Abstract
Purpose
Bone morphogenetic protein (BMP) signaling is essential for the induction and subsequent development of the lens. The purpose of this study was to analyze the function(s) of the type 1 BMP receptor, Acvr1, in lens development.
Methods
Acvr1 was deleted from the surface ectoderm of mouse embryos on embryonic day 9 using the Cre-loxP method. Cell proliferation, cell cycle exit, and apoptosis were measured in tissue sections by immunohistochemistry, immunofluorescence, and TUNEL staining.
Results
Lenses formed in the absence of Acvr1. However, Acvr1CKO (conditional knockout) lenses were small. Acvr1 signaling promoted proliferation at early stages of lens formation but inhibited proliferation at later stages. Inhibition of cell proliferation by Acvr1 was necessary for the proper regionalization of the lens epithelium and promoted the withdrawal of lens fiber cells from the cell cycle. In spite of the failure of all Acvr1CKO fiber cells to withdraw from the cell cycle, they expressed proteins characteristic of differentiated fiber cells. Although the stimulation of proliferation was Smad independent, the ability of Acvr1 to promote cell cycle exit later in development depended on classical R-Smad-Smad4 signaling. Loss of Acvr1 led to an increase in apoptosis of lens epithelial and fiber cells. Increased cell death, together with the initial decrease in proliferation, appeared to account for the smaller sizes of the Acvr1CKO lenses.
Conclusions
This study revealed a novel switch in the functions of Acvr1 in regulating lens cell proliferation. Previously unknown functions mediated by this receptor included regionalization of the lens epithelium and cell cycle exit during fiber cell differentiation.
Lens formation is one of the most widely studied examples of embryonic induction. 1,2 As a result of the precise localization of cell proliferation and differentiation in the developing lens, it has often been used to demonstrate the fundamental molecular mechanisms that control terminal cell differentiation and cell proliferation in all tissues.3-6 Lens morphogenesis begins on embryonic day (E) 9 of mouse development with the formation of the lens placode. After the lens placode invaginates and separates from the surface ectoderm to form the lens vesicle, cells in the posterior part of the vesicle stop proliferating and form primary lens fiber cells. The anterior epithelial cells continue to proliferate. Proliferation eventually becomes restricted to the epithelial cells near the lens equator, which subsequently differentiate into secondary fiber cells, accounting for lens growth throughout life.
Lens formation and its subsequent development are regulated by members of at least two major families of growth factors. Bone morphogenetic proteins (BMPs), which belong to the transforming growth factor-β (TGFβ) superfamily, are essential for lens induction. Targeted deletion or inactivation of the BMP ligands Bmp4 and Bmp7 results in the failure of lens formation.2,7-9 Lenses lacking the type 1 BMP receptor Bmpr1a (Alk3) are small, have thin epithelia, and degenerate fiber cells.10 Fibroblast growth factors (FGFs) are the other family of growth factors important for lens formation. Deletion of Fgfr2 results in defects in fiber cell terminal differentiation and reduced cell survival,11 and the deletion of Fgfr1, Fgfr2, and Fgfr3 in the lens vesicle prevents subsequent fiber cell formation.12 Finally, there is genetic evidence of interactions between the FGF and BMP signaling pathways during early lens development.13
Although BMPs are essential for lens development, little is known about the cellular events initiated by BMP signaling and the downstream signaling molecules that mediate these events during lens induction and subsequent development. The BMP signaling cascade is initiated by type 2 and type 1 cell surface serine/threonine kinase receptors. Ligand-activated receptors phosphorylate downstream signaling molecules, the receptor-activated Smads or R-Smads. Activated R-Smads complex with the common mediator Smad (Co-Smad) known as Smad4 and translocate to the nucleus to regulate gene expression.14
To gain an understanding of the molecular events mediating BMP signaling in the lens, we used the Cre-loxP approach to inactivate the type 1 BMP receptor Acvr1 (Alk2) in the lens-forming head ectoderm of the mouse embryo. Acvr1 is expressed in the prospective ectoderm at the time of lens induction,15 but its function in lens development has not been examined. Acvr1CKO (conditional knockout) lenses formed but were smaller than wild-type lenses. Analysis of the cause of the smaller lens size revealed that Acvr1 promoted proliferation at early stages (Rajagopal R et al., manuscript submitted) but inhibited epithelial cell proliferation later in lens development. Inhibition of cell proliferation by Acvr1 was necessary for the proper regionalization of the lens epithelium and promoted the withdrawal of lens fiber cells from the cell cycle. Deletion of the downstream Smad effector proteins showed that Acvr1 inhibited proliferation and promoted cell cycle exit by engaging the BMP-specific R-Smads (Smad1 and Smad5) and the Co-Smad (Smad4). Although Acvr1 is required for fiber cells to withdraw from the cell cycle, it is not required for the expression of proteins that are characteristic of differentiated fiber cells. Acvr1 signaling promoted the survival of lens epithelial and fiber cells. The initial decrease in proliferation, along with an increase in cell death in the Acvr1CKO lens epithelia and fiber cells, appears to account for the overall decrease in lens size.
Materials and Methods
Mice and Genotyping
Mice expressing Cre recombinase under the control of Pax6 P0 enhancer/promoter (Le-Cre) were described previously.16 Primers for genotyping mice carrying the Cre transgene or the floxed alleles used in this study, (Acvr1fx(exon7),17 Smad4fx(exon8),18 Smad1fx(exon2),19Smad5fx(exon2),20 and Smad2fx(exon2)21) are listed in Supplementary Table S1, online at http://www.iovs.org/cgi/content/full/49/11/4953/DC1. Mouse genomic DNA from the toe or embryonic tail tissue was extracted using the hot sodium hydroxide and Tris (HotSHOT) method,22 and DNA from P3 lenses was extracted using a purification kit (DNeasy Blood and Tissue Kit; Qiagen, Valencia, CA). PCR conditions were selected according to the universal PCR protocol.23 Mice that were homozygous floxed, one of which was Cre-positive, were mated to generate 50% Cre-positive (conditional knockout [CKO]) and 50% Cre-negative (wild-type [WT]) offspring. Cre-positive animals were always mated to Cre-negative animals, ensuring that Cre-positive offspring inherited only one copy of the Cre transgene.
Antibodies and Immunostaining
Embryos or postnatal day (P) 3 mouse heads were fixed in 10% formalin overnight at room temperature, processed and embedded in paraffin, and sectioned at 4 μm. For morphologic studies, sections were stained with hematoxylin and eosin (Surgipath, Richmond, IL). For antibody staining, sections were deparaffinized and rehydrated. Endogenous peroxidase activity was inactivated with 3% H2O2 in methanol for 30 minutes at room temperature for those samples that would be treated for horseradish peroxidase. Epitope retrieval was performed in 0.01 M citrate buffer (pH 6.0) either at 100°C for 20 minutes using a water bath or in a decloaking chamber (Biocare Medical, Walnut Creek, CA) for 3 minutes. Slides were then incubated in blocking solution containing 20% inactivated normal donkey serum for 30 minutes at room temperature followed by incubation in primary antibodies overnight at 4°C. Primary antibodies used were anti-Ki67 (BD PharMingen, San Diego, CA) at 1:400 dilution, anti–phospho-Histone H3 (Upstate Biotechnology, Lake Placid, NY) at 1:1000 dilution, anti-p57 (Santa Cruz Biotechnology, Santa Cruz, CA) at 1:1000 dilution, and anti– c-maf (Santa Cruz Biotechnology) at 1:500 dilution. Rabbit polyclonal antibody against major intrinsic protein (MIP) was kindly provided by Alan Shiels and was used at a dilution of 1:1000. Slides were then incubated for 1 hour at room temperature with Alexa-Fluor–labeled secondary antibodies (Molecular Probes, Eugene, OR) or biotinylated secondary antibodies (Vector Laboratories, Burlin-game, CA). Slides incubated with biotinylated secondary antibodies were treated with the ABC-peroxidase reagent (Vectastain Elite ABC Kit; Vector Laboratories) followed by treatment with diaminobenzidine (DAB; Sigma, St. Louis, MO) and H2O2 and were counterstained with hematoxylin (Surgipath).
Terminal deoxynucleotidyl transferase (TdT)–mediated deoxyuridine triphosphate nick end-labeling (TUNEL) was performed with an apoptosis detection kit (ApopTag; Chemicon, Temecula, CA). For BrdU staining, pregnant females or P3 mice were injected with 50 mg/kg body weight of 10 mM BrdU (Roche, Indianapolis, IN) containing 1 mM 5-fluoro-5′-deoxyuridine (Sigma) and were killed after 1 hour. A monoclonal anti–BrdU antibody (1:250; Dako, Carpinteria, CA) was used with an immunostaining kit (Vectastain Elite Mouse IgG ABC; Vector Laboratories), as described, or with the blocking reagent (Histostain-Plus Bulk Kit; Zymed Laboratories, San Francisco, CA). Sections were counterstained with hematoxylin.
Imaging
Whole lenses were imaged by dark-field illumination with a dissecting microscope (Stemi 2000-C; Carl Zeiss, Thornburgh, NY) fitted with a digital camera (Spot Diagnostic Instruments, Sterling Heights, MI). Lens diameters were measured from lens images using software provided with the Spot camera. Bright-field and fluorescent images of lens sections were taken using Olympus microscopes (BX60 and BX51, respectively; Olympus, Melville, NY) with a Spot camera.
Determination of the Patterns of Cell Proliferation and Death
Wild-type and knockout placodes were marked based on their thickened appearance compared with the flanking head ectoderm and based on contact with the underlying optic vesicle. Cell proliferation in wild-type and knockout lens epithelia was mapped by dividing the lens epithelia into radially arranged sectors, an approach modified from previous studies.13,24,25 To demarcate epithelial cells from fibers cells at the equator of the lens, the following criterion was used. The last cell to be considered as an epithelial cell was the one whose longitudinal axis was parallel to the equatorial line or, in other words, whose nucleus was horizontal. Beneath the last epithelial cell, a horizontal line was drawn across the equator of the lens section dividing the epithelium from the fibers (see Fig. 3A). A protractor was placed on the center of this line, and 12 radially arranged sectors of 15° were marked. The percentage of BrdU- or Ki67-labeled nuclei was counted within each sector. For the purpose of our analysis, the BrdU-labeling index was considered an indirect indication of the rate of cell proliferation. However, changes in the duration of S-phase in mutant lenses or at different stages in the development of wild-type lenses could yield overestimates or underestimates of the rate of cell proliferation. Similarly, our estimates of cell proliferation are averaged over the entire cell population. Since the percentage of cycling (Ki67 positive) cells decreased from 100% at E9.5 to lower values at each subsequent stage, the BrdU-labeling index sometimes did not reflect the proliferation rate of the cells that were actively cycling. For cell death analyses, the first two sectors (0°-15° and 15°-30°) were scored on both sides of lens sections. The remainder of the epithelium was regarded as central epithelium. There was no statistically significant difference between the percentages of labeled cells in corresponding sectors on either side of the lens. Therefore, cell counts from corresponding sectors on the left and right sides of the lens sections were pooled. For counting the percentage of labeled cells in the fiber mass, the entire fiber mass was included at E12.5 and E15.5. At E18.5 and onward, the fiber cells located in the center of the lens degraded their nuclei. Before denucleation, the fiber cell nuclei become rounded and compact. Fiber cells in the periphery of the lens (cortical fibers) contained nuclei that remained oval. Therefore, from E18.5 onward, only the cortical fiber cells with oval nuclei were analyzed for the purposes of determining the percentage of labeled cells. For all experiments, three to six sections from each of at least six wild-type and six knockout placodes or lenses were analyzed.
Figure 3.
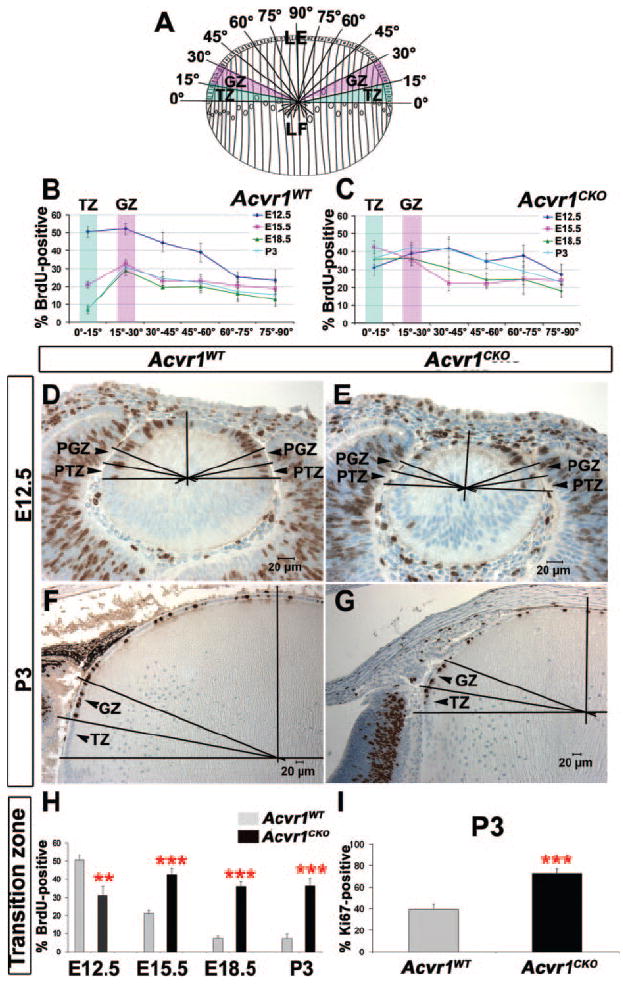
Acvr1CKO lens epithelial cells fail to form the transition zone (A) Schematic representation of the division of lens epithelia (LE) into 15° sectors, showing the location of the germinative zone (GZ) and the transition zone (TZ) of the LE and the lens fiber (LF) compartment. (B) Patterns of BrdU incorporation in different sectors of Acvr1WT lens epithelia at different stages of development. The BrdU-labeling index declined from E12.5 to P3 in Acvr1WT epithelia, particularly in the TZ and the central epithelium, establishing the location of the GZ. (C) Distribution of BrdU incorporation in different sectors of Acvr1CKO lens epithelia during development. No significant change in the BrdU-labeling index of the transition zone was detected from E12.5 to P3. (D, E) Representative images of BrdU-labeled sections of Acvr1WT (D) and Acvr1CKO (E) lenses at E12.5, showing the location of the prospective GZ (PGZ) and the prospective TZ (PTZ). (F, G) Representative images of BrdU-labeled sections of Acvr1WT (F) and Acvr1CKO (G) lenses at P3, showing the location of the GZ and the TZ. (H) BrdU-labeling index in the transition zone of Acvr1WT and Acvr1CKO lens epithelia from E12.5 to P3. (I) Ki67-labeling index in the transition zone of Acvr1CKO and Acvr1WT lenses at P3. ** P < 0.01; ***P < 0.001.
Statistical Analysis
For the statistical analysis of two groups, the unpaired t-test was used. Statistical tests were performed using commercial software (GraphPad InStat, Version 3.05; GraphPad Software, San Diego, CA). Error bars are ± SEM.
Results
Rise in Small Lenses due to Deletion of the Type 1 BMP Receptor Acvr1
BMP signaling is essential for the formation and proper differentiation of the lens.7,9,10,26,27 To determine the specific functions of each of the three type 1 BMP receptors in lens development, we deleted Acvr1 (also known as Alk2) from the head ectoderm beginning on E9. Mice expressing Cre recombinase under control of the Pax6 P0 promoter/enhancer (Le-Cre)16 were mated to mice harboring floxed alleles of Acvr1.17 The mating scheme generated mice that were homozygous for the floxed allele (Acvr1fx/fx) and were either Le-Cre-positive or -negative. For simplicity, homozygous floxed mice that expressed Cre were designated Acvr1CKO, and those that did not express Cre were designated Acvr1WT. Inactivation of the targeted alleles was complete or nearly so in all cases (Supplementary Fig. S1, http://www.iovs.org/cgi/content/full/49/11/ 4953/DC1). Although lenses formed in the absence of Acvr1 signaling, they were significantly smaller than lenses from wild-type littermates (Fig. 1). By P3, knockout lenses showed a variable extent of fiber cell swelling and disorganization and occasionally developed nuclear cataracts (Fig. 1D). Because all Acvr1CKO lenses were small, we focused our analysis on this aspect of the phenotype.
Figure 1.
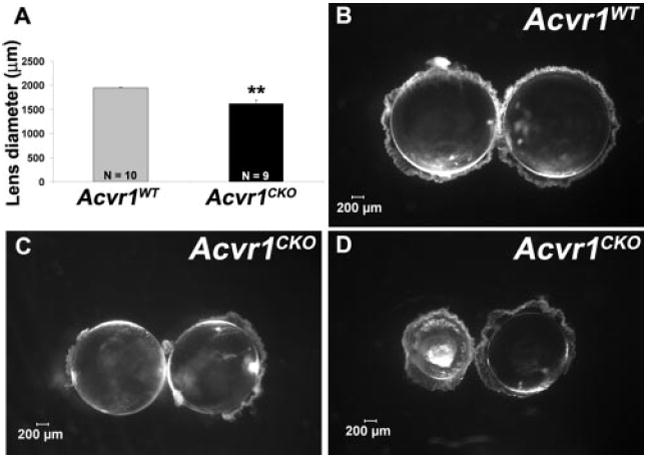
Acvr1CKO lenses are smaller (A) Acvr1CKO lenses had reduced diameters. Lenses from P22 Acvr1WT (B) and Acvr1CKO (C, D) mice were dissected and viewed with a dissecting microscope under dark-field illumination. Acvr1CKO lenses occasionally developed nuclear cataracts (D). **P < 0.01.
Biphasic Proliferation Defect in Acvr1CKO Lens Cells
To address the possibility that decreased cell proliferation was responsible for the smaller size of the Acvr1CKO lenses, we used BrdU labeling starting at E9.5, when lens morphogenesis began with the formation of the lens placode. In wild-type lenses, the BrdU-labeling index was high at E9.5 and decreased by approximately 50% in the lens epithelium by P3 (Fig. 2). As we anticipated, lens placode cells from Acvr1CKO mice had a significantly lower rate of proliferation than did wild-type placodes (P < 0.01; Rajagopal R et al., manuscript submitted). However, by E12.5, this difference disappeared. To our surprise, the BrdU-labeling index of Acvr1CKO lens epithelial cells did not decrease from E12.5 to P3 as it did in wild-type cells. Therefore, Acvr1CKO lens cells proliferated at a slower rate early in lens formation, but from E15.5 onward their proliferation was more rapid than in their Acvr1WT littermates. This indicated a switch in the regulation of lens cell proliferation by the Acvr1 receptor by E15.5.
Figure 2.
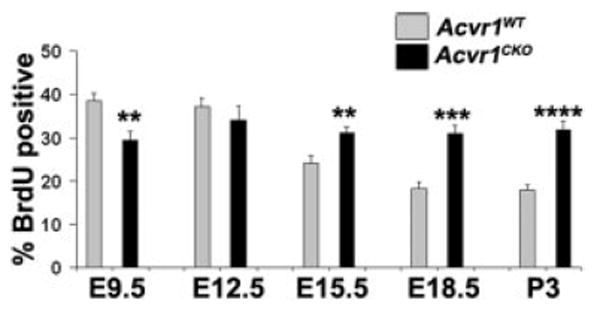
Compared with wild-type, Acvr1CKO cells have reduced proliferation during early lens formation but increased proliferation at later stages. Sections of embryo heads or postnatal eyes were labeled for BrdU, and the percentages of BrdU-labeled nuclei in Acvr1WT and Acvr1CKO lens placodes and epithelia were counted from E9.5 to P3. ** P < 0.01; ***P < 0.001; ****P < 0.0001.
Altered Pattern of Proliferation in Acvr1CKO Lens Epithelial Cells
The normal lens has well-recognized regional differences in the rate of epithelial cell proliferation.13,24,25 Cell proliferation is greatest just anterior to the lens equator, in a region called the germinative zone (GZ; Fig. 3A). Posterior to the germinative zone, in the transition zone (TZ), proliferation slows as epithelial cells begin to withdraw from the cell cycle in preparation for fiber cell terminal differentiation. Proliferation is also slower in epithelial cells anterior to the germinative zone, the central epithelium.
We determined whether differences in the rate of proliferation between Acvr1CKO and Acvr1WT lens epithelial cells localized to a specific region. In the wild-type lens epithelium at E12.5, there was no difference in the BrdU-labeling index between the prospective transition zone (PTZ) and the prospective germinative zone (PGZ). As reported previously, proliferation was slower in the central region at this stage.13 At E15.5 and later, the BrdU-labeling index clearly defined the transition, germinative, and central zones of the wild-type epithelium (Fig. 3B). As in the lens placode, the rate of proliferation in the PTZ and PGZ of the knockout lenses was significantly lower than wild-type lenses at E12.5 (P < 0.01 and P < 0.05 in PTZ and PGZ, respectively; Figs. 3C, 3E). By E15.5, proliferation in the germinative zone and central region of Acvr1CKO lenses was indistinguishable from that of wild-type (Fig. 3C). However, from E15.5 through P3, proliferation in the transition zone remained higher in the Acvr1CKO than in wild-type lenses (Figs. 3G, 3H). Proliferation also tended to be higher in the central epithelium of the knockout lenses after E15.5, effectively expanding the width of the germinative zone into the central epithelium (Fig. 3C). Consistent with these results, Acvr1CKO lenses had a higher percentage of cycling cells (Ki67-positive nuclei) in the transition zone at P3 than did the Acvr1WT lenses (Fig. 3I). Therefore, at E9.5 and E12.5, signaling through Acvr1 promotes cell proliferation in the lens placode and in the presumptive transition and germinative zones. At later stages, Acvr1 is required for lens cells to reduce their rate of proliferation in the transition zone and to withdraw from the cell cycle.
Failure of Acvr1CKO Fiber Cells to Withdraw from Cell Cycle
Lens fiber cells withdrew from the cell cycle early in their differentiation. As shown in Figures 4A and 4B, fiber cells from P3 Acvr1CKO lenses formed and elongated normally. However, some of these fiber cells later became vacuolated (Figs. 4B, 4D, 4F, 4H, 4J). Given that wild-type lens fiber cells are postmitotic, they rarely express Ki67 (Figs. 4C, 5A) and do not incorporate BrdU (Figs. 4E, 5B) or express phospho-Histone H3, a marker of mitotic cells (Fig. 4G). However, some Acvr1CKO fiber cells were positive for Ki67, BrdU, and phospho-Histone H3 (Figs. 4D, 4F, 4H). Some Acvr1CKO fibers failed to express the Cdk inhibitor, p57KIP2, which localizes to the nuclei of all wild-type fiber cells (Figs. 4I, 4J). Acvr1CKO lenses had significantly higher percentages of Ki67- and BrdU-positive and a statistically significant decrease in the percentage of p57KIP2-positive superficial fiber cells than Acvr1WT lenses (Figs. 5A–C). Therefore, Acvr1 signaling contributes to the withdrawal of fiber cells from the cell cycle during their terminal differentiation.
Figure 4.
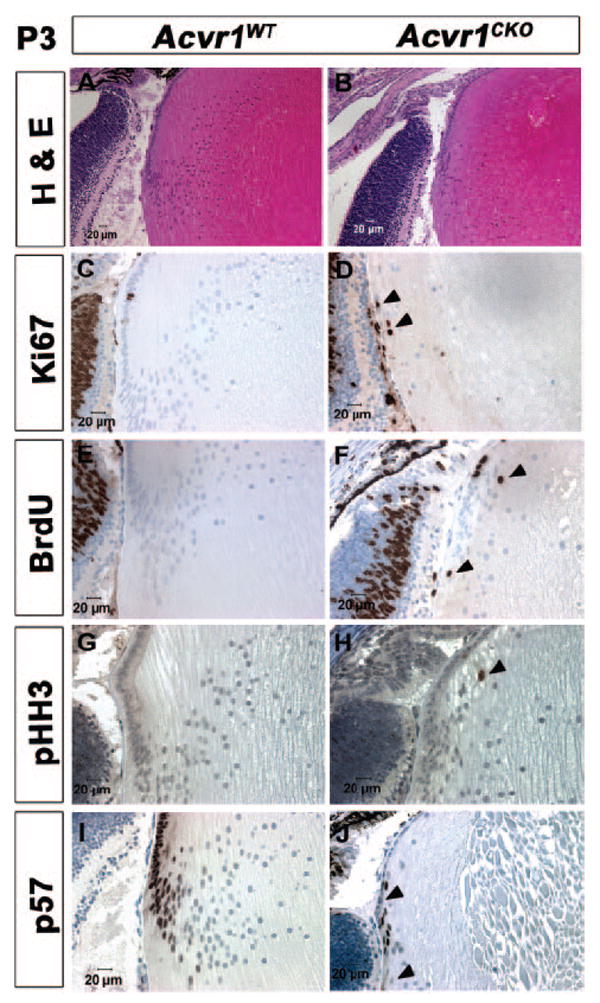
A subset of Acvr1CKO lens fiber cells fails to withdraw from the cell cycle. Acvr1WT (A, C, E, G, I) and Acvr1CKO (B, D, F, H, J) lens sections from P3 animals were stained with hematoxylin and eosin (A, B) or were labeled with antibodies against Ki67 (C, D), BrdU (E, F), phospho-Histone H3 (pHH3) (G, H), or p57KIP2 (I, J). Although Acvr1CKO fibers appeared to form and elongate normally, they developed vacuoles (B, D, F, H, J). Acvr1WT fiber cells seldom expressed Ki67 (C) and did not incorporate BrdU (E) or express pHH3 (G). A subset of Acvr1CKO fiber cells stained for Ki67 (D), incorporated BrdU (F), and expressed pHH3 (H). All the Acvr1WT fiber cell nuclei were stained by antibodies to the Cdk inhibitor p57KIP2 (I), whereas some of the Acvr1CKO fiber cell nuclei were not stained with antibodies to p57KIP2 (J). (D, F, H, arrowheads) Ki67-, BrdU-, and pHH3-positive fiber cells, respectively. (J, arrowheads) Fiber cells that did not express p57.
Figure 5.
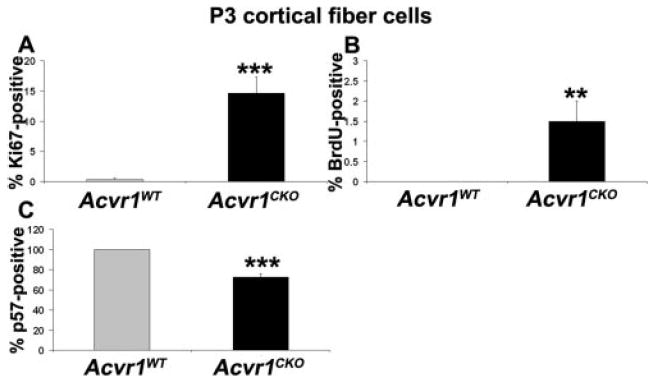
A subset of Acvr1CKO fiber cells fails to exit from the cell cycle. Sections of P3 Acvr1WT and Acvr1CKO eyes were labeled with antibodies against Ki67, BrdU, or p57, and the percentage of labeled nuclei in the cortical fiber cells was counted. There was a significant increase in the Ki67- (A) and BrdU-labeling index (B) and a significant decrease in the p57KIP2-labeling index (C) in Acvr1CKO cortical fiber cells compared with Acvr1WT fiber cells. **P < 0.01; ***P < 0.001.
Cessation of Cell Proliferation by Acvr1 through Canonical Smad Signaling Pathway
When BMPs bind to their receptors, they typically trigger phosphorylation of the receptor-activated Smad proteins (R-Smads), Smad1, Smad5, and Smad8,28 though examples of Smad-independent BMP signaling have been described (for a review, see Moustakas and Heldin29). R-Smads form a complex with the co-Smad Smad4 and are transported to the nucleus to regulate transcription.14 To determine whether Acvr1 decreases cell proliferation and cell cycle exit through the canonical Smad signaling pathway, we analyzed BrdU incorporation in the transition zone and cortical fiber cells in lenses lacking Smad4 or R-Smads (Fig. 6). Similar to Acvr1CKO lenses, P3 lenses lacking Smad4 showed a significant increase in the percentage of BrdU-positive cells in the transition zone compared with the lenses of wild-type littermates (Fig. 6A). Lenses lacking the BMP-specific R-Smads Smad1 and Smad5 also had increased percentages of BrdU-positive cells in the transition zone compared with lenses from wild-type littermates (Figs. 6B, 6C). As in fiber cells lacking Acvr1, some Smad4CKO, Smad1CKO, and Smad5CKO fiber cells incorporated BrdU (Figs. 6E–G). However, unlike the BMP-activated R-Smads, increased BrdU labeling was not seen in the transition zone of lenses lacking the TGFβ-activated Smad Smad2 (Fig. 6D), and no significant increase in BrdU labeling was detected in Smad2CKO fiber cells (Fig. 6H). The lack of any alteration of BrdU incorporation in the Smad2CKO lenses provided independent evidence that the Cre construct used in our studies did not contribute to the phenotypes seen in the Acvr1CKO and Smad1CKO, Smad4CKO, and Smad5CKO lenses.
Figure 6.
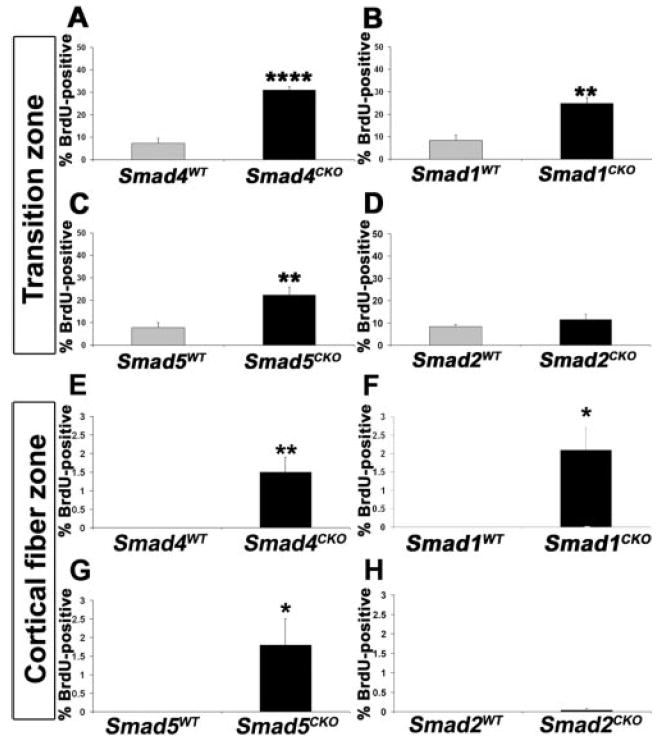
Cessation of cell proliferation in the transition zone is mediated by the canonical BMP-specific Smad pathway. Sections of P3 eyes were labeled with antibody to BrdU, and the labeling indices in the transition zone and the cortical fiber cell zone were determined. A significant increase in the BrdU-labeling index was seen in the transition zone and cortical fiber cells of lenses lacking the Co-Smad Smad4 (A, E) or the BMP-specific R-Smads Smad1 (B, F) and Smad5 (C, G). No abnormal proliferation was seen in the transition zone or cortical fiber cells of lenses lacking the non-BMP R-Smad Smad2 (D, H). *P < 0.05; ** P < 0.01; ****P < 0.0001.
Expression of Fiber Cell-Specific Markers by Acvr1CKO Fiber Cells
To determine whether failure of Acvr1CKO fiber cells to withdraw completely from the cell cycle affects other aspects of their differentiation, we analyzed the expression of c-maf, a transcription factor required for fiber cell differentiation (Figs. 7A, 7B).30-32 Both Acvr1CKO and Acvr1WT lens fiber cells expressed c-maf (Figs. 7A, 7B). This was also true for Acvr1CKO fiber cells that contained nuclei positive for Ki67 (Fig. 7B, arrowhead). Similarly, antibodies to the fiber cell-specific membrane protein MIP (Aquaporin0) stained all the Alk2CKO fiber cells, including those that expressed Ki67 (Fig. 7D, arrowhead). These tests show that failure to execute one aspect of the fiber cell differentiation program, withdrawal from cell cycle, does not prevent other aspects of this process. Thus, many of the morphologic and molecular aspects of fiber cell terminal differentiation can occur in the absence of Acvr1.
Figure 7.
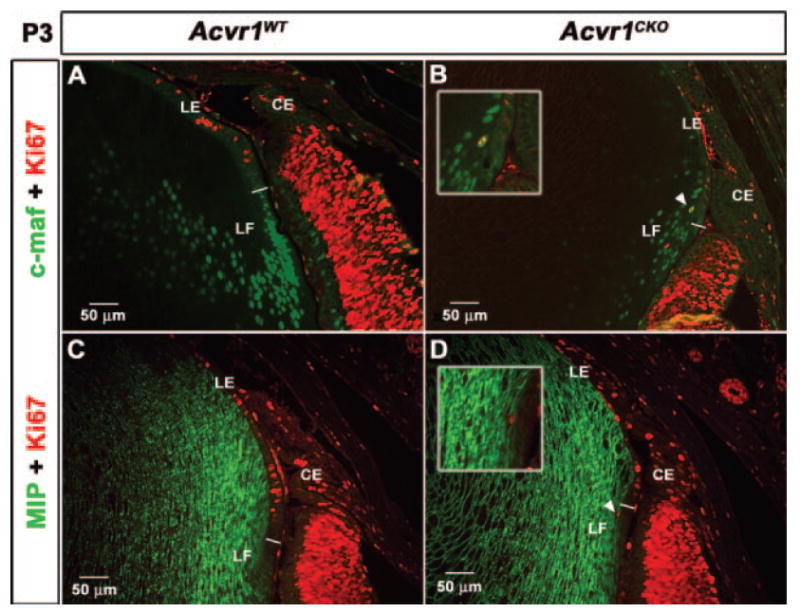
Acvr1CKO fiber cells that fail to withdraw completely from the cell cycle express fiber cell–specific markers Sections of P3 Acvr1WT and Acvr1CKO eyes were labeled with antibodies against markers characteristic of the terminally differentiated fibers c-Maf and MIP (Aquaporin0). Acvr1CKO fibers expressed c-Maf (B) and MIP (D), similar to Acvr1WT fibers (A, C). Some Acvr1CKO fiber cells expressed Ki67 and c-Maf (B, arrowhead, inset) or Ki67 and MIP (D, arrowhead, inset). LE, lens epithelium; LF, lens fiber cell; CE, ciliary epithelium. The white line separates LE from LF.
Increased Cell Death in Acvr1CKO Lenses
Given that loss of Acvr1 resulted in smaller lenses but increased lens cell proliferation, we quantified the apoptosis in lens epithelial and fiber cells. In wild-type lens epithelia at E12.5, substantial cell death was seen in the central epithelium but not in the prospective germinative or transition zone (Fig. 8A). Acvr1CKO lenses also had high levels of cell death in the central region of the epithelium. Unlike wild type, these lenses also had increased levels of cell death in the prospective transition and germinative zones (Fig. 8A). In wild-type lens epithelia at E15.5, the cell death that had been present in the central epithelium at E12.5 was greatly reduced, and the level of cell death in the newly formed transition and germinative zones remained low (Fig. 8B). However, the TUNEL-labeling index of Acvr1CKO central lens epithelial cells was now significantly higher than in wild-type lenses (Fig. 8B). By E18.5 and P3, increased cell death in epithelial cells localized exclusively in the transition zones of Acvr1CKO lenses (Figs. 8C, 8D). More cell death was seen in the fiber cells of Acvr1CKO lenses than in wild-type lenses at all stages from E12.5 to P3 (Fig. 8E). The failure of fiber cells and cells in the transition zone to withdraw from the cell cycle may add to the cell death seen in the Acvr1CKO lenses. Together, these data suggest that increased cell death contributes to the smaller sizes of Acvr1CKO lenses.
Figure 8.
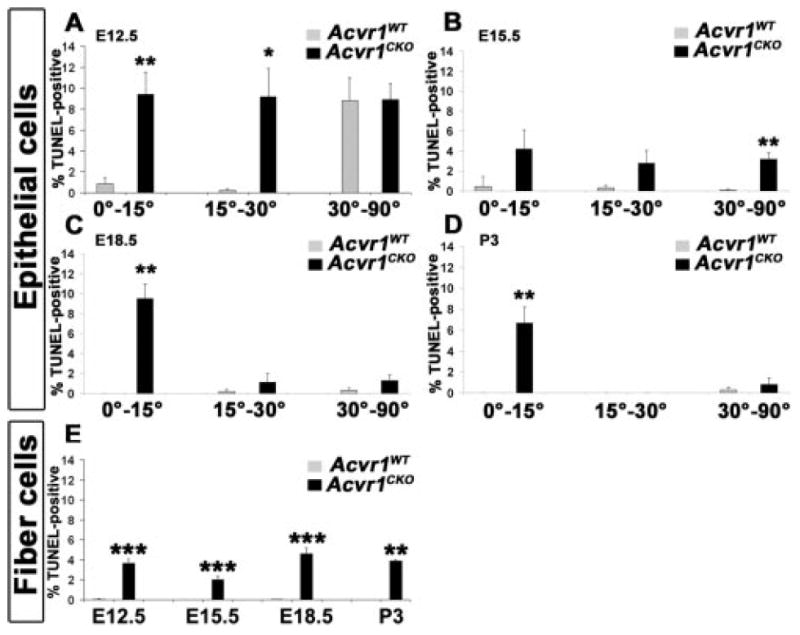
Acvr1CKO lens epithelial and fiber cells show increased cell death The percentage of TUNEL-positive nuclei was counted in the different sectors of epithelia and in fiber cells. (A) At E12.5, there was a significant increase in apoptosis in the Acvr1CKO prospective germinative zone (0°-15°) and the prospective transition zone (15°-30°). (B) At E15.5, apoptosis was higher in Acvr1CKO epithelia, particularly in the central region (30°-90°). At E18.5 (C) and P3 (D), apoptosis was significantly higher in the Acvr1CKO transition zone (0°-15°). (E) Acvr1CKO fibers showed increased cell death at all stages. *P < 0.05; ** P < 0.01; ***P < 0.001.
Discussion
Examination of the phenotype of Acvr1CKO lenses revealed that signals transmitted by this receptor, though not required for lens induction, are important for regulating cell proliferation, terminal differentiation, and survival. These studies also revealed a novel switch in the functions of the Acvr1 receptor with respect to the regulation of cell proliferation during the course of lens development.
Cell Cycle Regulation by Acvr1 during Lens Development
Analysis of proliferation in lens placodes and lenses lacking the Acvr1 receptor demonstrated that signals transmitted by Acvr1 promote proliferation during the lens placode stage. However, later in lens development, signaling through Acvr1 is required to reduce the rate of proliferation of the lens epithelial cells that form the transition zone and to promote the exit of lens fiber cells from the cell cycle. We believe that this ability of a receptor to mediate opposite effects on a cellular process during successive stages of development in a single tissue is a novel finding. It is unclear how Acvr1 promotes lens cell proliferation at early stages and inhibits proliferation later. It seems likely that a change in the downstream signaling cascade accounts for the biphasic effect of Acvr1 receptor on cell proliferation because deletion of Smad4 or Smad1 and Smad5 did not reduce proliferation at the lens placode stage but did increase proliferation later in lens development.
Stage-Specific Differences in Cell Proliferation and Cell Cycle Exit during Lens Development
The present study revealed distinct stages in lens development not noted previously. At E12.5, the transition zone anterior to the lens equator has not yet formed, and there is a high level of cell death in the central epithelium and minimal proliferation in the newly formed fiber cells. From E15.5 onward, the transition zone has formed, and there is little apoptosis in the central epithelium and no proliferation in the fiber cell compartment. These stages correspond to the switch in the function of Acvr1. At E12.5, Acvr1 provides proliferative cues to cells near the periphery of the lens epithelium, in the future transitional and germinative zones. From E15.5 onward, Acvr1 signaling suppresses epithelial cell proliferation, especially in the transition zone and fiber cells, thereby contributing to the regionalization of the lens epithelium and to fiber cell terminal differentiation.
Transduction of BMP or Activin Signals by Acvr1 to Suppress Cell Proliferation
Acvr1 may associate with either BMP or activin type 2 receptors.33 Several types of BMP and activin ligands are produced in the cells of the optic cup surrounding the lens.34 Therefore, one or more BMPs or activins may be responsible for the effects seen in the Acvr1 knockout lenses. TGFβs are well-known inhibitors of cell proliferation in many cell types, and it has been reported that Acvr1 may also mediate TGFβ signaling.35,36 TGFβs and activins signal through complexes of Smads 2/3 and Smad4, and targeted deletion of Acvr1 in the heart leads to reduced phosphorylation of Smad2.37 However, deletion of Smad2 did not increase the number of germinative zone or fiber cells that incorporated BrdU. Lenses overexpressing truncated TGFβ receptors in the fiber cells showed disrupted fiber cell differentiation.38 However, no developmental defects were reported in lenses lacking TGFβRII or Smad3.10,39 Furthermore, we detected no increase in BrdU incorporation in TGFβRIICKO or Smad3 germ line knockout mice (Garcia C, et al., unpublished results, 2006), suggesting that TGFβ signaling is not important for cell cycle withdrawal in lens epithelial cells or during fiber cell differentiation. In contrast, much of the Acvr1-dependent decrease in lens cell proliferation seen in the transition zone and fiber cells of older lenses occurred in a Smad-dependent manner because excessive proliferation in these compartments followed the deletion of Smad4 or the BMP-dependent R-Smads Smad1 and Smad5.
Novel Functions of Acvr1 in the Lens
Signaling by Acvr1 influences several aspects of vertebrate development. These are, most commonly, processes that involve epithelial-to-mesenchymal transformation (EMT), including mesoderm formation,33 generation of primordial germ cells,40 regression of the Müllerian ducts,41 and formation of the endocardial cushions,37 cardiac valves,42 cardiac outflow tract,43 cardiac fibroblasts, and components of the coronary vasculature from proepicardial cells.44 Acvr1 is also involved in the proper specification of the left-right axis in mice45 and in promoting cell proliferation in the neural crest-derived cells of Meckel cartilage.17 As in chondrocytes, Acvr1 signaling promoted lens cell proliferation at earlier stages of lens development. However, the later functions of Acvr1 in suppressing cell proliferation, mediating cell cycle withdrawal during terminal differentiation, and promoting cell survival have not been described in other tissues.
Multiple Processes in Lens Fiber Cell Differentiation
This study demonstrates that Acvr1 signaling contributes to the differentiation of lens fiber cells by promoting their withdrawal from the cell cycle. However, fiber cell terminal differentiation appears to involve parallel processes in which one aspect of differentiation may not depend on the accomplishment of a preceding aspect. Thus, even though withdrawal from the cell cycle normally precedes the expression of fiber cell-specific markers, such as c-maf and MIP, the Acvr1CKO fiber cells that failed to exit from the cell cycle still expressed these markers of terminal differentiation. Similar results have been seen in fiber cells lacking Fgfr211, Rb5, and or the cyclin-dependent kinase inhibitors p27KIP1 (Cdkn1b) and p57KIP2 (Cdkn1c).6 Future studies are required to dissect the intersecting pathways that mediate fiber cell differentiation.
Supplementary Material
Acknowledgments
The authors thank Ruth Ashery-Padan and Judy West-Mays for providing the Le-Cre mice; Chenghua Wu and Mary Feldmeier for their expert technical assistance with genotyping; Belinda McMahan and Jean Jones for their assistance with histology and immunohistochemistry; and Danny Huylebroeck for his support of this work.
Supported by an unrestricted grant from Research to Prevent Blindness and by National Institutes of Health Core Grant EY02687 (Department of Ophthalmology and Visual Sciences) and Grants EY04853 (DCB), 5R01HL074862 (VK), and 5R01DE013085 (VK).
Footnotes
Disclosure: R. Rajagopal, None; L.K. Dattilo, None; V. Kaartinen, None; C.-X. Deng, None; L. Umans, None; A. Zwijsen, None; A.B. Roberts, None; E.P. Bottinger, None; D.C. Beebe, None
References
- 1.Lang RA. Pathways regulating lens induction in the mouse. Int J Dev Biol. 2004;48:783–791. doi: 10.1387/ijdb.041903rl. [DOI] [PubMed] [Google Scholar]
- 2.Solursh M, Langille RM, Wood J, Sampath TK. Osteogenic protein-1 is required for mammalian eye development. Biochem Biophys Res Commun. 1996;218:438–443. doi: 10.1006/bbrc.1996.0078. [DOI] [PubMed] [Google Scholar]
- 3.Saavedra HI, Wu L, de Bruin A, et al. Specificity of E2F1, E2F2, and E2F3 in mediating phenotypes induced by loss of Rb. Cell Growth Differ. 2002;13:215–225. [PubMed] [Google Scholar]
- 4.Liu Y, Zacksenhaus E. E2F1 mediates ectopic proliferation and stage-specific p53-dependent apoptosis but not aberrant differentiation in the ocular lens of Rb-deficient fetuses. Oncogene. 2000;19:6065–6073. doi: 10.1038/sj.onc.1203996. [DOI] [PubMed] [Google Scholar]
- 5.Morgenbesser SD, Williams BO, Jacks T, DePinho RA. p53-Dependent apoptosis produced by Rb-deficiency in the developing mouse lens. Nature. 1994;371:72–74. doi: 10.1038/371072a0. [DOI] [PubMed] [Google Scholar]
- 6.Zhang P, Wong C, DePinho RA, Harper JW, Elledge SJ. Cooperation between the Cdk inhibitors p27(KIP1) and p57(KIP2) in the control of tissue growth and development. Genes Dev. 1998;12:3162–3167. doi: 10.1101/gad.12.20.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furuta Y, Hogan BL. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12:3764–3775. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jena N, Martin-Seisdedos C, McCue P, Croce CM. BMP7 null mutation in mice: developmental defects in skeleton, kidney, and eye. Exp Cell Res. 1997;230:28–37. doi: 10.1006/excr.1996.3411. [DOI] [PubMed] [Google Scholar]
- 9.Wawersik S, Purcell P, Rauchman M, Dudley AT, Robertson EJ, Maas R. BMP7 acts in murine lens placode development. Dev Biol. 1999;207:176–188. doi: 10.1006/dbio.1998.9153. [DOI] [PubMed] [Google Scholar]
- 10.Beebe D, Garcia C, Wang X, et al. Contributions by members of the TGFβ superfamily to lens development. Int J Dev Biol. 2004;48:845–856. doi: 10.1387/ijdb.041869db. [DOI] [PubMed] [Google Scholar]
- 11.Garcia CM, Yu K, Zhao H, et al. Signaling through FGF receptor-2 is required for lens cell survival and for withdrawal from the cell cycle during lens fiber cell differentiation. Dev Dyn. 2005;233:516–527. doi: 10.1002/dvdy.20356. [DOI] [PubMed] [Google Scholar]
- 12.Zhao H, Rossant J, Ornitz DM, Beebe DC, Robinson ML. Different FGFR genes play an essential but redundant role in post-induction lens development. Invest Ophthalmol Vis Sci. 2003;44:244–249. [Google Scholar]
- 13.Faber SC, Dimanlig P, Makarenkova HP, Shirke S, Ko K, Lang RA. Fgf receptor signaling plays a role in lens induction. Development. 2001;128:4425–4438. doi: 10.1242/dev.128.22.4425. [DOI] [PubMed] [Google Scholar]
- 14.Moustakas A, Souchelnytskyi S, Heldin CH. Smad regulation in TGF-beta signal transduction. J Cell Sci. 2001;114:4359–4369. doi: 10.1242/jcs.114.24.4359. [DOI] [PubMed] [Google Scholar]
- 15.Yoshikawa SI, Aota S, Shirayoshi Y, Okazaki K. The ActR-I activin receptor protein is expressed in notochord, lens placode and pituitary primordium cells in the mouse embryo. Mech Dev. 2000;91:439–444. doi: 10.1016/s0925-4773(99)00320-2. [DOI] [PubMed] [Google Scholar]
- 16.Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dudas M, Sridurongrit S, Nagy A, Okazaki K, Kaartinen V. Cranio-facial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mech Dev. 2004;121:173–182. doi: 10.1016/j.mod.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Yang X, Li C, Herrera PL, Deng CX. Generation of Smad4/Dpc4 conditional knockout mice. Genesis. 2002;32:80–81. doi: 10.1002/gene.10029. [DOI] [PubMed] [Google Scholar]
- 19.Huang S, Tang B, Usoskin D, et al. Conditional knockout of the Smad1 gene. Genesis. 2002;32:76–79. doi: 10.1002/gene.10059. [DOI] [PubMed] [Google Scholar]
- 20.Umans L, Vermeire L, Francis A, Chang H, Huylebroeck D, Zwijsen A. Generation of a floxed allele of Smad5 for Cre-mediated conditional knockout in the mouse. Genesis. 2003;37:5–11. doi: 10.1002/gene.10219. [DOI] [PubMed] [Google Scholar]
- 21.Ju W, Ogawa A, Heyer J, et al. Deletion of Smad2 in mouse liver reveals novel functions in hepatocyte growth and differentiation. Mol Cell Biol. 2006;26:654–667. doi: 10.1128/MCB.26.2.654-667.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and Tris (HotSHOT) BioTechniques. 2000;29:52–54. doi: 10.2144/00291bm09. [DOI] [PubMed] [Google Scholar]
- 23.Stratman JL, Barnes WM, Simon TC. Universal PCR genotyping assay that achieves single copy sensitivity with any primer pair. Transgenic Res. 2003;12:521–522. doi: 10.1023/a:1024225408961. [DOI] [PubMed] [Google Scholar]
- 24.McAvoy JW. Cell division, cell elongation and distribution of alpha-, beta- and gamma-crystallins in the rat lens. J Embryol Exp Morphol. 1978;44:149–165. [PubMed] [Google Scholar]
- 25.Mikulicich AG, Young RW. Cell proliferation and displacement in the lens epithelium of young rats injected with tritiated thymidine. Invest Ophthalmol. 1963;2:344–354. [PubMed] [Google Scholar]
- 26.Belecky-Adams TL, Adler R, Beebe DC. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development. 2002;129:3795–3802. doi: 10.1242/dev.129.16.3795. [DOI] [PubMed] [Google Scholar]
- 27.Faber SC, Robinson ML, Makarenkova HP, Lang RA. Bmp signaling is required for development of primary lens fiber cells. Development. 2002;129:3727–3737. doi: 10.1242/dev.129.15.3727. [DOI] [PubMed] [Google Scholar]
- 28.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 29.Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 30.Kim JI, Li T, Ho IC, Grusby MJ, Glimcher LH. Requirement for the c-Maf transcription factor in crystallin gene regulation and lens development. Proc Natl Acad Sci U S A. 1999;96:3781–3785. doi: 10.1073/pnas.96.7.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawauchi S, Takahashi S, Nakajima O, et al. Regulation of lens fiber cell differentiation by transcription factor c-Maf. J Biol Chem. 1999;274:19254–19260. doi: 10.1074/jbc.274.27.19254. [DOI] [PubMed] [Google Scholar]
- 32.Ring BZ, Cordes SP, Overbeek PA, Barsh GS. Regulation of mouse lens fiber cell development and differentiation by the Maf gene. Development. 2000;127:307–317. doi: 10.1242/dev.127.2.307. [DOI] [PubMed] [Google Scholar]
- 33.Gu Z, Reynolds EM, Song J, et al. The type I serine/threonine kinase receptor ActRIA (ALK2) is required for gastrulation of the mouse embryo. Development. 1999;126:2551–2561. doi: 10.1242/dev.126.11.2551. [DOI] [PubMed] [Google Scholar]
- 34.Belecky-Adams TL, Scheurer D, Adler R. Activin family members in the developing chick retina: expression patterns, protein distribution, and in vitro effects. Dev Biol. 1999;210:107–123. doi: 10.1006/dbio.1999.9268. [DOI] [PubMed] [Google Scholar]
- 35.Ward SM, Desgrosellier JS, Zhuang X, Barnett JV, Galper JB. Transforming growth factor beta (TGFβ) signaling via differential activation of activin receptor-like kinases 2 and 5 during cardiac development: role in regulating parasympathetic responsiveness. J Biol Chem. 2002;277:50183–50189. doi: 10.1074/jbc.M209668200. [DOI] [PubMed] [Google Scholar]
- 36.Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127:2021–2036. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Sridurongrit S, Dudas M, et al. Atrioventricular cushion transformation is mediated by ALK2 in the developing mouse heart. Dev Biol. 2005;286:299–310. doi: 10.1016/j.ydbio.2005.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Iongh RU, Lovicu FJ, Overbeek PA, et al. Requirement for TGFβ receptor signaling during terminal lens fiber differentiation. Development. 2001;128:3995–4010. doi: 10.1242/dev.128.20.3995. [DOI] [PubMed] [Google Scholar]
- 39.Saika S, Kono-Saika S, Ohnishi Y, et al. Smad3 signaling is required for epithelial-mesenchymal transition of lens epithelium after injury. Am J Pathol. 2004;164:651–663. doi: 10.1016/S0002-9440(10)63153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Sousa Lopes SM, Roelen BA, Monteiro RM, et al. BMP signaling mediated by ALK2 in the visceral endoderm is necessary for the generation of primordial germ cells in the mouse embryo. Genes Dev. 2004;18:1838–1849. doi: 10.1101/gad.294004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhan Y, Fujino A, MacLaughlin DT, et al. Mullerian inhibiting substance regulates its receptor/SMAD signaling and causes mesenchymal transition of the coelomic epithelial cells early in Mullerian duct regression. Development. 2006;133:2359–2369. doi: 10.1242/dev.02383. [DOI] [PubMed] [Google Scholar]
- 42.Desgrosellier JS, Mundell NA, McDonnell MA, Moses HL, Barnett JV. Activin receptor-like kinase 2 and Smad6 regulate epithelial-mesenchymal transformation during cardiac valve formation. Dev Biol. 2005;280:201–210. doi: 10.1016/j.ydbio.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 43.Kaartinen V, Dudas M, Nagy A, Sridurongrit S, Lu MM, Epstein JA. Cardiac outflow tract defects in mice lacking ALK2 in neural crest cells. Development. 2004;131:3481–3490. doi: 10.1242/dev.01214. [DOI] [PubMed] [Google Scholar]
- 44.Olivey HE, Mundell NA, Austin AF, Barnett JV. Transforming growth factor-beta stimulates epithelial-mesenchymal transformation in the proepicardium. Dev Dyn. 2006;235:50–59. doi: 10.1002/dvdy.20593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kishigami S, Yoshikawa S, Castranio T, Okazaki K, Furuta Y, Mishina Y. BMP signaling through ACVRI is required for left-right patterning in the early mouse embryo. Dev Biol. 2004;276:185–193. doi: 10.1016/j.ydbio.2004.08.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


