Abstract
A constellation of pharmacologic treatments for generalized anxiety disorder (GAD) have been developed over the past five decades, although each has a number of potential drawbacks in clinical practice. This review addresses one potentially new pharmacologic treatment for generalized anxiety disorder, the gamma-aminobutyric acid analogue pregabalin. We review the mechanism of action, and pharmacokinetic and pharmacodynamic properties of pregabalin as well as the results of 5 double-blind, placebo-controlled trials of pregabalin in the treatment of generalized anxiety disorder (GAD). Based entirely on data from these industry-sponsored (Pfizer), multi-site clinical trials in patients with GAD, pregabalin appears to be generally well tolerated and has rapid onset of action (approximately 1 week), comparable efficacy to benzodiazepines and lower discontinuation rates compared with other pharmacologic treatments. Thus in GAD, a disorder that is often suboptimally responsive to traditional psychotherapeutic and psychopharmacologic interventions – secondary to poor efficacy, tolerability, and/or side-effects – pregabalin may have a primary role in GAD patients, especially in those with certain psychiatric comorbidities or individuals who are on multi-drug regimens for medical comorbidities.
Keywords: GAD, pregabalin, panic attack, anxiety disorders, antidepressant, anxiolytic
Introduction
With a lifetime prevalence of 4%–7% in the general population, generalized anxiety disorder (GAD) is among the most common psychiatric disorders (Kessler et al 2001) and is associated with significant morbidity (World Health Organization Mental Health Survey 2004). A constellation of pharmacologic treatments for GAD has been developed over the past 5 decades, including benzodiazepines, selective-serotonin reuptake inhibitors (SSRIs), selective serotonin-norepinephrine reuptake inhibitors (SSNRIs), monoamine oxidase inhibitors, and tricyclic antidepressants. However, each of these modalities has a number of potential drawbacks in clinical practice. Benzodiazepines are associated with cognitive and motor impairment (van Laar et al 1992), pose a significant risk for dependence (Rickels et al 1990), and interact with alcohol. The SSRIs and SSNRIs, while efficacious in the treatment of GAD, have a delayed onset of treatment effect, are associated with sexual side-effects (Kennedy et al 2000), and are contraindicated as monotherapy in patients with comorbid bipolar disorder (Keck et al 2006) in whom anxiety disorders commonly co-occur (McElroy et al 2001; Simon et al 2004). Thus, there is a considerable need to expand our psychopharmacologic armamentarium. This review addresses one potentially new pharmacologic treatment for generalized anxiety disorder, the γ-aminobutyric acid analogue pregabalin, which is currently FDA-indicated for the management of neuropathic pain and post-herpetic neuralgia and for adjunctive therapy in patients with partial onset seizures.
Mechanism of action of pregabalin
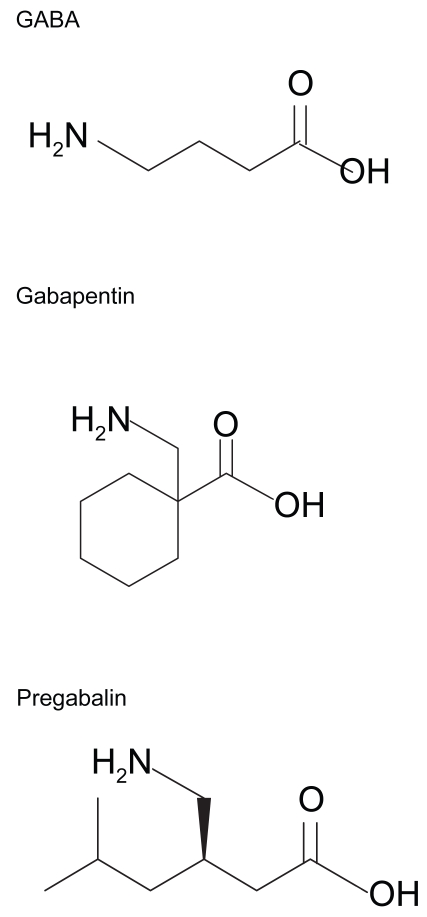
Through high affinity binding to the α2δ subunit of brain voltage-gated calcium channels (N-type), the γ-aminobutyric acid (GABA) analogue pregabalin (Figure 1) decreases presynaptic calcium currents (Finker et al 2002) and thus decreases calcium-dependent vesicle docking at the presynaptic membrane. Since this process is fundamental for all chemical synapses, pregabalin decreases the release of various neurotransmitters which have been pathophysiolgically implicated in anxiety disorders including glutamate (Dooley et al 2000a), substance P (Fehrenbacher et al 2003; Geracioti et al 2006), and norepinephrine (Dooley et al 2000b; Strawn and Geracioti). However, unlike anxiolytic compounds (eg, benzodiazepines) which exert their therapeutic effects through binding to GABAA, GABAB, and benzodiazepine receptors, pregabalin neither binds directly to these receptors nor augments GABAA currents or affects GABA metabolism (Pfizer Inc. 2006). Moreover, pregabalin does not appear to have functional activity at serotonin, dopamine, or norepinephrine receptors and does not alter in vitro reuptake kinetics of these monoamines as do traditional antidepressant medications (eg, selective serotonin reuptake inhibitors, tricyclic antidepressants)
Figure 1.
Chemical structures of γ-amino-butyric acid (GABA), gabapentin, and pregabalin.
Pharmacokinetics of pregabalin
Highly lipophilic, pregabalin is rapidly absorbed from the gastrointestinal tract and achieves maximum plasma concentrations within 1.5 hours of oral administration and has an oral bioavailability of ≥ 90% (Pfizer Inc. 2006). The Cmax is decreased by 25%–30% when pregabalin is taken with food but “has no clinically relevant effect on total absorption” (Pfizer Inc. 2006) and can therefore be taken with or without food. Because of the lipophilic properties related to substitution at the 3 position of the GABA molecule, pregabalin is thought to rapidly penetrate the central nervous system in humans based on cerebrospinal fluid and blood–brain barrier studies in lower animals (Feng et al 2001; Pfizer Inc. 2006). Moreover, pregabalin dose not exhibit any significant protein binding, has no known drug interactions and, even at high doses, does not interact with CYP1A2, CYP2A6, CYP2C9, CYP2D6, CYP2E1, or CYP3A4 systems in vivo (Pfizer Inc. 2006). Ninety per cent of pregabalin, which has a half-life of approximately 6 hours, is excreted renally as unchanged drug and it is recommended that dosage should be adjusted for patients with creatinine clearance <60 mL/min (see Pfizer Inc. 2006 for further details).
Pre-clinical studies of pregabalin in anxiety states
Lower animal studies have suggested that pregabalin may have anxiolytic properties. For example, in rats, pregabalin dose-dependently induced anxiolytic-like effects in both the conflict test and elevated plus maze tests compared with placebo (Field et al 2001).
Clinical studies of pregabalin in GAD
In a 3-armed study of pregabalin for the treatment of GAD (Feltner et al 2003), patients receiving high dose pregabalin (600 mg/day) or lorazepam (6 mg/day) but not low-dose pregabalin (150 mg/day) significantly improved compared with patients receiving placebo at 4 weeks (mean decrease in HAM-A score: 13.2 with high-dose pregabalin compared with 9.3 with placebo, p = 0.001). However, patients receiving high-dose pregabalin and lorazepam were significantly more likely to discontinue than those receiving low-dose pregabalin or placebo, though there was no statistically significant difference between the adverse events in patients treated with high-dose pregabalin compared with those receiving lorazepam (Feltner et al 2003).
Pande et al (2003) evaluated two doses of pregabalin (50 mg tid and 200 mg tid) in a double-blind, placebo-controlled trial which included a lorazepam arm (2 mg tid). This study consisted of a 1-week lead-in followed by 4 weeks of double blind treatment and a 1-week taper. Baseline-to-endpoint decreases in HAM-A score were significantly greater than placebo for both doses of pregabalin and for lorazepam. However, this study failed to identify a statistically significant difference between the two dosages of pregabalin with respect to outcome HAM-A score, nor were there significant differences in HAM-A scores between patients treated with lorazepam and those treated with high-dose pregabalin.
Pohl and colleagues (2005) compared multiple pregabalin dosing regimens (100 mg bid, 200 mg bid, 150 mg tid) in a double-blind, placebo-controlled trial in GAD and found that all three pregabalin groups had improvement in HAM-A scores compared with placebo. As with previous studies in this population, pregabalin was generally well tolerated.
Rickels et al (2005), examined 3 doses of pregabalin (300 mg/day, 450 mg/day, and 600 mg/day) and alprazolam (1.5 mg/day) in the treatment of 454 patients with GAD in a randomized, placebo-controlled, multi-site trial. All three doses of pregabalin and alprazolam were significantly superior to placebo in reduction of HAM-A scores and separation from placebo was observed at week one for all treatments.
A recent double-blind comparison of pregabalin (400 mg/day and 600 mg/day) and fixed-dose venlafaxine (75 mg/day) found both dosages of pregabalin and venlafaxine produced significantly greater improvement in HAM-A total score than did placebo (Montgomery et al 2006). HAM-A scores at week one were significantly greater than placebo for both dosages of pregabalin while venlafaxine-treated patients had statistically significant improvement at week two (Montgomery et al 2006)
Withdrawal effects of pregabalin in patients with anxiety disorders
Of note, clinical trials of pregabalin in the treatment of generalized anxiety and social anxiety disorder have included withdrawal measures such as the physician withdrawal checklist (PWC), a physician-administered instrument which measures 20 common withdrawal symptoms and has been used extensively to evaluate benzodiazepine withdrawal (Rickels et al 1990). Following 4 weeks of pregabalin treatment, Rickels and colleagues (2005) determined PWC scores during a 1-week taper and found similar PWC scores in all treatment groups (pregabalin 300 mg/day, 450 mg/day, and 600 mg/day; alprazolam 1.5 mg/day). However, in the subsequent week, during which subjects were medication free, significantly higher withdrawal anxiety was observed in patients who had been treated with pregabalin 600 mg/day (p < 0.04 vs placebo) (Rickels et al 2005). Interestingly, this study did not observe significant increases in PWC scores in those patients who had been treated with alprazolam, 1.5 mg/day (Rickels et al 2005). Pande et al (2003) found PWC scores to be significantly higher than placebo for patients receiving lorazepam (6 mg/day) and noted a statistical trend (p = 0.06) for patients receiving pregabalin 600 mg/day to have increased discontinuation-related anxiety symptoms, but found no difference in PWC score between pregabalin 150 mg/day and placebo (Pande et al 2003). Pohl et al (2005) found PWC scores following swift taper or abrupt discontinuation of pregabalin (200 mg/day) to be no different from placebo but found PWC scores to be higher than placebo in the patients who had been treated with pregabalin (450 mg/day), though the authors of this study did not consider these differences to be clinically significant (Pohl et al 2005).
Effects of pregabalin on mood in GAD patients
Because depressive-spectrum disorders commonly co-occur in patients with GAD, and anxiolytics can sometimes worsen or improve depressive symptoms, several of the clinical trials have included measures of depressive symptoms. Pohl and colleagues (2003) observed improvement in Hamilton Depression (HAM-D) Scale (17-item) scores for patients receiving pregabalin 200 mg tid but did not observe such a difference for patients receiving pregabalin 150 mg/day (5.5 points) or lorazepam 6 mg/day (5.6 points). In a 6-week trial comparing pregabalin (400 mg/day and 600 mg/day) with fixed-dose venlafaxine (75 mg/day) HAM-D scores were noted to improve (vs placebo) by 5.3 points (pregabalin 400 mg/day), 4.9 points (pregabalin 600 mg/day), and 5.1 points (venlafaxine 75 mg/day) (Montgomery et al 2006). Rickels et al (2005) excluded patients with depression in their study but did not observe any worsening in HAM-D scores in patients receiving pregabalin (300 mg/day, 450 mg/day, or 600 mg/day) or alprazolam. Both Feltner et al (2003) and Pande et al (2003) excluded patients with current major depression, though patients with dysthymia or a history of major depression were enrolled. Neither of these studies noted worsening of depressive symptoms in any treatment arm, though it might be noted that the HAM-D lacks sensitivity for the detection of atypical depression.
Adverse effects and safety of pregabalin in patients with anxiety disorders
The most frequently reported adverse effects in GAD patients included dizziness, somnolence, headache, dry mouth, amblyopia, and diarrhea, all of which were more frequent in patients receiving pregabalin 600 mg/day compared with those receiving pregabalin 150 mg/day (Pande et al 2003; Feltner et al 2003). A study of pregabalin 400 mg/day and 600 mg/day in GAD also found dizziness to be the most common side-effect, followed by somnolence (Montgomery et al 2006). A dose-effect with respect to these side-effects was observed (Montgomery et al 2006). Pohl and colleagues (2005) evaluated bid and tid dosing and found no significant difference in side-effects, though the overall adverse effects were similar to those described in previous studies: dizziness, somnolence and dry mouth, but also euphoria, inco-ordination, flatulence, and difficulty concentrating (Pohl et al 2005).
Given concern for somnolence and other possible central nervous system depressant effects, Hindmarch et al (2005) evaluated the cognitive and psychomotor effects of pregabalin (450 mg/day) compared with alprazolam (3 mg/day) and placebo in healthy volunteers. In this study pregabalin produced “only minor, transient impairment on some objective cognitive and psychomotor measures, suggesting a relatively benign CNS side-effect profile”, while alprazolam treatment resulted in significant decrement in all measures (Hindmarch et al 2005).
Weight gain has been associated with pregabalin in nearly all clinical trials of patients with anxiety disorders (Pande et al 2003, 2004; Pohl et al 2005; Rickels et al 2005; Montgomery et al 2006) and appears to be a dose-related effect. In a 4-week trial, mean increases in weight (± SEM) were 1.1 ± 0.2 kg for patients receiving 300 mg, 1.4 ± 0. 2 kg for patients receiving 450 mg, and 1.9 ± 0.3 kg for patients taking 600 mg, while increases of 0.9 ± 0.3 kg were observed for patients taking alprazolam (1.5 mg/day); all increases in weight were statistically significant compared with placebo (Rickels et al 2005). Pande et al (2003) similarly observed significant weight increases of 1.3 kg and 2.2 kg in patients treated for 4 weeks with pregabalin 150 mg/day and 600 mg/day respectively, but did not observe significant weight gain in patients treated with lorazepam (Pande et al 2003). The studies by Montgomery et al (2006) and Feltner et al (2003) noted that some pregabalin-treated patients did experience >7% increases in body mass but do not include statistical comparisons with placebo-treated patients. Finally, Pande et al observed mean increases of 0.6 kg and 1.7 kg in patients receiving pregabalin 150 mg/day and 600 mg/day for 4 weeks respectively, while patients treated with placebo had a mean weight loss of 0.1 kg; however, no statistical comparisons were reported (Pande et al 2004). The effects of pregabalin on lipid profiles are unknown.
Discussion
In a disorder that is often suboptimally responsive to traditional psychotherapeutic and psychopharmacologic interventions – secondary to poor efficacy, tolerability, and/or side-effects – pregabalin may have an emerging role in the treatment of GAD. Moreover, pregabalin may have a primary role in patients with certain psychiatric comorbidities (eg, bipolar disorder, substance dependence) and in patients with multiple medical comorbidities who may be taking multiple concomitant medications, as pregabalin has no known drug interactions (Brodie et al 2005; Pfizer Inc. 2006).
The efficacy of pregabalin in treating GAD is not surprising as a number of other antiepileptic drugs have also been shown to have anxiolytic properties and to be effective in treating patients with anxiety disorders. Valproate has been shown to have efficacy in the treatment of panic disorder (Primeau et al 1990; Woodman and Noyes 1994; Baetz and Bowen 1998) and blocks lactate-induced panic attacks (Keck et al 1993). The antiepileptic drug carbamazepine has efficacy in the treatment of panic disorder (Tondo et al 1989), post-traumatic stress disorder (PTSD) (Lipper et al 1986), and obsessive compulsive disorder (OCD) (Joffe and Swinson 1987). Lamotrigine is potentially effective in the treatment of PTSD (Hertzberg et al 1999) and may have an adjunctive role in the treatment of refractive OCD (Kumar and Khanna 2000). Topiramate has been shown to be efficacious in open-label trials for PTSD (Berlant and van Kammen 2002; Berlant 2004), social phobia disorder (Van Ameringen et al 2004) and may have an adjunctive role in treatment-resistant OCD (Van Ameringen et al 2006). However, unlike previous antiepileptic drugs which primarily block sodium and potassium channels or increase cerebral GABA concentrations, pregabalin decreases presynaptic calcium currents and in doing so decreases the release of several neurotransmitters, including glutamate (Dooley et al 2000a), substance P (Fehenbacher et al 2003), calcitonin-gene-related peptide (Fehenbacher et al 2003), and norepinephrine (Dooley et al 2002). Interestingly, many of these neurotransmitters have been implicated in the pathogenesis GAD or other anxiety disorders (Erikkson et al 1991; Geracioti et al 2001; Olsson et al 2004; Geracioti et al 2006). As might be expected, agents that pharmacologically dampen these systems have therapeutic roles in a number of anxiety disorders (Peet and Ali 1986; Furmark et al 2005; Strawn and Geracioti 2006). Also, decreases in the activity of these or related “fear circuits” that underlie the pathophysiology of certain anxiety disorders (Stahl 2004) could explain the efficacy of pregabalin in patients with GAD. It will be of interest to examine the effects of pregabalin in other anxiety disorders such as PTSD, panic disorder, or even meal-related anxiety in anorexia nervosa (wherein additional benefit may be conferred by pregabalin-associated weight gain).
Conclusion
Based entirely on data from industry-sponsored (Pfizer), multi-site clinical trials, pregabalin appears to be generally well-tolerated in adult patients with generalized anxiety disorder and has rapid onset of action (approximately one week) as well as comparable efficacy to benzodiazepines (Feltner et al 2003; Rickels et al 2005). Moreover, discontinuation rates observed in the five published double-blind, placebo-controlled trials of pregabalin in the treatment of GAD have generally been lower than those observed for both benzodiazepines (Geltner et al 2003; Pande et al 2003; Rickels et al 2005) and venlafaxine, 75 mg/day (Montgomery et al 2006).
Table 1.
Placebo-controlled trials of pregabalin in generalized anxiety disorder
| Study | Population | Design | Comparator dose
(mg/d) |
Na | Results | |
|---|---|---|---|---|---|---|
| Montgomery et al 2006 | Outpatients with GAD Baseline HAM-A >20 and Covi Anxiety Score >9 | Double-blind, placebo- controlled comparison to venlafaxine 6 weeks, LOCF | venlafaxine
(75 mg/day, fixed) |
400
600 |
97/8
1110/81 |
HAM-A separation from placebo at week 1 for both pregabalin doses and separation from placebo at week 2 for venlafaxine. |
| Rickels et al 2005 | Outpatients with GAD, baseline HAM-A score >20 | Double-blind, placebo-controlled.
4-weeks, LOCF, excluded current depressive disorder |
alprazolam
(1.5 mg/day) |
300
450 600 |
89/81
87/72 85/66 |
Separation from placebo at week 1 for all pregabalin groups and an alprazolam group in HAM-A psychic anxiety symptoms |
| Pande et al 2003 | Outpatients with GAD, Baseline HAM-A score >20 and Covi Anxiety Score >9 | Double-blind, placebo-controlled.
4-week treatment with1 week placebo lead in. |
lorazepam
(6 mg/day) |
150
600 |
69/62
70/50 |
Separation from placebo at week 1 for both pregabalin groups. HAM-A decreases were 9.5 (pregabalin 150 mg/day), 10.3 (pregabalin, 600 mg/day), 12.0 (lorazepam) and 6.8 (placebo). No serious adverse effects were reported. |
| Feltner et al 2003 | Outpatients with GAD, Baseline HAM-A score 20 and Covi Anxiety Score >9 | Double-blind, placebo-controlled 4-week. | lorazepam
(6 mg/day) |
150
600 |
70/
66/ |
Separation from placebo in HAM-A at week 1 for pregabalin 600 mg/day but not for pregabalin 150 mg/day. |
| Pohl et al 2005 | Outpatients with GAD, Baseline HAM-A score >20 and Covi Anxiety Score >9 | Double-blind, placebo-controlled 6-week. Pairwise comparison of bid vs tid dosing with LOCF | none | 200b 400b 450c |
78/55
89/64 88/66 |
Separation from placebo in HAM-A at week 1 for all pregabalin arms. BID dosing provided similar efficacy and tolerability compared with tid dosing. |
Left number indicates number of patients randomized to a particular arm; right number denotes the number of patients completing the arm.
bid dosing regimen.
tid dosing regimen.
Abbreviations: GAD, generalized anxiety disorder; LOCF, last observation carried forward.
Acknowledgments
The authors thank Dr. Joseph Wise (Walter Reed Army Medical Center, Washington DC) for critical comments on the manuscript and Mrs. Shannon Kennedy (The Ohio State University, Columbus, Ohio) for assistance in the preparation of figures.
Footnotes
Disclosures
Drs. Strawn and Geracioti report no conflicts of interest.
References
- Baetz M, Bowen RC. Efficacy of divalproex sodium in patients with panic disorder and mood instability who have not responded to conventional therapy. Can J Psychiatry. 1998;43:73–7. doi: 10.1177/070674379804300109. [DOI] [PubMed] [Google Scholar]
- Brodie MJ, Wilson EA, Wesche DL, et al. Pregabalin drug interaction studies: lack of effect on the pharmacokinetics of carbamazepine, phenytoin, lamotrigine, and valproate in patients with partial epilepsy. Epilepsia. 2005;46:1407–13. doi: 10.1111/j.1528-1167.2005.19204.x. [DOI] [PubMed] [Google Scholar]
- Dooley DJ, Donovan CM, Pugsley TA. Stimulus-dependent modulation of [3H]norepinephrine release from rat neocortical slices by gabapentin and pregabalin. J Pharmacol Exp Ther. 2000b;295:1086–1093. [PubMed] [Google Scholar]
- Dooley DJ, Mieske CA, Borosky SA. Inhibition of K+-evoked glutamate release from rat neocortical and hippocampal slices by gabapentin. Neurosci Lett. 2000a;280:107–110. doi: 10.1016/s0304-3940(00)00769-2. [DOI] [PubMed] [Google Scholar]
- Eriksson E, Westberg P, Alling C, et al. Cerebrospinal fluid levels of monoamine metabolites in panic disorder. Psychiatry Res. 1991;36:243–51. doi: 10.1016/0165-1781(91)90023-i. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher JC, Taylor CP, Vasko MR. Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain. 2003;105:133–41. doi: 10.1016/s0304-3959(03)00173-8. [DOI] [PubMed] [Google Scholar]
- Feltner DE, Crockatt JG, Dubovsky SJ, et al. A randomized, double-blind, placebo-controlled, fixed-dose, multicenter study of pregabalin in patients with generalized anxiety disorder. J Clin Psychopharmacol. 2003;23:240–9. doi: 10.1097/01.jcp.0000084032.22282.ff. [DOI] [PubMed] [Google Scholar]
- Feng MR, Turluck D, Burleigh J, et al. Brain microdialysis and PK/PD correlation of pregabalin in rats. Eur J Drug Metab Pharmacokinet. 2001;26:123–8. doi: 10.1007/BF03190385. [DOI] [PubMed] [Google Scholar]
- Field MJ, Oles RJ, Singh L. Pregabalin may represent a novel class of anxiolytic agents with a broad spectrum of activity. Br J Pharmacol. 2001;132:1–4. doi: 10.1038/sj.bjp.0703794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink K, Dooley DJ, Meder WP, et al. Inhibition of neuronal Ca2+ influx by gabapentin and pregablin in the human neocortex. Neuropharmacology. 2002;42:229–36. doi: 10.1016/s0028-3908(01)00172-1. [DOI] [PubMed] [Google Scholar]
- Furmark T, Appel L, Michelgard A, et al. Cerebral blood flow changes after treatment of social phobia with the neurokinin-1 antagonist GR205171, citalopram, or placebo. Biol Psychiatry. 2005;58:132–42. doi: 10.1016/j.biopsych.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Geracioti TD, Jr, Baker DG, Ekhator NN, et al. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158:1227–30. doi: 10.1176/appi.ajp.158.8.1227. [DOI] [PubMed] [Google Scholar]
- Geracioti TD, Jr, Carpenter LL, Owens MJ, et al. Elevated cerebrospinal fluid substance P concentrations in posttraumatic stress disorder and major depression. Am J Psychiatry. 2006;163:637–43. doi: 10.1176/ajp.2006.163.4.637. [DOI] [PubMed] [Google Scholar]
- Hertzberg MA, Butterfield MI, Feldman ME, et al. A preliminary study of lamotrigine for the treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;45:1226–9. doi: 10.1016/s0006-3223(99)00011-6. [DOI] [PubMed] [Google Scholar]
- Hindmarch I, Trick L, Ridout F. A double-blind, placebo- and positive-internal-controlled (alprazolam) investigation of the cognitive and psychomotor profile of pregabalin in healthy volunteers. Psychopharmacology (Berl) 2005;183:133–43. doi: 10.1007/s00213-005-0172-7. [DOI] [PubMed] [Google Scholar]
- Joffe RT, Swinson RP. Carbamazepine in obsessive-compulsive disorder. Biol Psychiatry. 1987;22:1169–71. doi: 10.1016/0006-3223(87)90061-8. [DOI] [PubMed] [Google Scholar]
- Keck PE, Strawn JR, McElroy SL. Pharmacologic treatment considerations in co-occuring bipolar and anxiety disorders. J Clin Psychiatry. 2006;67:8–15. [PubMed] [Google Scholar]
- Kennedy SH, Eisfeld BS, Dickens SE, et al. Antidepressant-induced sexual dysfunction during treatment with moclobemide, paroxetine, sertraline and venlafaxine. J Clin Psychiatry. 2000;61:276–281. doi: 10.4088/jcp.v61n0406. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Keller MB, Wittchen HU. The epidemiology of generalized anxiety disorder. Psychiatr Clin North Am. 2001;24:19–39. doi: 10.1016/s0193-953x(05)70204-5. [DOI] [PubMed] [Google Scholar]
- Kumar TC, Khanna S. Lamotrigine augmentation of serotonin re-uptake inhibitors in obsessive-compulsive disorder. Aust N Z J Psychiatry. 2000;34:527–8. doi: 10.1080/j.1440-1614.2000.0751c.x. [DOI] [PubMed] [Google Scholar]
- Lipper S, Davidson JR, Grady TA, et al. Preliminary study of carbamazepine in post-traumatic stress disorder. Psychosomatics. 1986;27:849–54. doi: 10.1016/S0033-3182(86)72590-5. [DOI] [PubMed] [Google Scholar]
- Keck PE, Jr, Taylor VE, Tugrul KC, et al. Valproate treatment of panic disorder and lactate-induced panic attacks. Biol Psychiatry. 1993;33:542–6. doi: 10.1016/0006-3223(93)90010-b. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Altshuler LL, Suppes T, et al. Axis I psychiatric comorbidity and its relationship to historical illness variables in 288 outpatients with bipolar disorder. Am J Psychiatry. 2004;158:420–6. doi: 10.1176/appi.ajp.158.3.420. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Tobias K, Zornberg GL, et al. Efficacy and safety of pregabalin in the treatment of generalized anxiety disorder: a 6-week, multicenter, randomized, double-blind, placebo-controlled comparison of pregabalin and venlafaxine. J Clin Psychiatry. 2006;67:771–82. doi: 10.4088/jcp.v67n0511. [DOI] [PubMed] [Google Scholar]
- Olsson A, Regnell G, Traskman-Bendz L, et al. Cerebrospinal neuropeptide Y and substance P in suicide attempters during long-term antidepressant treatment. Eur Neuropsychopharmacol. 2004;14:479–85. doi: 10.1016/j.euroneuro.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Pande AC, Crockatt JG, Feltner DE, et al. Pregabalin in generalized anxiety disorder: a placebo-controlled trial. Am J Psychiatry. 2003;160:533–40. doi: 10.1176/appi.ajp.160.3.533. [DOI] [PubMed] [Google Scholar]
- Peet M, Ali S. Propranolol and atenolol in the treatment of anxiety. Int Clin Psychopharmacol. 1986;1:314–9. doi: 10.1097/00004850-198610000-00005. [DOI] [PubMed] [Google Scholar]
- Pfizer Incorporated. Package insert: pregabalin. New York, NY, USA: 2006. [Google Scholar]
- Pohl RB, Feltner DE, Fieve RR, et al. Efficacy of pregabalin in the treatment of generalized anxiety disorder: double-blind, placebo-controlled comparison of BID versus TID dosing. J Clin Psychopharmacol. 2005;25:151–8. doi: 10.1097/01.jcp.0000155820.74832.b0. [DOI] [PubMed] [Google Scholar]
- Primeau F, Fontaine R, Beauclair L. Valproic acid and panic disorder. Can J Psychiatry. 1990;35:248–50. doi: 10.1177/070674379003500309. [DOI] [PubMed] [Google Scholar]
- Rickels K, Pollack MH, Feltner DE, et al. Pregabalin for treatment of generalized anxiety disorder: a 4-week, multicenter, double-blind, placebo-controlled trial of pregabalin and alprazolam. Arch Gen Psychiatry. 2005;62:1022–30. doi: 10.1001/archpsyc.62.9.1022. [DOI] [PubMed] [Google Scholar]
- Rickels K, Schweizer E, Case WG, et al. Long-term therapeutic use of benzodiazepines. I. Effects of abrupt discontinuation. Arch Gen Psychiatry. 1990;47:899–907. doi: 10.1001/archpsyc.1990.01810220015002. [DOI] [PubMed] [Google Scholar]
- Simon NM, Otto MW, Wisniewski SR, et al. Anxiety disorder comorbidity in bipolar patients: data from the first 500 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Am J Psychiatry. 2004;161:2222–9. doi: 10.1176/appi.ajp.161.12.2222. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Anticonvulsants as anxiolytics, part 2: Pregabalin and gabapentin as alpha(2)delta ligands at voltage-gated calcium channels. J Clin Psychiatry. 2004;65:460–1. doi: 10.4088/jcp.v65n0401. [DOI] [PubMed] [Google Scholar]
- Strawn JR, Geracioti TD., Jr Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2007 Mar 12; doi: 10.1002/da.20292. [Epub a head of print] [DOI] [PubMed] [Google Scholar]
- Tondo L, Burrai C, Scamonatti L, et al. Carbamazepine in panic disorder. Am J Psychiatry. 1989;146:558–9. doi: 10.1176/ajp.146.4.558b. [DOI] [PubMed] [Google Scholar]
- Van Ameringen M, Mancini C, Pipe B, et al. An open trial of topiramate in the treatment of generalized social phobia. J Clin Psychiatry. 2004;65:1674–8. doi: 10.4088/jcp.v65n1213. [DOI] [PubMed] [Google Scholar]
- Van Ameringen M, Mancini C, Patterson B, et al. Topiramate augmentation in treatment-resistant obsessive-compulsive disorder: a retrospective, open-label case series. Depress Anxiety. 2006;23:1–5. doi: 10.1002/da.20118. [DOI] [PubMed] [Google Scholar]
- Woodman CL, Noyes R., Jr Panic disorder: treatment with valproate. J Clin Psychiatry. 1994;55:134–6. [PubMed] [Google Scholar]
- The World Health Organization Mental Health Survey Consortium. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA. 2004;291:2581–90. doi: 10.1001/jama.291.21.2581. [DOI] [PubMed] [Google Scholar]