Abstract
External guidance cues play a role in controlling neuronal cell turning in the developing brain, but little is known about whether intrinsic programs are also involved in controlling the turning. In this study, we examined whether granule cells undergo autonomous changes in the direction of migration in the microexplant cultures of the early postnatal mouse cerebellum. We found that granule cells exhibit spontaneous and periodical turning without cell-cell contact and in the absence of external guidance cues. The frequency of turning was increased by stimulating the Ca2+ influx and the internal Ca2+ release, or inhibiting the cAMP signaling pathway, while the frequency was reduced by inhibiting the Ca2+ influx. Granule cell turning in vitro was classified into four distinct modes, which were characterized by the morphological changes in the leading process and the trailing process, such as bifurcating, turning, withdrawing, and changing the polarity. The occurrence of the 1st and 2nd modes of turning was differentially affected by altering the Ca2+ and cAMP signaling pathways. Collectively, the results demonstrate that intrinsic programs regulate the autonomous turning of cerebellar granule cells in vitro. Furthermore, the results suggest that extrinsic signals play a role as essential modulators of intrinsic programs.
Keywords: mouse, migration, cerebellum, neuron, intrinsic program, granule cell, microexplant cultures, Ca2+ signaling, cAMP signaling
Introduction
On the way to their final destination, immature neurons often change the direction of their movement (Rakic, 1990; Britanova et al., 2006; Kawauchi et al., 2006; Tanaka et al., 2006; Nakajima, 2007). For example, in the developing cerebellum, postmitotic granule cells first migrate tangentially at the middle of the external granular layer (EGL), and 20–30 hours later, change the direction of migration from tangential to radial at the interface of the EGL and the molecular layer (ML) (Rakic, 1971; Komuro et al., 2001). Subsequently, granule cells migrate radially through the ML and Purkinje cell layer (PCL) to their final destination within the internal granular layer (IGL) (Komuro and Rakic, 1995, 1998a,Komuro and Rakic, b; Komuro et al., 2001). The turning of granule cells at the EGL-ML border is essential for reaching their final destination (Yacubova and Komuro, 2003; Kumada et al., 2007).
The accumulated evidence suggests that contact with the surfaces of neighboring cells and external guidance cues play a role in directing the turning of neurons (Rakic et al., 1994; Wu et al., 1999; Alcantara et al., 2000; Bagri and Tessier-Lavigne, 2002; Marin et al., 2003). For example, it has been reported that stromal-cell derived factor 1α, CXCR4, Netrin1 and Sema6A affect the turning of cerebellar granule cells (Ma et al., 1998; Zou et al., 1998; Lu et al., 2001; Kerjan et al., 2005). Although cell-cell contact and external guidance cues are essential for the selection of migratory paths, the turning of neurons may also depend, at least in part, on an internal clock or intrinsic programs (Trenkner et al., 1984; Yacubova and Komuro, 2002a). This is because cerebellar granule cells in vitro exhibit sequential and stereotypical behaviors without the external guidance molecules (Nakatsuji and Nagata, 1989; Nagata and Nakatsuji, 1990). Furthermore, it has been shown that intrinsic programs play a role in autonomous changes in the rate of granule cell migration over timewithout cell-cell contact (Yacubova and Komuro, 2002a; Komuro and Yacubova, 2003). Based on these previous studies, we hypothesized that intrinsic programs play a role in controlling the turning of cerebellar granule cells. To test this hypothesis, we used the microexplant cultures of the early postnatal mouse cerebellum. In the cultures, granule cells actively migrate without cell-cell contact (Yacubova and Komuro, 2002a). In this study, using the time-lapse imaging of cell movement, we examined whether granule cells exhibit autonomous changes in the direction of migration.
Materials and methods
All procedures were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic Foundation.
Microexplant culture of early postnatal mouse cerebellum
Cerebella of postnatal day (P) 0–3 mice (CD-1, both sexes) were quickly removed from the skull, placed in cold Hanks’ balanced salt solution (Sigma), and freed from meninges and choroid plexus (Komuro and Rakic, 1999; Yacubova and Komuro, 2002a,b; Cameron et al., 2007). Cerebellar slices were then made with a surgical blade from which white matter and deep cerebellar nuclei were removed. Rectangular pieces (50–100 μm) were dissected out from the remaining tissue, which mainly consisted of the cerebellar gray matter, using a surgical blade under a dissecting microscope. Such microexplants were rinsed with the culture medium and placed on 35 mm-glass bottom microwell dishes (1 microexplant/dish, Mat-Tec Corporation) with 50 μl of the culture medium. The culture medium consisted of DMEM/F12 with N2 supplement, 90 U/ml penicillin and 90 μg/ml streptomycin. Each dish was placed in a CO2 incubator (37°C, 95% and 5% CO2). The glass bottom microwell dishes were coated with poly-L-lysine (100 μg/ml)/laminin (20 μg/ml, Sigma) before use. Two hours after plating, 1.5 ml of the culture medium was added to each dish. In this culture more than 95% of migrating cells were granule cells, which were easily distinguished from other neurons by the small size of their cell bodies (Komuro and Rakic, 1996; Yacubova and Komuro, 2002a). Although granule cells were prepared from the EGL and the IGL of all lobules of the cerebellum, the vast majority of granule cells were derived from the EGL, since at the age of P0–P3 the IGL contains only very small numbers of postmigratory granule cells (Miale and Sidman, 1961; Fujita et al., 1966; Fujita, 1967; Altman, 1972). Therefore, the majority of granule cells were at the same developmental stage (Yacubova and Komuro, 2002a). In some experiments, to determine whether granule cell turning depends on the type of adhesive substrates, we used a fibronectin (20 μg/ml, Sigma) as a substitute for laminin. Furthermore, to determine whether the use of different concentrations of laminin results in the alterations of granule cell turning, we used lower (4 μg/ml) and higher (100 μg/ml) concentrations of laminin in separate experiments. Moreover, to examine whether the molecules released from the other cells in the microexplant cultures affect granule cell turning, in some experiments, we replaced the culture medium with the fresh culture medium immediately before the initiation of the observation.
Methods for real-time observation of granule cell turning in vitro
Twenty hours after plating, dishes were transferred into the chamber of a micro-incubator (PDMI-2, Harvard Apparatus)attached to the stage of a confocal microscope (TCS SP, Leica). The migratory behavior of granule cells is closely related to the temperature of the medium; lowering the medium temperature slows cell movement (Rakic and Komuro, 1995). Therefore, the chamber temperature was kept at 37.0 ± 0.5°C using a temperature controller (TC-202, Harvard Apparatus) during the observation of migration. The cells were provided with constant gas flow (95% air, 5% CO2). A laser scanning confocal microscope was used to visualize migrating granule cells in the microexplant cultures (Yacubova and Komuro, 2002a; Kumada et al., 2006). Granule cells were illuminated with a 488-nm wavelength light from an argon laser through an inverted microscope equipped with a 20x oil-immersion objective or a 40x oil-immersion objective, and the light transmitted through granule cells was detected by a photomultiplier (Yacubova and Komuro, 2002a). To protect granule cells from cytotoxic effects of the laser beam, the light level was reduced by 99%. Images of granule cells in a single focal plane were collected with laser scans every 60 seconds for up to 15 hours. The distance traveled by granule cells was defined as the absolute value of the change in its position during the entire time-lapse session.
Examination of granule cell turning in the early postnatal mouse cerebella
Fifty postnatal 10-days-old mice (CD-1, both sexes) were injected with 5 μl of saline, NMDA (0.01 mg/kg body weight), caffeine (2 mg/kg body weight), Rp-cAMPS (0.4 mg/kg body weight), or Br-cGMP (0.4 mg/kg body weight) into the subarachnoid space between the skull and the surface of the cerebellum in separate experiments. Sex hours after injection, all animals were deeply anesthetized with ether and killed by decapitation. Cerebella were quickly removed from the skull and frozen with isopentane precooled to −70°C with dry ice. Then, cerebella were sectioned transversely or sagittally into 90-μm-thick sections on a cryostat. Golgi staining was performed by using an FD Rapid GolgiStain kit (FD NeuroTechnologies) according to the instructions of the manufacturer. After staining, the sections were examined with a bright field light microscope (DM 4000B, Leica), and photographed with x63 oil-immersion objective lens using digital camera (Xli, XL Imaging Ltd.). Images of the segments of Golgi-staining-positive granule cells were obtained at different focal planes in order to have a clear definition of the whole cell morphology. The photomontage of Golgi-staining-positive granule cells was created from multiple images using Photoshop software (Adobe Systems).
In this study, to examine the role of intrinsic programs in controlling granule cell turning in vivo, we determined the number and the mode of granule cell turning at the EGL-ML border with the use of Golgi-staining. Although it is known that the appearance of granule cells using Golgi-staining is sporadic (Ono et al., 1997), we assumed that the frequency of Golgi-stained granule cells with a particular morphological feature among the total Golgi-stained granule cells is reflected the proportion of granule cells with the same morphology in the early postnatal mouse cerebella. We also assumed that the application of NMDA, caffeine, Rp-cAMP and Br-cGMP does not affect the efficiency of the Golgi-staining.
Statistical analysis
Statistical differences were determined using ANOVA. Statistical significance was defined at P <0.05 or P < 0.01.
Results
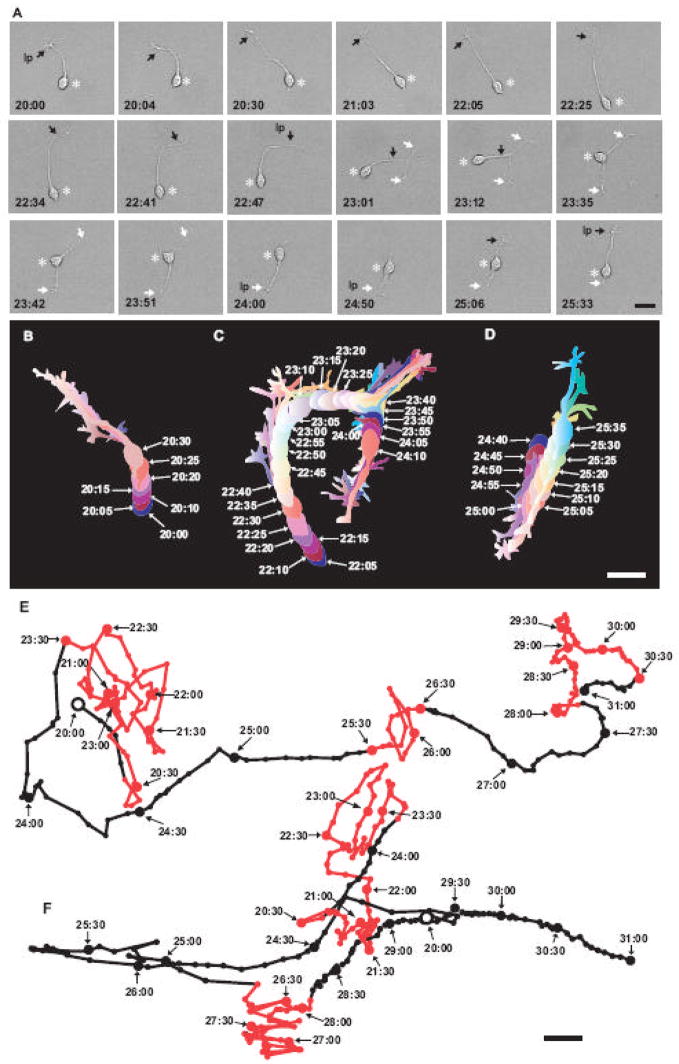
In this series of the experiments, we started to monitor granule cell turning at 20 hours after in vitro for up to 15 hours (until 35 hours after in vitro), when the cells moved at the fastest rate (Yacubova and Komuro, 2002a). We selected granule cells that were located farthest from the cerebellar microexplant and were at least 300 μm away from other cells and neuronal processes at the time of the start of the observation. This selection allowed us to examine the migration of granule cells that had the least contact with other cells prior to the observation. If granule cells contacted with other cells or processes during the observation, the data were excluded from this study. We first examined whether granule cells change the direction of their migration without cell-cell contact. Fig. 1A represents a typical example showing the turning of a granule cell over time. During the period of 20:00–22:05 after in vitro, the tip of the leading process turned to the left side of the photograph, and then the soma moved in the direction of the process extension (Fig. 1A and B). During the period of 22:25–23:12 after in vitro, the tip of the leading process turned to the right side, bifurcated, and then became a T-shape (marked by white arrows in Fig. 1A). Meanwhile, the soma moved towards the junction of two branches of the leading process, and advanced to the junction at 23:35 after in vitro (Fig. 1A and C). During the period of 23:42–23:51 after in vitro, the branch extending towards the bottom of the photograph continuously extended, whereas the branch extending towards the top of the photograph collapsed and retracted (Fig. 1A and C). Thereafter, the soma changed orientation and moved towards the branch extending towards the bottom of the photograph during the period of 24:00–24:50 after in vitro (Fig. 1A and C). During the period of 24:50–25:06 after in vitro, the leading process retracted towards the soma, and then became short and thin, while the trailing process extended and became long and wide (Fig. 1A and C). These morphological changes indicated that the leading process transformed to the trailing process, and the trailing process became the leading process. Upon the completion of reversing the polarity of the cell, the soma started to move in the direction of the extension of the new leading process at 25:10 after in vitro (Fig. 1A and D). These results indicated that granule cells exhibit autonomous changes in the direction of migration without cell-cell contact.
Fig. 1.
Autonomous changes in the direction of granule cell migration in vitro. (A) Time-lapse series of images showing the turning of a granule cell in the microexplant cultures of P2 mouse cerebella over time. Elapsed time after in vitro is indicated on the bottom of each photograph. Black and white arrows indicate granule cell processes. Asterisks represent the granule cell soma. Bar: 15 μm. (B) Turning of the granule cell (shown in A) towards the left side. Seven pseudocolor images of the cell taken every 5 minutes during the period of 20:00–20:30 after in vitro are superimposed. (C) Turning of the granule cell (shown in A) towards the right side and then towards the bottom. Twenty-four pseudocolor images of the cell taken every 5 minutes during the period of 22:10–24:10 after in vitro are superimposed. (D) Reversing the direction of granule cell migration (shown in A). Twelve pseudocolor images of the cell taken every 5 minutes during the period of 24:40–25:35 after in vitro are superimposed. Bar: 12 μm in B–D. (E and F) Line drawings represent the trajectory of the migration of two granule cells in the microexplant cultures of P2–P3 mouse cerebella over time. The migration of these cells was observed in separate experiments. Black open circles represent the position of each granule cell at the beginning of observation (at 20:00 after in vitro). The interval between large filled circles (black and red) represents 30 minutes. The interval between small filled circles (black and red) represents 3 minutes. Red circles and red lines represent the frequent turning periods, while black circles and black lines represent the less frequent turning periods. The numbers in the figure represent the elapsed time after in vitro. Bar: 15 μm in E and F.
How often do granule cells exhibit the turning? Do granule cells turn randomly or regularly? To address these questions, we examined the occurrence, rhythmicity and periodicity of the autonomous turning of granule cells. Analyzing the trajectory of cell movement revealed that granule cells repeatedly undergo the frequent and less frequent turning periods over time. The frequent turning periods were defined as the periods of when granule cells exhibited more than three turnings within consecutive 60 minutes whereas the less frequent turning periods were defined as the periods of when the cells exhibited less than three turnings within consecutive 60 minutes. Fig. 1E and F represents typical examples of the periodical turnings of granule cells over time. For example, a granule cell shown in Fig. 1E exhibited the frequent turning periods (indicated by red circles and red lines) three times and the less frequent turning periods (indicated by black circles and black lines) twice during 11 hours of observation. Furthermore, a granule cell shown in Fig. 1F exhibited the frequent turning periods twice and the less frequent turning periods three times during 11 hours of observation. The average cycle of the frequent and less frequent turning periods was 4.0±0.8 hours (n=75).
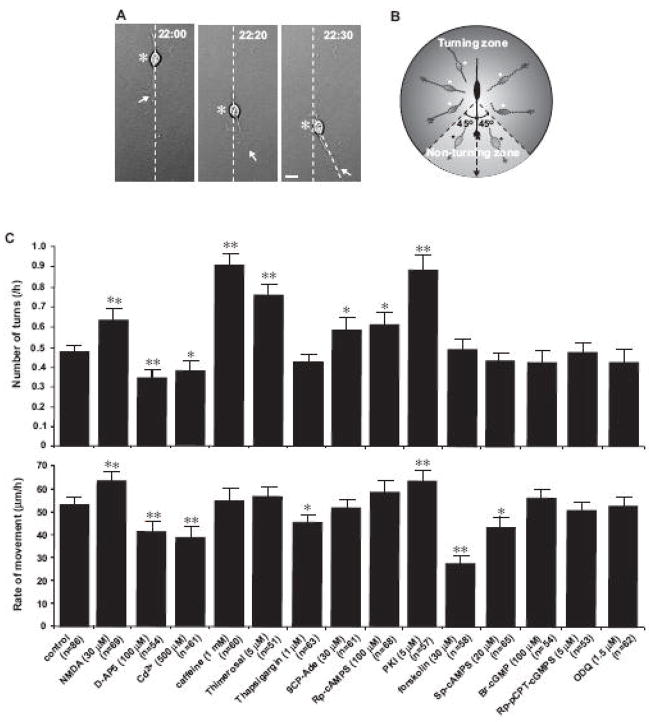
One can assume that the occurrence and periodicity of granule cell turning depend on the type and the concentrations of adhesive substrates used. To answer this assumption, we used the fibronectin (20 μg/ml)/poly-L-lysine (100 μg/ml), the low laminin (4 μg/ml)/poly-L-lysine (100 μg/ml), and the high laminin (100 μg/ml)/poly-L-lysine (100 μg/ml) as substrates for granule cell migration, in separate experiments. Analysis of the periodicity of autonomous turning revealed that there is no significant difference in the average cycles of the frequent and less frequent turning periods among each group. For example, the average cycles of the frequent and less frequent turning periods were 3.8±0.8 hours (n=63) in the fibronectin/poly-L-lysine group, 3.9±0.8 hours (n=69) in the low laminin/poly-L-lysine group, and 4.1±0.9 hours (n=72) in the high laminin/poly-L-lysine group, respectively. Furthermore, we examined the average numbers of autonomous turning during 10 hours of observation in each group. In this series of experiments, granule cells were considered to turn if the direction of their migration deviates more than 45° from previous movement of their cell body within consecutive 30 minutes (Fig. 2A and B). The average numbers of turning during 10 hours of observation were 4.8±0.4 turns (n=63) in the fibronectin/poly-L-lysine group, 4.7±0.5 turns (n=69) in the low laminin/poly-L-lysine group, and 4.9±0.4 turns (n=72) in the high laminin/poly-L-lysine group, respectively. There was no statistical significance in the average numbers of autonomous turning during 10 hours of observation among each group. These results indicated that the occurrence and periodicity of granule cell turning are independent of the type and the concentrations of adhesive substrates.
Fig. 2.
Modulation of the frequency of turning and the rate of migration of granule cells in vitro by altering the Ca2+ and cAMP signaling pathways. (A) Measurement of granule cell turning. In each photograph arrows and asterisks indicate the leading process and the soma of a granule cell, respectively. The numbers at the top of each photograph represent the elapsed time after in vitro. Bar: 10 μm.(B) Schematic representation showing how to identify and count the turning of granule cell. (C) Histograms showing the effects of the alterations of the Ca2+ and cyclic nucleotide signaling pathways on the frequency of turning (top histogram) and the rate of migration (bottom histogram) of granule cells. Each column represents the average frequency of turning and the average rate of migration obtained during a 10 hour-period of observation between 20–30 hours after in vitro. Each reagent was added to the culture medium at 20 hour after in vitro. Single (p <0.05) and double (p <0.01) asterisks indicate statistical significance. Bar: S.D.
Do soluble guidance (attractive or repulsive) molecules, which are potentially released from other cells, affect the occurrence and periodicity of granule cell turning? To test this question, we replaced the culture medium with the fresh culture medium immediately before the start of the observation. The average cycle of the frequent and less frequent turning periods in the control group (no replacement of the medium) was 4.0±0.7 hours (n=68), whereas the average cycle in the replacement group was 4.1±0.9 hours (n=59). Furthermore, the average numbers of turning during 10 hours of observation were 4.9±0.4 turns (n=68) in the control group (no replacement of the medium), and 4.7±0.5 turns (n=59) in the replacement group. There was no statistical significance in the average cycles of the frequent and less frequent turning periods and the average numbers of turning during 10 hours of observation among each group. These results indicated that granule cell turning examined in this study is not influenced by soluble guidance (attractive or repulsive) molecules released from other cells. Collectively, these results suggest that the occurrence and periodicity of granule cell turning are controlled by intrinsic (inherent) programs.
How do intrinsic programs control the turning of granule cells? Our working hypothesis is that intrinsic programs regulate granule cell turning by altering the Ca2+ and cyclic nucleotide (such as cAMP and cGMP) signaling pathways. This is because the migratory behavior of granule cells is highly sensitive to the changes in these signaling pathways (Komuro and Rakic, 1992, 1993, 1996, 1998b; Yacubova and Komuro, 2002b; Kumada and Komuro, 2004; Komuro and Kumada, 2005; Kumada et al., 2006, 2007; Botia et al., 2007; Cameron et al., 2007). Therefore, we examined whether the alterations of the Ca2+ and cyclic nucleotide signaling pathways affect the autonomous turning of granule cells. First, stimulating the Ca2+ influx through the NMDA type-glutamate receptors with NMDA (30 μM) significantly increased the frequency of turning, while inhibiting the Ca2+ influx through the NMDA receptors with D-AP5 (a NMDA receptor antagonist, 100 μM) significantly decreased the frequency of turning (Fig. 2C). Second, inhibiting the Ca2+ influx with CdCl2 (a voltage-gated Ca2+ channel inhibitor, 500 μM) significantly decreased the frequency of turning (Fig. 2C). Third, stimulating the internal Ca2+ release through the ryanodine receptor with caffeine (1 mM) or the IP3 receptor with thimerosal (5 μM) significantly increased the frequency of turning (Fig. 2C). Fourth, inhibiting the production of cAMP with 9CP-Ade (an adenylyl cyclase inhibitor, 30 μM)significantly increased the frequency of turning (Fig. 2C). Fifth, inhibiting the cAMP signaling with Rp-cAMPS (a competitive cAMP antagonist, 100 μM) or inhibiting the activity of protein kinase A (PKA) with PKI (5 μM) significantly increased the frequency of turning (Fig. 2C). Finally, stimulating the cGMP signaling with Br-cGMP (a cGMP analogue, 100 μM), or inhibiting the cGMP signaling with Rp-8-pCPT-cGMPS (a cGMP antagonist, 5 μM) and ODQ (a guanylyl cyclase inhibitor, 1.5 μM) did not alter the frequency of turning (Fig. 2C). Collectively, these results indicated that the frequency of granule cell turning is increased by stimulating the Ca2+ influx and the internal Ca2+ release or inhibiting the cAMP signaling pathways, while the frequency is reduced by inhibiting the Ca2+ influx. These results also suggest that intrinsic programs regulate the frequency of autonomous turning of granule cells by altering the activity of the Ca2+ and cAMP signaling pathways.
Next, we examined the relationship between the frequency of autonomous turning of granule cells and the speed of migration. Based on the response to the alterations of the Ca2+ and cAMP signaling pathways, the relationships between the frequency of turning and the speed of migration were categorized into three groups. The 1st group was that the speed of migration and the frequency of turning changed in the same direction. For example, increasing the Ca2+ influx with NMDA or inhibiting the activity of PKA with PKI increased both the frequency of turning and the speed of migration, while inhibiting the Ca2+ influx with D-AP5 and CdCl2 reduced both the turning and the speed (Fig. 2C). The 2nd group was that the frequency of turning altered, but the speed of migration did not change. For example, stimulating the internal Ca2+ release with caffeine and thimerosal, inhibiting the adenylyl cyclase with 9CP-Ade, or inhibiting the cAMP signaling with Rp-cAMPS increased the frequency of turning, but did not change the speed of migration (Fig. 2C). The 3rd group was that the speed of migration altered, but the frequency of turning did not change. For example, inhibiting the internal Ca2+ release with thapsigargin (1 μM), stimulating the adenylyl cyclase with forskolin (30 μM), or stimulating the cAMP signaling with Sp-cAMPS (20 μM) reduced the speed of migration, but did not change the frequency of turning (Fig. 2C). Collectively, these results indicated that the cellular mechanisms of controlling the frequency of autonomous turning of granule cells partially share those of controlling the speed of migration. These results also suggest that intrinsic programs control the frequency of granule cell turning in cell motility-dependent and -independent manners.
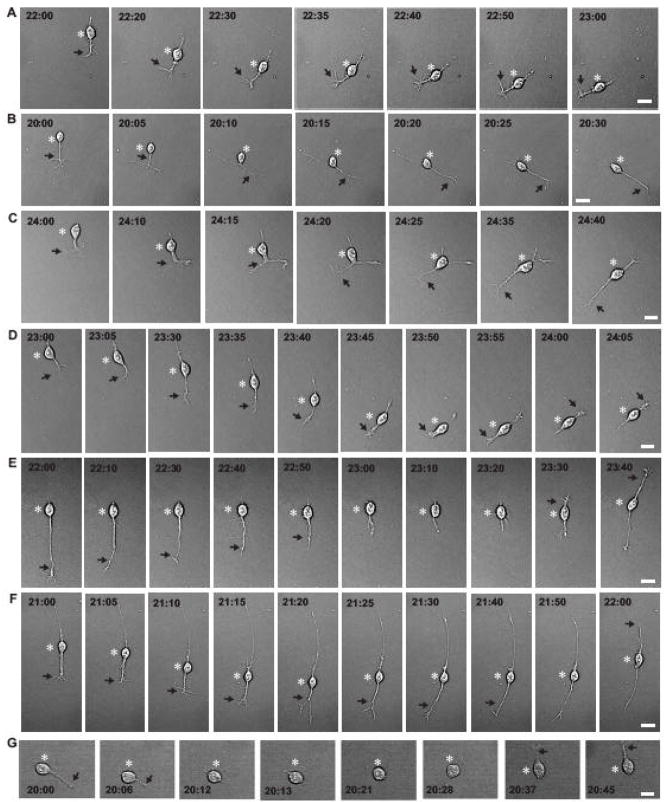
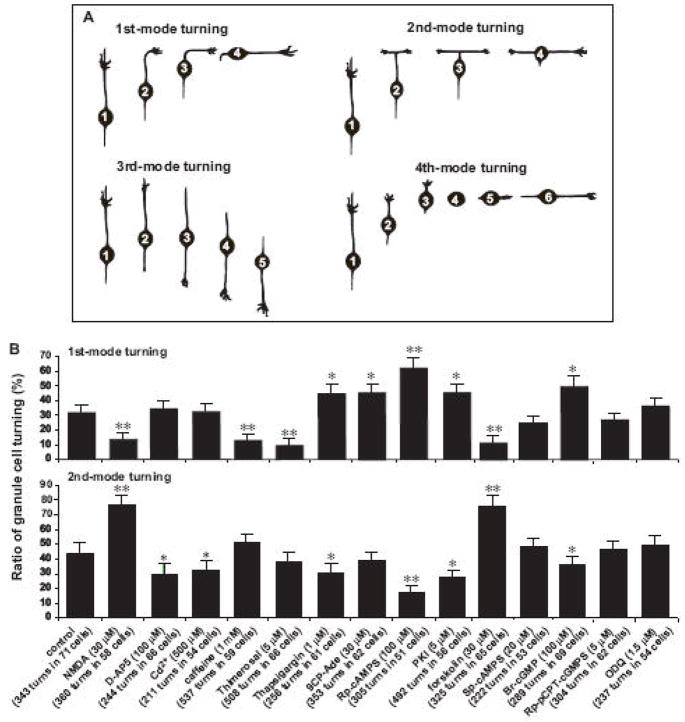
To further determine the features of autonomous turning of granule cells, we examined the modes of turning. The time-lapse observation of cell movement revealed that granule cells exhibit four distinct modes of turning in vitro. The 1st mode of turning was characterized by the turning of the tip of the leading process to a new direction, which was followed by the soma (Fig. 3A). The 2nd mode was initiated by the bifurcation of the tip of the leading process. Both branches extended in the opposite direction, and then one of the branches collapsed and retracted. The cell body followed the direction of extension of the remaining branch (Fig. 3B and C). The 3rd mode was a reversal of the cell polarity: the leading process transformed to the trailing process, the trailing process became the leading process, and then cells started to migrate in the direction of extension of the new leading process (Fig. 3D, E and F). The 4th mode was initiated by withdrawing the leading process, which was followed by extending the new leading process towards the direction of upcoming movement (Fig. 3G). Each mode of granule cell turning was schematically presented in the Fig.4A. Individual granule cells exhibited multiple modes of turning over time, but there was preference in the occurrence of each mode of turning. For example, during the period of 20–35 hours after in vitro, granule cells most frequently exhibited the 2nd mode of turning. The occurrence of each mode of turning decreased in the following order: the 2nd mode (44.1±6.7 %) > the 1st mode (31.8±6.0 %) > the 4th mode (16.3±3.9 %) > the 3rd mode (7.8±3.0 %). These results indicated that granule cells preferentially exhibit the 2nd mode and the 1st mode of turning, suggesting that intrinsic programs tend to induce granule cell turning by bifurcating or turning the tip of leading processes.
Fig. 3.
Four distinct modes of granule cell turning in vitro. (A)Sequential images of a granule cell represent the 1st mode of turning. The tip of the leading process turned to the left side and then, the cell body followed the changes. (B, C) Sequential images of granule cells represent the 2nd mode of turning. The tip of the leading process bifurcated, and both branches extended in the opposite direction. Subsequently, one of the branches collapsed and retracted, and the cell body followed the direction of extension of the remaining branch. (D) Sequential images of a granule cell represent the 1st and 3rd modes of turning. The tip of the leading process of the granule cell turned to the left side and then, the cell body followed the changes. Thereafter, the growth cone of the tip of the leading process collapsed. The trailing process then started to develop a new growth cone at the tip and converted into a new leading process. Sequentially, the soma started to migrate in the direction of the extension of the new leading process. (E and F)Sequential images of granule cells represent the 3rd mode of turning. The leading process of the granule cells transformed to the trailing process and the trailing process became the leading process. Thereafter, the cells started to migrate in the reversed direction. (G) Sequential images of a granule cell represent the 4th mode of turning. The granule cell completely withdrew the leading process, and extended a new leading process. Subsequently, the cell migrated toward the direction of the extension of the new leading process. Bar: 10 μm in A, D, E, F, 12 μm in B, and 8 μm in C, G. In A–G, asterisks and arrows indicate the granule cell somata and the leading processes, respectively. The numbers in each photograph represent the elapsed time after in vitro.
Fig. 4.
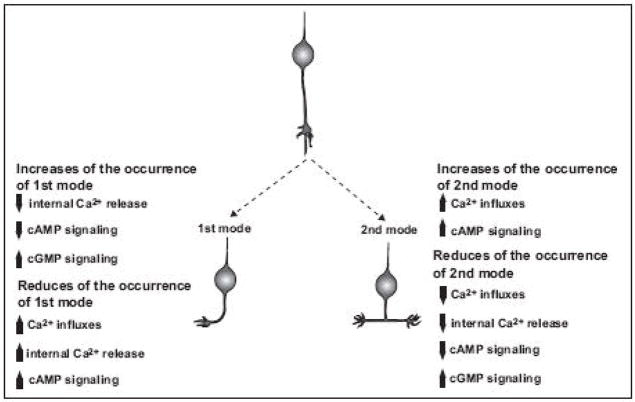
Changes in the ratio of the 1st and 2nd modes of granule cell turning in vitro by altering the Ca2+ and cyclic nucleotide signaling pathways. (A) Schematic representation of four distinct modes of granule cell turning in vitro. The numbers in granule cell somata indicate the order in each mode of turning. (B) Histograms showing the effects of the alterations of the Ca2+ and cyclic nucleotide signaling pathways on the ratio of the 1st and 2nd modes of granule cell turning in vitro. Each column represents the ratio in the occurrences of the 1st and 2nd modes of granule cell turning during a 10 hour-period of observation between 20–30 hours after in vitro. Each reagent was added to the culture medium at 20 hour after in vitro. Single (p <0.05) and double (p <0.01) asterisks indicate statistical significance. Bar: S.D.
Next, we examined the cellular mechanisms underlying the mode of granule cell turning. We hypothesized that the occurrence of each mode of granule cell turning is differentially controlled by the activity of the Ca2+ and cyclic nucleotide signaling pathways. To test this hypothesis, we focused on the 1st and 2nd modes of turning because the low rates of the occurrence of the 3rd and 4th modes of turning prevented us from analyzing the effects of the alterations of the Ca2+ and cyclic nucleotide signaling pathways. So, we examined whether the alterations of the Ca2+ and cyclic nucleotide signaling pathways affect the occurrence of the 1st and 2nd modes of granule cell turning. First, stimulating the Ca2+ influxes with NMDA significantly decreased the occurrence of the 1st mode, but significantly increased the occurrence of the 2nd mode (Fig. 4B). Second, inhibiting the Ca2+ influxes with D-AP5 and CdCl2 significantly reduced the occurrence of the 2 mode (Fig. 4B). Third, stimulating the internal Ca2+ release with caffeine and thimerosal significantly decreased the occurrence of the 1st mode (Fig. 4B). Fourth, inhibiting the internal Ca2+ release with thapsigargin significantly increased the occurrence of the 1st mode, but significantly reduced the occurrence of the 2nd mode (Fig. 4B). Fifth, inhibiting the cAMP signaling with Rp-cAMPS, or inhibiting the PKA with PKI significantly increased the occurrence of the 1st mode of turning, but significantly decreased the occurrence of the 2nd mode (Fig. 4B). Sixth, stimulating the adenylyl cyclase with forskolin significantly decreased the occurrence of the 1st mode, but significantly increased the occurrence of the 2nd mode (Fig. 4). Seventh, inhibiting the adenylyl cyclase with 9CP-Ade significantly increased the occurrence of the 1st mode (Fig. 4B). Finally, stimulating the cGMP signaling with Br-cGMP significantly increased the occurrence of the 1st mode, but significantly decreased the occurrence of the 2nd mode (Fig. 4B). Collectively, these results indicated that stimulating the Ca2+ influxes and the internal Ca2+ release reduces the occurrence of the 1st mode of granule cell turning but increases the occurrence of the 2nd mode, while inhibiting the Ca2+ influxes and the internal Ca2+ release increases the occurrence of the 1st mode but reduces the occurrence of the 2nd mode. These results also indicated that inhibiting the cAMP signaling pathways or stimulating the cGMP signaling pathways increases the occurrence of the 1st mode of turning but decreases the occurrence of the 2nd mode, while stimulating the cAMP signaling pathways reduces the occurrence of the 1st mode but increases the occurrence of the 2nd mode. The effects of the alterations of the Ca2+ and cyclic nucleotide signaling pathways on the 1st and 2nd modes of granule cell turning were summarized in Fig. 5.
Fig. 5.
The summary of the effects of altering the Ca2+ and cyclic nucleotide signaling pathways on the 1st and 2nd modes of granule cell turning in vitro.
The present results indicated that the 1st and 2nd mode of granule cell turning is controlled by balancing the activity of the Ca2+ and cyclic nucleotide signaling pathways. The present results also indicated that the Ca2+ and cyclic nucleotide signaling pathways reciprocally control the occurrence of the 1st and the 2nd modes of turning. Taken together, the present results suggest that intrinsic programs control the occurrence of the 1st and 2nd modes of autonomous turning of granule cells in vitro by altering the Ca2+ and cyclic nucleotide signaling pathways.
Our in vitro studies indicated that intrinsic programs control the autonomous turning of granule cells. The next question was whether intrinsic programs also play a role in directing the turning of granule cells in vivo. As schematically presented in Fig. 6A, after final mitosis, granule cells start to migrate tangentially at the middle of the EGL (Komuro et al., 2001; Komuro and Yacubova, 2003; Yacubova and Komuro, 2003). Subsequently, granule cells changed the direction of migration from tangential to radial at the EGL-ML border, and enter the ML (Komuro et al., 2001). In the ML, granule cells migrate radially along the Bergmann glial processes (Rakic, 1971, Komuro and Rakic, 1995). Upon entering the PCL, granule cells detach from the glial processes and became stationary for a couple of hours (Komuro and Rakic, 1998). Thereafter, granule cells resume their radial migration and cross the PCL-IGL border. In the IGL, the majority of granule cells migrate radially towards the bottom of the IGL until attaining their final destination (Komuro and Rakic, 1998). On the way to their final destination, all granule cells exhibit the right angle turn at the EGL-ML border of the developing cerebellum (Rakic, 1971; Ono et al., 1997; Komuro et al., 2001). To test the role of intrinsic programs in controlling granule cell turning in vivo, we first determined the morphology of migrating granule cells and the mode of granule cell turning at the EGL-ML border in the P10 mouse cerebella. The use of Golgi-staining revealed the alterations of granule cell morphology during their migration and differentiation (Fig. 6B). For example, at the top of the EGL, granule cell precursors had a polygonal cell body with a couple of short processes (a–j of Fig. 6B). At the middle and bottom of the EGL, tangentially migrating granule cells had a horizontally-oriented soma with two horizontally-extending processes (a leading process and a trailing process) (k–z of Fig. 6B). In the ML, the PCL and the IGL, radially migrating granule cells had a vertically-oriented soma with a voluminous leading process and a thin trailing process (a1–e1, g1–j1, l1–q1of Fig. 6B). After completing their migration within the IGL, differentiating granule cells extended several short processes (future dendrites) from the somata (k1 and r1–y1 of Fig. 6B).
Fig. 6.
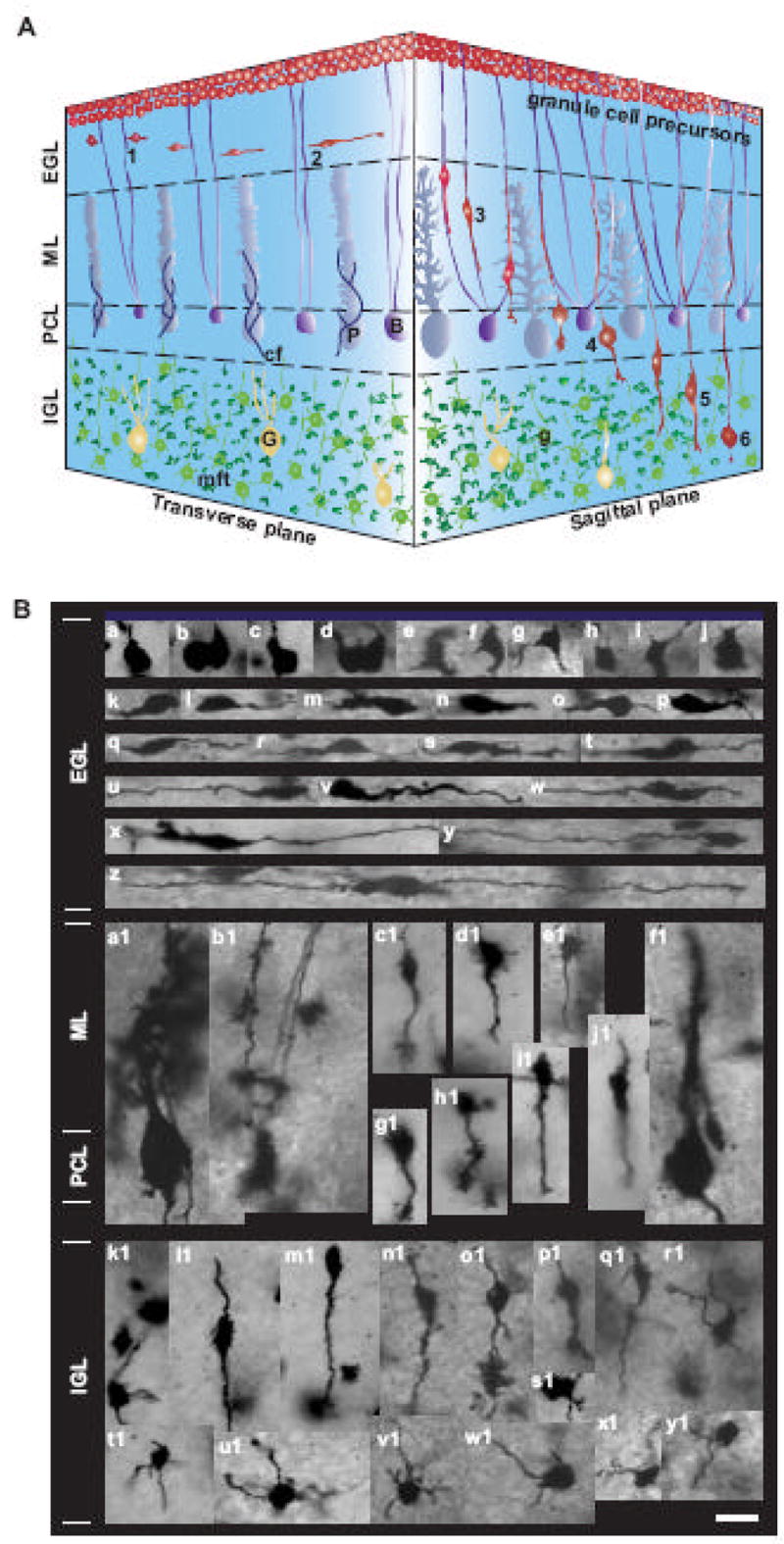
Migration and differentiation of cerebellar granule cells in vivo. (A) The three-dimensional representation of granule cell migration from the EGL to the IGL in the developing cerebellum. 1, Extension of two uneven horizontal processes near the top of the EGL; 2, Tangential migration in the middle and bottom of the EGL; 3, Bergmann glia-associated radial migration in the ML; 4, Stationary state in the PCL; 5, Glia-independent radial migration in the IGL; 6, Completion of migration in the middle or the bottom of the IGL. Abbreviations: P, Purkinje cell; B, Bergmann glia; G, Golgi cell, g, postmigratory granule cell; cf, climbing fiber; mft, mossy fiber terminal. (B) Micrographs showing the changes in the morphology of granule cells in the P10 mouse cerebellum revealed by Golgi-staining. a–j, granule cell precursors at the top of the EGL. k–z, tangentially migrating granule cells at the middle and bottom of the EGL. a1 and f1, Purkinje cells. b1, Bergmann glial cells. c1–e1 and g1–j1, radially migrating granule cells in the ML. k1 and r1–y1, postmigratory granule cells in the IGL. l1–q1, radially migrating granule cells in the IGL. Bar: 10 μm in a-y1.
The morphological analysis revealed that granule cell turning at the EGL-ML border is classified into three distinct modes (Fig. 7). Based on the morphological features, we called them T-, L-, Y-shape turning of granule cells. T-shape turning was characterized by the extension of vertical processes into the ML from the soma of tangentially-oriented granule cells, which had two horizontally-extended axon-like processes (a1–a25 in Fig. 7). We assumed that the cell body follows the direction of extension of newly-developed vertical processes. L-shape turning was characterized by the turning of the tip of horizontally-extended leading processes towards the ML (b1–b37 in Fig. 7). We assumed that the soma follows the direction of the turning of the leading process, and enters the ML. Y-shape turning characterized by the bifurcation of the tip of the horizontally-oriented leading process (c1–c25 in Fig. 7). The one of the branches extended horizontally, while the other extended vertically into the ML. We assumed that the soma follows the direction of extension of the vertical process. There was preference in the occurrence of each mode of turning. For example, granule cells most frequently exhibited the L-shape turning at the EGL-ML border of the P10 mouse cerebellum. The occurrence of each mode of turning decreased in the following order: L-shape turning (41.8±4.5 %) > T-shape turning (30.6±3.5 %) > Y-shape turning mode (27.6±2.9 %).
Fig. 7.
Micrographs showing three different modes of granule cell turning at the EGL-ML border of the P10 mouse cerebellum. (A) T-shape tuning of granule cells. a1–a25, Micrographs showing the extension of the vertical processes into the ML from the cell body of tangentially-oriented granule cells, which had two horizontally-extended axon-like processes. (B) L-shape turning of granule cells. b1–b34, Micrographs showing the turning of the tip of the horizontally-oriented leading process towards the ML. (C) Y-shape turning of granule cells. c1–c25, Micrographs showing the bifurcation of the tip of the horizontally-oriented leading process. Bar: 15 μm in a1-c25.
Next, to examine the role of intrinsic programs in controlling granule cell turning in vivo, we examined whether the alterations of the Ca2+ and cyclic nucleotide signaling pathways affects the number of granule cell turning at the EGL-ML border of the P10 mouse cerebellum. This is because the alteration of these signaling pathways significantly affects the numbers of the autonomous turning of granule cells in vitro (Fig. 2C). To this end, in separate experiments, we injected with 5 μl of saline (as a control), caffeine (2 mg/kg body weight), NMDA (0.01 mg/kg body weight), Rp-cAMPS (0.4 mg/kg body weight), or Br-cGMP (0.4 mg/kg body weight)into the subarachnoid space between the skull and the surface of the P10 mouse cerebellum. Six hours after injection, the cerebella were removed. With the use of Golgi-staining, we determined the number of granule cells, which exhibited the characteristic feature of turning towards ML at the EGL-ML border. We found that the injection of NMDA, caffeine, or Rp-cAMPS significantly increases the average number of turning granule cells towards the ML at the EGL-ML border, while the injection of Br-cGMP fails to alter the average number of turning granule cells (Fig. 8). These results indicated that stimulating the Ca2+ influx through the NMDA type-glutamate receptors, stimulating the internal Ca2+ release through the ryanodine receptor, or inhibiting the cAMP signaling results in an accelerated (or premature) turning of tangentially-migrating granule cells at the EGL-ML border of the cerebellum. Because the frequency of autonomous turning of granule cells in vitro was significantly increased by the application of NMDA, caffeine or Rp-cAMPS but not by the application of Br-cGMP (Fig. 2C), these results suggest that intrinsic programs participate in controlling the timing of granule cell turning towards the ML at the EGL-ML border of the cerebellum.
Fig. 8.
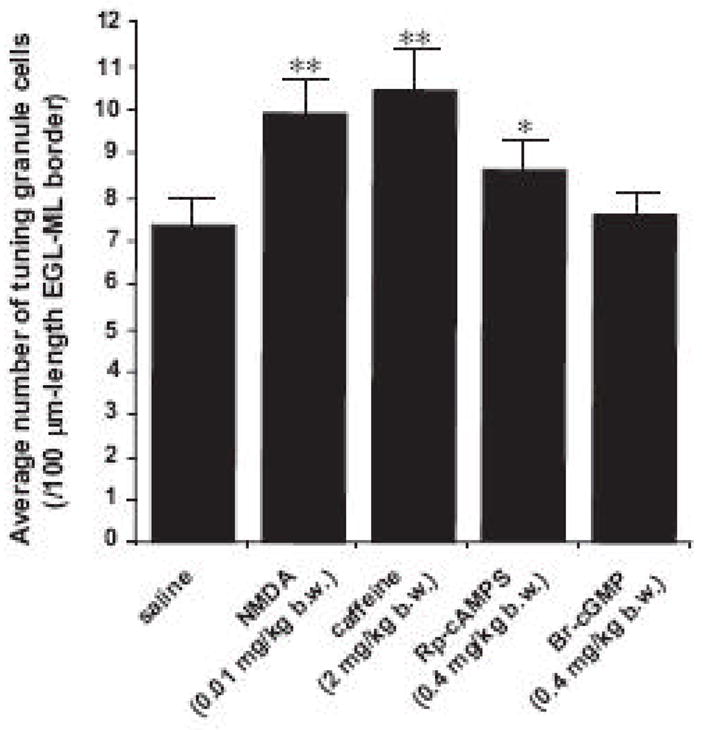
Changes in the average number of turning granule cells at the EGL-ML border of the P10 mouse cerebella by the injection of saline, NMDA, caffeine, Rp-cAMP, or Br-cGMP. Each column represents the average number of turning granule cells at the 100 μm-length EGL-ML border obtained from at least 500 cells. Single (p <0.05) and double (p <0.01) asterisks indicate statistical significance. Bar: S.D.
To further examine the role of intrinsic programs in directing granule cell turning in vivo, we examined whether the mode of granule cell turning in vivo is affected by the alterations of the Ca2+ and cyclic nucleotide signaling pathways. First, the application of NMDA or caffeine significantly increased the occurrence of the T- and Y-shape turning of granule cells at the EGL-ML border, but significantly decreased the occurrence of the L-shape turning (Fig. 9A and B). In contrast, the application of Rp-cAMPS significantly increased the occurrence of the L-shape turning of granule cells, but significantly decreased the occurrence of the T- and Y-shape turning (Fig. 9A and B). Furthermore, the application of Br-cGMP significantly increased the occurrence of the L-shape turning of granule cells, but significantly decreased the occurrence of the Y-shape turning (Fig. 9A and B). Collectively, theses results demonstrate that the mode of granule cell turning at the EGL-ML border of the P10 mouse cerebella depends on the activity of the Ca2+ and cyclic nucleotide signaling pathways.
Fig. 9.
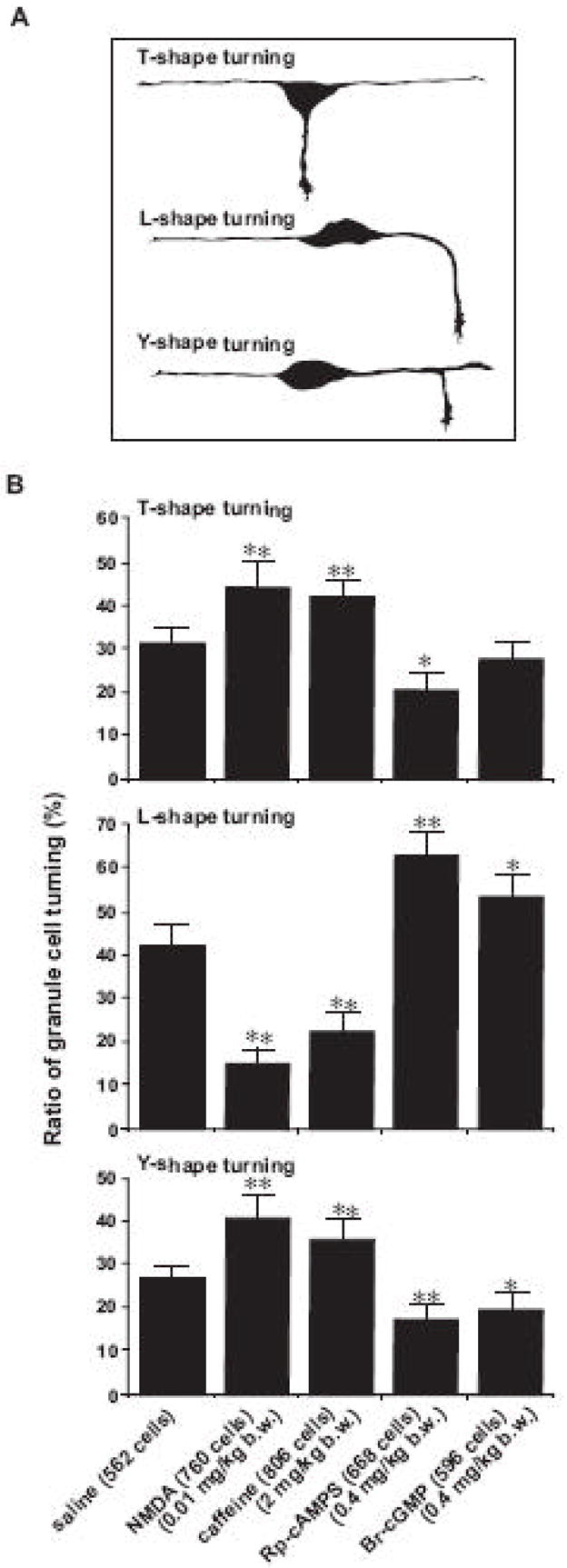
Changes in the ratio of the T-, L-, and Y-shape granule cell turning at the EGL-ML border of the P10 mouse cerebella by the application of saline, NMDA, caffeine, Rp-cAMP, or Br-cGMP. (A) Schematic representation of the T-, L-, and Y-shape granule cell turning in vivo. (B) Histograms showing the effects of the injection of saline, NMDA, caffeine, Rp-cAMP, or Br-cGMP on the ratio of the T-, L-, and Y-shape granule cell turning at the EGL-ML border. Single (p <0.05) and double (p <0.01) asterisks indicate statistical significance. Bar: S.D.
The occurrence of the 1st mode of granule cell turningin vitro, which is characterized by the turning of the tip of the leading process to a new direction, is significantly increased by inhibiting the cAMP signaling or stimulating the cGMP signaling, but is significantly decreased by stimulating the Ca2+ influx or the internal Ca2+ release (Fig. 4B). Interestingly, the occurrence of the L-shape turning of granule cells in vivo, which is characterized by the turning of the tip of the horizontally-oriented leading process towards the ML, is significantly increased by inhibiting the cAMP signaling or stimulating the cGMP signaling, but is significantly decreased by stimulating the Ca2+ influx or the internal Ca2+ release (Fig. 9B). The similarities in the changes in the morphology during the initiation of turning and the responses to the alterations of the Ca2+ and cyclic nucleotide signaling pathways between the 1st mode of turningin vitro and the L-shape turning in vivo suggest that intrinsic programs control the occurrence of the 1st mode turning in vitro and the L-shape turning in vivo by altering the similar signaling pathways. Furthermore, the occurrence of the 2nd mode of granule cell turningin vitro, which is characterized by the bifurcation of the tip of the leading process, is significantly increased by stimulating the Ca2+ influx, but is significantly decreased by inhibiting the cAMP signaling or stimulating the cGMP signaling (Fig. 4B). Likewise, the occurrence of the Y-shape turning of granule cells in vivo, which is characterized by the bifurcation of the tip of the horizontally-oriented leading process, is significantly increased by stimulating the Ca2+ influx and internal Ca2+ release, but is significantly decreased by inhibiting the cAMP signaling or stimulating the cGMP signaling (Fig. 9B). These similarities observed in the 2nd mode of turningin vitro and the Y-shape turning in vivo suggest that intrinsic programs play role in controlling the Y-shape turning of granule cells in vivo by altering the intracellular signaling pathways, which is responsible for controlling the 2nd mode of turning in vitro.
Taken together, these results suggest that intrinsic programs are involved in controlling the timing and the mode of granule cell turning in vivo by altering the Ca2+ and cyclic nucleotide signaling pathways. We also assume that the extrinsic signals (such as cell-cell contact and guidance molecules) and intrinsic programs cooperate in directing the turning of granule cells in vivo. For example, it seems that the occurrence of the T-shape turning of granule cells is regulated by the intrinsic programs through the alterations of the Ca2+ and cyclic nucleotide signaling pathways and by the contact with the processes of Bergmann glial cells as previously reported by Rakic (1971).
Discussion
In this series of the experiments, we examined whether granule cells autonomously change the direction of migration. To minimize the effect of cell-cell contact on granule cell turning, we monitored the turning of granule cells which were located at least 300 μm away from other cells and the processes at the start of the observation. Furthermore, to eliminate the possibility that molecules released from other cells induce granule cell turning, we replaced the culture medium with the fresh culture medium immediately before the observation. Moreover, to examine whether the cell adhesion molecules affect autonomous changes in granule cell turning, we used the different types and the concentrations of cell adhesion molecules as a substrate. With the use of the time-lapse observation of cell movement, the present study revealed that granule cells exhibit spontaneous and periodical turning without cell-cell contact and in the absence of potential external guidance cues. The frequency of turning was increased by stimulating the Ca2+ influx and the internal Ca2+ release, or inhibiting the cAMP signaling pathway, while the frequency was reduced by inhibiting the Ca2+ influx. Granule cell turning in vitro was classified into four distinct modes. The occurrence of the 1st and 2nd mode of turning, which were characterized by turning and bifurcating the leading processes, was affected by altering the Ca2+ and cAMP signaling pathways. Collectively, these results suggest that autonomous turning of granule cells in vitro is controlled by intrinsic (inherent) programs through altering the activity of the Ca2+ and cAMP signaling pathways.
The Ca2+ and cyclic nucleotide signaling pathways are known to play a role in controlling neuronal growth cone turning (Gomez et al., 2001; Gorbunova and Spitzer, 2002; Conklin et al., 2005: Spitzer, 2006), but little is known about their roles in granule cell turning. The questions of how these signaling pathways control the frequency and mode of autonomous turning of granule cells remain to be examined, but there are possible scenarios. First, the Ca2+ signaling pathways play a role in organizing the assembly and disassembly of cytoskeletal components (Henley and Poo, 2004). So, the changes in the Ca2+ signaling pathways may be essential for the initiation of autonomous turning of granule cells by altering the organization of cytoskeletal components. Second, it has been shown that transient elevations of intracellular Ca2+ levels control the formation of focal adhesion (Conklin et al., 2005). Therefore, the Ca2+ transients may be required for the formation of new focal adhesion sites, which is essential for changing the direction of cell movement. Third, the cAMP and cGMP signaling pathways affect the distribution of F-actin in the somata of migrating neurons (Haase and Bicker, 2003), suggesting that alterations of the cAMP and cGMP signaling pathways are prerequisite for the reorientation of the soma to a new direction of migration. Fourth, phosphorylation by PKA can switch off the activity of oncoprotein 18, a regulator of microtubule dynamics (Gradin et al., 1998). So, the changes in the PKA activity may play a role in changing the direction of cell movement by controlling the behavior of the leading process by altering the microtubule dynamics. Fifth, the Ca2+ signals localized to one side of the neuronal growth cone can cause asymmetric activation of effecter enzymes to steer the growth cone (Petersen and Cancela, 2000; Henley and Poo, 2004). Therefore, the localized changes in the Ca2+ signaling pathways in the leading process may be crucial for the 1st mode of granule cell turning in vitro. Finally, changes in the ratio of cAMP/cGMP or the cytoplasmic cAMP gradients affect neuronal growth cone turning (Song et al., 1998; Nishiyama et al., 2003; Munck et al., 2004), suggesting that the alterations of the cAMP and cGMP signaling pathways induce the turning of the leading process of granule cells.
The involvement of the Ca2+ and cyclic nucleotide signaling pathways in the autonomous turning suggests that intrinsic programs affect the role of extracellular guidance molecules in controlling granule cell turning. This is because these signaling pathways play a role in converting extracellular guidance signals to intracellular signals for controlling the migration of granule cells (Bix and Clark, 1998; Zou et al., 1998; Yuen and Mobley, 1999; Klein et al., 2001; Borghesani et al., 2002; Roland et al., 2003; Du et al., 2006: Botia et al., 2007; Cameron et al., 2007; Jiang et al., 2008). Therefore, intrinsic programs may change the role of external guidance molecules in granule cell turning by altering the Ca2+ and cyclic nucleotide signaling pathways. It has been reported that the response of growing axons to external guidance cues is altered by the basal activity of the Ca2+ and cyclic nucleotide signaling pathways (Ming et al., 2001; Nishiyama et al., 2003; Wen et al., 2004; Jin et al., 2005). Moreover, granule cells frequently exhibit spontaneous Ca2+ transients (Ca2+ spikes) during their migration (Komuro and Rakic, 1996; Kumada and Komuro, 2004). Immature neurons frequently exhibit spontaneous elevations of cAMP levels, which interact with the Ca2+ transients (Gorbunova and Spitzer, 2002). Collectively, the previous and present studies suggest that intrinsic programs and external guidance molecules interact with each other for directing the turning of granule cells through the alterations of the Ca2+ and cyclic nucleotide signaling pathways.
The uneven distribution of the cell adhesion molecules affects the migration of neurons and neuronal processes (Gomez et al., 2001; Adams et al., 2005). In this study, we tried to coat cell adhesion molecules evenly on the glass-bottom culture dishes. However, we can not rule out the possibility that the undetectably small and localized differences in the concentrations of the laminin and/or poly-L-lysine on the bottom surface of the dishes affect the turning of granule cells in part. Furthermore, the questions of whether and how intrinsic programs control the turning of migrating neurons other than cerebellar granule cells remain open.
Acknowledgments
This work was supported by a Pilot Research Award (PP1450) from the National Multiple Sclerosis Society and a grant (R01 ES015612) from National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams DN, Kao EY, Hypolite CL, Distefano MD, Hu WS, Letourneau PC. Growth cones turn and migrate up an immobilized gradient of the laminin IKVAV peptide. J Neurobiol. 2005;62:134–147. doi: 10.1002/neu.20075. [DOI] [PubMed] [Google Scholar]
- Alcantara S, Ruiz M, De Castro F, Soriano E, Sotelo C. Netrin 1 acts as an attractive or as a repulsive cue for distinct migrating neurons during the development of the cerebellar system. Development. 2000;127:1359–1372. doi: 10.1242/dev.127.7.1359. [DOI] [PubMed] [Google Scholar]
- Altman J. Postnatal development of the cerebellar cortex in the rat. I The external germinal layer and the transitional molecular layer. J Comp Neurol. 1972;145:353–398. doi: 10.1002/cne.901450305. [DOI] [PubMed] [Google Scholar]
- Bagri A, Tessier-Lavigne M. Neuropilins as Semaphorin receptors: in vivo functions in neuronal cell migration and axon guidance. Adv Exp Med Biol. 2002;515:13–31. [PubMed] [Google Scholar]
- Bix GJ, Clark GD. Platelet-activating factor receptor stimulation disrupts neuronal migration in vitro. J Neurosci. 1998;18:307–318. doi: 10.1523/JNEUROSCI.18-01-00307.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botia B, Basille M, Allais A, Raoult E, Falluel-Morel A, Galas L, Jolivel V, Wurtz O, Komuro H, Fournier A, Vaudry H, Burel D, Gonzalez B, Vaudry D. Neurotrophic effects of PACAP in the cerebellar cortex. Peptides. 2007;28:1746–1752. doi: 10.1016/j.peptides.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Borghesani PR, Peyrin JM, Klein R, Rubin J, Carter AR, Schwartz PM, Luster A, Corfas G, Segal RA. BDNF stimulates migration of cerebellar granule cells. Development. 2002;129:1435–1442. doi: 10.1242/dev.129.6.1435. [DOI] [PubMed] [Google Scholar]
- Britanova O, Alifragis P, Junek S, Jones K, Gruss P, Tarabykin V. A novel mode of tangential migration of cortical projection neurons. Dev Biol. 2006;298:299–311. doi: 10.1016/j.ydbio.2006.06.040. [DOI] [PubMed] [Google Scholar]
- Cameron DB, Galas L, Jiang Y, Raoult E, Vaudry D, Komuro H. Cerebellar cortical-layer-specific control of neuronal migration by pituitary adenylate cyclase-activating polypeptide. Neuroscience. 2007;146:697–712. doi: 10.1016/j.neuroscience.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin MW, Lin MS, Spitzer NC. Local calcium transients contribute to disappearance of pFAK, focal complex removal and deadehesion of neuronal growth cones and fibroblasts. Dev Biol. 2005;287:201–212. doi: 10.1016/j.ydbio.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Du Y, Lercher LD, Zhou R, Dreyfus CF. Mitogen-activated protein kinase pathway mediates effects of brain-derived neurotrophic factor on differentiation of basal forebrain oligodendrocytes. J Neurosci Res. 2006;84:1692–1702. doi: 10.1002/jnr.21080. [DOI] [PubMed] [Google Scholar]
- Fujita S. Quantitative analysis of cell proliferation and differentiation in the cortex of the postnatal mouse cerebellum. J Cell Biol. 1967;32:277–287. doi: 10.1083/jcb.32.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita S, Shimada M, Nakamura T. H3-thymidine autoradiographic studies on the cell proliferation and differentiation in the external and the internal granular layers of the mouse cerebellum. J Comp Neurol. 1966;128:191–208. doi: 10.1002/cne.901280206. [DOI] [PubMed] [Google Scholar]
- Gomez TM, Robles E, Poo MM, Spitzer NC. Filopodial calcium transients promote substrate-dependent growth cone turning. Science. 2001;291:1983–1987. doi: 10.1126/science.1056490. [DOI] [PubMed] [Google Scholar]
- Gorbunova YV, Spitzer NC. Dynamic interactions of cyclic AMP transients and spontaneous Ca2+ spikes. Nature. 2002;418:93–96. doi: 10.1038/nature00835. [DOI] [PubMed] [Google Scholar]
- Gradin HH, Larsson N, Marklund U, Gullberg M. Regulation of microtubule dynamics by extracellular signals: cAMP-dependent protein kinase switches off the activity of oncoprotein 18 in intact cells. J Cell Biol. 1998;140:131–141. doi: 10.1083/jcb.140.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A, Bicker G. Nitric oxide and cyclic nucleotides are regulators of neuronal migration in an insect embryo. Development. 2003;130:3977–3987. doi: 10.1242/dev.00612. [DOI] [PubMed] [Google Scholar]
- Henley J, Poo MM. Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol. 2004;14:320–330. doi: 10.1016/j.tcb.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Kumada T, Cameron DB, Komuro H. Cerebellar granule cell migration and the effects of alcohol. Dev Neurosci. 2008;30:7–23. doi: 10.1159/000109847. [DOI] [PubMed] [Google Scholar]
- Jin M, Guan CB, Jiang YA, Chen G, Zhao CT, Cui K, Song YQ, Wu CP, Poo MM, Yuan XB. Ca2+-dependent regulation of Rho GTPases triggers turning of nerve growth cones. J Neurosci. 2005;25:2338–2347. doi: 10.1523/JNEUROSCI.4889-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi D, Taniguchi H, Watanabe H, Saito T, Murakami F. Direct visualization of nucleogenesis by precerebellar neurons: involvement of ventricle-directed, radial fiber-associated migration. Development. 2006;133:1113–1123. doi: 10.1242/dev.02283. [DOI] [PubMed] [Google Scholar]
- Kerjan G, Dolan J, Haumaitre C, Schneider-Maunoury S, Fujisawa H, Mitchell KJ, Chedotal A. The transmembrane semapholin Sema6A controls cerebellar granule cell migration. Nat Neurosci. 2005;8:1516–1524. doi: 10.1038/nn1555. [DOI] [PubMed] [Google Scholar]
- Klein RS, Rubin JB, Gibson HD, DeHaan EN, Alvarez-Hernandez X, Segal RA, Luster AD. SDF-1α induces chemotaxis and enhances Sonic hedgehog-induced proliferation of cerebellar granule cells. Development. 2001;128:1971–1981. doi: 10.1242/dev.128.11.1971. [DOI] [PubMed] [Google Scholar]
- Komuro H, Kumada T. Ca2+ transients control CNS neuronal migration. Cell Calcium. 2005;37:387–393. doi: 10.1016/j.ceca.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Selective role of N-type calcium channels in neuronal migration. Science. 1992;257:806–809. doi: 10.1126/science.1323145. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Modulation of neuronal migration by NMDA receptors. Science. 1993;260:95–97. doi: 10.1126/science.8096653. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Dynamics of granule cell migration: a confocal microscopic study in acute cerebellar slice preparations. J Neurosci. 1995;15:1110–1120. doi: 10.1523/JNEUROSCI.15-02-01110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Intracellular Ca2+ fluctuations modulate the rate of neuronal migration. Neuron. 1996;17:275–285. doi: 10.1016/s0896-6273(00)80159-2. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Distinct modes of neuronal migration in different domains of developing cerebellar cortex. J Neurosci. 1998a;18:1478–1490. doi: 10.1523/JNEUROSCI.18-04-01478.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Orchestration of neuronal migration by activity of ion channels, neurotransmitter receptors, and intracellular Ca2+ fluctuations. J Neurobiol. 1998b;37:110–130. [PubMed] [Google Scholar]
- Komuro H, Rakic P. In vitro analysis of signal mechanisms involved in neuronal migration. In: Haynes LW, editor. The neuron in tissue culture. John Wiley & Sons; New York: 1999. pp. 57–69. [Google Scholar]
- Komuro H, Yacubova E. Recent advances in cerebellar granule cell migration. Cell Mol Life Sci. 2003;60:1084–1098. doi: 10.1007/s00018-003-2248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H, Yacubova E, Yacubova E, Rakic P. Mode and tempo of tangential cell migration in the cerebellar external granular layer. J Neurosci. 2001;21:527–540. doi: 10.1523/JNEUROSCI.21-02-00527.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumada T, Komuro H. Completion of neuronal migration regulated by loss of Ca2+ transients. Proc Natl Acad Sci USA. 2004;101:8479–8484. doi: 10.1073/pnas.0401000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumada T, Lakshmana MK, Komuro H. Reversal of neuronal migration in a mouse model of fetal alcohol syndrome by controlling second-messenger signalings. J Neurosci. 2006;26:742–756. doi: 10.1523/JNEUROSCI.4478-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumada T, Jiang Y, Cameron DB, Komuro H. How does alcohol impair neuronal migration? J Neurosci Res. 2007;85:465–470. doi: 10.1002/jnr.21149. [DOI] [PubMed] [Google Scholar]
- Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- Lu Q, Sun EE, Klein RS, Flanagan JG. Ephrin-B signaling is mediated by a novel PDZ-RGS protein and selectively inhibits G protein-coupled chemoattraction. Cell. 2001;105:69–79. doi: 10.1016/s0092-8674(01)00297-5. [DOI] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O, Plump AS, Flames N, Sanchez-Camacho C, Tessier-Lavigne M, Rubenstein JL. Directional guidance of interneuron migration to the cerebral cortex relies on subcortical Slit1/2-indipendent repulsion and cortical attraction. Development. 2003;130:1889–1901. doi: 10.1242/dev.00417. [DOI] [PubMed] [Google Scholar]
- Miale IL, Sidman RL. An autoradiographic analysis of histogenesis in the mouse cerebellum. Exp Neurol. 1961;4:277–296. doi: 10.1016/0014-4886(61)90055-3. [DOI] [PubMed] [Google Scholar]
- Ming G, Henley J, Tessier-Lavigne M, Song H, Poo MM. Electrical activity modulates growth cone guidance by diffusible factors. Neuron. 2001;29:441–452. doi: 10.1016/s0896-6273(01)00217-3. [DOI] [PubMed] [Google Scholar]
- Munck S, Bedner P, Bottaro T, Harz H. Spatiotemporal properties of cytoplasmic cyclic AMP gradients can alter the turning behavior of neuronal growth cones. Eur J Neurosci. 2004;19:791–797. doi: 10.1111/j.0953-816x.2004.03118.x. [DOI] [PubMed] [Google Scholar]
- Nagata I, Nakatsuji N. Granule cell behavior on laminin in cerebellar microexplant cultures. Dev Brain Res. 1990;52:63–73. doi: 10.1016/0165-3806(90)90222-k. [DOI] [PubMed] [Google Scholar]
- Nakajima K. Control of tangential/non-radial migration of neurons in the developing cerebral cortex. Neurochem Int. 2007;51:121–131. doi: 10.1016/j.neuint.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Nakatsuji N, Nagata I. Paradoxical perpendicular contact guidance displayed by mouse cerebellar granule cell neurons in vitro. Development. 1989;106:441–447. doi: 10.1242/dev.106.3.441. [DOI] [PubMed] [Google Scholar]
- Nishiyama M, Hoshino A, Tsai L, Henley JR, Goshima Y, Tessier-Lavigne M, Poo MM, Hong K. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature. 2003;423:990–995. doi: 10.1038/nature01751. [DOI] [PubMed] [Google Scholar]
- Petersen OH, Cancela JM. Attraction or repulsion by local Ca2+ signals. Curr Biol. 2000;10:R311–R314. doi: 10.1016/s0960-9822(00)00438-3. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A golgi and electron microscopic study in Macacus rhesus. J Comp Neurol. 1971;141:283–312. doi: 10.1002/cne.901410303. [DOI] [PubMed] [Google Scholar]
- Rakic P. Principles of neuronal cell migration. Experientia. 1990;46:882–891. doi: 10.1007/BF01939380. [DOI] [PubMed] [Google Scholar]
- Rakic P, Komuro H. The role of receptor/channel activity in neuronal cell migration. J Neurobiol. 1995;26:299–315. doi: 10.1002/neu.480260303. [DOI] [PubMed] [Google Scholar]
- Rakic P, Cameron RS, Komuro H. Recognition, adhesion, transmembrane signaling and cell motility in guided neuronal migration. Curr Opin Neurobiol. 1994;4:63–69. doi: 10.1016/0959-4388(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Roland J, Murphy BJ, Ahr B, Robert-Hebmann V, Delauzun V, Nye KE. Role of the intracellular domains of CXCR4 in SDF-1-mediated signaling. Blood. 2003;101:399–406. doi: 10.1182/blood-2002-03-0978. [DOI] [PubMed] [Google Scholar]
- Ryder EF, Cepko CL. Migration patterns of clonally related granule cells and their progenitors in the developing chick cerebellum. Neuron. 1994;12:1011–1028. doi: 10.1016/0896-6273(94)90310-7. [DOI] [PubMed] [Google Scholar]
- Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo MM. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- Spitzer NC. Electrical activity in early neuronal development. Nature. 2006;444:707–712. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- Tanaka DH, Maekawa K, Yanagawa Y, Obata K, Murakami F. Multidirectional and multizonal tangential migration of GABAergic interneurons in the developing cerebral cortex. Development. 2006;133:2167–2176. doi: 10.1242/dev.02382. [DOI] [PubMed] [Google Scholar]
- Trenkner E, Smith D, Segil N. Is cerebellar granule cell migration regulated by an internal clock? J Neurosci. 1984;4:2850–2855. doi: 10.1523/JNEUROSCI.04-11-02850.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Guirland C, Ming G, Zheng JA. CaMKII/Calcineurin switch controls the direction of Ca2+-dependent growth cone guidance. Neuron. 2004;43:835–846. doi: 10.1016/j.neuron.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Wu W, Wong K, Jiang Z, Dupuis S, Wu JY, Rao Y. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature. 1999;400:331–336. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacubova E, Komuro H. Intrinsic program for migration of cerebellar granule cells in vitro. J Neurosci. 2002a;22:5966–5981. doi: 10.1523/JNEUROSCI.22-14-05966.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacubova E, Komuro H. Stage-specific control of neuronal migration by somatostatin. Nature. 2002b;415:77–81. doi: 10.1038/415077a. [DOI] [PubMed] [Google Scholar]
- Yacubova E, Komuro H. Cellular and molecular mechanisms of cerebellar granule cell migration. Cell Biochem Biophys. 2003;37:213–234. doi: 10.1385/cbb:37:3:213. [DOI] [PubMed] [Google Scholar]
- Yuen EC, Mobley WC. Early BDNF, NT-3, and NT-4 signaling events. Exp Neurol. 1999;159:297–308. doi: 10.1006/exnr.1999.7148. [DOI] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]