
- 10.Mochizuki M, Watanabe T, Yamaguchi K, et al. HTLV-I uveitis: a distinct clinical entity caused by HTLV-I. Jpn J Cancer Res. 1992;83:236–239. doi: 10.1111/j.1349-7006.1992.tb00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merien F, Perolat P, Mancel E, et al. Detection of leptospira DNA by polymerase chain reaction in aqueous humor of a patient with unilateral uveitis. J Infect Dis. 1993;168:1335–1336. doi: 10.1093/infdis/168.5.1335. [DOI] [PubMed] [Google Scholar]
- 12.Kotake S, Kimura K, Yoshikawa K, et al. Polymerase chain reaction for the detection of Mycobacterium tuberculosis in ocular tuberculosis. Am J Ophthalmol. 1994;117:805–806. doi: 10.1016/s0002-9394(14)70328-9. [DOI] [PubMed] [Google Scholar]
- 13.Karma A, Seppala I, Mikkila H, et al. Diagnosis and clinical characteristics of ocular Lyme borreliosis. Am J Ophthalmol. 1995;119:127–135. doi: 10.1016/s0002-9394(14)73864-4. [DOI] [PubMed] [Google Scholar]
- 14.Rickman LS, Freeman WR, Green WR, et al. Brief report: uveitis caused by Tropheryma whippelii (Whipple’s bacillus) N Engl J Med. 1995;332:363–366. doi: 10.1056/NEJM199502093320604. [DOI] [PubMed] [Google Scholar]
- 15.Alexandrakis G, Sears M, Gloor P. Postmortem diagnosis of Fusarium panophthalmitis by the polymerase chain reaction. Am J Ophthalmol. 1996;121:221–223. doi: 10.1016/s0002-9394(14)70594-x. [DOI] [PubMed] [Google Scholar]
- 16.Montoya JG, Parmley S, Liesenfeld O, et al. Use of the polymerase chain reaction for diagnosis of ocular toxoplasmosis. Ophthalmology. 1999;106:1554–1563. doi: 10.1016/S0161-6420(99)90453-0. [DOI] [PubMed] [Google Scholar]
- 17.Bodaghi B, LeHoang P. Testing ocular fluids in uveitis. Ophthalmol Clin North Am. 2002;15:271–279. doi: 10.1016/s0896-1549(02)00037-8. [DOI] [PubMed] [Google Scholar]
- 18.Aouizerate F, Cazenave J, Poirier L, et al. Detection of Toxoplasma gondii in aqueous humour by the polymerase chain reaction. Br J Ophthalmol. 1993;77:107–109. doi: 10.1136/bjo.77.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villard O, Filisetti D, Roch-Deries F, et al. Comparison of enzyme-linked immunosorbent assay, immunoblotting, and PCR for diagnosis of toxoplasmic chorioretinitis. J Clin Microbiol. 2003;41:3537–3541. doi: 10.1128/JCM.41.8.3537-3541.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knox CM, Chandler D, Short GA, et al. Polymerase chain reaction-based assays of vitreous samples for the diagnosis of viral retinitis. Use in diagnostic dilemmas. Ophthalmology. 1998;105:37–44. doi: 10.1016/s0161-6420(98)71127-2. discussion 45. [DOI] [PubMed] [Google Scholar]
- 21.Kumar SR, Gill PS, Wagner DG, et al. Human T-cell lymphotropic virus type I-associated retinal lymphoma. A clinicopathologic report. Arch Ophthalmol. 1994;112:954–959. doi: 10.1001/archopht.1994.01090190102028. [DOI] [PubMed] [Google Scholar]
- 22.Mochizuki M, Ono A, Ikeda E, et al. HTLV-I uveitis. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13 Suppl 1:S50–S56. doi: 10.1097/00042560-199600001-00010. [DOI] [PubMed] [Google Scholar]
- 23.de Smet MD, Shen de F, Pepose J, et al. Microdissection combined with the polymerase chain reaction to identify potentiating viral co-infection in patients with HIV/AIDS with ocular infection. Can J Ophthalmol. 2003;38:207–213. doi: 10.1016/s0008-4182(03)80062-5. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell SM, Fox JD, Tedder RS, et al. Vitreous fluid sampling and viral genome detection for the diagnosis of viral retinitis in patients with AIDS. J Med Virol. 1994;43:336–340. doi: 10.1002/jmv.1890430404. [DOI] [PubMed] [Google Scholar]
- 25.Levinson RD, Hooks JJ, Wang Y, et al. Triple viral retinitis diagnosed by polymerase chain reaction of the vitreous biopsy in a patient with Richter’s syndrome. Am J Ophthalmol. 1998;126:732–733. doi: 10.1016/s0002-9394(98)00138-x. [DOI] [PubMed] [Google Scholar]
- 26.Dabil H, Boley ML, Schmitz TM, et al. Validation of a diagnostic multiplex polymerase chain reaction assay for infectious posterior uveitis. Arch Ophthalmol. 2001;119:1315–1322. doi: 10.1001/archopht.119.9.1315. [DOI] [PubMed] [Google Scholar]
- 27.Al-Attar L, Berrocal A, Warman R, et al. Diagnosis by polymerase chain reaction of ocular posttransplant lymphoproliferative disorder after pediatric renal transplantation. Am J Ophthalmol. 2004;137:569–571. doi: 10.1016/j.ajo.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Barza M, Pavan PR, Doft BH, et al. Evaluation of microbiological diagnostic techniques in postoperative endophthalmitis in the Endophthalmitis Vitrectomy Study. Arch Ophthalmol. 1997;115:1142–1150. doi: 10.1001/archopht.1997.01100160312008. [DOI] [PubMed] [Google Scholar]
- 29.Therese KL, Anand AR, Madhavan HN. Polymerase chain reaction in the diagnosis of bacterial endophthalmitis. Br J Ophthalmol. 1998;82:1078–1082. doi: 10.1136/bjo.82.9.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anand AR, Madhavan HN, Therese KL. Use of polymerase chain reaction (PCR) and DNA probe hybridization to determine the Gram reaction of the infecting bacterium in the intraocular fluids of patients with endophthalmitis. J Infect. 2000;41:221–226. doi: 10.1053/jinf.2000.0731. [DOI] [PubMed] [Google Scholar]
- 31.Meisler DM, Mandelbaum S. Propionibacterium-associated endophthalmitis after extracapsular cataract extraction. Review of reported cases. Ophthalmology. 1989;96:54–61. doi: 10.1016/s0161-6420(89)32939-3. [DOI] [PubMed] [Google Scholar]
- 32.Bodaghi B, LeHoang P. Ocular tuberculosis. Curr Opin Ophthalmol. 2000;11:443–448. doi: 10.1097/00055735-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Dutly F, Altwegg M. Whipple’s disease and “Tropheryma whippelii.”. Clin Microbiol Rev. 2001;14:561–583. doi: 10.1128/CMR.14.3.561-583.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishimura JK, Cook BE, Jr, Pach JM. Whipple disease presenting as posterior uveitis without prominent gastrointestinal symptoms. Am J Ophthalmol. 1998;126:130–132. doi: 10.1016/s0002-9394(98)00084-1. [DOI] [PubMed] [Google Scholar]
- 35.Lohmann CP, Heeb M, Linde HJ, et al. Diagnosis of infectious endophthalmitis after cataract surgery by polymerase chain reaction. J Cataract Refract Surg. 1998;24:821–826. doi: 10.1016/s0886-3350(98)80138-7. [DOI] [PubMed] [Google Scholar]
- 36.Knox CM, Cevallos V, Margolis TP, et al. Identification of bacterial pathogens in patients with endophthalmitis by 16S ribosomal DNA typing. Am J Ophthalmol. 1999;128:511–512. doi: 10.1016/s0002-9394(99)00181-6. [DOI] [PubMed] [Google Scholar]
- 37.Buggage RR, Callanan DG, Shen DF, et al. Propionibacterium acnes endophthalmitis diagnosed by microdissection and PCR. Br J Ophthalmol. 2003;87:1190–1191. doi: 10.1136/bjo.87.9.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okhravi N, Adamson P, Matheson MM, et al. PCR-RFLP-mediated detection and speciation of bacterial species causing endophthalmitis. Invest Ophthalmol Vis Sci. 2000;41:1438–1447. [PubMed] [Google Scholar]
- 39.Kinnear FB, Kirkness CM. Advances in rapid laboratory diagnosis of infectious endophthalmitis. J Hosp Infect. 1995;30 Suppl:253–261. doi: 10.1016/0195-6701(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 40.Okhravi N, Adamson P, Mant R, et al. Polymerase chain reaction and restriction fragment length polymorphism mediated detection and speciation of Candida spp causing intraocular infection. Invest Ophthalmol Vis Sci. 1998;39:859–866. [PubMed] [Google Scholar]
- 41.Jaeger EE, Carroll NM, Choudhury S, et al. Rapid detection and identification of Candida, Aspergillus, and Fusarium species in ocular samples using nested PCR. J Clin Microbiol. 2000;38:2902–2908. doi: 10.1128/jcm.38.8.2902-2908.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anand A, Madhavan H, Neelam V, et al. Use of polymerase chain reaction in the diagnosis of fungal endophthalmitis. Ophthalmology. 2001;108:326–330. doi: 10.1016/s0161-6420(00)00517-0. [DOI] [PubMed] [Google Scholar]
- 43.Spencer WH, Chan CC, Shen DF, et al. Detection of histoplasma capsulatum DNA in lesions of chronic ocular histoplasmosis syndrome. Arch Ophthalmol. 2003;121:1551–1555. doi: 10.1001/archopht.121.11.1551. [DOI] [PubMed] [Google Scholar]
- 44.Saiki RK, Bugawan TL, Horn GT, et al. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986;324:163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- 45.Uryu N, Maeda M, Ota M, et al. A simple and rapid method for HLA-DRB and -DQB typing by digestion of PCR-amplified DNA with allele specific restriction endonucleases. Tissue Antigens. 1990;35:20–31. doi: 10.1111/j.1399-0039.1990.tb01751.x. [DOI] [PubMed] [Google Scholar]
- 46.Shindo Y, Ohno S, Yamamoto T, et al. Complete association of the HLA-DRB1*04 and -DQB1*04 alleles with Vogt-Koyanagi-Harada’s disease. Hum Immunol. 1994;39:169–176. doi: 10.1016/0198-8859(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 47.Mizuki N, Ota M, Katsuyama Y, et al. HLA-B*51 allele analysis by the PCR-SBT method and a strong association of HLA-B*5101 with Japanese patients with Behçet’s disease. Tissue Antigens. 2001;58:181–184. doi: 10.1034/j.1399-0039.2001.580306.x. [DOI] [PubMed] [Google Scholar]
- 48.Mizuki N, Ota M, Katsuyama Y, et al. Sequencing-based typing of HLA-B*51 alleles and the significant association of HLA-B*5101 and -B*5108 with Behçet’s disease in Greek patients. Tissue Antigens. 2002;59:118–121. doi: 10.1034/j.1399-0039.2002.590207.x. [DOI] [PubMed] [Google Scholar]
- 49.Duymaz-Tozkir J, Gul A, Uyar FA, et al. Tumour necrosis factor-alpha gene promoter region −308 and −376 G ≥ A polymorphisms in Behçet’s disease. Clin Exp Rheumatol. 2003;21 Suppl 30:S15–S18. [PubMed] [Google Scholar]
- 50.Murray PI, Clay CD, Mappin C, et al. Molecular analysis of resolving immune responses in uveitis. Clin Exp Immunol. 1999;117:455–461. doi: 10.1046/j.1365-2249.1999.00993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hooks JJ, Chan CC, Detrick B. Identification of the lymphokines, interferon-gamma and interleukin-2, in inflammatory eye diseases. Invest Ophthalmol Vis Sci. 1988;29:1444–1451. [PubMed] [Google Scholar]
- 52.Chan CC, Li Q. Immunopathology of uveitis. Br J Ophthalmol. 1998;82:91–96. doi: 10.1136/bjo.82.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Smet MD, Chan CC. Regulation of ocular inflammation—what experimental and human studies have taught us. Prog Retin Eye Res. 2001;20:761–797. doi: 10.1016/s1350-9462(01)00011-8. [DOI] [PubMed] [Google Scholar]
- 54.Li B, Yang P, Zhou H, et al. T-bet expression is upregulated in active Behçet’s disease. Br J Ophthalmol. 2003;87:1264–1267. doi: 10.1136/bjo.87.10.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silverman MD, Zamora DO, Pan Y, et al. Constitutive and inflammatory mediator-regulated fractalkine expression in human ocular tissues and cultured cells. Invest Ophthalmol Vis Sci. 2003;44:1608–1615. doi: 10.1167/iovs.02-0233. [DOI] [PubMed] [Google Scholar]
- 56.Imai Y, Sugita M, Nakamura S, et al. Cytokine production and helper T cell subsets in Vogt-Koyanagi-Harada’s disease. Curr Eye Res. 2001;22:312–318. doi: 10.1076/ceyr.22.4.312.5510. [DOI] [PubMed] [Google Scholar]
- 57.Lim W-K, Nussenblatt RB, Chan CC. Immunopathology of inflammatory Coats’ disease. Arch Ophthalmol. 2005;123:279–281. doi: 10.1001/archopht.123.2.279. [DOI] [PubMed] [Google Scholar]
- 58.Chan CC, Nussenblatt RB, Fujikawa LS, et al. Sympathetic ophthalmia. Immunopathological findings. Ophthalmology. 1986;93:690–695. doi: 10.1016/s0161-6420(86)33694-7. [DOI] [PubMed] [Google Scholar]
- 59.Chan CC, Palestine AG, Kuwabara T, et al. Immunopathologic study of Vogt-Koyanagi-Harada syndrome. Am J Ophthalmol. 1988;105:607–611. doi: 10.1016/0002-9394(88)90052-9. [DOI] [PubMed] [Google Scholar]
- 60.Shen DF, Zhuang Z, LeHoang P, et al. Utility of microdissection and polymerase chain reaction for the detection of immunoglobulin gene rearrangement and translocation in primary intraocular lymphoma. Ophthalmology. 1998;105:1664–1669. doi: 10.1016/S0161-6420(98)99036-4. [DOI] [PubMed] [Google Scholar]
- 61.Chan CC. Molecular pathology of primary intraocular lymphoma. Trans Am Ophthalmol Soc. 2003;101:275–292. [PMC free article] [PubMed] [Google Scholar]
- 62.Coupland SE, Bechrakis NE, Anastassiou G, et al. Evaluation of vitrectomy specimens and chorioretinal biopsies in the diagnosis of primary intraocular lymphoma in patients with masquerade syndrome. Graefes Arch Clin Exp Ophthalmol. 2003;241:860–870. doi: 10.1007/s00417-003-0749-y. [DOI] [PubMed] [Google Scholar]
- 63.Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp Eye Res. 2003;76:397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- 64.Tuo J, Bojanowski CM, Chan CC. Genetic factors of age-related macular degeneration. Prog Retin Eye Res. 2004;23:229–249. doi: 10.1016/j.preteyeres.2004.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tuo J, Smith BC, Bojanowski CM, et al. The involvement of sequence variation and expression of CX3CR1 in the pathogenesis of age-related macular degeneration. FASEB J. 2004;18:1297–1299. doi: 10.1096/fj.04-1862fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnson LV, Leitner WP, Rivest AJ, et al. The Alzheimer’s A beta-peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc Natl Acad Sci USA. 2002;99:11830–11835. doi: 10.1073/pnas.192203399. [DOI] [PMC free article] [PubMed] [Google Scholar]