Abstract
Context
Asthma and chronic obstructive pulmonary disease (COPD) are prevalent respiratory conditions with overlapping disease characteristics. Differentiation between asthma and COPD is important because several aspects of the guideline-recommended management strategies differ for these conditions. This review identifies the epidemiologic, etiologic, and clinical distinctions of these diseases to assist physicians and other clinicians in differentiating between asthma and COPD. Key components of the guideline-recommended management approaches for these conditions are also reviewed.
Search Strategies
Relevant articles were found by searching the MEDLINE database for “asthma” and “chronic obstructive pulmonary disease OR COPD” in association with the terms “diagnostic criteria” and “differential diagnosis”. Recent statistical summaries (meta-analyses), reviews, and consensus-type documents were also included.
Synthesis
A review of relevant articles found that, although asthma and COPD may occur simultaneously, differences between these diseases are frequently recognized in terms of age at onset, prevalence in relation to age and sex, potential for reversibility of airway obstruction, pathophysiology, and typical symptom presentation. A thorough clinical history in conjunction with lung function testing usually aids in diagnostic distinction and choice of therapeutic interventions. Radiologic imaging and inflammatory marker testing may also aid in the differentiation of these conditions. Over time, disease progression often differs between asthma and COPD.
Conclusions
Although overlaps exist in the disease characteristics of asthma and COPD, careful history, physical examination, and lung function testing often reveal information that facilitates distinction between these diseases, allowing physicians and other clinicians to better tailor their therapy.
A Snapshot of Obstructive Lung Disease
Asthma and chronic obstructive pulmonary disease (COPD) are characterized by a reduced rate of pulmonary airflow resulting from increased inflammation.[1] Airway obstruction is typically fully or nearly fully reversible in patients with asthma,[2] whereas COPD is characterized by airway obstruction that is not fully reversible.[3] Patients with either disease may develop acute exacerbations characterized by increased inflammation of the airway and worsening airway obstruction.[2,3]
Asthma most often presents at a younger age as recurrent episodes of increased airway obstruction that may have varying frequency and intensity, which then become recognized as a chronic pattern of reversible airway obstruction.[2] In a subset of patients with long-term disease, reversibility of airway obstruction is diminished (due to airway remodeling), and a disease pattern similar to COPD may ensue.[4] When the onset of asthma occurs in the sixth or seventh decade of life, recognition is more difficult because symptoms may be similar to those of cardiac disease and COPD, and patients often accept problems as secondary to aging rather than disease.[5]
COPD is a progressive disease of declining lung function observed mainly in older adults with a history of cigarette smoking.[3] COPD includes emphysema and chronic bronchitis, and identification of the clinical signs of these different conditions often occur simultaneously.[6,7] The overlap between asthma and COPD has been recognized and its treatment delineated (ie, manage overlapping COPD and asthma as “asthma”).[8] However, the combination can complicate diagnosis and treatment.[6,9]
Although asthma and COPD share some degree of clinical overlap, they are frequently viewed as separate diseases.[9] The most difficult group of patients in which to distinguish the 2 conditions are those who are current or former smokers with a 15- to 20-pack-year history. In smokers as young as 30 to 40 years, clinical and pathologic manifestations resembling early-stage COPD may be present, which is suggestive of a mixed or intermediary condition.[10] Many older patients, either with recently diagnosed asthma or long-term asthma, have decreased lung function beyond that expected with aging and are at increased risk for fixed airflow obstruction compared with younger patients.[11–14] Long-term follow-up studies show that up to 23% of patients with a history of asthma develop the COPD hallmark of fixed airflow obstruction.[15,16]
Epidemiology
The rates for asthma prevalence have been consistently higher in children than in adults.[17] The overall prevalence in patients younger than 18 years was 8.9% in the United States in 2005,[18] which translates to approximately 6.5 million children. Asthma is more common in boys than in girls; estimates for the prevalence of asthma by sex in patients younger than 18 years in the United States in 2004 were approximately 10% for boys and 7% for girls.[19] During adolescence, asthma incidence increases for girls and decreases for boys,[20] resulting in a gender reversal in prevalence among adults. Estimates from 2004 show that approximately 5.1 million men (5%) and 9.2 million women (8%) had asthma.[19]
In 2002, COPD was the fourth leading cause of death in the United States among people 65 years or older.[21] An estimated 20% of smokers may eventually develop COPD.[22] In an investigation of 169.3 million adults in the United States, obstructive lung disease was found to be present in 12.5% of smokers, 9.4% of former smokers, and 5.8% of people who had never smoked.[14] Genetics also plays a role in the development of COPD.[3] Currently, alpha1-antitrypsin deficiency is the only genetic change that has been directly associated with COPD, but this disorder accounts for fewer than 5% of cases of COPD in the United States.[23] Other genetic associations are likely to be discovered in the future that help explain why approximately 80% of long-term smokers do not develop COPD. Other causes of COPD include industrial or occupational exposure to air pollutants[24] and exposure to wood or cooking fire smoke.[25]
In 2006, an estimated 12.1 million adults in the United States were diagnosed with COPD.[26] However, the overall prevalence of COPD has been estimated at 24 million,[27] suggesting that many people with COPD remain unrecognized and undiagnosed. The prevalence of COPD is higher among whites than blacks and among women; rising rates of smoking have been accompanied by a rising prevalence of COPD – mortality from COPD is now higher for white women than for white men.[28]
Although asthma is more prevalent than COPD, COPD imposes a heavier disease burden due to the greater number of hospitalizations, greater severity of exacerbations, uniform progression of the disease, and poorer overall prognosis. The estimated total cost of asthma in the United States in 2004 was $16.1 billion ($11.5 billion in direct medical expenditures, and $4.6 billion in indirect costs), whereas the total cost of COPD was more than twice as high at $37.2 billion ($20.9 billion in direct medical expenditures, and $16.3 billion in indirect costs).[21]
Natural History
Asthma is most often associated with onset during childhood, prior atopic reactions, and a family history of atopy or asthma.[2] The initial clinical presentation varies, most often with intermittent symptoms but sometimes with constant wheezing, cough, or shortness of breath. Wheezing on expiration is the classic symptom, but some patients present primarily with cough, especially at night.[2] Symptoms typically increase with exposure to allergens and triggers, such as viral upper respiratory infections, pollen, dust mites, animal dander, environmental irritants (most commonly tobacco smoke), cold air, and perhaps physical exertion.[29] In some cases, asthma symptoms diminish after childhood.[30]
In contrast, COPD is essentially unknown in children and is rare in younger adults without a history of alpha1-antitrypsin deficiency. After age 40, however, the prevalence of COPD increases substantially with aging, and the prevalence of patient-reported asthma declines slightly.[31] In general, risk for COPD increases with pack-years of smoking or, less often, with ongoing occupational exposure to inhaled toxins or irritants.[32] Although daily symptoms are present in only 27% of people with asthma,[33] symptoms in COPD are more likely to be constant and progressive, reflecting the fact that airway obstruction in COPD is not due to the reversible airway constriction and inflammation of asthma but rather to structural changes and mechanical derangements with abnormal elastic recoil.[34]
The nature and extent of the systematic components of COPD remain unclear. However, it is clear that COPD often coexists with cardiac disease, depression, and other chronic conditions.[32] Some of the coexisting conditions may be unique disease processes secondary to long-term tobacco exposure or may be part of the same inflammatory process that affects the lungs in COPD.[34–36] The importance of the coexisting conditions was highlighted recently in a review article in which lung cancer and cardiovascular disease were listed as the leading causes of death in patients with mild to moderate COPD.[37]
Pathophysiology
Airway obstruction in asthma results from bronchial smooth muscle constriction, airway hyperreactivity to allergens and irritants, and inflammation accompanied by increased eosinophils and CD4+ lymphocytes.[38] Both asthma and COPD are associated with inflammatory changes.[9] However, the airway mucosa often appears markedly different between the 2 conditions, particularly in regard to the appearance of the surface epithelium and the reticular basement membrane (Figure).[39]
Figure.
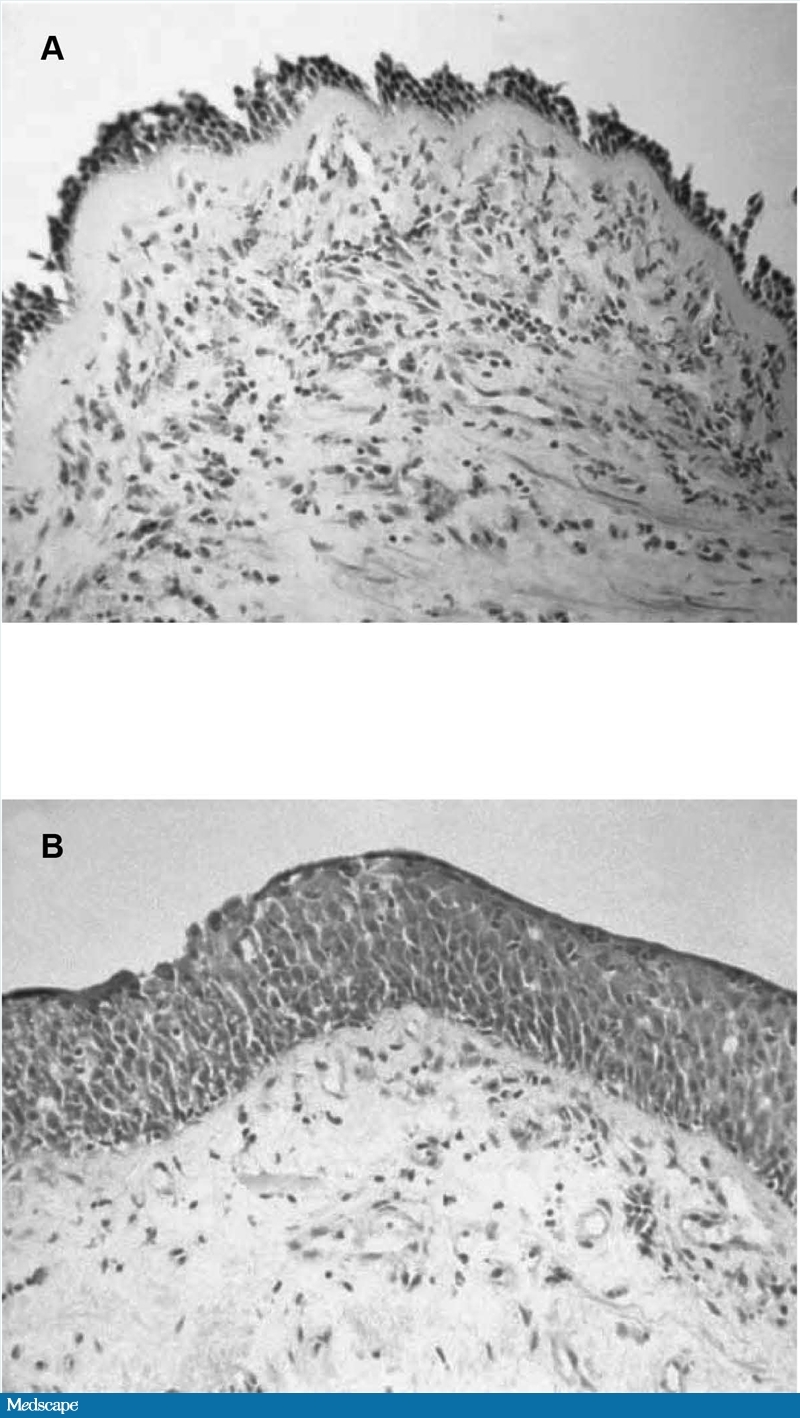
A, Airway mucosa (bronchial biopsy) from a patient with asthma, showing loss of surface epithelium and early basement membrane thickening. B, Airway mucosa (bronchial biopsy) from a heavy smoker with COPD with an FEV1 of 40% predicted, showing epithelial squamous metaplasia. Adapted with permission from Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164:S28-S38.
Airway obstruction in COPD is associated with cellular damage and mucus hypersecretion; inflammation becomes more prominent in exacerbations of severe disease.[38] Cellular damage is a progressive process initiated by inflammatory changes induced by smoking and other environmental toxins to pulmonary tissues.[22] Emphysema is characterized by loss of lung elastic recoil and loss of alveolar structure resulting from inflammatory cell-mediated damage to bronchioles, alveolar ducts, and alveoli.[22] In chronic bronchitis, chronic cough and increased sputum production occur as a consequence of mucosal infiltration by inflammatory cells that lead to cellular damage to the airway mucosa.[22] Most ominously, the cellular destruction and structural changes associated with COPD interfere with oxygenation and pulmonary circulation,[40] which may cause an increased burden on the right side of the heart.[41]
Clinical Assessment
The classic clinical presentation of asthma is a younger patient with recurrent episodes of wheezing and coughing that may be accompanied by chest tightening or breathlessness.[29] Acute symptoms usually respond quickly to bronchodilators.[1,29,32] In contrast, the classic presentation of COPD is an older smoker with progressively worsening shortness of breath and possible cough (with copious mucus in chronic bronchitis) that is often assumed to be a sign of aging and accompanied with decreasing physical activity; COPD may only be diagnosed after a moderate to severe exacerbation.[32] In the classic presentation, reversing pulmonary obstruction after bronchodilator treatment does not return lung function to normal or nearly normal levels and is lower than that observed with asthma,[1] although recent data show that considerable reversibility can be achieved by patients with moderate to severe COPD (stages II–IV).[42] Furthermore, patients with COPD can experience day-to-day variability in both pre-[43,44] and postbronchodilator[43,44] forced expiratory volume in one second (FEV1) reversibility.
When the clinical history of a patient who is suspected to have or is at high risk for obstructive airway disease is being taken, a proactive approach is warranted. Patients with mild symptoms, including those with early-stage COPD, may not volunteer information about symptoms that they do not consider serious and may ignore symptoms or regard them as a sign of aging.[32] To facilitate differentiation of COPD from asthma, the patient history should include specific queries regarding the following[9]:
Age at onset of symptoms – younger with asthma, older with COPD. Reporting of symptoms may require specific prompts, such as “What were you able to do 2 to 5 years ago that you cannot do now because of breathing problems?”
Any history of atopy – such conditions as allergy, eczema, and rhinitis are associated mainly with asthma[32]; asthma is also suggested when symptoms are acutely triggered by exercise or exposure to cold air.[2]
Characteristics of cough – more likely to be dry in asthma, productive in COPD.
History of smoking – pack-years likely to be lower in asthma than in COPD.
Symptom variability – daily changes in symptom frequency or intensity are common in asthma; COPD symptoms are less variable.
In addition, note that viral upper respiratory tract infection can result in exacerbations of either condition,[45] and this factor is therefore of little help in distinguishing asthma from COPD. However, exacerbations associated with bacterial respiratory infection are more suggestive of COPD than asthma.[46] The clinical assessment should also elicit evidence of comorbid conditions. For example, gastroesophageal reflux disease is present in patients with asthma at 3 times the rate of the general population,[29] and the conditions exacerbate each other. Patients with COPD have an increased risk for right-side congestive heart failure, as previously mentioned,[41] and are at increased risk for all types of cardiovascular disease; muscle wasting; weight loss; chronic hypoxia; and depression related to marked and progressive functional impairment, systemic effects of tobacco smoking, and perhaps to the systemic inflammation associated with COPD.[47–49]
Lung Function Testing
Spirometry is the most commonly performed noninvasive test of lung function[50] and is considered the most practical and reliable tool for establishing the presence and severity of obstructive airway diseases, including asthma and COPD.[32,51] Standardized guidelines for obtaining accurate and interpretable spirometry tests are available (eg, the American Thoracic Society/European Respiratory Society Standardization of Lung Function Testing[52]) and should be followed to ensure reproducible and clinically meaningful results. Commonly used spirometry measurements of relevance for the differentiation of asthma from COPD include the volume of air that can be forcibly exhaled in a single breath after a maximum inspiration (forced vital capacity [FVC]), the FEV1 of this maneuver, and the ratio of these measurements (FEV1/FVC). Much emphasis is placed on FEV1 and FEV1/FVC measures because abnormal levels of each (ie, FEV1/FVC ratio <70% predicted is required for a diagnosis of COPD) are considered indicators of airflow obstruction.[2,32] FEV1 and FEV1/FVC measurements must also be measured after 2 puffs of a short-acting bronchodilator to complete reversibility testing that is useful in differentiating between classical presentations of asthma (ie, often fully reversible airway obstruction) and COPD (ie, not fully reversible or irreversible airflow obstruction).[2,32] Table 1 summarizes the criteria for interpretation of spirometry results in the diagnosis of these diseases on the basis of FEV1 readings and as percentages of expected normal values for the FEV1/FVC ratio.[2,32] However, the frequent variability in reversibility over time[43,44] and overlap of asthma and COPD (eg, fixed airflow obstruction in elderly patients with asthma[11,14] and significant bronchodilator response in patients with COPD[42]) limit the use of bronchodilator reversibility testing as the sole criterion for differentiating asthma from COPD. Although these measures are not perfect, they improve the ability to identify reversible and nonreversible airway obstruction compared with clinical impression alone.
Table 1.
Spirometry Essentials for Asthma and COPD
| Adult Lung Function Parameters | ||
|---|---|---|
| Condition and Severity | FEV1 | FEV1/FVC |
| Asthma | ||
| Intermittent | >80% predicted | Normal* |
| Mild persistent | >80% predicted | Normal* |
| Moderate persistent | 60%–79% predicted | Reduced ≤5% from normal* |
| Severe persistent | <60% predicted | Reduced >5% from normal* |
| COPD† | ||
| Mild | ≥80% predicted | <0.7 |
| Moderate | 50%–79% predicted | <0.7 |
| Severe | 30%–49% predicted | <0.7 |
| Very severe | <30% predicted or <50% predicted plus chronic respiratory failure | <0.7 |
COPD = chronic obstructive pulmonary disease; FEV1=forced expiratory volume in 1 s; FVC=forced vital capacity
Normal FEV1/FVC = 85% for patients aged 8 to 19 y; 80% for patients aged 20 to 39 y; 75% for patients aged 40 to 59 y; 70% for patients aged 60 to 80 y.
Postbronchodilator FEV1 recommended for diagnosis and assessment.
Other less commonly used spirometry measures that may be helpful in differentiating between asthma and COPD include total lung capacity, residual volume, and functional residual capacity. These measures are reported to be lower in patients with asthma than in those with COPD,[53,54] although none has been shown to be good predictors of asthma vs COPD.[54] The diffusing capacity for carbon monoxide is another lung function measure that may assist in distinguishing asthma from COPD. This measure has been reported to be higher in patients with asthma than in those with COPD.[53–55] Exhaled nitric oxide has also been shown to be a good predictor of asthma vs COPD in that levels greater than 16 parts per billion suggested a history of asthma, whereas exhaled nitric oxide levels below 16 ppb were suggestive of a history of COPD.[54] Methacholine challenge testing may be used in some specialty settings but is seldom done in primary care. The appropriate use of bronchoprovocation testing, its exact meaning, and the best provocative agent to use remain controversial.[56–58]
Hyperinflation is an elevation in resting functional residual capacity that results in dyspnea and limits exercise capacity.[32,59] It is also associated with a reduction in inspiratory capacity.[50] Hyperinflation is often observed in COPD patients at rest, with worsening during exercise or exacerbation.[60] In patients with asthma, hyperinflation is generally restricted to periods of moderate to severe exacerbations, although a few patients may maintain elevated residual volumes at rest.[60]
Other Diagnostic Modalities
When the diagnosis remains unclear after full clinical and lung function testing assessments, a number of other diagnostic modalities may be used. A chest radiograph can be performed and is often normal in asthma, whereas signs of hyperinflation, bronchial thickening, and increased basilar markings may be observed in COPD.[61] Other useful diagnostic modalities include those based on differences in cellular inflammation profiles for asthma and COPD (Table 2).[62] For example, eosinophil levels are higher and neutrophil levels are lower in bronchoalveolar lavage fluid and sputum samples from patients with asthma vs COPD.[62] In some countries, such as Japan, as well as in some subspecialty offices, more advanced imaging studies are being used to diagnose COPD, especially in confusing or atypical cases.[63]
Table 2.
Differences in Inflammation Profiles Between Asthma and COPD
| Asthma | COPD |
|---|---|
| Cell types infiltrating airways | |
| CD4+ lymphocytes | CD8+ lymphocytes |
| ↑ Ratio of activated CD4+/CD8+ | ↓ Ratio of activated CD4+/CD8+ |
| Eosinophils | Neutrophils |
| Mast cells | Macrophages |
| Neutrophils (severe asthma) | Eosinophils (exacerbation) |
| Upregulated cytokines/chemokines observed | |
| IL-4, IL-5, IL-13 | IL-8, IL-1 |
| RANTES, eotaxins, MCP-1 | Leukotriene B4, interferon-γ |
COPD = chronic obstructive pulmonary disease; IL = interleukin; MCP-1 = monocyte chemoattractant protein-1; RANTES = regulated on activation, normal T-cell expressed and secreted
Adapted with permission from Mauad T, Dolhnikoff M. Pathologic similarities and differences between asthma and chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2008;14:31–38.
Disease Management
The most important reason to accurately diagnose asthma vs COPD is probably because management approaches and goals for these conditions differ. However, some elements of management are similar – for example, both asthma and COPD involve lifestyle modification concomitant with pharmacotherapy tailored to the severity of the condition.[2,32] Treatment of comorbid conditions is crucial in both disorders to differentiate symptoms and to ensure that appropriate treatment is being provided. Patient education also plays a key role in both conditions in optimizing compliance with lifestyle adjustment and pharmacotherapy.[2,32]
Lifestyle Modification
Lifestyle modification begins with smoking cessation, regardless of the diagnosis. Asthma patients must be reminded that smoking exacerbates their condition.[64] Continued smoking may accelerate loss of lung function in COPD patients,[65] whereas smoking cessation can significantly reduce this decline. Thus, for the patient with obstructive airway disease, smoking cessation is a high priority.[65]
Avoidance of environmental air pollution is helpful to minimize exacerbation risk in patients with asthma or COPD, whereas allergen avoidance is more effective for reducing exacerbation risk in patients with asthma.[46] Although total avoidance may not be possible, any meaningful reduction in exposure is beneficial. Other avoidance measures, such as using high-efficiency particulate air filters in the home and changing the furnace/air conditioner filters every 3 months; cleaning the home thoroughly on a weekly basis; and remaining indoors when pollen or mold counts are highest, are recommended.[29]
Another lifestyle modification that may be considered is weight normalization, which may benefit acute or chronic obstructive airway disease and any comorbid cardiovascular disease. Furthermore, exercise as part of a pulmonary rehabilitation program may be important for maintaining and improving health-related quality of life in patients with COPD.[66–68] Aerobic exercises, such as walking, swimming, or bicycling, are important and have been shown to improve cognition and reduce depression.[69] In addition, low-intensity weight training may decrease or delay muscle wasting and improve functional capacity.[32]
Pulmonary rehabilitation that includes disease-specific education, exercise training, nutritional education, and social support can improve most aspects of COPD and its comorbid conditions, such as depression.[66–68] This is the most comprehensive type of COPD therapy and addresses all aspects of the burden on individuals and families.
Pharmacotherapy
Because asthma is a chronic, eosinophil-based inflammatory disorder of the airways, reduction of airway inflammation is a fundamental goal of asthma pharmacotherapy.[2] The types of pharmacotherapy and the doses used are based on assessment of asthma control as measured by symptom burden and effect of symptoms on daily life and numbers and severity of exacerbations and can include spirometry assessment.[2,29] Inhaled corticosteroids (ICSs) reduce airway inflammation and are the cornerstone of pharmacotherapy for treatment of persistent asthma.[2] These drugs do not elicit immediate relief during episodes of acute bronchospasm – their role is mainly to control symptoms by minimizing the inflammatory component of asthma on an ongoing basis.[70] ICS therapy should be titrated up or down based on assessment of control.[2,29] Thus, short-acting bronchodilators, such as short-acting beta2-adrenergic agonists, are required in conjunction with ICS therapy to treat breakthrough symptoms. For patients whose asthma is not well controlled on ICS therapy alone, adding long-acting beta2-adrenergic agonists may be considered. For patients aged 12 years or older whose asthma is not well controlled with low-dose ICS therapy, asthma treatment guidelines recommend adding a long-acting beta2-adrenergic agonist to low-dose ICS treatment or increasing ICSs to a medium dose.[2] Other types of anti-inflammatory therapy include leukotriene inhibitors or modifiers, cromoglycates, and theophylline.[2,29] In both the National Heart Lung and Blood Institute/National Asthma Education and Prevention Program and Global Initiative for Asthma guidelines, ICSs are listed as the preferred therapy for asthma inflammation but other considerations, such as allergic disease, adherence, and acceptability, should be considered when individualizing therapy.[2,29] Anti-IgE therapy is available in an injection form and should be considered in patients whose asthma is difficult to control and who have a confirmed allergic basis for their asthma. Few primary care physicians initiate anti-IgE therapy.[2,71–73] Oral steroids are a major part of therapy for moderate to severe asthma exacerbations and are usually given in a short 5- to 10-day burst of the equivalent of 10 mg of prednisone daily. No tapering is required for this dosing, and no advantage has been found for use of intramuscular or intravenous corticosteroids in persons not experiencing respiratory failure.[2]
Although anticholinergic drugs can be used as alternative reliever therapy in asthma patients who cannot tolerate short-acting beta2-agonists,[2] bronchodilatory response in asthma patients after anticholinergic treatment has been shown to be more variable than with beta2-adrenergic agonist treatment.[74] Furthermore, no long-acting anticholinergic medications are currently indicated for use in the United States for the treatment of asthma.
Treatment of COPD with bronchodilator medications is fundamental for symptomatic management of the disease. Selective inhaled beta2-adrenergic agonists relax bronchial smooth muscle and may help improve mucociliary activity.[70] Short-acting bronchodilators (eg, short-acting beta2-agonists and short-acting anticholinergics) are recommended as short-term therapy for patients with COPD at any level of severity (stages I-IV).[32] Long-acting bronchodilators (eg, anticholinergics, beta2-adrenergic agonists, or methylxanthines) are more effective than short-acting bronchodilators,[75–77] and COPD guidelines recommend treatment with one or more of these medications to control symptoms in patients with moderate to severe COPD (stages II-IV) who do not respond to as-needed short-acting beta2-agonist therapy.[32] Because COPD is typically associated with neutrophilic vs eosinophilic inflammation (the latter is more commonly observed in asthma),[38] ICS treatment is not a first-line therapy for COPD and is reserved for use in combination with a long-acting bronchodilator in patients with severe to very severe COPD (stages III-IV) who have severe or frequent exacerbations.[32] In COPD therapy, ICS is not titrated on the basis of control but rather on exacerbation rates. Usually the dose is only titrated upward or may be discontinued if it does not reduce exacerbation rates.[5,8,22] Systematic corticosteroids are used to treat COPD exacerbations in short bursts of oral steroids usually lasting 10 days and in doses equivalent to 10 to 20 mg of prednisone per day.[5,8] There is no role for daily oral steroid maintenance in COPD with the exception of very severe and late-stage disease in which any and all types of therapy may be tried.[5,8]
Controlled oxygen support may be used in advanced cases of COPD.[32,78] Current recommendations are for oxygen supplementation when oxygen saturation (SaO2) is 88% or lower.[32] This therapy is also recommended for use in asthma but only for management of exacerbations to maintain SaO2 at 90% or greater.[2] Recommendations for earlier use of oxygen supplementation cannot be made because it has not been sufficiently studied.
Conclusions
Although asthma and COPD often overlap in clinical features, they are distinct conditions whose differences influence both management and prognosis. Concurrent assessment of clinical history, current clinical symptoms, lung function tests, and other diagnostic modalities (eg, radiographic imagery and cellular markers of inflammation) can help facilitate an accurate diagnosis of asthma vs COPD. Establishing the correct diagnosis is the essential first step in developing an individualized disease management plan that will optimize quality of life and physical functioning for patients with asthma or COPD.
Acknowledgments
The author acknowledges Tracy J. Wetter, PhD, and Rhonda L. Croxton, PhD, from Complete Healthcare Communications, Inc., for providing medical writing support funded by AstraZeneca LP.
Footnotes
Reader Comments on: Differential Assessment and Management of Asthma vs Chronic Obstructive Pulmonary Disease See reader comments on this article and provide your own.
References
- 1.Welte T, Groneberg DA. Asthma and COPD. Exp Toxicol Pathol. 2006;57(suppl 2):35–40. doi: 10.1016/j.etp.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 2.National Heart Lung and Blood Institute, National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda: MD; 2007. NIH Publication No. 07-4051. [Google Scholar]
- 3.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 4.Braman SS, Kaemmerlen JT, Davis SM. Asthma in the elderly: a comparison between patients with recently acquired and long-standing disease. Am Rev Respir Dis. 1991;143:336–340. doi: 10.1164/ajrccm/143.2.336. [DOI] [PubMed] [Google Scholar]
- 5.Global Initiative for Asthma. Global strategy for asthma management and prevention. Bethesda, MD: National Heart, Lung, and Blood Institute; 2006. NIH Publication No. 02-3659. [Google Scholar]
- 6.Honig EG, Ingram RH. Chronic bronchitis, emphysema, and airways obstruction. In: Braunwald E, Fauci AS, Kasper DL, et al., editors. Harrison's Principles of Internal Medicine. 15th ed. New York: McGraw-Hill; 2001. pp. 1491–1499. [Google Scholar]
- 7.Sciurba FC. Physiologic similarities and differences between COPD and asthma. Chest. 2004;126(2 suppl):117S–124S. doi: 10.1378/chest.126.2_suppl_1.117S. [DOI] [PubMed] [Google Scholar]
- 8.Celli BR, MacNee W, Force AET. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 9.Beeh KM, Kornmann O, Beier J, Ksoll M, Buhl R. Clinical application of a simple questionnaire for the differentiation of asthma and chronic obstructive pulmonary disease. Respir Med. 2004;98:591–597. doi: 10.1016/j.rmed.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Boulet LP, Lemiere C, Archambault F, et al. Smoking and asthma: clinical and radiologic features, lung function, and airway inflammation. Chest. 2006;129:661–668. doi: 10.1378/chest.129.3.661. [DOI] [PubMed] [Google Scholar]
- 11.Brinke AT, Zwinderman AH, Sterk PJ, Rabe KF, Bel EH. Factors associated with persistent airflow limitation in severe asthma. Am J Respir Crit Care Med. 2001;164:744–748. doi: 10.1164/ajrccm.164.5.2011026. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ. 1977;1:1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 14.Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2000;160:1683–1689. doi: 10.1001/archinte.160.11.1683. [DOI] [PubMed] [Google Scholar]
- 15.Ulrik CS, Backer V. Nonreversible airflow obstruction in life-long nonsmokers with moderate to severe asthma. Eur Respir J. 1999;14:892–896. doi: 10.1034/j.1399-3003.1999.14d27.x. [DOI] [PubMed] [Google Scholar]
- 16.Vonk JM, Jongepier H, Panhuysen CIM, et al. Risk factors associated with the presence of irreversible airflow limitation and reduced transfer coefficient in patients with asthma after 26 years of follow up. Thorax. 2003;58:322–327. doi: 10.1136/thorax.58.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudd RA, Moorman JE. Asthma incidence: data from the national health interview survey, 1980–1996. J Asthma. 2007;44:65–70. doi: 10.1080/02770900601125896. [DOI] [PubMed] [Google Scholar]
- 18.Influenza vaccination coverage among children with asthma – United States, 2004–05 influenza season. MMWR Morb Mortal Wkly Rep. 2007;56:193–196. [PubMed] [Google Scholar]
- 19.American Lung Association. Trends in Asthma Morbidity and Mortality. New York, NY: American Lung Association Epidemiology & Statistics Unit; 2006. [Google Scholar]
- 20.Brunner WM, Lindgren PG, Langner DM, Williams AN, Yawn BP. Asthma among rural Minnesota adolescents. J Asthma. 2005;42:787–792. doi: 10.1080/02770900500308460. [DOI] [PubMed] [Google Scholar]
- 21.National Heart Lung and Blood Institute. Morbidity and Mortality Chartbook. Bethesda, MD: US Department of Health and Human Services; 2004. [Google Scholar]
- 22.Sutherland ER, Martin RJ. Airway inflammation in chronic obstructive pulmonary disease: comparisons with asthma. Allergy Clin Immunol. 2003;112:819–827. doi: 10.1016/S0091. quiz, 828. [DOI] [PubMed] [Google Scholar]
- 23.American Thoracic Society. American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med. 2003;168:818–900. doi: 10.1164/rccm.168.7.818. [DOI] [PubMed] [Google Scholar]
- 24.Viegi G, Scognamiglio A, Baldacci S, Pistelli F, Carrozzi L. Epidemiology of chronic obstructive pulmonary disease (COPD) Respiration. 2001;68:4–19. doi: 10.1159/000050456. [DOI] [PubMed] [Google Scholar]
- 25.Ekici A, Ekici M, Kurtipek E, et al. Obstructive airway diseases in women exposed to biomass smoke. Environ Res. 2005;99:93–98. doi: 10.1016/j.envres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 26.American Lung Association. Trends in COPD (chronic bronchitis and emphysema): morbidity and mortality American Lung Association epidemiology & statistics unit research and program services. 2007.
- 27.Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance–United States, 1971-2000. MMWR Surveill Summ. 2002;51:1–16. [PubMed] [Google Scholar]
- 28.Hoyert DL, Heron MP, Murphy SL, Kung HC. Deaths: final data for 2003. Natl Vital Stat Rep. 2006;54:1–120. [PubMed] [Google Scholar]
- 29.Global Initiative for Asthma. Pocket Guide for Asthma Management and Prevention. Available at: www.ginasthma.org/download.asp?intId=215 Accessed November 21, 2007.
- 30.Jenkins MA, Hopper JL, Bowes G, et al. Factors in childhood as predictors of asthma in adult life. BMJ. 1994;309:90–93. doi: 10.1136/bmj.309.6947.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Schayck CP, Levy ML, Chen JC, Isonaka S, Halbert RJ. Coordinated diagnostic approach for adult obstructive lung disease in primary care. Prim Care Respir J. 2004;13:218–221. doi: 10.1016/j.pcrj.2004.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Available at: http://www.goldcopd.com/Guidelineitem.asp?l1=2&l2=1&intId=989 Accessed April 14, 2008.
- 33.American Lung Association. ALA Asthma Survey: Executive Summary. Available at: http://www.lungusa.org/site/apps/s/content.asp?c=dvLUK9O0E&b=34706&ct=67427 Accessed November 21, 2007.
- 34.Walter RE, Wilk JB, Larson MG, et al. Systemic inflammation and COPD: the Framingham Heart Study. Chest. 2008;133:19–25. doi: 10.1378/chest.07-0058. [DOI] [PubMed] [Google Scholar]
- 35.Groenewegen KJ, Postma DS, Hop WC, Wielders PL, Schlosser NJ, Wouters EF. Increased systemic inflammation is a risk factor for COPD exacerbations. Chest. 2008;133:350–357. doi: 10.1378/chest.07-1342. [DOI] [PubMed] [Google Scholar]
- 36.Eisner MD, Blanc PD, Yelin EH, et al. COPD as a systemic disease: impact on physical functional limitations. Am J Med. 2008;121:789–796. doi: 10.1016/j.amjmed.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: role of comorbidities. Eur Respir J. 2006;28:1245–1257. doi: 10.1183/09031936.00133805. [DOI] [PubMed] [Google Scholar]
- 38.Decramer M, Selroos O. Asthma and COPD: differences and similarities. With special reference to the usefulness of budesonide/formoterol in a single inhaler (Symbicort) in both diseases. Int J Clin Pract. 2005;59:385–398. doi: 10.1111/j.1368-5031.2005.00509.x. [DOI] [PubMed] [Google Scholar]
- 39.Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164(10 Pt 2):S28–S38. doi: 10.1164/ajrccm.164.supplement_2.2106061. [DOI] [PubMed] [Google Scholar]
- 40.Rogers DF. Mucus pathophysiology in COPD: differences to asthma, and pharmacotherapy. Monaldi Arch Chest Dis. 2000;55:324–332. [PubMed] [Google Scholar]
- 41.Naeije R. Pulmonary hypertension and right heart failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:20–22. doi: 10.1513/pats.200407-037MS. [DOI] [PubMed] [Google Scholar]
- 42.Tashkin DP, Celli B, Decramer M, et al. Bronchodilator responsiveness in patients with COPD. Eur Respir J. 2008;31:742–750. doi: 10.1183/09031936.00129607. [DOI] [PubMed] [Google Scholar]
- 43.Calverley PMA, Burge PS, Spencer S, Anderson JA, Jones PW. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax. 2003;58:659–664. doi: 10.1136/thorax.58.8.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dorinsky PM, Reisner C, Ferguson GT, et al. The combination of Ipratropium and albuterol optimizes pulmonary function reversibility testing in patients with COPD. Chest. 1999;115:966–971. doi: 10.1378/chest.115.4.966. [DOI] [PubMed] [Google Scholar]
- 45.Mallia P, Contoli M, Caramori G, et al. Exacerbations of asthma and chronic obstructive pulmonary disease (COPD): focus on virus induced exacerbations. Curr Pharm Des. 2007;13:73–97. doi: 10.2174/138161207779313777. [DOI] [PubMed] [Google Scholar]
- 46.Pauwels RA. Similarities and differences in asthma and chronic obstructive pulmonary disease exacerbations. Proc Am Thorac Soc. 2004;1:73–76. doi: 10.1513/pats.2306024. [DOI] [PubMed] [Google Scholar]
- 47.Agusti AG. Systemic effects of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:367–370. doi: 10.1513/pats.200504-026SR. discussion, 371-372. [DOI] [PubMed] [Google Scholar]
- 48.Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome? Lancet. 2007;370:797–799. doi: 10.1016/S0140-6736(07)61383-X. [DOI] [PubMed] [Google Scholar]
- 49.Yawn BP, Kaplan A. Co-morbidities in people with COPD: a result of multiple diseases, or multiple manifestations of smoking and reactive inflammation? Prim Care Respir J. 2008 doi: 10.3132/pcrj.2008.00021. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pierce R. Spirometry: an essential clinical measurement. Aust Fam Physician. 2005;34:535–539. [PubMed] [Google Scholar]
- 51.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 52.Aggarwal AN, Agarwal R. The new ATS/ERS guidelines for assessing the spirometric severity of restrictive lung disease differ from previous standards. Respirology. 2007;12:759–762. doi: 10.1111/j.1440-1843.2007.01117.x. [DOI] [PubMed] [Google Scholar]
- 53.Boulet LP, Turcotte H, Hudon C, Carrier G, Maltais F. Clinical, physiological and radiological features of asthma with incomplete reversibility of airflow obstruction compared with those of COPD. Can Respir J. 1998;5:270–277. doi: 10.1155/1998/780739. [DOI] [PubMed] [Google Scholar]
- 54.Fabbri LM, Romagnoli M, Corbetta L, et al. Differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:418–424. doi: 10.1164/rccm.200203-183OC. [DOI] [PubMed] [Google Scholar]
- 55.Saydain G, Beck KC, Decker PA, Cowl CT, Scanlon PD. Clinical significance of elevated diffusing capacity. Chest. 2004;125:446–452. doi: 10.1378/chest.125.2.446. [DOI] [PubMed] [Google Scholar]
- 56.Liem JJ, Kozyrskyj AL, Cockroft DW, Becker AB. Diagnosing asthma in children: what is the role for methacholine bronchoprovocation testing? Pediatr Pulmonol. 2008;43:481–489. doi: 10.1002/ppul.20801. [DOI] [PubMed] [Google Scholar]
- 57.Anderson SD. Provocative challenges to help diagnose and monitor asthma: exercise, methacoline, adenosine, and mannitol. Curr Opin Pulm Med. 2008;14:39–45. doi: 10.1097/MCP.0b013e3282f197f6. [DOI] [PubMed] [Google Scholar]
- 58.Birnbaum S, Barreiro TJ. Methacholine challenge testing: identifying its diagnostic role, testing, coding, and reimbursement. Chest. 2007;131:1932–1935. doi: 10.1378/chest.06-1385. [DOI] [PubMed] [Google Scholar]
- 59.Ferguson GT. Why does the lung hyperinflate? Proc Am Thorac Soc. 2006;3:176–179. doi: 10.1513/pats.200508-094DO. [DOI] [PubMed] [Google Scholar]
- 60.Chang J, Mosenifar Z. Differentiating COPD from asthma in clinical practice. J Intensive Care Med. 2007;22:300–309. doi: 10.1177/0885066607304445. [DOI] [PubMed] [Google Scholar]
- 61.Dewar M, Curry RW., Jr Chronic obstructive pulmonary disease: diagnostic considerations. Am Fam Physician. 2006;73:669–676. [PubMed] [Google Scholar]
- 62.Mauad T, Dolhnikoff M. Pathologic similarities and differences between asthma and chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2008;14:31–38. doi: 10.1097/MCP.0b013e3282f19846. [DOI] [PubMed] [Google Scholar]
- 63.Sverzellati N, Molinari F, Pirronti T, et al. New insights on COPD imaging via CT and MRI. Int J Chron Obstruct Pulmon Dis. 2007;2:301–312. [PMC free article] [PubMed] [Google Scholar]
- 64.Tonnesen P, Carrozzi L, Fagerstrom KO, et al. Smoking cessation in patients with respiratory diseases: a high priority, integral component of therapy. Eur Respir J. 2007;29:390–417. doi: 10.1183/09031936.00060806. [DOI] [PubMed] [Google Scholar]
- 65.Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272:1497–1505. [PubMed] [Google Scholar]
- 66.Paz-Diaz H, Montes de Oca M, Lopez JM, Celli BR. Pulmonary rehabilitation improves depression, anxiety, dyspnea and health status in patients with COPD. Am J Phys Med Rehabil. 2007;86:30–36. doi: 10.1097/phm.0b013e31802b8eca. [DOI] [PubMed] [Google Scholar]
- 67.Guell R, Resqueti V, Sangenis M, et al. Impact of pulmonary rehabilitation on psychosocial morbidity in patients with severe COPD. Chest. 2006;129:899–904. doi: 10.1378/chest.129.4.899. [DOI] [PubMed] [Google Scholar]
- 68.Foglio K, Bianchi L, Bruletti G, et al. Seven-year time course of lung function, symptoms, health-related quality of life, and exercise tolerance in COPD patients undergoing pulmonary rehabilitation programs. Respir Med. 2007;101:1961–1970. doi: 10.1016/j.rmed.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 69.Rochester CL. Exercise training in chronic obstructive pulmonary disease. J Rehabil Res Dev. 2003;40(5 Suppl 2):59–80. doi: 10.1682/jrrd.2003.10.0059. [DOI] [PubMed] [Google Scholar]
- 70.McFadden ER. Asthma. In: Braunwald E, Fauci AS, Kasper DL, et al., editors. Harrison's Principles of Internal Medicine. 15th ed. New York: McGraw-Hill; 2001. pp. 1457–1463. [Google Scholar]
- 71.Marcus P. Incorporating anti-IgE (omalizumab) therapy into pulmonary medicine practice: practice management implications. Chest. 2006;129:466–474. doi: 10.1378/chest.129.2.466. [DOI] [PubMed] [Google Scholar]
- 72.Fahy JV. Anti-IgE: lessons learned from effects on airway inflammation and asthma exacerbation. J Allergy Clin Immunol. 2006;17:1230–1232. doi: 10.1016/j.jaci.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 73.Holgate S, Casale T, Wenzel S, Bousquet J, Deniz Y, Reisner C. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J Allergy Clin Immunol. 2005;115:459–465. doi: 10.1016/j.jaci.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 74.Restrepo RD. Use of inhaled anticholinergic agents in obstructive airway disease. Respir Care. 2007;52:833–851. [PubMed] [Google Scholar]
- 75.Dahl R, Greefhorst LA, Nowak D, et al. Inhaled formoterol dry powder versus ipratropium bromide in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:778–784. doi: 10.1164/ajrccm.164.5.2007006. [DOI] [PubMed] [Google Scholar]
- 76.Mahler DA, Donohue JF, Barbee RA, et al. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest. 1999;115:957–965. doi: 10.1378/chest.115.4.957. [DOI] [PubMed] [Google Scholar]
- 77.Vincken W, van Noord JA, Greefhorst AP, et al. Improved health outcomes in patients with COPD during 1 yr's treatment with tiotropium. Eur Respir J. 2002;19:209–216. doi: 10.1183/09031936.02.00238702. [DOI] [PubMed] [Google Scholar]
- 78.Calverley PM. Respiratory failure in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2003;47:26s–30s. doi: 10.1183/09031936.03.00030103. [DOI] [PubMed] [Google Scholar]


