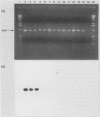
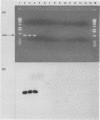
Abstract
A rapid polymerase chain reaction test was developed for specific identification of the human middle ear pathogen Alloiococcus otitis. Primers for the enzymatic amplification reaction were designed from highly specific sequences within the 16S rRNA gene. In addition, a confirmatory test based on hybridization of the polymerase chain reaction products to a specific internal probe was developed.
Full text
PDF

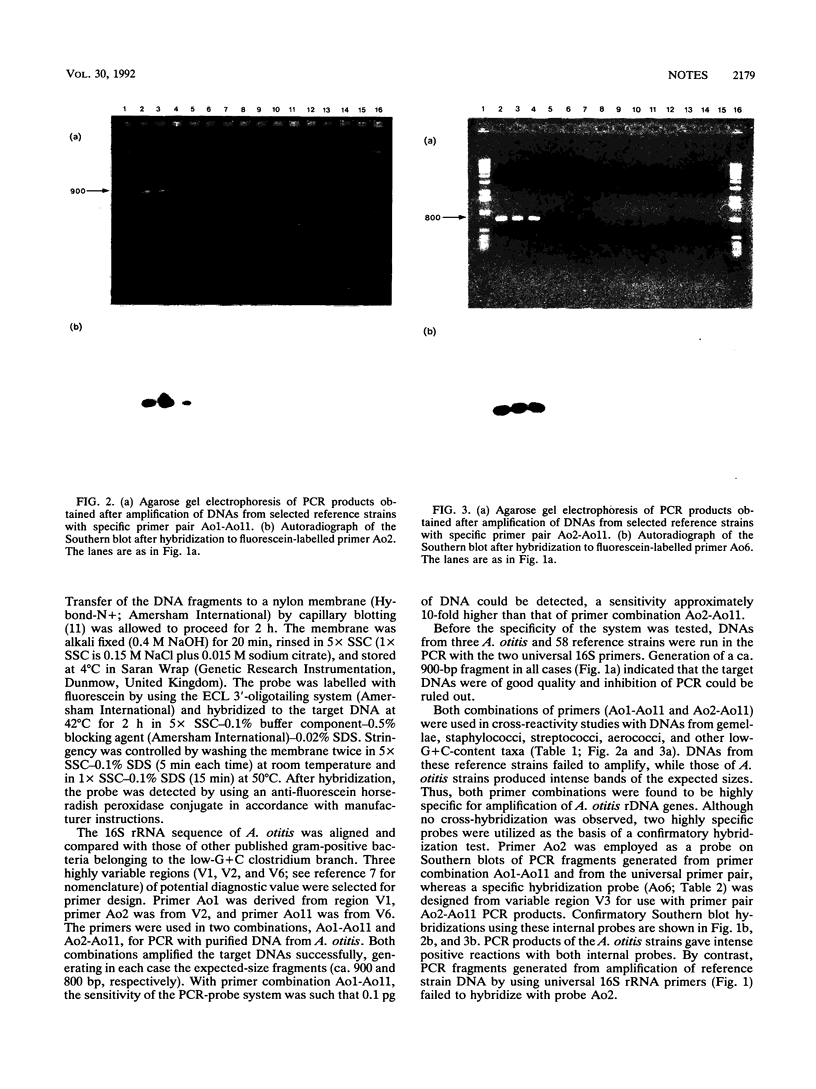

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguirre M., Collins M. D. Phylogenetic analysis of Alloiococcus otitis gen. nov., sp. nov., an organism from human middle ear fluid. Int J Syst Bacteriol. 1992 Jan;42(1):79–83. doi: 10.1099/00207713-42-1-79. [DOI] [PubMed] [Google Scholar]
- Barry T., Powell R., Gannon F. A general method to generate DNA probes for microorganisms. Biotechnology (N Y) 1990 Mar;8(3):233–236. doi: 10.1038/nbt0390-233. [DOI] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faden H., Dryja D. Recovery of a unique bacterial organism in human middle ear fluid and its possible role in chronic otitis media. J Clin Microbiol. 1989 Nov;27(11):2488–2491. doi: 10.1128/jcm.27.11.2488-2491.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klijn N., Weerkamp A. H., de Vos W. M. Identification of mesophilic lactic acid bacteria by using polymerase chain reaction-amplified variable regions of 16S rRNA and specific DNA probes. Appl Environ Microbiol. 1991 Nov;57(11):3390–3393. doi: 10.1128/aem.57.11.3390-3393.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefs J. M., Van de Peer Y., Hendriks L., De Wachter R. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res. 1990 Apr 25;18 (Suppl):2237–2317. doi: 10.1093/nar/18.suppl.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pütz J., Meinert F., Wyss U., Ehlers R. U., Stackebrandt E. Development and application of oligonucleotide probes for molecular identification of Xenorhabdus species. Appl Environ Microbiol. 1990 Jan;56(1):181–186. doi: 10.1128/aem.56.1.181-186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama M., Sandine W., Giovannoni S. Development and application of oligonucleotide probes for identification of Lactococcus lactis subsp. cremoris. Appl Environ Microbiol. 1991 May;57(5):1313–1318. doi: 10.1128/aem.57.5.1313-1318.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wang R. F., Cao W. W., Johnson M. G. Development of a 16S rRNA-based oligomer probe specific for Listeria monocytogenes. Appl Environ Microbiol. 1991 Dec;57(12):3666–3670. doi: 10.1128/aem.57.12.3666-3670.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]