Abstract
Quetiapine was developed in 1985 by scientists at AstraZeneca (formerly Zeneca) Pharmaceuticals. It received official US Food and Drug Administration approval in September 1997 and approval in Germany in 2000. Since then, quetiapine has been used in the treatment of severe mental illness in approximately 70 countries including Canada, most Western European countries, and Japan. Quetiapine is a dibenzothiazepine derivative with a relatively broad receptor binding profile. It has major affinity to cerebral serotonergic (5HT2A), histaminergic (H1), and dopaminergic D1 and D2 receptors, moderate affinity to α1- und α2-adrenergic receptors, and minor affinity to muscarinergic M1 receptors; it demonstrates a substantial selectivity for the limbic system. This receptor occupancy profile with relatively higher affinity for the 5HT2A receptor compared with the D2 receptor is in part responsible for the antipsychotic characteristics and low incidence of extrapyramidal side-effects of quetiapine. The efficacy of quetiapine in reducing positive and negative symptoms of schizophrenia has been proven in several clinical trials with placebo-controlled comparators. Quetiapine has also demonstrated robust efficacy for treatment of cognitive, anxious-depressive, and aggressive symptoms in schizophrenia. Long-term trials show sustained tolerability for a broad spectrum of symptoms. Quetiapine has also proven efficacy and tolerability in the treatment of moderate to severe manic episodes, and in the treatment of juveniles with oppositional-defiant or conduct disorders, and in the geriatric dementia population. Recent data indicate that quetiapine may also be effective in the treatment of bipolar depressive symptoms without increasing the risk of triggering manic episodes, and in borderline personality disorder. In comparison with other antipsychotics, quetiapine has a favorable side-effect profile. In clinical trials only small insignificant prolongations of the QT interval were observed. Weight-gain liabilities and new-onset metabolic side-effects occupy a middle-ground among newer antipsychotics. As a result of its good efficacy and tolerability profile quetiapine has become well established in the treatment of schizophrenia and manic episodes.
Keywords: schizophrenia, antipsychotic, quetiapine, efficacy, tolerability
Introduction
The discovery of the first antipsychotics such as chlorpromazine led to a significant change in the treatment of schizophrenia, especially with respect to “positive” symptoms of schizophrenia. However, it soon became obvious that this efficacy came at the price of high incidence for extrapyramidal symptoms such as parkinsonism, dyskinesia, or dystonia; and akathisia (Nemeroff et al 2002). Additionally, conventional antipsychotics seem to be less effective in the treatment of “negative” symptoms characterized by anergia, avolition, alogia, or affective flattening.
The first “atypical”, antipsychotic, clozapine, became available in the 1960s. While efficacy on both positive and negative symptoms of schizophrenia was comparably better than that of the previously available antipsychotics, in 1%–2% of cases severe agranulocytosis developed; consequently clozapine had to be removed from the market in 1975. Only the standard use of expensive continuous hematologic monitoring allowed for readmission to the market in 1990 with the indication to ameliorate the most treatment-resistent schizophrenia (Nemeroff et al 2002).
A variety of “new” atypical antipsychotics have since been developed, to meet requirements for better tolerability and comparable efficacy. While older agents still have a place in the treatment of schizophrenia symptomatology (Ganguli and Strassnig 2006), newer agents have been the mainstay of treatment. Quetiapine was synthesized at AstraZeneca (formerly Zeneca) in 1985 (Lieberman et al 2002). Company scientists combined perlapin and fluperlapin, two benzodiazepine-derived components with structural analogy to clozapine, but with molecular discrepancies explaining the lack of agranulocytosis risk (Nemeroff et al 2002). Quetiapine was approved in September 1997 by the US Food and Drug Administration (FDA) and has since been introduced in Canada, most Western European countries, Japan as well as in 70 other countries worldwide for the treatment of schizophrenia and other psychiatric illnesses (Nemeroff et al 2002).
Pharmaceutical formulation
Quetiapine is available in 25, 100, 200, and 300 mg film-coated tablets. Due to a relatively short half-life of 7 hours, quetipaine should be administered in 2 daily doses. The following initial titration schedule for schizophrenic patients should be adhered to: 1st day 50 mg, 2nd day 100 mg, 3rd day 200 mg, and 4th day 300 mg. After that, the dose may be gradually raised further up to 750 mg per day, the recommended high dose. In the treatment of manic episodes in bipolar disorder the following titration schedule is recommended: 1st day 100 mg, 2nd day 200 mg, 3rd day 300 mg, and 4th day 400 mg. Further dose adjustments up to 800 mg at day 6 should not be conducted in steps greater than 200 mg per day. It should be noted, however, that clinical experience may justify titration up to 1600 mg per day in selected cases (Nagy 2003).
Indication
Quetiapine is approved for the treatment of schizophrenia and moderate to severe acute manic episodes. No long-term trial data are available yet to confirm efficacy on the prevention of manic or depressive episodes.
Mechanisms of action
Quetiapine is a dibenzothiazepin-derivate (Nemeroff 2002) with a relative broad receptor affinity profile. Its relatively higher affinity to serotonergic (5HT2A)- than to dopaminergic D1- and D2-receptors (Bandelow and Ruther 2000) has been hypothesized to be responsible for the antipsychotic characteristics and the relatively low risk for extrapyramidal side-effects. Quetiapine also has high affinity to histaminergic and alpha-1 adrenergic receptors with a lower affinity to alpha-2-adrenergic receptors, but no appreciable affinity to muscarinergic acetylcholine- or GABA receptors (Table 1) (Richelson and Souder 2000).
Table 1.
In vitro receptor binding profile of quetiapine (derived from data of Richelson and Souder 2000)
| Receptor | Mean Kda (nM) |
|---|---|
| α1-adrenergic | 8.1 |
| α2-adrenergic | 80 |
| Dopamine D2 | 770 |
| HistamineH1 | 19 |
| Muscarinergic | 1400 |
| 5HT1A | 300 |
| 5HT2D | 560 |
| 5HT2A | 31 |
| 5HT2C | 3500 |
A lower Kd value indicates higher receptor affinity.
Dopamine receptors
Clinical studies have shown that quetiapine has lower affinity to dopaminergic D2-receptors than other comparable antipsychotics. PET studies (positron emmission tomography) show regional differences in CNS receptor affinity. Secondary to low affinity for the dopaminergic D2-receptor, the receptor binding curve shows an early peak with a rapid reduction in receptor occupancy. This relatively low D2-receptor occupancy could be an explanation for the low incidence of extrapyramidal symptoms and only mild prolactin elevations (Kapur et al 2000).
A preclinical study comparing effects of the atypical antipsychotics quetiapine, olanzapine and risperidone on CNS dopamine-receptor-density in rodents showed that after a 28-day treatment period, olanzapine and risperidone, but not quetiapine, significantly increased D2-receptor binding throughout different cortex areas including caudate, putamen, nucleus accumbens, and hippocampus. Moreover, dopamine D4-receptor density increased during treatment with olanzapine or risperidone. D1- and D3-receptor density remained unchanged in all investigated areas (Tarazi et al 2001).
Gefvert et al (2001) studied the relationship between different quetiapine dosages and the D2- and 5HT2A-receptor occupancy by PET scans in a small sample of 5 patients. Dosed clinically, quetiapine showed only a relatively low D2-receptor occupancy, leading the authors to conclude that lack of extrapyramidal symptoms during the treatment phase were secondary to this low occupancy (Gefvert et al 2001).
Serotonin receptors
Quetiapine, in line with other “atypical” antipsychotics, exhibits a higher affinity for 5HT2A-receptors than conventional compounds (Gefvert et al 2001). Thus, quetiapine can partially balance disadvantages of a relatively moderate dopamine receptor blockade and effect a reduction in extra-pyramidal symptoms, have good efficacy in improving negative symptoms, and have insignificant prolactin elevation, all distinct therapeutic improvements (Stahl 2005).
Whereas a 5HT2A-receptor-blockade is opposed to an increased D2 antagonism, especially in nigrostriatal, mesocortical, and tuberoinfundibular cortex areals, it is assumed that the same mechanism cannot abolish a desired D2-receptor blockade in the mesolimbic system (Stahl 2005).
Effects on other neurotransmitters
Relatively strong affinity to histaminergic and α1-adrenergic receptors may cause side-effects such as somnolence, stupor, dry mouth, asthenia, constipation, tachycardia, orthostatic hypotension, and dyspepsia. A case of quetiapine-associated malignant neuroleptic syndrome has been reported.
Pharmacokinetics
After oral intake quetiapine is rapidly absorbed; the main site of metabolism is the liver. Peak plasma levels are reached after approximately 1–2 hours, and half-life is 7 hours. Bioavailability of quetiapine is not significantly affected by simultaneous ingestion of food (Goldstein 1999). Plasma levels and dosage follow a nearly linear gradient (DeVane and Nemeroff 2001). Pharmakokinetic data seem to be unaffected by gender, ethnicity, body weight, or nicotine consumption; no difference between adolescents and adults has been detected (McConville et al 2000). The CYP3A4 isoenzyme of the liver cytochrome P450 system is mainly responsible for quetiapine metabolism; in vitro examinations have revealed that CYP2D6 may also be significanlty involved (Lin et al 2004). In declining order, CYP3A7 (11.8%), CYP3A5 (8.7%), and CYP2C19 (4.5%) also contribute (Lin et al 2004). Thus far, 11 metabolites have been identified, among which only 2 show intrinsic receptor activity (7-hydroxy-quetiapine and 7-hydroxy-N-dealkyl-quetiapine). Quetiapine sulfoxide represents the quantitatively largest proportion of inactive metabolites (Li et al 2004). Ongoing research is investigating the pharmacologic potential of these metabolites. Seventy-three per cent of radio-ligand tagged quetiapine is excreted by the kindneys, and 21% through bowel (DeVane and Nemeroff 2001).
In several clinical subpopulations, dose adjustments may be warranted. Geriatric patients, for example, show a reduction in plasma clearance by 30%–50%; the quetiapine target dose should thus be reduced by an equal proportion (Thyrum et al 2000). Patients with decreased kidney function (creatinine clearance <30 mL/min/1.73 m2) show approximately a 25% reduction in plasma clearance. Patients with reduced liver function show an approximately 25% reduction in plasma clearance. A dose reduction may be indicated in these cases.
Interaction potential
Quetiapine neither inhibits nor induces known liver CYP-450 isoenzymes. Other medications with activity on the P450 system may however alter quetiapine plasma levels. For example, potent inhibitors of the CYP3A4-system, such as erythromycin (Li et al 2005), may elevate quetiapine plasma levels, and inductors including carbamazepine and phenytoin (DeVane and Nemeroff 2001) may reduce quetiapine levels.
Potential medication interactions have been examined in various clinical studies. Concurrent administration of the CYP2D6-inhibitors imipramine and fluoxetine did not significantly alter quetiapine metabolism (Potkin et al 2002a). Concurrent administration of haloperidol or risperidone (Potkin et al 2002b), cimetidine (Strakowksi et al 2002), lithium, and sodium valproate (Potkin et al 1997) does not change quetiapine metabolism. Thioridazine lowers quetiapine plasma levels and requires increase in quetiapine dose (Potkin et al 2002b). Interactions between quetiapine and cardiovascular drugs have not been studied.
Clinical efficacy trials
Overview
Short- and long-term efficacy has been established through several double-blind, placebo-controlled trials. There are also indications that quetiapine may be efficacious in the treatment of a variety of disorders apart from schizophrenia, such as bipolar disorders, dementia, and in child and adolescent psychiatry. Economic aspects of quetiapine treatment have been investigated; efficacy for treatment of aggression and agitation during acute schizophrenic episodes, and efficacy in the treatment of iatrogenically induced psychotic symptoms in Parkinson’s patients has been proven.
Recent short-term trials
Efficacy
Efficacy of quetiapine was shown in 6- to 16-week double-blind, placebo-controlled and in comparative studies. Usual psychometric instruments employed to establish efficacy on positive and negative symptoms of schizophrenia are the
Positive And Negative Syndrome Scale (PANSS; Kay et al 1987),
Its derivative BPRS (Brief Psychiatric Rating Scale; Overall and Gorham 1962), and the
SAPS/SANS (Scale for the Assessment of Positive/Negative Symptoms; Andreasen 1982).
Buckley et al (2004a) published a small meta-analysis of 3 placebo-controlled trials and reported equal efficacy of quetiapine compared with haloperidol, chlorpromazine, and risperidone in the treatment of positive and negative symptoms, mood symptoms, and aggression, agitation, and irritability in schizophrenia patients. Quetiapine dosed at 400 mg showed better efficacy, especially in the treatment of positive symptoms, than at lower doses (Buckley 2004a). Conversely, Potkin et al (2006), referring to results from another blinded, placebo-controlled, randomized controlled trial (RCT), found superior efficacy of risperidone to quetiapine and placebo in all measured domains during a 2-week monotherapy phase; their sample consisted of acutely exacerbated DSM-IV schizophrenia patients requiring inpatient hospitalization. However, no significant differences in efficacy between both atypical antipsychotic treatment groups were seen during the following additive therapy phase.
Another meta-analysis by Schulz et al (2003) looked at 3 placebo-controlled and 5 comparative studies of quetiapine with haloperidol and found significantly better efficacy (p < 0.05) as compared to placebo and equal efficacy to haloperidol (Schulz et al 2003).
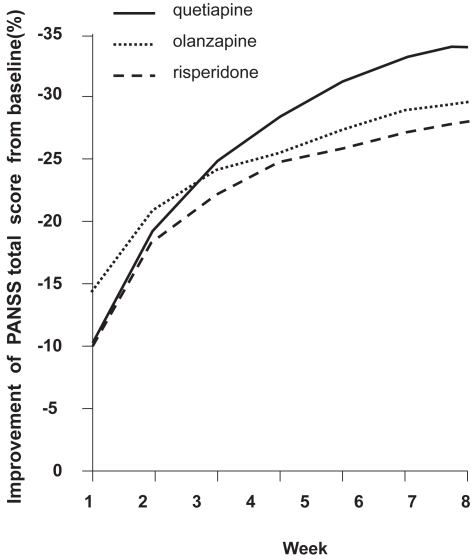
Sacchetti et al (2004) reported equal short-term efficacy of qetiapine, olanzapine, and risperidone; and recommended all three medications as first-line treatments in acute schizophrenia (Figure 2).
Figure 2.
Positive and Negative Syndrome Scale (PANSS) total score changes from baseline (mean values: 103.5, 98.5, and 96.0 for quetiapine-, olanzapine-, and risperidone-treated patients) (compiled from data of Sacchetti et al 2004).
In a recently published meta-analysis Tandon and Jibson (2005) came to a similar conclusion. They reviewed published short-term, RCTs of first-line atypical antipsychotics (risperidone, olanzapine, quetiapine, ziprasidone, and aripiprazole) in the treatment of schizophrenia and schizoaffective-disorder that used the PANSS to assess efficacy and found similar improvements among all first-line atypical antipsychotics for all efficacy parameters considered.
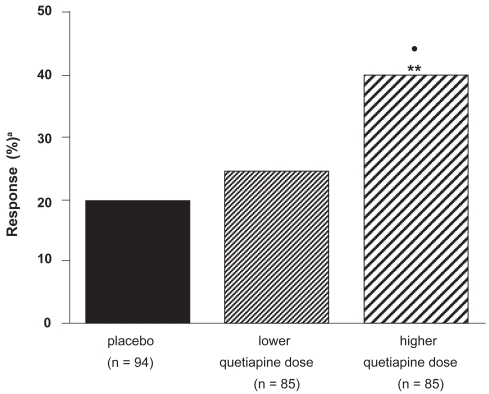
Small et al (2002), in a 6-week study examining quetiapine dose-response relationships, found significantly better efficacy on positive, negative, and affective symptoms for higher than lower doses. An initial target dose of 400 mg was recommended, and higher dosing according to individual requirements and tolerability was suggested (Small et al 2002; Figure 3).
Figure 3.
Percentage of patients responding to higher (mean 439 mg/d) and lower (mean 229 mg/day) quetiapine doses, and placebo (compiled from data of Small et al 2002).
Note: patients receiving <150 mg/d of quetiapine were not analyzed.
•p < 0.05, quetiapine: higher vs lower dose; **p < 0.01, quetiapine: higher dose vs placebo; a≥40% reduction in Brief Psychiatric Rating Scale (BPRS) total score.
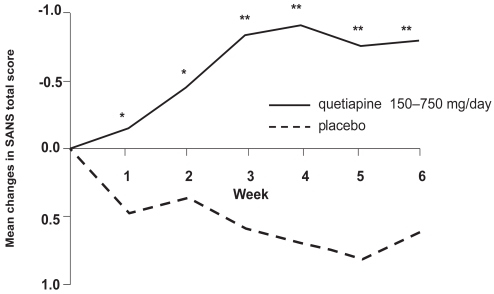
Some evidence for better efficacy of atypical antipsychotics in the treatment of negative symptoms of schizophrenia exists (Möller 1999). A meta-analysis of 4 RCTs by Tandon (2004) containing data on 1106 patients explored quetiapine’s direct effects on schizophrenic negative symptoms. Treatment effected a significantly greater improvement in SANS-scores in the quetiapine group compared with placebo (p < 0.001). A direct effect of quetiapine on schizophrenic negative symptoms was suggested, since indirect contributors such as antidepressant effects or lack of extrapyramidal-motor side-effects (EPS) were statistically excluded (Figure 4).
Figure 4.
Quetiapine significantly improved negative symptoms in a pooled analysis of three double-blind randomized controlled trials within 3 weeks (compiled from data of Tandon 2004).
*p < 0.05, **p < 0.01 vs placebo.
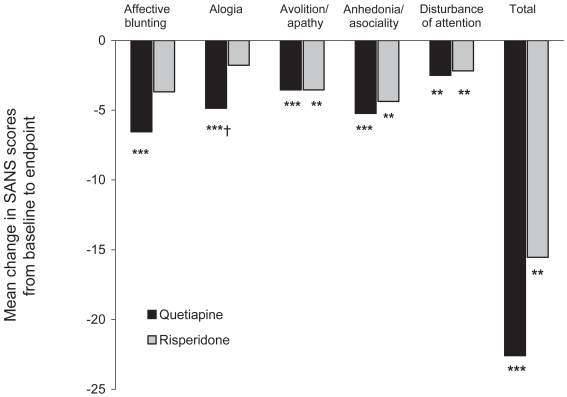
A 12-week RCT (quetiapine, n = 22; risperidone, n = 22) by Riedel et al (2005) showed equal efficacy in the treatment of negative symptoms of schizophrenia with quetiapine tending to improve the SANS-“Alogia” subscale to a greater extent (Figure 5).
Figure 5.
Mean change in Scale for the Assessment of Positive/Negative Symptoms (SANS) scores from baseline to week 12 following treatment with quetiapine or risperidone.
**p < 0.01 vs baseline, ***p < 0.001 vs, baseline (paired t-tests), †p = 0.065 vs risperidone (unpaired t-test).
Zhong et al (2006) reported results from a 8-week randomized, double-blind, fixed-dose trial comparing quetiapine and rsiperidone in the treatment of schizophrenia. Patients (n = 338 quetiapine, mean dose 525 mg; risperidone n = 335, mean dose 5.2 mg) were evaluated with the Positive and Negative Syndrome Scale (PANSS) and the Clinical Global Impression Scale (CGI). Improvements with both treatments were comparable on PANSS total, negative, and general psychopathology subscale, Risperidone-treated patients had a significantly greater (p = 0.03) improvement on positive subscores. EPS were significantly more frequent with risperidone treatment (n = 22) than with quetiapine (n = 13; p = 0.01). Somnolence was more common with quetiapine (26%) than risperidone (20%; p = 0.04). The authors conclude that both agents improved the patients’ symptomatolgy, with advantages for risperidone in efficacy and for quetiapine in tolerability.
Short-term efficacy on agitation/aggression
Nasrallah et al (2002) showed in a meta-analysis better efficacy of quetiapine than placebo and haloperidol on aggression and agitation. Aggression and agitation were estimated via the BPRS hostility-cluster subscore (items “anxiety”, “tension”, “hostility”, “suspiciousness”, “uncooperativeness”, and “excitement”).
A 6-week double-blind placebo-controlled trial by Chengappa et al (2003) compared the efficacy of quetiapine versus haloperidol on hostility, agitation, and aggression in a total of 257 patients (quetiapine, n = 175; haloperidol, n = 42, placebo, n = 40). Main outcome measure was the observed change in BPRS scores. A clear benefit of quetiapine in the treatment of all three symptoms was shown. Path analysis revealed direct effects of quetiapine on these symptoms independent of a more general improvement of psychopathology.
A naturalistic open-label pilot study with 36 acutely psychotic patients by Ganesan et al (2005) showed good applicability of quetiapine; after the first treatment day, improvements in BPRS scores and the Overt Aggression Scale (OAS), especially on the item “aggression against others” was observed. Mean doses of quetiapine administered on Days 1, 2, 3, 4, and 5 were 203, 276, 351, 373, and 384 mg/day, respectively. The authors urged further examination of quetiapine’s efficacy in treating acute agitation and aggression through RCTs.
A beneficial effect of a treatement with quetiapine on aggression, anxiety, and hostility could also have been supported by the CATIE study in which quetiapine exhibited a comparable sedative effect as olanzapine (Lieberman et al 2005).
Short-term efficacy in depression/anxiety
Quetiapine’s efficacy in the treatment of schizophrenia-related depressive symptoms has been established by several studies. Meltzer and Lee (2001) conducted 2 comparative analyses: 4 randomized-double blind 6- to 12-week trials (quetiapine, n = 676; haloperidol, n = 559) and 3 randomized, placebo-controlled, double-blind, 6-week trials (quetiapine, n = 284; placebo, n = 116). Treatment with quetiapine was significantly superior to haloperidol on BPRS factor I (“somatic concern”, “anxiety”, “guilt feelings”, “depressive mood”) and Kay′s “Depressive Factor” (sum of PANSS-items: “anxiety”, “guilt feelings”, “depression”, “somatic concern”, and “preoccupation”). In the placebo-controlled trials, quetiapine was significantly superior to placebo in improving the BPRS-mood cluster and BPRS factor I.
A double-blind, randomized, multi-center trial analyzed by Emsley et al (2003) compared the efficacy of quetiapine (600 mg/day) and high doses of haloperidol (20 mg/day) in partially remitted schizophrenia patients. Depressive symptoms were estimated with a change in the Positive and Negative Syndrome Scale depression factor score from baseline to endpoint. Quetiapine was reported to be superior to haloperidol in treating depressive symptoms. The path analyses indicated that this was a direct effect on depressive symptoms, and it was concluded that there is evidence for an antidepressant effect of quetiapine in schizophrenia and that its use is not limited to psychotic patients.
Short-term efficacy in treatment-resistant patients
There is an approximate rate of 10%–30% of partial treatment resistance in schizophrenia. More efficacious treatment for this subgroup is especially important. A post-hoc subanalysis of a trial by Emsley et al (2001) on efficacy and tolerability of quetiapine compared with high-dose haloperidol revealed a significantly better response to treatment with quetiapine than the comparator (response defined as CGI-Score ≤3; 51% vs 25%; p = 0.023) in previously non-responsive patients to a 4-week treatment course with fluphenazine (Guy et al 1970). According to the authors, the results may be interpreted in context of a better tolerability of quetiapine; they conclude that a switch to quetiapine may be a benefical option when faced with acute treatment-resistant schizophrenia symptomatology (Buckley et al 2004b).
Current long-term trials
Efficacy
A small 2-phase open-label trial by Nagy (2003) incorporating 25 schizophrenics investigated efficacy and tolerability over 16 months of treatment. The trial consisted of a first phase, a 4-week trial, which examined quetiapine’s efficacy in relatively high doses, up to 1600 mg daily, and an ensuing second phase, a 15-month relapse prevention part, with dose reduction to an average of 600 mg/day. Good short-term efficacy and tolerability of the high initial doses was reported; the relapse-prevention part was well tolerated as well. Neither EPS including akathisia nor significant weight gain was observed.
Buckley (2004b) reported results of a subanalysis of three pooled double-blind randomized trials (n = 259), incorporating robust responders to 6-week quetiapine administration who were subsequently observed further for a total of 156 weeks. More than half of the patients sustained their initial 6-week treatment response and some improved further.
A 16-week, randomized, multicenter, double-blind trial reported by Mullen et al (2001; n = 728) compared the efficacy of quetiapine and risperidone in the treatment of outpatients with schizophrenia and related disorders. Both drugs were found to be similar in efficacy but quetiapine showed a numerically but not statistically superior efficacy in ameliorating depressive symptoms while retaining good tolerability.
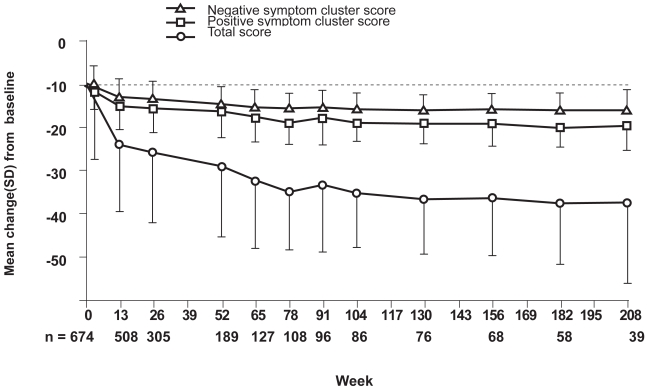
Kasper et al (2004) published a pooled data analysis of 674 patients from 4 long-term, open-label, extension phase IIIa trials. The authors argue that quetiapine therapy (mean dose 472.4 mg) over 208 weeks successfully treated positive and negative BPRS symptoms, CGI, and SANS scores. This led the authors to conclude that quetiapine is efficacious in long-term relapse prevention of schizophrenia (Figure 6).
Figure 6.
Mean changes in the Brief Psychiatric Rating Scale (BPRS) total score, Positive and Negative Symptom Cluster Score from baseline (compiled from data of Kasper et al 2004).
p < 0.001: Changes from baseline in total, positive, and negative symptom scores at all timepoints except week 4 (total score; p < 0.01).
SD, standard deviation.
In a large-scale, double-blind trial comparing multiple atypical antipsychotics against perphenazine (Clinical Antipsychotic Trials of Intervention Effectiveness [CATIE]) (Lieberman et al 2005), the efficacy of treatment was determined by means of discontinuation of treatment. It demonstrated that the time to the discontinuation of treatment for any cause was significantly longer in the olanzapine group than in the quetiapine or risperidone group. However, in this study olanzapine was administered above the recommended upper limit.
Cochrane meta-analysis
According to a meta-analysis looking at quetiapine for the treatment of schizophrenia, Srisurrapanont et al (2004) concluded that quetiapine is efficacious in ameliorating acute psychotic exacerbations. No differences in short-term efficacy and overall tolerability between quetiapine and either conventional antipsychotics or risperidone were reported. Quetiapine was associated with lower short-term risk for EPS, but higher risk for dizziness, asthenia, and dry mouth. The authors conclude that while short-term tolerability and efficacy are established, further pragmatic, randomized, comparative trials covering topics such as “usual clinical practice”, “mid-” and “long-term efficacy”, “cost-benefit analysis”, and “quality of life” are warranted, as these aspects have not been examined sufficiently.
Long-term efficacy on depressive and anxiety symptoms
A pooled analysis of three randomized, open-label, extension phase trials (n = 415) published by Kasper (2004) revealed good long-term efficacy of quetiapine in the treatment of depressive and anxiety symptoms in schizophrenia patients. The BPRS-Cluster I (“somatic concern”, “anxiety”, “guilt feelings”, “depression) was used as a proxy measure.
Other indications than schizophrenia core symptoms
Cognition
Cognitive deficits such as executive function, attention, working and verbal memory, visual memory, reaction time, and vigilance are important characteristics of the schizophrenic disorder. They have been found to be a better predictor of long-term outcome than positive symptoms, as they are closely related to functional outcomes. However, since cognitive deficits often “smolder” in the background of more expressive positive and negative symptoms and may persist even after sufficient treatment of acute psychotic episodes, they have not received as much clinical attention. This perspective seems to change, and some authors have suggested expanding the notion of antipsychotic efficacy to include changes in cognitive function (Velligan et al 2002).
It has been shown that newer antypsychotics may have superior efficacy in addressing schizophrenia-associated cognitive deficits to older agents (Mishara and Goldberg 2004). A small study (n = 22; n = 10, quetiapine; n = 12, controls) reported by Sax et al (1998) compared the effects of a 2-month treatment course of medication versus placebo and found a significant improvement in attention span in the quetiapine-treated group.
Another small, double-blind, randomized comparative trial was published by Purdon et al (2001) on cognitive effects of quetiapine (n = 13, 468 ± 114.6 mg/day) and high-dose haloperidol treatment (n = 12, 15.5 ± 3.3 mg/day). After a 6-month observation period, there was a significant trend towards improvement on the general cognitive index for quetiapine. Quetiapine also had beneficial effects on cognitive skills, particularly verbal reasoning and fluency skills and immediate recall, with additional improvements on executive skills and visuomotor tracking and on the average of the 6 cognitive domains with sustained treatment. Positive, negative, and depressive symptoms of schizophrenia also improved and quetiapine was the better tolerated medication overall. The authors caution, however, that the results may not be generalizable, as only very few patients completed the entire trial (n = 8, quetiapine; n = 3, haloperidol).
Velligan et al (2003) compared the effects of 24 weeks of treatment with quetiapine (300 and 600 mg) and haloperidol (12 mg) in 58 patients. Subsets of cognition including verbal fluency (p < 0.04), attention (p < 0.03) and verbal memory (p < 0.02) improved more robustly with the 600 mg quetiapine dose than either in the 300 mg quetiapine group or 12 mg haloperidol group.
An interim-analysis of an open-label, 2-year study examining the effects of quetiapine on cognition in patients with a first episode of schizophrenia and related disorders was conducted by Good et al (2002). One-year data for 13 patients was available (mean dose 517.9 ± 225.8 mg). Statistically significant improvements were noted on measures of attention, verbal fluency, and executive function after 6 and 12 months of treatment.
In 2 short-term, double-blind, randomized trials conducted by Riedel and colleagues (2007a, b) comparing quetiapine with risperidone and olanzapine, all three compounds improved cognition; however, the improvement in cognitive index scores was more marked in patients receiving quetiapine. Furthermore, quetiapine produced a significantly greater improvement in reaction quality/attention than risperidone and olanzapine.
Switching studies
A single, large (n = 509), open-label, naturalistic, industry-supported, multicenter study to examine the efficacy of quetiapine after a switch from another neuroleptic or combinations thereof was conducted by de Nayer et al (2003) (SPECTRUM trial). Non-responders and those poorly tolerant to the previous antipsychotics were included. Quetiapine was efficacious overall, in both non-responders and non-tolerators, especially with respect to improving depressive symptoms. Quetiapine showed, in certain cases, marked amelioration of acute EPS induced by the previously administered medication. Furthermore these results could have been confirmed by a recently published post hoc analysis of the SPECTRUM trial which was carried out to evaluate whether the improvements in efficacy and tolerability gained on switching to quetiapine occurred consistently for patients previously treated with an antipsychotic monotherapy (Larmo et al 2005). Switching to quetiapine showed a significant improvement in terms of efficacy and tolerability regardless of whether their previous antipsychotic was haloperidol, olanzapine, or risperidone. Among another chronically ill group of schizophrenia patients who had just discontinued treatment with an atypical antipsychotic (n = 168), quetiapine was moderately effective as measured by discontinuation for any reason (Stroup et al 2006). In patients who prospectively failed to improve with a variety of newer antipsychotics, clozapine may be more effective than switching to another newer antipsychotic (McEvoy et al 2006).
Place in the treatment of dementia-related behavior
In a 10-week, double-blind, placebo-controlled, multicenter trial, Zhong et al (2004) examined the efficacy of quetiapine on agitated behavior problems in geriatric inpatients with dementia. Patients (n = 333) were randomized to either quetiapine or placebo. Dose escalation to 200 mg occurred within 8 days and was generally well tolerated. A mean dose of 200 mg quetiapine was significantly superior to placebo in controlling anxiety and improving CGI. During the 10-week treatment duration, no cerebrovascular accidents were observed. Similarly, Tariot et al (2000) reported on a 52-week, open-label, multicenter trial of quetiapine administered to geriatric patients. Quetiapine was efficacious and safe. Slower uptitration was better tolerated. The authors recommended starting treatment at 25 mg and increasing slowly up to 100–150 mg.
To further examine cerebrovascular risks of quetiapine, Schneider et al (2004) analyzed data from 2 double-blind, placebo-controlled, randomized, multicenter trials incorporating a total of 684 patients (quetiapine n = 355, haloperidol n = 116, placebo n = 213). No increased CVA risk in either group was found.
Treatment of borderline personality disorder
A pilot study conducted by Bellino et al (2006) suggests that quetiapine is efficacious and well tolerated in treating patients who have borderline personality disorder, particularly when impulsivity and aggression-related symptoms were prominent. These results are in line with many clinical observations of beneficial effects of quetiapine in the treatment of borderline patients.
Use in child and adolescent psychiatry
Shaw et al (2001) conducted a 8-week open label study incorporating 15 adolescents with diagnosable DSM-IV schizophrenia (mean age 15.1 years). A significant improvement under quetiapine treatment was reported. BPRS, PANSS, Young-Mania (Young et al 1978), and CGI scale scores all showed improvements. Moderate to marked weight gain of approximately 4.1 kg was observed. A non-significant reduction in thyroxin (T4) and increased thyroid-stimulating hormone (TSH) were seen. Quetiapine was well tolerated overall.
A small, 88-week, open-label study McConville et al (2003) reported results of quetiapine treatment on long-term efficacy and tolerability in 7 schizoaffective and 3 bipolar adolescents. Significant improvements in BPRS, CGI, and SANS were noted. Neither acute EPS nor tardive dyskinesias were observed. Weight gain was nonsignificant.
Delbello et al (2002) conducted a randomized, double-blind, placebo controlled trial on the efficacy of quetiapine as adjuvant medication in the treatment of adolescent mania. Quetiapine was added to valproate. Thirty adolescents aged 12–18 years with acute manic or mixed episode in context of a DSM-IV bipolar disorder were included; half of the patients received either add-on quetiapine or add-on placebo. The main outcome parameter was the change in Young Mania Rating Scale (YMRS). Quetiapine add-on was associated with significantly better improvement of YMRS scores than placebo add-on. The quetiapine-valproate combination was well tolerated.
Treatment of bipolar disorder
Two randomized, double-blind, placebo-controlled trials looking at efficacy and tolerability of quetiapine as add-on to standard of care in the treatment of acute bipolar disorder have been published (Sachs et al 2004; Calabrese et al 2005).
The trial by Sachs et al (2004) was a brief (3-week), multicenter, add-on study of quetiapine in patients with DSM-IV acute bipolar mania (n = 91). Quetiapine (600–800 mg/day) or placebo were added to either lithium or valproate medication. Quetiapine add-on was associated with significantly greater improvement in several areas. More patients receiving quetiapine showed reduction in mania scores (≥50% YMRS-Score reduction), remission of mania (defined as YMRS <12), and improvements in CGI. Mean quetiapine dose was 584 mg/day. Somnolence and dry mouth were significantly more commonly associated with quetiapine, however did not translate into higher drop-out rates.
In a combined analysis of two 12-week placebo-controlled trials (n = 403) about quetiapine monotherapy (up to 800 mg/day) for mania associated with bipolar disorder, Vieta et al (Vieta et al 2005) found a significant improvement in YMRS score observed from Day 4 (p = 0.021) onward in the quetiapine group compared with placebo. The treatment advantage of quetiapine over placebo continued to increase to Day 21 (p < 0.001) and Day 84 (p < 0.001). Of adverse events occurring in ≥5% of patients, quetiapine-treated patients had a significantly greater incidence versus placebo of somnolence (16.3% vs 4.0%), dry mouth (15.8% vs 3%), weight gain (9.1% vs 1.5%), and dizziness (6.7% vs 2.5%).
A study of Calabrese et al (2005) indicated good efficacy of either 300 or 600 mg of quetiapine in the treatment of bipolar depression (n = 542). Results from this short-term trial (8 weeks) indicated significantly greater efficacy of either quetiapine dose than placebo on Montgomery Asberg Depression Rating Scale (MADRS) and Hamilton Depression Rating Scale (HAM-D) (Hamilton 1960) scores. Quetiapine was not associated with induction of mania or hypomania. Frequent side-effects were dry mouth, dizziness, sedation, and constipation.
Treatment of Parkinson’s patients for iatrogenically induced psychotic symptoms
Iatrogenically induced psychotic symptoms are a common complication in the treatment of Parkinson’s disease.
Morgante et al (2004) conducted a 12-week, randomized, open-label, trial of quetiapine versus clozapine in 40 patients with Parkinson’s disease who had developed psychotic symptoms as a result of their primary treatment. BPRS and Clinical Global Impression-Severity Scale (GCI-S) scores were used to track changes in psychopathology. Quantification of potential movement disorders was done with the Unified Parkinson’s Disease Rating Scale (UPDRS) III (UPDRS, 2003) and the AIMS (Guy 1976). Low-dose quetiapine and clozapine were equally efficacious per BPRS and CGI changes (p < 0.001); tolerability of low-dose quetiapine was good. Neither medication worsened symptoms of the primary Parkinson’s disease in low doses. However, the authors recommended not exceeding 100 mg of quetiapine because in higher doses, adverse motor symptoms were observed.
Augmentation with quetiapine of patients treated with selective serotonin reuptake inhibitors (SSRIs)
SSRIs are the mainstay of treatment of depressive and anxiety disorders. A small subset of patients, however, continues to suffer from anxiety symptoms in the long run.
Adson et al (2004) conducted a small (n = 11), 9-week, open-label trial adding quetiapine to pre-existing SSRI treatment. HAM-D and HAM-A (Hamilton 1960) and State Anxiety Inventory (SAI Ferreira and Murray 1983) were used to measure associated psychopathology changes. Patients were required to have been pretreated with and been only partially responsive to SSRI after at least 6 weeks of SSRI exposure. A significant symptomatic improvement was seen in 10 of the 11 patients; however caution should be used in generalizing the results given the small sample size.
Tolerability
Overview
Side-effects most commonly associated with quetiapine (>10% per clinical trials) are somnolence, dizziness, dry mouth, asthenia, constipation, tachycardia, orthostatic hypotension, dyspepsia, and weight gain. Furthermore weight gain, syncopal episodes, leucopenias, neutropenias, and peripheral angioedema are sporadically associated with quetiapine treatment.
Mullen et al (2001) published results from the industry-sponsored QUEST study comparing the efficacy and tolerability of quetiapine with risperidone. The 16-week, open-label, multicenter trial (n = 728) revealed that significant proportions of patients receiving quetiapine developed somnolence (31.3% vs 15.4% on risperidone), dry mouth (14.5% vs 6.9%), and dizziness (12.7% vs 6.9%) (Table 2). Overall tolerance to side-effects with the two drugs, measured by dropout rates, was comparable. The EPS profile was more favorable for quetiapine (329 mg/day) than for risperidone (5 mg/day).
Table 2.
Quetiapine side-effect profile (Mullen et al 2001)
| Side-effecta | Quetiapine (n = 553) |
|---|---|
| Overall | 400 (72.3%) |
| Somnolence | 173 (31.3%) |
| Dry mouth | 80 (14.5%) |
| Dizziness | 70 (12.7%) |
| Insomnia | 65 (11.8%) |
| Headache | 52 (9.4%) |
| Agitation | 34 (6.1%) |
side-effects reported with >5% frequency.
EPS
Most of the newer agents have better tolerability with respect to acute EPS. Especially older agents, and the newer agent aripiprazole have been associated with akathisia; other EPS including dyskinesias, dystonias, and parkinsonoid have predominantly been observed with the older agents. Although long-term data are still scarce, it appears that older antipsychotics are associated with higher risks for tardive dyskinesias than newer ones, which are therapeutically difficult to ameliorate.
Incidence and quantification of EPS is usually done with standard rating scales, including the
Simpson Angus Scale (SAS; Simpson and Angus 1970), a rater-administered 10-item scale to assess medication-induced Parkinson-like symptoms
Abnormal Involuntary Movement Scale (AIMS), a 12-item scale assessing movement disorders including akathisia and tardive dyskinesia.
Barnes Akathisia Scale (BAS; Barnes 1989) a 4-item scale to quantify medication-induced akathisia symptoms.
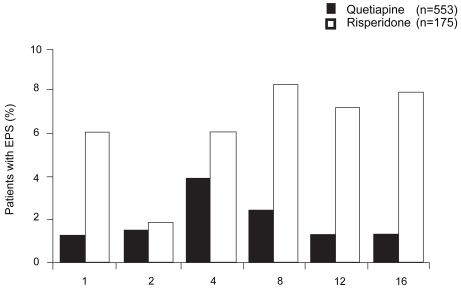
Quetiapine has an overall low risk for inducing EPS (Aravantis et al 1997; Simpson and Angus 1970). The QUEST study (Mullen et al 2001), a comparative efficacy and safety trial of quetiapine (n = 553) versus risperidone (n = 175), showed equal inital rates for acute EPS of 53% for quetiapine and 52% for risperidone. Intensity of acute EPS gradually decreased during the 4-month trial period. EPS were less severe in quetiapine-treated patients, but remained present throughout in both groups. However, antipsychotic dose reductions and additional prescription of anticholinergics were significanlty less common in quetiapine-treated patients (p < 0.001; Figure 7).
Figure 7.
Percentage of patients with extrapyramidal-motor side-effects (EPS) (compiled from data of Mullen et al 2001).
Better tolerability of quetiapine (with respect to induction of acute EPS, prolactin elevations, and weight gain) was also reported by Riedel et al (2005) in a 12-week comparative RCT of quetiapine and risperidone in the treatment of predominantly negative-symptom schizophrenia (n = 44).
Kasper et al (2004) reported results from a pooled analysis of 4 commercially funded, open label, phase IIIa extension trials on the long-term efficacy and tolerability (208 weeks) of quetiapine (n = 674). EPS occurred in approximately 9% of patients, predominantly akathisia (3.1%), other acute EPS (2.5%), and tremors (1.6%). Three patients had to be withdrawn from the protocols secondary to serious EPS.
A recent 12-month, double blind, RCT of quetiapine versus high-dose haloperidol reported by Emsley et al (2004), examined the potential of quetiapine to favorably influence pre-existing tardive dyskinesias in schizophrenia patients. Fifty-five patients (quetiapine n = 22; haloperidol, n = 23) were examined for changes in tardive dyskinesia severity via the Extrapyramidal Symptom Rating Scale (ESRS) (Chouinard et al 1980). Compared with the haloperidol-treated group, quetiapine patients experienced significant improvement in ESRS scores (p < 0.01) leading the authors to conclude that quetiapine may have the potential to positively influence tardive dyskinesia.
Prolactin
Medication-induced prolactin elevations may induce side-effects such as galactorrhoea, gynecomastia, menstrual cycle abnormalities, and sexual dysfunctions. They can strongly influence medication adherence rates. A short-term study (6 weeks) by Atmaca et al (2002) in 35 female schizophrenia patients treated with either quetiapine (n = 18) or high-dose haloperidol (n = 17) found a doubling of haloperidol-associated prolactin levels (baseline 15.4 ± 4.2 ng/mL to 31.4 ± 10.2 ng/mL; p < 0.001) whereas quetiapine did not change prolactin levels (15.3 ± 4.4 ng/mL to 15.7 ± 4.8 ng/mL). Conversely, Stevens et al (2005) reported mild dose-dependent increases in serum prolactin levels for quetiapine, but with an insignificant effect of exposure time. Results are consistent with previous reports (Hamner et al 1996).
Weight gain
Patients are well aware of antipsychotic-induced weight gain (Strassnig et al 2005), and experience weight gain as an impairment to their overall quality of life (Strassnig et al 2003). Importantly, marked weight gain is a predictor for medication nonadherence in schizophrenia patients (Perkins, 1999). Available antipsychotics have different weight gain liabilities (Sussman, 2001; Brecher et al 2000). Compared to other newer antipsychotics, quetiapine treatment induces moderate short-term weight gain (Allison et al 1999); two 6-week clinical trials reported a mean weight gain of 2.08 kg (Jones et al 2000). Similarly, Zhong et al (2006) reported mean weight gain of 2.33 kg in a 8-week comparative study of quetiapine with risperidone (2.06 kg for ris, p = ns). Long-term data are scarce. An analysis of pooled data (n = 674 patients) of long-term treatment with quetiapine over 208 weeks revealed moderate weight gain potential, mostly during the inital 26 weeks of treatment and at a lower rate thereafter (compared to baseline, 26th week 3.2 ± 6.88 kg, 52nd week 3.4 ± 8.6 kg, 208th week 4.9 ± 11.89 kg). In the CATIE study, Lieberman and colleagues found that weight gain in patients treated with quetiapine was similar to risperidone (mean weight change was 1.1 ± 0.9 and 0.8 ± 0.9 lb, respectively), whereas olanzapine was associated with greater weight gain (9.4 ± 0.9 lb) and increases in measures of glucose and lipid metabolism (Lieberman et al 2005).
Hyperglycemia, new-onset type II diabetes
Glucose intolerance, frank hyperglycemia, new-onset type II diabetes, and diabetic ketoacidosis (DKA) are more common in patients when they are treated with atypical antipsychotics compared with classic agents (Jin et al 2004). Concerns about medication-related type II diabetes is particularly high in schizophrenia patients, as there is evidence that patients may be predisposed and more susceptible to developing the insulin resistance syndrome (Mukherjee et al 1996). Biological and retrospective studies generally find abnormal glucose tolerance during extended treatment (Jin et al 2004).
Quetiapine seems to occupy a “middle-ground” among newer antipsychotics with respect to its propensity to induce hyperglycemia/diabetes type II. Leslie and Rosenheck (2004) analyzed the VA Connecticut Mental Health prescription database for new-onset diabetes secondary to antipsychotic administration. 4132 (7.3%) of patients had developed diabetes mellitus and among these, 88 (0.2 %) required inpatient hospitalization for ketoacidosis. The relative risk for new onset diabetes under quetiapine treatment was not different to that with risperidone and conventional antipsychotics, and lower than with either olanzapine or clozapine. The authors conclude that the absolute risk for development of diabetes secondary to treatment with atypical antipsychotics is relatively low (lowest for risperidone 0.05%, highest for clozapine 2.03%).
Gianfrancesco et al (2003) analyzed a large health insurance database for new-onset diabetes in schizophrenia patients treated with newer antipsychotics. Among 922 patients receiving quetiapine, 117 developed new-onset diabetes. Odds ratios were extrapolated for 12-month exposure, and were significantly elevated only for olanzapine, but not for risperidone, quetiapine, or conventional antipsychotics.
In a recently published review article Chu and Cheung (2006) concluded that an increased risk of diabetes with some atypical antipsychotics should not deter physicians from using these agents in patients with schizophrenia or bipolar disorder, but they recommended that antipsychotic therapy should be carefully selected in those patients at greatest risk of developing diabetes or metabolic syndrome. Appropriate management and regular monitoring of patients receiving antipsychotics should minimize the risk of patients with schizophrenia or bipolar disorder developing diabetes.
Hematological and blood chemistry changes
While much of the literature has focused on weight gain and glucose intolerance, the importance of recognizing and treating hyperlipidemia in schizophrenia patients cannot be overemphasized (Meyer and Koro 2004). Data are scarce on quetiapine. The available data suggest that quetiapine shares the propensity with other benzodiazepine-derived atypical antipsychotics to primarily elevate serum triglycerides, and to a lesser extent cholesterol levels (Meyer and Koro 2004).
Potkin et al (2006) reported increased risk for thyroid dysregulation in quetiapine-treated inpatients; however, changes in thyroid hormones occurred mainly during an additive therapy phase with other antipsychotics in this study. Changes were reversible with treatment cessation. TSH increased significantly in 0.4 % of patients. Clinically relevant hypothyreosis was not observed in the majority of patients.
Goldstein (1999) described transient, clinically asymptomatic liver enzyme elevations, predominantly alanine transaminase (ALT) during quetiapine treatment. Most increases appeared within 3 weeks of treatment initiation, and subsequently subsided.
No cases of persistent clincially significant neutropenia or agranulocytosis have been reported. Previous experience shows that neutropenia and leukopenia induded by quetiapine were reversible after removal of the drug. Risk factors are a history of medication-induced neutropenia and pre-existent low white blood count.
Cardiovascular system
Reilly et al (2000) reported increased risks for QTc prolongation effected by several antipsychotics including thioridazine, droperidol, and ziprasidone. Prolongation of the QT interval (determined by ECG) and the QTc – corrected for heart rate – may lead to serious cardiac arrhythmias and sudden death.
A review by Goldstein (1999) on quetiapine-induced ECG changes showed a small QTc time prolongation with quetiapine independent of quetiapine plasma levels.
The incidence of QTc time prolongation and associated risk for torsades de pointes arrhythmia and ensuing cardiac arrest was examined by Glassman and Bigger (2001). Haloperidol, pimozide, sertindole, and droperidol all showed increased risk for torsades de pointes arrhythmia; the highest risk was associated with thiroidazine. No association with olanzapine, quetiapine, or risperidone was found. Among the newer agents, only ziprasidone prolonged QTc time.
A review by von Vieweg (2003) on the association of newer antipsychotics and QTc time prolongation concluded that up to 2002 there was no convincing evidence for involvement of these agents in triggering torsades de pointes arrhythmia; but non-emergence of QTc time prolongation could not safely be excluded either. Still, based on available evidence, the reviewer argued that no special precautions are necessary in patients without pre-existing risk for ECG changes started on newer medications.
However, according to the CATIE trial the mean change in QTc interval from baseline to last observation was 5.9 ± 1.9 ms in quetiapine-treated patients in comparison with other atypicals (risperidone 0.2 ± 1.8 ms; olanzapine 1.2 ± 1.8 ms; ziprasidone 1.3 ± 2.2 ms; and perphenazine 1.4 ± 2.0 ms) (Lieberman et al 2005).
In a randomized evaluation of the effects of 6 antipsychotic agents (haloperidol 15 mg/d [n = 27], thioridazine 300 mg/d [n = 30], ziprasidone 160 mg/d [n = 31], quetiapine 750 mg/d [n = 27], olanzapine 20 mg/d [n = 24], or risperidone 6–8 mg/d increased to 16 mg/d [n = 25/20]) on the QTc time, Harrigan et al (2004) found that mean QTc intervals did not exceed 500 ms in any patient taking any of the antipsychotics studied, a threshold generally accepted as being clinically important for the association with the development of torsades de pointes (Bednar et al 2001). The authors concluded that the theoretical risk of cardiotoxicity associated with QTc prolongation should be balanced against the substantial clinical benefits associated with atypical antipsychotics and the likelihood of other toxicities.
Conclusion
Efficacy of quetiapine in reduction of positive and negative schizophrenia symptoms has been shown in several short- to intermediate-term comparative trials and double-blind RCTs. Long-term efficacy on a variety of symptoms has also been established. Cognitive, depressive, and aggressive symptoms in the context of schizophrenia have been successfully treated. Other indications, such as treatment of moderate to severe manic episodes, treatment of aggressive behavior in children and adolescents, and behavioral disturbances in patients with dementia, have also been explored and have been successfully carried out. More recent data point to good efficacy of quetiapine in the treatment of depressive episodes in the context of bipolar disorder, while lacking the potential to trigger switch into mania. Borderline personality disorder is another diagnostic category in which quetiapine has successfully been applied, although results are preliminary.
Quetiapine’s preferred dose ranges from 150 to 750 mg daily; yet clinical trials indicate that – individually and according to clinical presentation – a far higher target dose (>1000 mg daily) may be used. For relapse-prevention, a dose of at least 400 mg/day should be prescribed. Switching to quetiapine secondary to lack of efficacy or tolerability of the previous drug may be effective and is usually well tolerated.
The most prominent side-effects associated with quetiapine treatment are somnolence, dizziness, dry mouth, asthenia, constipation, tachycardia, orthostatic hypotension, and dyspepsia (≥10% in placebo-controlled trials). The majority of clinical trials emphasize the favorable tolerability of quetiapine with regards to extrapyramidal symptoms and only mild prolactin elevation. Among newer antipsychotics, quetiapine occupies a middle ground with respect to its weight gain potential and liability to induce metabolic side-effects such as hyperglycemia and hyperlipidemia. QTc prolongation is usually not of clinical significance.
Figure 1.
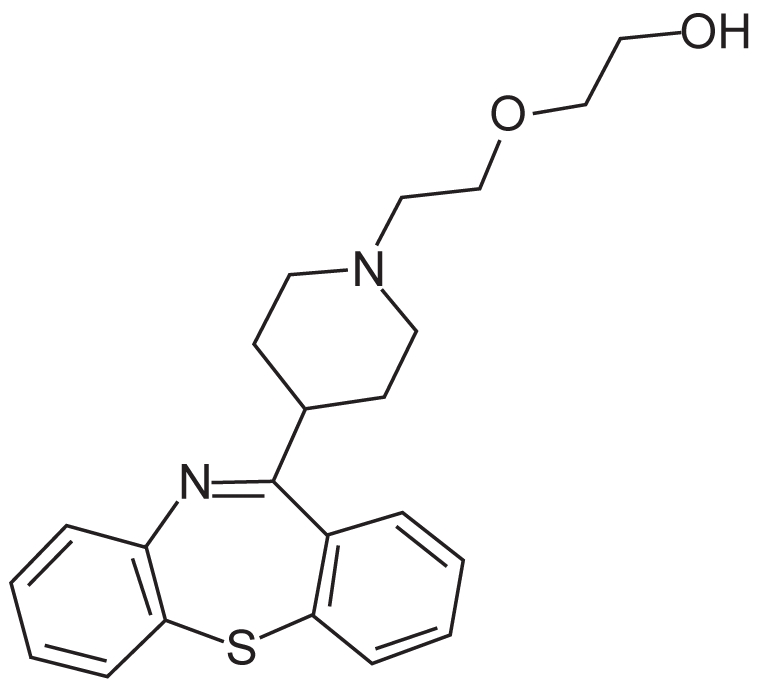
Chemical structure of quetiapine.
References
- Adson DE, Kushner MG, Eiben KM, et al. Preliminary Experience With Adjunctive Quetiapine In Patients Receiving Selective Serotonin Reuptake Inhibitors. Depr Anxiety. 2004;19:121–6. doi: 10.1002/da.10137. [DOI] [PubMed] [Google Scholar]
- Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–96. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Negative symptoms in schizophrenia. Arch Gen Psychiatry. 1982;39:784–8. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- Arvanitis LA, Miller BG the Seroquel Trial 13 Study Group. Multiple fixed doses of “Seroquel” (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. Biol Psychiatry. 1997;42:233–46. doi: 10.1016/s0006-3223(97)00190-x. [DOI] [PubMed] [Google Scholar]
- Atmaca M, Kuloglu M, Tezcan E, et al. Quetiapine is not associated with increase in prolactin secretion in contrast to haloperidol. Arch Med Res. 2002;33:562–5. doi: 10.1016/s0188-4409(02)00403-4. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Rüther E. Quetiapin – ein neues atypisches Antipsychotikum. Fundamenta Psychiatrica. 2000;14:127–31. [Google Scholar]
- Barnes TRE. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–6. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- Bednar MM, Harrigan EP, Anziano RJ, et al. The QT interval. Prog Cardiovasc Dis. 2001;43:1–45. doi: 10.1053/pcad.2001.21469. [DOI] [PubMed] [Google Scholar]
- Brecher M, Rak IW, Melvin K, et al. The long-term effect of quetiapine (Seroquel) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract. 2000;4:287–91. doi: 10.1080/13651500050517849. [DOI] [PubMed] [Google Scholar]
- Buckley PF. Efficacy of quetiapine for the treatment of schizophrenia: a combined analysis of three placebo-controlled trials. Curr Med Res Opin. 2004;20(9):1357–63. doi: 10.1185/030079904125004510. [DOI] [PubMed] [Google Scholar]
- Bellino S, Paradiso E, Bogetto F. Efficacy and tolerability of quetiapine in the treatment of borderline personality disorder: a pilot study. J Clin Psychiatry. 2006;67:1042–6. doi: 10.4088/jcp.v67n0705. [DOI] [PubMed] [Google Scholar]
- Borison RL, Arvanitis LA, Miller BG the U.S. SEROQUEL Study Group. ICI 204, 636, an atypical antipsychotic: efficacy and safety in a multicenter, placebo-controlled trial in patients with schizophrenia. J Clin Psychopharmacol. 1996;16:158–69. doi: 10.1097/00004714-199604000-00008. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Goldstein JM, Emsley RA. Efficacy and tolerability of quetiapine in poorly responsive, chronic schizophrenia. Schiz Res. 2004;66:143–50. doi: 10.1016/j.schres.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Buckley PF. Maintenance treatment for schizophrenia with quetiapine. Hum Psychopharmacol Clin Exp. 2004;19:121–4. doi: 10.1002/hup.573. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Keck PE, Macfadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of Bipolar I or II depression. Am J Psychiatry. 2005;162:1351–60. doi: 10.1176/appi.ajp.162.7.1351. [DOI] [PubMed] [Google Scholar]
- Chengappa KN, Goldstein JM, Greenwood M, et al. A post hoc analysis of the impact on hostility and agitation of quetiapine and haloperidol among patients with schizophrenia. Clin Therapeutics. 2003;25:530–41. doi: 10.1016/s0149-2918(03)80094-2. [DOI] [PubMed] [Google Scholar]
- Chouinard G, et al. Extrapyramidal Symptom Rating Scale. Can J Neurol Sci. 1980;7:233. [Google Scholar]
- Chue P, Cheung R. Minimising the risk of diabetes in patients with schizophrenia and bipolar disorder. Int J of Psychiatr in Clin Pract. 2006;10:105–16. doi: 10.1080/13651500600579084. [DOI] [PubMed] [Google Scholar]
- DelBello MP, Schwiers ML, Rosenberg HL, et al. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2002;41:1216–23. doi: 10.1097/00004583-200210000-00011. [DOI] [PubMed] [Google Scholar]
- De Nayer A, Windhager E, Irmansyah, et al. Efficacy and tolerability of quetiapine in patients with schizophrenia switched from other antipsychotics. Int J Psych Clin Pract. 2003;7:59–66. doi: 10.1080/13651500310001095. [DOI] [PubMed] [Google Scholar]
- DeVane CL, Nemeroff CB. Clinical pharmakokinetics of quetiapine: an atypical antipsychotic. Clin Pharmacokinet. 2001;40:509–22. doi: 10.2165/00003088-200140070-00003. [DOI] [PubMed] [Google Scholar]
- Emsley R, Buckley P, Jones AM, et al. Differential effect of quetiapine on depressive symptoms in patients with partially responsive schizophrenia. J Psychopharmacol. 2003;17(2):210–5. doi: 10.1177/0269881103017002010. [DOI] [PubMed] [Google Scholar]
- Emsley R, Turner HJ, Schronen J, et al. A single-blind, randomized trial comparing quetiapine and haloperidol in the treatment of tardive dyskinesia. J Clin Psychiatry. 2004;65:696–701. doi: 10.4088/jcp.v65n0516. [DOI] [PubMed] [Google Scholar]
- Ferreira R, Murray J. Spielberger’s State-Trait Anxiety Inventory: Measuring anxiety with and without an audience during performance on a stabilometer. Percept Mot Skills. 1983;57:15–18. doi: 10.2466/pms.1983.57.1.15. [DOI] [PubMed] [Google Scholar]
- Ganesan S, Levy M, Bilsker D, et al. Effectiveness of quetiapine for the management of aggressive psychosis in the emergency psychiatric setting: a naturalistic uncontrolled trial. Int J Psychiatr Clin Pract. 2005;9:199–203. doi: 10.1080/13651500510029011. [DOI] [PubMed] [Google Scholar]
- Ganguli R, Strassnig M. Are older antipsychotic drugs obsolete? BMJ. 2006;332:1346–1347. doi: 10.1136/bmj.332.7554.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefvert O, Lundberg T, Wieselgren IM, et al. D2 and 5HT2A receptor occupancy of different doses of quetiapine in schizophrenia: a PET study. Eur Neuropsychopharmacol. 2001;11:105–10. doi: 10.1016/s0924-977x(00)00133-4. [DOI] [PubMed] [Google Scholar]
- Gianfrancesco F, White R, Wang RH, et al. Antipsychotic-induced type 2 diabetes: evidence from a large health plan database. J Clin Psychopharmacol. 2003;23:328–35. doi: 10.1097/01.jcp.0000085404.08426.3a. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Bigger JT., Jr Antipsychotic drugs:prolonged QTc interval, torsade de pointes, and sudden death. Am J Psychiatry. 2001;158:1774–82. doi: 10.1176/appi.ajp.158.11.1774. [DOI] [PubMed] [Google Scholar]
- Goldstein J. Quetiapine fumarate (Seroquel): a new atypical antipsychotic. Drugs Today. 1999;35:193–210. doi: 10.1358/dot.1999.35.3.533849. [DOI] [PubMed] [Google Scholar]
- Good KP, Kiss I, Buiteman C, et al. Improvement in cognitive functioning in patients with first-episode psychosis during treatment with quetiapine: an interim analysis. Br J Psychiatry. 2002;181(Suppl 43):s45–s49. doi: 10.1192/bjp.181.43.s45. [DOI] [PubMed] [Google Scholar]
- Guy W, Bonato RR, editors. Manual for the ECDEU Assessment Battery. 2nd Revised ed . Chevy Chase, Md: National Institute of Mental Health; 1970. pp. 12-1–12-6. [Google Scholar]
- Guy W, editor. ECDEU Assessment Manual for Psychopharmacology Revised ed. Washington DC: US Department of Health, Education and Welfare; 1976. [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neursurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamner MB, Arvanitis LA, Miller BG, et al. Plasma prolactin in schizophrenia subjects treated with Seroquel (ICI 204, 636) Psychopharmacol Bull. 1996;32:107–10. [PubMed] [Google Scholar]
- Harrigan EP, Miceli JJ, Anziano R, Watsky E, et al. A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibition. J Clin Psychopharmacol. 2004;24:62–9. doi: 10.1097/01.jcp.0000104913.75206.62. [DOI] [PubMed] [Google Scholar]
- Jin H, Meyer JM, Jeste DV. Atypical antipsychotics and glucose dysregulation: a systematic review. Schizophr Res. 2004;71:195–212. doi: 10.1016/j.schres.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Jones A, Rak I, Raniwalla J, et al. Weight changes in patients treated with quetiapine. Presented at the 153rd annual meeting of the American Psychiatric Association; May 13–18; Chicago, Ill. 2000. [Google Scholar]
- Kapur S, Zipursky R, Jones C, et al. A positron emission tomography study of quetiapine in schizophrenia: a preliminary finding of an antipsychotic effect with only transiently high dopamine D2 receptor occupancy. Arch Gen Psychiatry. 2000;57:553–9. doi: 10.1001/archpsyc.57.6.553. [DOI] [PubMed] [Google Scholar]
- Kasper S, Brecher M, Fitton L, et al. Maintenance of long-term efficacy and safety of quetiapine in the open-label treatment of schizophrenia. Int Clin Psychopharmacol. 2004;19:281–9. doi: 10.1097/01.yic.0000138822.62874.fd. [DOI] [PubMed] [Google Scholar]
- Kasper S. Quetiapine is effective against anxiety and depressive symptoms in long-term treatment of patients with schizophrenia. Depress Anxiety. 2004;20:44–7. doi: 10.1002/da.20017. [DOI] [PubMed] [Google Scholar]
- Koller EA, Weber J, Doraiswamy PM, et al. A survey of reports of quetiapine-associated hyperglycemia and diabetes mellitus. J Clin Psychiatry. 2004;65:857–63. doi: 10.4088/jcp.v65n0619. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Larmo I, de Nayer A, Windhager E, et al. Efficacy and tolerability of quetiapine in patients with schizophrenia who switched from haloperidol, olanzapine or risperidone. Human Psychopharmacol Clin Exp. 2005;20:573–581. doi: 10.1002/hup.723. [DOI] [PubMed] [Google Scholar]
- Leslie DL, Rosenheck RA. Incidence of newly diagnosed diabetes attributable to atypical antipsychotic medications. Am J Psychiatry. 2004;161:1709–11. doi: 10.1176/appi.ajp.161.9.1709. [DOI] [PubMed] [Google Scholar]
- Leucht S, Pitschel-Walz G, Abraham D. Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo. A meta-analysis of randomized controlled trials. Schizophr Res. 1999;35:51–68. doi: 10.1016/s0920-9964(98)00105-4. [DOI] [PubMed] [Google Scholar]
- Li KY, Li X, Cheng ZN, et al. Multiple dose pharmakokinetics of quetiapine and some of its metabolites in Chinese suffering from schizophrenia. Acta Pharmacol Sin. 2004;25(3):390–4. [PubMed] [Google Scholar]
- Li KY, Li X, Cheng ZN, et al. Effect of erythromycin on metabolism of quetiapine in Chinese suffering from schizophrenia. Eur J Clin Pharmacol. 2005;60(11):791–5. doi: 10.1007/s00228-004-0853-x. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Perkins DO. Quetiapine: A 5-Year Update. J Clin Psychiatry. 2002;63(suppl 13) [Google Scholar]
- Lieberman JA, Strout TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Lin SH, Chang Y, Moody DE, Foltz RL. A liquid chromatographic-electrospray-tandem mass spectrometric method for quantitation of quetiapine in human plasma and liver microsomes: Application to a Study of In Vitro Metabolism. J Analyt Toxicol. 2004;28 doi: 10.1093/jat/28.6.443. [DOI] [PubMed] [Google Scholar]
- McConville BJ, Arvanits LA, Thyrum PT, et al. Pharmakokinetics, tolerability, and clinical effectiveness of quetiapine fumarate: an open-label-trial in adolescents with psychotic disorders. J Clin Psychiatry. 2000;61:252–60. doi: 10.4088/jcp.v61n0403. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163:600–10. doi: 10.1176/ajp.2006.163.4.600. [DOI] [PubMed] [Google Scholar]
- McConville B, Carrero L, Sweitzer D, et al. Long-term safety, tolerability, and clinical efficacy of quetiapine in adolescents: an open-label extension trial. J Child Adolesc Psychopharmacol. 2003;13:73–80. doi: 10.1089/104454603321666216. [DOI] [PubMed] [Google Scholar]
- Meltzer H, Lee M. Quetiapine is significantly superior to haloperidol and placebo in improving mood in patients with schizophenia. Presented at the 7th World Congress of Biological Psychiatry; Berlin, Germany. 2001. [Google Scholar]
- Meyer JM, Koro CE. The effects of antipsychotic therapy on serum lipids: a comprehensive review. Schizophr Res. 2004;70:1–17. doi: 10.1016/j.schres.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Mishara AL, Goldberg TE. A meta-analysis and critical review of the effects of conventional neuroleptic treatment on cognition in schizophenia: opening a closed book. Biol Psychiatry. 2004;55:1013–22. doi: 10.1016/j.biopsych.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Möller HJ. Atypical neuroleptics: a new approach in the treatment of negative symptoms. Eur Arch Psychiatry Clin Neurosci. 1999;249(Suppl 4):99–107. doi: 10.1007/pl00014191. [DOI] [PubMed] [Google Scholar]
- Morgante L, Epifanio A, Spina E, et al. Quetiapine and Clozapine in Parkinsonian patients with dopaminergic psychosis. Clin Neuropharmacol. 2004;27:153–6. doi: 10.1097/01.wnf.0000136891.17006.ec. [DOI] [PubMed] [Google Scholar]
- Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2004;18:738–50. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Decina P, Boccola V, et al. Diabetes mellitus in schizophrenic patients. Compr Psychiatry. 1996;37:68–73. doi: 10.1016/s0010-440x(96)90054-1. [DOI] [PubMed] [Google Scholar]
- Mullen J, Jibson MD, Sweitzer D. A comparison of the relative safety, efficacy, and tolerability of quetiapine and risperidone in outpatients with schizophrenia and other psychotic disorders: The Quetiapine Experience with Safety and Tolerability (QUEST) Study. Clin Therapeutics. 2001;23:1839–54. doi: 10.1016/s0149-2918(00)89080-3. [DOI] [PubMed] [Google Scholar]
- Nagy J. Effectiveness of Quetiapine up to 1.600 mg/day: Short-term Results with 14–month Follow-up. Poster presented at the 16th Congress of the European College of Neuropsychopharmacology; September 20–24, 2003; Prague, Czech Republic. 2003. [Google Scholar]
- Nasrallah HA, Tandon R. Efficacy, Safety, and Tolerability of Quetiapine in Patients With Schizophrenia. J Clin Psychiatry. 2002;63(Suppl 13):12–20. [PubMed] [Google Scholar]
- Nemeroff CB, Kinkead B, Goldstein J. Quetiapine: preclinical studies, pharmakokinetics, drug interactions, and dosing. J Clin Psychiatry. 2002;63(Suppl 13):5–11. [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- Perkins DO. Adherence to antipsychotic medications. J Clin Psychiatry. 1999;60(Suppl 21):25–30. [PubMed] [Google Scholar]
- Peuskens J, Link CG. A comparison of quetiapine and chlorpromazine in the treatment of schizophrenia. Acta Psychiatr Scand. 1997;96:265–73. doi: 10.1111/j.1600-0447.1997.tb10162.x. [DOI] [PubMed] [Google Scholar]
- Potkin S, Thyrum P, Bera R, et al. Pharmakokinetics and safety of lithium coadministered with Seroquel (quetiapine) [abstract] Schizophr Res. 1997;24:199. [Google Scholar]
- Potkin SG, Thyrum PT, Alva G, et al. The safety and pharmakokinetics of quetiapine when coadministered with haloperidol, risperidone, or thioridazine. J Clin Psychopharmacol. 2002a;22:121–30. doi: 10.1097/00004714-200204000-00004. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Thyrum PT, Alva G, et al. Effect of fluoxetine and imipramine on the pharmakokinetics and tolerability of the antipsychotic quetiapine. J Clin Psychopharmacol. 2002b;22:174–82. doi: 10.1097/00004714-200204000-00011. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Gharabawi GM, Greenspan AJ, et al. A double-blind comparison of risperidone, quetiapine and placebo in patients with schizophrenia experiencieng an acute exacerbation requring hospitalization. Schizophr Res. 2006;85:254–65. doi: 10.1016/j.schres.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Purdon SE, Malla A, Labelle A, et al. Neuropsychological change in patients with schizophrenia after treatment with quetiapine or haloperidol. J Psychiatry Neurosci. 2001;26:137–49. [PMC free article] [PubMed] [Google Scholar]
- Riedel M, Muller N, Strassnig M, et al. Quetiapine has equivalent efficacy and superior tolerability to risperidone in the treatment of schizophrenia with predominantly negative symptoms. Eur Arch Psychiatry Clin Neurosci. 2005;255:432–7. doi: 10.1007/s00406-005-0622-6. [DOI] [PubMed] [Google Scholar]
- Riedel M, Spellmann I, Strassnig M, et al. Effects of risperidone and quetiapine on cognition in patients with schizophrenia and predominantly negative symptoms. Eur Arch Psychiatry Clin Neurosci. 2007a doi: 10.1007/s00406-007-0739-x. [DOI] [PubMed] [Google Scholar]
- Riedel M, Spellmann I, Engel RR, et al. Efficacy of olanzapine versus quetiapine on cognitive dysfunctions in patients with an acute episode of schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2007b doi: 10.1007/s00406-007-0748-9. [DOI] [PubMed] [Google Scholar]
- Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors: focus on newer generation compounds. Life Sci. 2000;68:29–39. doi: 10.1016/s0024-3205(00)00911-5. [DOI] [PubMed] [Google Scholar]
- Sacchetti E, Valsecchi P, Regini C, et al. Comparison of quetiapine (“Seroquel”), olanzapine and risperidone in a randomized study in patients with schizophrenia. Presented at the XXIV Collegium Internationale Neuro-Psychopharmacologicum; June 20–24, 2004; Paris, France. 2004. [Google Scholar]
- Sachs G, Chengappa KNR, Suppes T, et al. Quetiapine with lithium or divalproex for the treatment of bipolar mania: a randomized, double-blind, placebo-controlled study. Bipolar Disord. 2004;6:213–23. doi: 10.1111/j.1399-5618.2004.00115.x. [DOI] [PubMed] [Google Scholar]
- Sax KW, Strakowski SM, Keck PE., Jr Attentional improvement following quetiapine fumarate treatment in schizophrenia. Schizophr Res. 1998;33:151–5. doi: 10.1016/s0920-9964(98)00067-x. [DOI] [PubMed] [Google Scholar]
- Schneider L, Tariot P, Mintzer J, et al. Cerebrovascular adverse events and quetiapine: a pooled analysis in elderly patients with dementia. Presented at the 9th International Conference on Alzheimer′s Disease and Related Disorders; July 17–22, 2004; Philadelphia, PA, USA. 2004. [Google Scholar]
- Schulz S, Thomson R, Brecher M. The efficacy of quetiapine vs. haloperidol and placebo: a meta-analytic study of efficacy. Schizophr Res. 2003;62:1–12. doi: 10.1016/s0920-9964(02)00522-4. [DOI] [PubMed] [Google Scholar]
- Shaw JA, Lewis JE, Pascal S, et al. A study of quetiapine: efficacy and tolerability in psychotic adolescents. J Child Adolesc Psychopharmacol. 2001;11:415–24. doi: 10.1089/104454601317261591. [DOI] [PubMed] [Google Scholar]
- Simpson GN, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;212(Suppl 44):11–9. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Small JG, Hirsch SR, Arvanitis LA, et al. Quetiapine in patients with schizophrenia: a high- and low-dose double-blind comparison with placebo. Arch Gen Psychiatry. 1997;54:549–57. doi: 10.1001/archpsyc.1997.01830180067009. [DOI] [PubMed] [Google Scholar]
- Small JG, Kellams JJ, Kolar MC. Relationship between quetiapine dose and efficacy. Presented at the 15th European College of Neuropsychopharmacology Congress; October 5–9, 2002; Barcelona, Spain. 2002. [Google Scholar]
- Srisurapanont M, Maneeton B, Maneeton N. Quetiapine for schizophrenia. Cochr Datab Syst Rev. 2004;2:CD000967. doi: 10.1002/14651858.CD000967.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl S. Essential psychopharmacology Neuroscientific basis and practical applications. 2nd ed. Cambridge University Press; 2005. pp. 415–24. [Google Scholar]
- Stevens JR, Kyrmissis PI, Baker AJL. Elevated Prolactin Levels in Male Youths Treated with Risperidone an Quetiapine. J Child Adolesc Psychopharmacol. 2005;15:893–900. doi: 10.1089/cap.2005.15.893. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Keck PE, Jr, Wong YW, et al. The effect of multiple dose of cimetidine on the steady-state pharmacokinetics of quetiapine in men with selected psychotic disorders. J Clin Psychopharmacol. 2002;22:201–5. doi: 10.1097/00004714-200204000-00015. [DOI] [PubMed] [Google Scholar]
- Strassnig M, Brar JS, Ganguli R. Quality of life and body weight in community-dwelling schizophrenic patient. Schizophr Res. 2003;62:73–6. doi: 10.1016/s0920-9964(02)00441-3. [DOI] [PubMed] [Google Scholar]
- Strassnig M, Brar JS, Ganguli R. Self-reported body weight perception and dieting practices in community-dwelling schizophrenia patients. Schizophr Res. 2005;75:425–32. doi: 10.1016/j.schres.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Stroup TS, Lieberman JA, McEvoy JP, et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry. 2006;163:611–22. doi: 10.1176/ajp.2006.163.4.611. [DOI] [PubMed] [Google Scholar]
- Sussman N. Review of atypical antipsychotics and weight gain. J Clin Psychiatry. 2001;62(Suppl 23):5–12. [PubMed] [Google Scholar]
- Tandon R. Quetiapine has a direct effect on the negative symptoms of schizophrenia. Hum Psychopharmacol. 2004;19:559–63. doi: 10.1002/hup.642. [DOI] [PubMed] [Google Scholar]
- Tandon R, Jibson MD. Comparing efficacy of first-line atypical antipsychotics: no evidence of differential efficacy between risperidone, olanzapine, quetiapine, ziprasidone, and aripiprazole. Intern J Psychiatr in Clin Pract. 2005;9:204–12. doi: 10.1080/13651500510029192. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Zhang K, Baldessarini RJ. Long-term effects of olanzapine, risperidone, and quetiapine on dopamine receptor types in regions of rat brain: implications for antipsychotic drug treatment. J Pharmacol Exp Ther. 2001;297:711–17. [PubMed] [Google Scholar]
- Tariot PN, Salzman C, Yeung PP, et al. Long-term use of quetiapine in elderly patients with psychotic disorders. Clin Therapeutics. 2000;22:1068–84. doi: 10.1016/s0149-2918(00)80085-5. [DOI] [PubMed] [Google Scholar]
- Thyrum PT, Wong YW, Yeh C. Single-dose pharmakokinetics of quetiapine in subjects with renal or hepatic impairment. Prog Neuro-psychopharmacol Biol Psychiatry. 2000;24:521–33. doi: 10.1016/s0278-5846(00)00090-7. [DOI] [PubMed] [Google Scholar]
- Reilly JG, Ayis SA, Ferrier IN, et al. QTc-interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet. 2000;355:1048–52. doi: 10.1016/s0140-6736(00)02035-3. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Prihoda TJ, Sui D, et al. Effectiveness of quetiapine versus conventional antipsychotics in improving cognitive and functional outcomes in standard treatment settings. J Clin Psychiatry. 2003;64:524–31. doi: 10.4088/jcp.v64n0505. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Newcomer J, Pultz J, et al. Does cognitive function improbe with quetiapine in comparison to haloperidol? Schizophr Res. 2002;53:239–48. doi: 10.1016/s0920-9964(01)00268-7. [DOI] [PubMed] [Google Scholar]
- Vieta E, Mullen J, Brecher M, et al. Quetiapine monotherapy for mania associated with bipolar disorder: combined analysis of two international, double-blind, randomised, placebo-controlled studies. Curr Med Res Opin. 2005;21:923–34. doi: 10.1185/030079905X46340. [DOI] [PubMed] [Google Scholar]
- Vieweg WVR. New generation antipsychotic drugs and QTc interval prolongation. Prim Care Comp J Clin Psychiatry. 2003;5:205–15. doi: 10.4088/pcc.v05n0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Zhong K, Tariot P, Minkwitz MC, et al. Quetiapine for the treatment of agitation in elderly institutionalized patients with dementia: a randomized, double-blind trial. Presented at the 9th International Conference on Alzheimer’s Disease and Related Disorders; July 17–22, 2004; Philadelphia, PA, USA. 2004. [Google Scholar]
- Zhong KX, Sweitzer DE, Hamer RM, et al. Comparison of quetiapine and risperidone in the treatment of schizophrenia: a randomized, double-blind, flexible-dose, 8-week study. J Clin Psychiatry. 2006;67:1093–103. doi: 10.4088/jcp.v67n0712. [DOI] [PubMed] [Google Scholar]