Abstract
Androgens are important regulators of body composition and promote myogenic differentiation and inhibit adipogenesis of mesenchymal, multipotent cells. Here, we investigated the mechanisms by which androgens induce myogenic differentiation of mesenchymal multipotent cells. Incubation of mesenchymal multipotent C3H 10T1/2 cells with testosterone and dihydrotestosterone promoted nuclear translocation of androgen receptor (AR)/β-catenin complex and physical interaction of AR, β-catenin, and T-cell factor-4 (TCF-4). Inhibition of β-catenin by small inhibitory RNAs significantly decreased testosterone-induced stimulation of myogenic differentiation. Overexpression of TCF-4, a molecule downstream of β-catenin in Wnt signaling cascade, in C3H 10T1/2 cells significantly up-regulated expression of myoD and myosin heavy chain II proteins and of follistatin (Fst), which binds and antagonizes native ligands of the TGF-β/Smad pathway. Gene array analysis of C3H 10T1/2 cells treated with testosterone revealed that testosterone up-regulated the expression of Fst and modified the expression of several signaling molecules involved in the TGF-β/Smad pathway, including Smad7. Lowering of testosterone levels in mice by orchidectomy led to a significant decrease in Fst and Smad7 expression; conversely, testosterone supplementation in castrated mice up-regulated Fst and Smad7 mRNA expression in androgen-responsive levator ani muscle. Testosterone-induced up-regulation of MyoD and myosin heavy chain II proteins in C3H 10T1/2 cells was abolished in cells simultaneously treated with anti-Fst antibody, suggesting an essential role of Fst during testosterone regulation of myogenic differentiation. In conclusion, our data suggest the involvement of AR, β-catenin, and TCF-4 pathway during androgen action to activate a number of Wnt target genes, including Fst, and cross communication with the Smad signaling pathway.
Androgen-induced myogenic differentiation in mouse multipotent C3H 10T1/2 cells is mediated through androgen receptor/β-catenin signaling pathway to upregulate follistatin and cross-communication with TGF-β/Smad signaling pathway.
Testosterone supplementation increases skeletal muscle mass by inducing skeletal muscle hypertrophy in young (1,2,3,4) and older men (5,6,7,8,9,10), in androgen-deficient (11,12,13,14,15) and androgen-replete men (1,2,3,4), and in men with chronic illnesses (7,8,9,10,16,17,18); these effects of testosterone on muscle mass are correlated with testosterone dose and circulating concentrations (4,19). Androgens promote the differentiation of mesenchymal multipotent cells into myogenic lineage (20). Thus, in C3H 10T1/2 multipotent cells, testosterone induces key myogenic proteins, MyoD and myosin heavy chain II (MHC II) and inhibits adipogenic differentiation factors, CCAAT enhancer-binding protein-α and peroxisome proliferator-activated receptor-γ2, in a dose-dependent manner (20). The effects of testosterone on myogenic and adipogenic differentiation in this model are blocked by bicalutamide, an androgen receptor (AR) antagonist (20), suggesting that AR signaling plays an important role in regulating myogenic differentiation of mesenchymal multipotent cells. However, the mechanisms and signaling pathways that mediate testosterone’s effects on myogenic differentiation are poorly understood and were the subject of this investigation.
β-Catenin serves as an important link between several signaling pathways, including the Wnt signaling pathway that acts as a molecular switch in the regulation of adipogenesis as well as myogenesis in multipotent mesenchymal cells (21,22,23,24,25,26). Association of AR with cytosolic β-catenin causes the latter to translocate to the nucleus and activate Wnt-target genes, which are implicated in the regulation of adipogenic differentiation (24). However, we do not know whether interaction of AR and β-catenin is essential in mediating androgen effects on myogenic differentiation. Furthermore, the downstream mechanisms by which AR-β-catenin interaction regulates myogenic differentiation are unknown and were investigated in this report. We show in this paper that androgen treatment led to physical interaction of AR with β-catenin and T-cell factor-4 (TCF-4) in mouse C3H 10T1/2 grown in myogenic conditions. Overexpression of full-length TCF-4 cDNA in C3H 10T1/2 cells was associated with up-regulation of a number of Wnt-target genes, including follistatin (Fst) that has a TCF-4 binding site in its promoter region (27). Fst binds to a number of TGF-β family members, including myostatin, and antagonizes myostatin activity (28,29,30,31). TGF-β signaling inhibits myogenesis in several biological systems (32,33,34,35,36); therefore, cross communication of AR signaling to the TGF-β pathway through TCF-4 and Fst could potentially explain androgen effects on myogenic differentiation. Accordingly, we tested the hypothesis that testosterone modulates myogenic differentiation of multipotent cells by activation of Fst, resulting in the inhibition of the TGF-β signaling pathway.
Materials and Methods
Cell culture
Mouse C3H 10T1/2 cells were grown in DMEM with 10% fetal bovine serum growth medium at 37 C, treated with 20 μm 5′-azacytidine for 3 d, split 1:2, allowed to recover for 2 d, and seeded at 70% confluence in six-well plates or chamber slides and grown with test agents for 0–14 d (20).
Detection of AR and β-catenin by immunofluorescence
The localization of AR and β-catenin was carried out in C3H 10T1/2 cells treated with or without testosterone (100 nm) and dihydrotestosterone (DHT) (10 nm) for 24 h. For AR immunofluorescence, cells were blocked with normal goat serum and incubated with rabbit anti-AR antibody (N20; Santa Cruz Biotechnology, Santa Cruz, CA), followed by incubation with antirabbit biotinylated secondary antibody (Calbiochem, La Jolla, CA). Subsequent reaction was carried out by treating the cells in streptavidin-fluorescein isothiocyanate (Vector Laboratories, Burlingame, CA), followed by incubation in 10% normal horse serum and a 1:500 dilution of anti-β-catenin monoclonal antibody (BD Biosciences PharMingen, San Diego, CA). Fluorescence labeling was performed with secondary antibody linked to Texas Red. The slides were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and mounted in Prolong antifade (Molecular Probes, Eugene, OR) (24).
Cell fractionation and Western blot analysis to evaluate β-catenin nuclear translocation
Cells were treated with or without testosterone (100 nm) for 0–60 min, harvested, and separated into cytoplasmic and nuclear fractions (24). In some cases, cells treated with DHT (10 nm) or testosterone (100 nm) (data not shown) were also incubated with 10-fold molar excess of AR antagonist bicalutamide for 60 min. Twenty micrograms of nuclear and cytoplasmic fractions were separated on 10% SDS-PAGE, and proteins were transferred onto polyvinylidene difluoride membrane (Amersham Biosciences, Inc., Chicago, IL). Membranes were probed with anti-β-catenin antibody (1:500 dilutions, C19220) for 2 h at room temperature. Detection of immunoreactive bands was achieved with antimouse-horseradish peroxidase-linked secondary antibody and enhanced chemiluminescence detection agents (Amersham).
Western blot analyses
Cell lysates (50–100 μg) were subjected to Western blot analyses after various treatments by 7.5–12% polyacrylamide gel electrophoresis using 1:500 anti-MyoD, 1:500 anti-myogenin (Santa Cruz), 1:500 anti-β-catenin, 1:200 anti-MHC II (fast; Vector), 1:200 anti-Smad7 (Santa Cruz), or 1:10,000 anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Chemicon International Inc., Temecula, CA). The filters were incubated with a 1:3000 dilution of secondary antibodies linked to horseradish peroxidase. Immunoreactive bands were detected by enhanced chemiluminescence detection system.
Immunoprecipitation and immunoblot analyses to detect AR, β-catenin, and TCF-4 interaction
Cells were treated with DHT (0–30 nm) alone or in combination with 100 nm bicalutamide for 24 h. The interaction between β-catenin, AR, and TCF-4 was studied by immunoprecipitation as described before (24). Briefly, cells were lysed using lysis buffer containing 20 mm Tris-HCl (pH 7.8), 0.5% Nonidet P-40, and protease inhibitors (Roche Diagnostics, Manheim, Germany). Cell lysates were passed through a 30.5-gauge needle to disrupt nuclei. Protein extracts (500 μg) were precleared by adding rabbit IgG together with protein A/G-Sepharose at 4 C for 30 min followed by incubated with anti-AR (N20; Santa Cruz) antibody overnight at 4 C, followed by 1 h incubation with protein A/G-Sepharose (Calbiochem). After three washes with 0.5 ml lysis buffer, the pellets were suspended in SDS sample buffer, boiled for 5 min, and analyzed on SDS-PAGE. Proteins were transferred to a polyvinylidene difluoride membrane and blotted with mouse anti-β-catenin (C19220; BD Biosciences PharMingen) or rabbit anti-AR or anti-TCF-4 (Santa Cruz Biotechnology; Upstate, Charlottesville, VA) antibodies. We also performed control immunoprecipitation experiments using cell lysates with 2 μg rabbit IgG instead of anti-AR antibody under similar conditions to detect any nonspecific interactions.
Real-time quantitative PCR
Real-time quantitative PCR was performed using PCR primers MyoD (130 bp) 801–821/930-911 on M84918, GAPDH (152 bp) 606-626/758-738 on BC023196, and Smad7 (67 bp) 575-597/623-641 on BC074818.2 and primer pairs from SABioscience (Frederick, MD) including Fst (150 bp on NM_008046), FKBP2.1 (130 bp on NM_016863), and BMP-10 (167 bp on NM_009756) as described previously (24). Human MyoD and MHC II were also purchased from SuperArray. The QIAGEN Sybr-green PCR kit with HotStar Taq DNA polymerase was used (QIAGEN, Valencia, CA) with i-Cycler PCR thermocycler and fluorescent detector lid (Bio-Rad, Hercules, CA). The protocol includes melting for 15 min at 95 C, 40 cycles of three-step PCR including melting for 15 sec at 95 C, annealing for 30 sec at 58 C, and elongation for 30 sec at 72 C with an additional detection step of 15 sec at 79 C, followed by a melting curve from 55–95 C at the rate of 0.5 C per 10 sec except that for Fst primers, annealing was at 55 C and detection was at 76 C. Samples of 25 ng cDNA were analyzed in quadruplicate in parallel with GAPDH controls as described (20,24).
Silencing of endogenous β-catenin in C3H 10T1/2 cells by small inhibitory RNA (SiRNA)
Two SiRNA sequences targeting mouse β-catenin gene were constructed using Dharmacon siDESIGN Center (Dharmacon Inc., Dallas, TX): SiRNA1 5′-GAACGCAGCAGCAGTTTGT-3′ (1876–1894 on NM_001904.2) and SiRNA2 5′-GCAAGTAGCTGATATTGAC-3′ (443–461 on NM-001904.2). These SiRNA were used at 100 nm concentrations with standard transfection technique using oligofectamine (Invitrogen). As a control, we also used 100 nm random SiRNA. We were able to get about 75–70% inhibition of β-catenin using SiRNA1 and SiRNA2, respectively. Cells were analyzed for β-catenin (after 3 d), MyoD (after 3 d), and MHC II (after 12 d) protein expression and phenotypic analysis (after 12 d).
Overexpression of full-length TCF-4 by nucleofection using amaxa
Full-length TCF-4 cDNA was kindly provided by Dr. M. Wierman (Veterans Affairs Medical Center, Denver, CO). We employed the amaxa-based nucleofection system to overexpress full-length TCF-4 construct, with approximately 75–80% efficiency as described (24).
SuperArray analysis
Total cellular RNA, isolated from C3H 10T1/2 cells treated with or without testosterone (100 nm) for 3 h was analyzed by mouse TGF-β pathway gene array (GE Array Q Series, MM-023; SuperArray). Biotin-labeled cDNA probes were synthesized from total RNA, denatured, and hybridized overnight at 60 C in GE hybridization solution. Membranes were washed, and chemiluminescent analysis was performed according to manufacturer’s instructions. Raw data were analyzed using GEArray Expression Analysis Suite (SuperArray), and fold changes in gene expression are presented after background correction and normalization with a housekeeping gene. The array analysis was performed twice, and relative changes in expression levels were confirmed in three independent experiments by RT and real-time PCR.
Animal experiments
The Institutional Animal Care and Use Committee at Charles Drew University approved the protocols for animal experiments. C57 BL6J mice (∼6 wk old and ∼20 g; Charles River Laboratories, Wilmington, MA) were housed under controlled temperature (22 C), humidity (40%), and light (12-h light, 12-h dark cycle) in a pathogen-free vivarium according to Institutional Animal Care and Use Committee guidelines. The animals had ad libitum access to laboratory chow and water. The orchidectomies were performed under ketamine (100 mg/kg) and xylazine (10 mg/kg) anesthesia.
The animals were randomly assigned to one of four groups: sham-operated, orchidectomized with sc implants of blank SILASTIC brand (Dow Corning, Midland, MI) capsules, orchidectomized implanted with 1-cm testosterone SILASTIC brand capsules, and orchidectomized implanted with 2-cm testosterone SILASTIC brand capsules. After 14 d treatment, mice were euthanized, and levator ani and gastrocnemius muscles were harvested.
Immunocytochemical analyses of MyoD and MHC II
Cells grown in eight-well chamber slides were fixed in 2% paraformaldehyde, quenched with H2O2, blocked with normal horse serum, and incubated with MyoD (1:200) or MHC II (1:200) primary antibodies. Detection was based on a secondary biotinylated antibody (1:200), followed by the addition of the streptavidin-horseradish peroxidase ABC complex (1:100, Vectastain Elite ABC System; Novocastra Laboratories, Newcastle upon Tyne, UK) and 3,3′-diaminobenzidine. The cells were counterstained with Meyer’s hematoxylin.
Image analysis
The number of MyoD-positive cells per field was counted against the total number of cells determined by hematoxylin counterstaining. MHC II immunostaining was quantified by image analysis using Image Pro 4.01 software (Media Cybernetics, Silver Spring, MD), coupled to a Leica DMLB microscope/VCC video camera. We analyzed integrated OD (IOD = area × average intensity) from at least 20 fields per treatment group as described previously (24).
Statistical analysis
Data are presented as mean ± sem. Student’s t test was performed for comparison between two groups. For statistical analysis of more than two groups, data were analyzed by ANOVA, and pairwise comparisons were performed by Tukey’s posttest procedure. P values <0.05 were considered statistically significant.
Results
Androgens, testosterone, and DHT up-regulate myogenesis and induce nuclear translocation of β-catenin and its colocalization with AR in C3H 10T1/2 cells
We used mesenchymal multipotent C3H 10T1/2 cells for studying the effects of testosterone and DHT on myogenic differentiation as well as study the interaction of AR and β-catenin because these cells have multipotentiality and express AR. We analyzed the effects of both testosterone and nonaromatizable DHT, the two prototypical androgens. Additionally, unlike testosterone, DHT is not aromatized to estradiol, and its effects likely represent androgenic effects not mediated through its conversion to estradiol by aromatase. Cells were treated with testosterone (100 nm) or DHT (10 nm) for various time points, and key myogenic differentiation markers such as MyoD (after 3 d treatment), myogenin (after 6 d treatment), and MHC II (after 12 d treatment) were analyzed by Western blot analysis. Testosterone treatment of these cells led to a significant up-regulation of key myogenic proteins (Fig. 1A). To study the subcellular localization of AR and β-catenin after testosterone and DHT treatment, C3H 10T1/2 cells were incubated with DHT (10 nm), testosterone (100 nm), or medium alone for various time periods and analyzed for immunofluorescence of β-catenin, a key protein that translocates to the nucleus after activation of Wnt signaling. β-Catenin was localized mainly in the cytoplasm and cytoskeleton in cells treated with medium alone, whereas β-catenin was translocated to the nucleus after testosterone (100 nm) and DHT (10 nm) treatment for 24 h (Fig. 1B). AR and β-catenin were colocalized in the nucleus in both testosterone-treated (100 nm) and DHT-treated (10 nm) cells after 24 h (Fig. 1B) as observed by merging the AR- and β-catenin-stained images (yellow). The respective positions of the nuclei were detected by counterstaining the cells with DAPI (blue). Concomitant treatment of cells with 10-fold molar excess of AR antagonist bicalutamide inhibited testosterone-induced translocation of β-catenin to the nucleus (data not shown).
Figure 1.
Testosterone induced myogenic differentiation and promoted nuclear translocation of β-catenin and its colocalization with AR in C3H 10T1/2 cells. A, Top, C3H 10T1/2 cells, pretreated with 5′-azacytidine, as described in Materials and Methods, were treated with medium alone (Con) or 100 nm testosterone (T) for 3 d (for MyoD), 6 d (for myogenin), or 12 d (for MHC II), and Western blot analysis was performed using 30 μg total cell lysate using anti-MyoD, myogenin, or MHC II antibodies. One representative gel has been shown of three independent experiments. Bottom, Quantitative densitometric analysis of Western blots from three independent gels after normalization with GAPDH. Data are mean ± sem. Student’s t test was performed for statistical analysis (*, P ≤ 0.05; **, P ≤ 0.01). B, Double-immunofluorescence analysis was performed to detect AR (green) and β-catenin (red). Colocalization of AR and β-catenin is indicated by yellow immunofluorescence in the merged fields. DAPI (blue) counterstain was used to localize the nuclei. Three independent experiments were performed, and a representative photograph is shown. C, Top, Cells were treated with or without testosterone (100 nm) for various time points as indicated. Three independent experiments were performed, and a representative gel is shown. Bottom, DHT-induced (10 nm DHT) nuclear translocation of β-catenin was restored to control levels by coincubation with AR antagonist bicalutamide (Bic). The experiment was repeated twice, and a representative gel is shown. Bic, Bicalutamide; Con, control wells; Cyto, cytoplasmic; Nuc, nuclear. D, Physical interaction of AR, β-catenin, and TCF-4 proteins in DHT-treated (0–30 nm) C3H 10T1/2 cells. Cells were also coincubated with 300 nm bicalutamide (Bic) along with DHT (10 nm) in some cases. The immunoprecipitation was performed using either anti-AR antibody or a nonspecific IgG, and the immunoprecipitates were analyzed by Western blot analysis using anti-AR, anti-β-catenin, or anti-TCF-4 antibodies. Three independent experiments were performed, and data from a representative experiment are presented.
Time course of nuclear translocation of β-catenin in C3H 10T1/2 cells by testosterone treatment
β-Catenin nuclear translocation was analyzed using nuclear and cytoplasmic fractions obtained after testosterone (100 nm) or DHT (10 nm) treatment for 0–60 min in C3H 10T1/2 cells. β-Catenin immunoreactivity was detectable in the nuclear fraction as early as 30 min after testosterone treatment and there was a concomitant decrease in β-catenin protein in the cytoplasmic fraction (Fig. 1C, top panel). Coincubation of DHT-treated cells with bicalutamide for 60 min resulted in lower β-catenin immunoreactivity in the nuclear fraction than in cells treated with DHT alone (Fig. 1C, bottom panel). These data indicate that testosterone promotes the nuclear translocation of β-catenin through an AR-mediated mechanism in C3H 10T1/2 cells.
Physical interaction of AR, β-catenin, and TCF-4 in DHT-treated C3H 10T1/2 cells
Protein extracts from C3H 10T1/2 cells after DHT (0–30 nm) treatment were immunoprecipitated using either IgG or anti-AR antibody, and protein complexes were detected by Western blot analyses using anti-β-catenin, anti-AR, or anti-TCF-4 antibodies (Fig. 1D). We did not detect β-catenin, AR, or TCF-4 bands when we performed immunoprecipitation reaction in which anti-AR antibody was replaced by nonspecific IgG (Fig. 1D, left). In cells not treated with 5′-azacytidine, the basal AR expression in these cells was below the limit of detection, and we were unable to detect any AR/β-catenin/TCF-4 interaction (data not shown) (20). Treatment of these cells with 5′-azacytidine up-regulated AR expression, consistent with our previous report (20); furthermore, incubation with DHT induced AR/β-catenin/TCF-4 interaction (Fig. 1D). The AR/β-catenin/TCF-4 interaction was significantly inhibited when DHT-treated cells were simultaneously incubated in the presence of 10-fold molar excess of bicalutamide (100 nm), an AR antagonist (Fig. 1D). Thus, the immunoprecipitation studies are consistent with our findings from immunofluorescence studies (Fig. 1B) and provide further evidence that after ligand binding, AR forms a complex with β-catenin and TCF-4.
Essential role of β-catenin in mediating testosterone’s effects on myogenic differentiation in C3H 10T1/2 cells
To determine whether β-catenin involvement is essential for mediating androgen effects on myogenic differentiation, we used two separate SiRNAs to block endogenous β-catenin expression and minimize the possibility of nonspecific off-target effects. Endogenous β-catenin protein expression was reduced by approximately 70–75% with both SiRNAs (Fig. 2B) as determined by densitometric analysis of β-catenin bands in control (random SiRNA) and SiRNA (SiRNA1 and SiRNA2) treatment groups (densitometric data not shown). Compared with control cells treated with random SiRNA, C3H 10T1/2 cells treated with β-catenin-specific SiRNAs (SiRNA1 and SiRNA2) exhibited a greater degree of adipogenic differentiation (Fig. 2A). The inhibition of β-catenin by SiRNAs not only limits the ability of testosterone to up-regulate myogenesis as indicated by significant reduction in the number of multinucleated myotubes but also results in significant induction of adipogenesis (Fig. 2A). We further analyzed the effects of β-catenin SiRNA on MyoD and MHC II, key myogenic differentiation markers in cells treated with and without testosterone. β-Catenin SiRNA treatments significantly decreased both MyoD and MHC II protein expression and blocked testosterone’s expected up-regulation of MyoD and MHC II expression in these cells (Fig. 2C). These data indicate that β-catenin plays an essential role in mediating testosterone’s effects on myogenic differentiation of C3H 10T1/2 cells, which was associated with a concomitant regulation of adipogenic differentiation.
Figure 2.
The effect of SiRNA-mediated inhibition of β-catenin on myogenic differentiation. A, Random SiRNA and β-catenin SiRNA (SiRNA1 and SiRNA2) transfected cells were treated with or without 100 nm testosterone (T, 100 nm) for 12 d, and the images of cells were obtained using a bright-field microscope. B, Cells were transfected with 100 nm either random SiRNA (Ran SiRNA) or β-catenin-specific SiRNA (SiRNA1 and SiRNA2) for 16 h and subsequently maintained in regular growth medium for 56 h for Western blot analysis of β-catenin levels. The experiment was repeated three times. C, Cells were transfected either with Ran SiRNA or β-catenin-specific SiRNA1 or SiRNA2 as described in B and treated with 100 nm testosterone (T). Cells were analyzed for MHC II (after 12 d) and MyoD (after 3 d) protein levels by Western blot. Three independent experiments were performed, and representative data are shown.
TCF-4 overexpression induces myogenic differentiation in C3H 10T1/2 cells
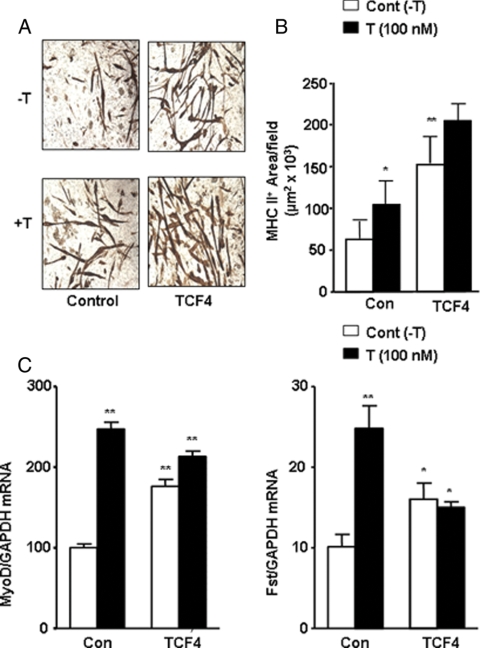
To investigate the role of AR/β-catenin/TCF-4-mediated pathways during testosterone’s action in C3H10T1/2 cells, we overexpressed full-length TCF-4 cDNA and tested the ability of testosterone to modulate myogenic differentiation under these conditions. We reasoned that if testosterone-induced AR and β-catenin interaction activates downstream Wnt signaling through TCF-4, then overexpression of full-length TCF-4 should directly up-regulate myogenesis. Under these conditions of TCF-4 overexpression, addition of testosterone would not be expected to produce any additional stimulation of myogenesis. In C3H 10T1/2 cells transfected with the TCF-4 cDNA expression construct, there was a significant increase in the area of MHC II+ myotubes (Fig. 3, A and B) and in MyoD expression, as analyzed by RT and real-time quantitative PCR (Fig. 3C, left). Testosterone treatment of cells transfected with TCF-4 cDNA did not significantly further increase MHC II+ myotube formation (Fig. 3B) and MyoD expression (Fig. 3C).
Figure 3.
TCF-4 overexpression in C3H 10T1/2 cells promotes myogenesis and up-regulates Fst mRNA expression. A, Cells were transfected with full-length TCF-4 or a control vector (control) and were allowed to differentiate in the absence (−T) or presence of 100 nm testosterone (T) for 12 d. Immunocytochemical staining was performed using anti-MHC II antibody. Magnification, ×100. B, Quantitative image analysis of MHC II-positive myotubes was performed by plotting total area of immunopositive cells per field. Three different replicates per group were performed, and data are represented as mean ± sem. Student’s t test was performed for comparison of control and testosterone-treated cells (*, P ≤ 0.05; **, P ≤ 0.01, testosterone vs. control group). C, RT and quantitative real-time PCR analysis demonstrating the effects of testosterone on MyoD and Fst mRNA in C3H 10T1/2 cells transfected with control vector or TCF-4 expression cDNA construct. Cells, transfected with control vector (con) or full-length TCF-4 (TCF-4) were allowed to differentiate for 3 d. Total cellular RNA was isolated, and MyoD and Fst mRNA expression was analyzed by RT and real-time PCR. Three different replicates were performed, and data are represented as mean ± sem. Student’s t test was performed for statistical analysis (*, P ≤ 0.05; **, P ≤ 0.01 compared with control group without testosterone treatment).
TCF-4 overexpression up-regulates Fst expression in C3H 10T1/2 cells
Testosterone (100 nm) treatment significantly up-regulated Fst expression (∼2.5-fold) compared with medium alone (Fig. 3C, right). Similarly, TCF-4 overexpression alone up-regulated Fst expression (∼1.7-fold) compared with control group; testosterone treatment of cells transfected with TCF-4 was unable to further induce Fst expression (Fig. 3D). These data show that both testosterone treatment and TCF-4 activation up-regulate Fst expression. Our data, therefore, suggest that Fst and MyoD act downstream of the TCF-4 pathway and that activation of TCF-4 by testosterone plays a central role during myogenesis.
Testosterone modulates the expression of several TGF-β signaling pathway genes
We considered the possibility that testosterone-induced up-regulation of Fst may inhibit TGF-β/Smad signaling. Accordingly, we performed gene-expression analysis of RNA samples obtained from 5′-azacytidine-treated C3H 10T1/2 cells in the presence or absence of testosterone (100 nm), using a TGF-β/Smad signaling pathway-specific gene array. Testosterone treatment of these cells for 3 h led to significant changes in several key genes in the TGF-β signaling pathway, including BMP10, FKBP 1B, Fst, LAP, IGF-I, and Smad7 (Fig. 4, A and B).
Figure 4.
Regulation of TGF-β signaling pathway genes by testosterone treatment. A, C3H 10T1/2 cells were treated with (+T) or without 100 nm testosterone (Con) for 3 h. Total cellular RNA was isolated, and mouse TGF-β signaling pathway genes were analyzed by SuperArray. Open circular spots represent some of the TGF-β signaling pathway target genes, which were regulated by testosterone treatment. The experiment was repeated two times, and a representative array is shown. B, Relative changes in some TGF-β signaling pathway target genes after normalization with GAPDH intensity. C, Verification of SuperArray data by RT and quantitative real-time PCR analysis. Total RNA isolated from C3H 10T1/2 cells treated with medium alone (con, black bars) or 100 nm testosterone (+T, white bars) were analyzed by RT and real-time PCR, using gene-specific primers for FKBP, BMP10, SMAD7, and FST. Three independent experiments were performed, and data shown are mean ± sem. Statistical analysis was performed by Student’s t test. **, P ≤ 0.01 compared with control group.
We also performed RT and real-time quantitative PCR analysis for selected genes (Fig. 4C) to validate SuperArray data. These quantitative RT-PCR analyses revealed concordant increases in BMP10, FKBP 1B, Fst, and Smad7.
Testosterone induces Smad7 protein expression in C3H 10T1/2 cells
Smad7 is an inhibitor transducer of TGF-β signaling that is down-regulated by TGF-β. To determine testosterone’s effects on the TGF-β-mediated expression of Smad7, we treated C3H 10T1/2 cells with recombinant TGF-β1 (5 ng/ml) either alone or in combination with 100 nm testosterone for 3 h. Testosterone treatment significantly increased Smad7 expression compared with controls (Fig. 5A). Incubation of cells with TGF-β1 alone down-regulated Smad7 expression, as expected. Cells treated with TGF-β1 and testosterone had higher Smad7 expression than cells treated with TGF-β1 alone or medium alone (Fig. 5A). Immunocytochemical staining using anti-Smad7 antibody confirmed a significant increase in Smad7 expression after testosterone treatment (Fig. 5B).
Figure 5.
Testosterone up-regulates Smad 7 and Fst protein expression both in vitro and in vivo. A, Top, Control or TGF-β1-treated (5 ng/ml) C3H 10T1/2 cells were incubated with or without testosterone (T) for 6 h, and 40 μg total protein lysates were analyzed by Western blot analysis using anti-Smad7 or anti-GAPDH antibody. The experiment was repeated three times, and a representative blot is shown. Bottom, Quantitative densitometric analysis of Western blots from three independent experiments after normalization with GAPDH is shown. Data are mean ± sem. *, P ≤ 0.05 vs. control (Con) group; #, P ≤ 0.05 vs. testosterone (T) group; ##, P ≤ 0.05 vs. TGF-β group. ANOVA was used to compare the difference between different groups, and individual groups were compared by using Tukey’s procedure. B, Immunocytochemical staining of C3H 10T1/2 cells for Smad7 expression. Control or TGF-β1-treated (5 ng/ml) C3H 10T1/2 cells were incubated with or without testosterone (T) for 6 h in six-well chamber slides, fixed with 2% paraformaldehyde, and stained with anti-Smad7 antibody. C and D, Quantitative real-time RT-PCR analysis of Fst (C) and Smad7 (D) mRNA levels in levator ani muscle. Total RNA isolated from levator ani muscle of 6-wk-old C57BL/6J male mice were subjected to RT and quantitative real-time PCR using gene-specific primers. Sham indicates sham operated; Cast, castrated with blank SILASTIC brand implants; 1 cm, castrated with 1-cm testosterone implants; and 2 cm, castrated with 2-cm testosterone implants. Treatment duration was 14 d. RNA was isolated from levator ani muscle of different animals, and quantitative real-time PCR was performed three times. Quadruplicate samples were analyzed each time for each treatment group. Data are presented as mean ± sem. Statistical analysis was performed by using ANOVA; pairwise comparisons between groups were performed by using Tukey’s procedure.
Surgical orchidectomy is associated with lower Fst and Smad7 expression, and testosterone supplementation of orchidectomized mice increases Fst and Smad7 expression dose dependently
To confirm the effects of testosterone on Fst and Smad7 expression in vivo, we determined the effects of surgical orchidectomy with and without testosterone replacement for 14 d in 6-wk-old sexually mature male C57BL/6J mice. Surgical orchidectomy was associated with a significant decrease in Fst (82 ± 9%) (Fig. 5C) and Smad7 (72 ± 12%) (Fig. 5D) mRNA expression in AR-abundant levator ani muscle. Fst and Smad7 expression in orchidectomized mice treated with 1-cm testosterone implants was greater than that in orchidectomized mice but not significantly different from that in saline-treated sham-operated mice (Fig. 5, C and D). Fst and Smad7 expression in orchidectomized mice treated with 2-cm testosterone implants was significantly higher than in sham-operated saline-treated mice.
In contrast to levator ani, an androgen-responsive muscle group in rodents (37), Fst and Smad 7 expression in gastrocnemius, an androgen-unresponsive muscle group in the mouse, showed little or no response to androgen deprivation or supplementation (data not shown).
Fst plays an essential role in mediating testosterone effects on myogenic differentiation
To determine whether Fst is essential for mediating testosterone’s stimulatory effects on myogenesis, C3H 10T1/2 cells maintained in myogenic conditions were treated with testosterone in the absence and presence of anti-Fst antibody (0.5 μg/ml). These cells were also treated with human recombinant Fst protein (0.2 μg/ml) and allowed to differentiate for 3 d (MyoD) or 12 d (MHC II). Testosterone and recombinant Fst both up-regulated the expression of myogenic differentiation markers MyoD and MHC II (Fig. 6, A and C). The stimulatory effects of testosterone on MyoD and MHC II expression were blocked by coincubation with anti-Fst antibody (Fig. 6, B and D) as analyzed by quantitative image analysis, indicating that Fst plays an essential role in mediating testosterone’s effects on myogenic differentiation.
Figure 6.
Inhibition of testosterone induction of MyoD and MHC II protein expression by anti-Fst antibody and induction of these proteins by recombinant Fst protein treatment. A, C3H 10T1/2 cells were treated as indicated in Materials and Methods and allowed to differentiate in myogenic medium for 3 d. Cells were fixed with 2% paraformaldehyde, and immunocytochemical analysis was performed using anti-MyoD antibody. The experiment was performed three times, and a representative photograph is shown. B, Quantitative image analysis of MyoD II+ cells. Three independent experiments were performed, and data are mean ± sem. **, P ≤ 0.001 for comparisons of groups, as shown in the figure. Statistical analysis was performed by using ANOVA; pairwise comparisons between groups were performed by Tukey’s procedure. C, Cells were treated for 12 d, and immunocytochemical analysis was performed using anti-MHC II antibody. The experiment was performed three times, and a representative photograph is shown. Magnification, ×400. D, Quantitative image analysis of MHC II+ cells. IOD, Integrated OD (area × average intensity). Three independent experiments were performed, and data are mean ± sem. **, P ≤ 0.01; #, P ≤ 0.05. Statistical analysis was performed by ANOVA; pairwise comparisons between groups were performed by Tukey’s procedure. Con, Control group with vehicle; T, 100 nm testosterone; T+Fst ab, 100 nm testosterone plus 0.5 μg/ml anti-Fst antibody; rFst, recombinant Fst protein (0.2 μg/ml).
Discussion
We have used mouse C3H 10T1/2 cells as our in vitro model system for investigating the mechanism of androgen-induced myogenesis. The C3H 10T1/2 multipotent cell line has been demonstrated to differentiate into various lineages including muscle cells after treatment with 5′-azacytidine (38,39). Several lines of evidence presented in this manuscript demonstrate that the anabolic effects of testosterone on myogenic differentiation are transduced through cross communication of AR signaling to Wnt and TGF-β pathways through mediation of β-catenin, TCF-4, and Fst. Androgens promote the association of AR with β-catenin, causing β-catenin to translocate into the nucleus. These data also provide evidence of the physical interaction of AR with β-catenin and TCF-4 after androgen treatment during myogenic differentiation of C3H 10T1/2 cells. Testosterone up-regulates Fst expression in vitro in C3H 10T1/2 cells as well as in vivo in the castrated rodent model. Orchidectomy lowers the expression of Fst and Smad7 mRNA, an effect that is reversed by testosterone supplementation. We have also demonstrated that testosterone modulates the expression of a number of key molecules in the TGF-β signaling pathway, including Smad7. Testosterone’s stimulatory effects on myogenic differentiation require the essential participation of β-catenin and Fst. Thus, the pro-myogenic effects of testosterone are transduced through cross talk among AR signaling, Wnt, and TGF-β pathways through mediation of β-catenin, TCF-4, and Fst.
Pioneering studies in the mid-20th century had suggested that testosterone increases muscle mass by promoting nitrogen retention (40). More recent studies using stable isotopes provided additional evidence that testosterone stimulates muscle protein synthesis and increases the efficiency of amino acid reuse by the skeletal muscle (7,10,12). However, the muscle protein synthesis hypothesis did not explain readily the reciprocal changes in fat mass that attended testosterone administration in men. In this regard, the hypothesis that androgens regulate the differentiation of mesenchymal stem cells (20) is attractive because it provides a unifying explanation for the reciprocal changes in skeletal muscle and fat mass induced by testosterone administration. The data presented in this manuscript provide a mechanistic framework of how androgens regulate mesenchymal multipotent cell differentiation. However, these data do not preclude the possibility that androgens have additional effects on muscle protein synthesis and satellite cell activation, as has been reported (41).
MacDougald and colleagues (23) have demonstrated a distinct role of the Wnt pathway in myogenic and adipogenic commitment of mesenchymal multipotent cells. Wnt/β-catenin signaling has been demonstrated to regulate myogenesis (26,42,43), and inhibition of β-catenin expression in mesenchymal cells results in a switch from myogenesis to adipogenesis (43). Dominant-negative β-catenin inhibits the expression of several myogenesis-specific transcription factors in the developing somite (44). Our data demonstrate that disruption of β-catenin expression by SiRNA blocks testosterone’s stimulatory effects on myotube formation and myogenic differentiation markers. We infer from these data that β-catenin-dependent mechanisms play an essential role during androgen regulation of myogenic differentiation in multipotent cells.
Consistent with studies in other systems (27,30), our data demonstrate that β-catenin interacts with AR (21,22,25) in C3H 10T1/2 cells and that AR and β-catenin are colocalized to the nucleus in a ligand-dependent manner. The signal generated by androgen binding to AR is communicated to the Wnt pathway through association of AR with β-catenin (24) and TCF-4, resulting in activation of a number of Wnt target genes, including Fst. Thus, androgen activation of Wnt target genes through β-catenin and TCF-4 bypasses the earlier steps in the canonical Wnt pathway that are proximal to β-catenin.
Our studies constitute the first evidence of an important role of Fst in mediating testosterone’s effects on myogenic differentiation in multipotent cells. Fst is known to antagonize several members of the TGF-β superfamily (28,45,46,47,48), including myostatin with which it interacts with high affinity to block its inhibitory effect on muscle growth and differentiation (28). Our in vitro data in C3H 10T1/2 cells, corroborated by in vivo data from the castrated mouse model, suggest that several key genes involved in TGF-β signaling, such as Fst and Smad7, are modulated by testosterone. Observations that testosterone’s effects on myogenic differentiation are blocked by using anti-Fst antibodies suggest that Fst plays an essential role in mediating these myogenic effects.
Although C3H 10T1/2 cells under myogenic conditions robustly form MHC II+ myotubes, the myogenic differentiation of these cells in vitro does not replicate the terminal stages of skeletal muscle fiber formation observed in vivo. Also, these cells require treatment with a demethylating agent, 5′-azacytidine, to induce myogenic differentiation (38,39). These data should be viewed in the context of these caveats. The concentrations of testosterone used in these experiments are similar to those observed in men receiving supraphysiological doses of testosterone (3).
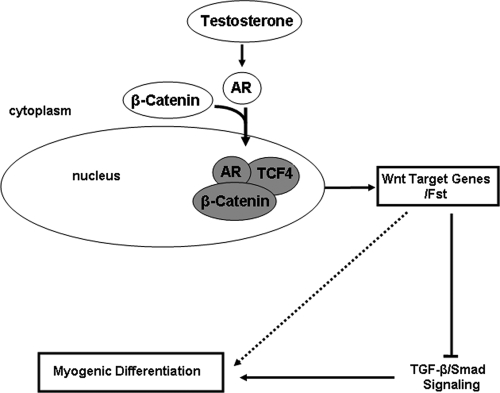
In summary, androgens regulate myogenic differentiation by inducing AR/β-catenin interaction followed by their nuclear localization to activate TCF-4 target genes in mesenchymal multipotent cells (Fig. 7). AR signal is cross communicated to the Wnt and TGF-β signaling pathways through mediation of β-catenin and Fst. Thus, β-catenin, TCF-4, and Fst play pivotal roles in linking AR signaling, Wnt target genes, and TGF β-regulated genes and mediating androgen effects on myogenesis. Our data do not exclude the possible involvement of additional androgen-dependent myogenic pathways (e.g. increased muscle protein synthesis mediated via IGF-I) (49,50). These data have therapeutic implications for the development of anabolic drugs; an understanding of the signaling cascades should facilitate discovery of pharmacophores that modulate these pathways.
Figure 7.
Proposed mechanism of testosterone action on muscle mass.
Acknowledgments
We thank Drs. Margaret E. Wierman (University of Colorado, Denver, CO) for TCF-4 construct and Dr. Alan Schneyer (University of Massachusetts, Boston, MA) for antimouse Fst antibody. We also thank Astra Zeneca for providing bicalutamide.
Footnotes
This work was supported by National Institutes of Health Grants 1S06-GM068510-03 and 1SC1AG033407-01A1 (R.S.) and in part by 1RO1DK70431-01 (S.B.), 1RO3-HD53888 (S.P.), 3S06-GM068510- 2S1 (R.J.), Research Centers in Minority Institutions Grant G12RR03026 and U54 RR019234.
Disclosure Statement: The authors have nothing to disclose.
First Published Online October 23, 2008
Abbreviations: AR, Androgen receptor; DAPI, 4′,6-diamidino-2-phenylindole; DHT, dihydrotestosterone; Fst, follistatin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MHC II, myosin heavy chain II; SiRNA, small inhibitory RNA; TCF-4, T-cell factor-4.
References
- Wilson JD 1988 Androgen abuse by athletes. Endocr Rev 9:181–199 [DOI] [PubMed] [Google Scholar]
- Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R 1996 The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med 335:1–7 [DOI] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Casaburi R., Singh AB, Bhasin D, Berman N, Chen X, Yarasheski KE, Magliano L, Dzekov C, Dzekov J, Bross R, Phillips J, Sinha-Hikim I, Shen R, Storer TW 2001 Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab 281:E1172–E1181 [DOI] [PubMed] [Google Scholar]
- Woodhouse LJ, Reisz-Porszasz S, Javanbakht M, Storer TW, Lee M, Zerounian H, Bhasin S 2003 Development of models to predict anabolic response to testosterone administration in healthy young men. Am J Physiol Endocrinol Metab 284:E1009–E1017 [DOI] [PubMed] [Google Scholar]
- Blackman MR, Sorkin JD, Munzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE, Jayme J, O'Connor KG, Christmas C, Tobin JD, Stewart KJ, Cottrell E, St Clair C, Pabst KM, Harman SM 2002 Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA 288:2282–2292 [DOI] [PubMed] [Google Scholar]
- Herbst K, Bhasin S 2004 Testosterone action on skeletal muscle. Curr Opin Clin Nutr Metab Care 7:271–277 [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ 2002 Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab 282:E601–E607 [DOI] [PubMed] [Google Scholar]
- Sih R, Morley JE, Kaiser FE, Perry 3rd HM, Patrick P, Ross C 1997 Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab 82:1661–1667 [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Peachy H, Hannoush P, Berlin JA, Loh L, Lenrow DA, Holmes JH, Dlewati A, Santanna J, Rosen CJ, Strom BL 1999 Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab 84:2647–2653 [DOI] [PubMed] [Google Scholar]
- Urban RJ, Bodenburg YH, Gilkison C, Foxworth J, Coggan AR, Wolfe RR, Ferrando A 1995 Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol 269:E820–E826 [DOI] [PubMed] [Google Scholar]
- Bhasin S, Storer TW, Berman N, Yarasheski K, Clevenger B, Phillips J, Lee M, Bunnell TJ, Casaburi R 1997 Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J Clin Endocrinol Metab 82:407–413 [DOI] [PubMed] [Google Scholar]
- Brodsky IG, Balgopal P, Nair KS 1996 Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men: a clinical research center study. J Clin Endocrinol Metab 81:3469–3475 [DOI] [PubMed] [Google Scholar]
- Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A 1996 Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab 81:4358–4365 [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Peachy H, Berlin JA, Hannoush P, Haddad G, Dlewati A, Santanna J, Loh L., Lenrow DA, Holmes JH, Kapoor SC, Atkinson LE, Strom BL 2000 Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab 85:2670–2677 [DOI] [PubMed] [Google Scholar]
- Wang C, Swerdloff RS, Iranmanesh A, Dobs A, Snyder PJ, Cunningham G, Matsumoto AM, Weber T, Berman N 2000 Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 85:2839–2853 [DOI] [PubMed] [Google Scholar]
- Kenny AM, P. K., Gruman CA, Marcello KM, Raisz LG 2001 Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci 56:M266–M272 [DOI] [PubMed] [Google Scholar]
- Morley JE, Perry HM 3rd, Kaiser FE, Kraenzle D, Jensen J, Houston K, Mattammal M, Perry Jr HM 1993 Effects of testosterone replacement therapy in old hypogonadal males: a preliminary study. J Am Geriatr Soc 41:149–152 [DOI] [PubMed] [Google Scholar]
- Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL 2005 Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab 90:1502–1510 [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I, Artaza JN, Woodhouse L, Gonzalez-Cadavid N, Singh AB, Lee MI, Storer TW, Casaburi R, Shen R, Bhasin S 2002 Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab 283:E154–E164 [DOI] [PubMed] [Google Scholar]
- Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S 2003 Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology 144:5081–5088 [DOI] [PubMed] [Google Scholar]
- Mulholland DJ, Cheng H, Reid K, Rennie PS, Nelson CC 2002 The androgen receptor can promote β-catenin nuclear translocation independently of adenomatous polyposis coli. J Biol Chem 277:17933–17943 [DOI] [PubMed] [Google Scholar]
- Pawlowski JE, Ertel JR, Allen MP, Xu M, Butler C, Wilson EM, Wierman ME 2002 Liganded androgen receptor interaction with β-catenin: nuclear co-localization and modulation of transcriptional activity in neuronal cells. J Biol Chem 277:20702–20710 [DOI] [PubMed] [Google Scholar]
- Ross SE, Hemati N, Longo KA, Bebbett CN, Jucas PC, Erickson RL, MacDougald OA 2000 Inhibition of adipogenesis by Wnt signaling. Science 289:950–953 [DOI] [PubMed] [Google Scholar]
- Singh R, Artaza JN, Taylor WE, Braga M, Yuan X, Gonzalez-Cadavid N, Bhasin S 2006 Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with β-catenin and T-cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology 1:141–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Li X, Sharma M, Sasaki CY, Longo DL, Lim B, Sun Z 2002 Linking β-catenin to androgen-signaling pathway. J Biol Chem 277:11336–11344 [DOI] [PubMed] [Google Scholar]
- Vertino AM, Taylor-Jones JM, Longo KA, Bearden ED, Lane TF, MacGehee Jr RE, MacDougald OA, Peterson CA 2005 Wnt10b deficiency promotes coexpression of myogenic and adipogenic programs in myoblasts. Mol Cell Biol 16:2039–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert J, Epping M, Pollack JR, Brown PO, Nusse R 2002 A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Dev Biol 2:2–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor H, Nicholas G, McKinnell I, Kemp CF, Sharma M, Kambadur R, Patel K 2004 Follistatin complexes myostatin and antagonizes myostatin-mediated inhibition of myogenesis. Dev Biol 270:19–30 [DOI] [PubMed] [Google Scholar]
- Hill JJ, Davies MV, Pearson AA, Wang JH, Hewick RM, Wolfman NM, Qiu Y 2002 The myostatin propeptide and the follistatin-related gene are inhibitory binding proteins of myostatin in normal serum. J Biol Chem 277:40735–40741 [DOI] [PubMed] [Google Scholar]
- Welt C, Sidis Y, Keutmann H, Schneyer A 2002 Activins, inhibins, and follistatins: from endocrinology to signaling. A paradigm for the new millennium. Exp Biol Med 227:724–752 [DOI] [PubMed] [Google Scholar]
- Zimmer TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ 2002 Induction of cachexia in mice by systemically administered myostatin. Science 296:1486–1488 [DOI] [PubMed] [Google Scholar]
- De Angelis L, Borghi S, Melchionna R, Berghella L, Baccarani-Contri M, Parise F, Ferrari S, Cossu G 1998 Inhibition of myogenesis by transforming growth factor β is density-dependent and related to the translocation of transcription factor MEF2 to the cytoplasm. Proc Natl Acad Sci USA 95:12358–12363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florini JR, Roberts AB, Ewton DZ, Falen SL, Flanders KC, Sporn MB 1986 Transforming growth factor-β. A very potent inhibitor of myoblast differentiation, identical to the differentiation inhibitor secreted by Buffalo rat liver cells. J Biol Chem 261:16509–16513 [PubMed] [Google Scholar]
- Liu D, Kang JS, Derynck R 2004 TGF-β-activated Smad3 represses MEF2-dependent transcription in myogenic differentiation. EMBO J 23:1557–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J, Cheifetz S, Endo T, Nadal-Ginard B 1986 Type β transforming growth factor is an inhibitor of myogenic differentiation. Proc Natl Acad Sci USA 83:8206–8210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs ML, Nagarajan R, Taylor-Jones JM, Nolen G, Macnicol M, Peterson CA 2004 Alterations in the TGFβ signaling pathway in myogenic progenitors with age. Aging Cell 3:353–361 [DOI] [PubMed] [Google Scholar]
- Monks DA, O'Bryant EL, Jordan CL 2004 Androgen receptor immunoreactivity in skeletal muscle: enrichment at the neuromuscular junction. J Comp Neurol 473:59–72 [DOI] [PubMed] [Google Scholar]
- Lassar AB, Paterson BM, Weintraub H 1986 Transfection of a DNA locus that mediates the conversion of 10T1/2 fibroblasts to myoblasts. Cell 47:649–656 [DOI] [PubMed] [Google Scholar]
- Taylor SM, Jones PA 1979 Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell 17:771–779 [DOI] [PubMed] [Google Scholar]
- Kochakian CD, Tillotson C 1957 Influence of several C19 steroids on the growth of individual muscles of the guinea pig. Am J Physiol 189:425–427 [DOI] [PubMed] [Google Scholar]
- Nnodim JO 2001 Testosterone mediates satellite cell activation in denervated rat levator ani muscle. Anat Rec 263:19–24 [DOI] [PubMed] [Google Scholar]
- Pan W, Jia Y, Wang J, Tao D, Gan X, Tsiokas L, Jing N, Wu D, Li L 2005 β-Catenin regulates myogenesis by relieving I-mfa-mediated suppression of myogenic regulatory factors in P19 cells. Proc Natl Acad Sci USA 102:17378–17383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango NA, Szotek PP, Manganaro TF, Oliva E, Donahoe PK, Teixeira J 2005 Conditional deletion of β-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol 288:276–283 [DOI] [PubMed] [Google Scholar]
- Petropoulos H, Skerjanc IS 2002 β-Catenin is essential and sufficient for skeletal myogenesis in P19 cells. J Biol Chem 277:15393–15399 [DOI] [PubMed] [Google Scholar]
- Harrington AE, Morris-Triggs SA, Ruotolo BT, Robinson CV, Ohnuma S, Hyvonen M 2006 Structural basis for the inhibition of activin signalling by follistatin. EMBO J 25:1035–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Takio K, Eto Y, Shibai H, Titani K, Sugino H 1990 Activin-binding protein from rat ovary is follistatin. Science 247:836–838 [DOI] [PubMed] [Google Scholar]
- Sidis Y, M. A., Keutmann H, Delbaere A, Sadatsuki M, Schneyer A 2006 Biological activity of follistatin isoforms and follistatin-like-3 is dependent on differential cell surface binding and specificity for activin, myostatin, and bone morphogenetic proteins. Endocrinology 147:3586–3597 [DOI] [PubMed] [Google Scholar]
- Link BA, Nishi R 1997 Opposing effects of activin A and follistatin on developing skeletal muscle cells. Exp Cell Res 233:350–362 [DOI] [PubMed] [Google Scholar]
- Ravaglia G, Forti P, Maioli F, Pratelli L, Cucinotta D, Bastagli L, Cavalli G 2000 Body composition, sex steroids, IGF-1, and bone mineral status in aging men. J Gerontol A Biol Sci Med Sci 55:M516–M521 [DOI] [PubMed] [Google Scholar]
- Sheffield-Moore M, Paddon-Jones D, Casperson SL, Gilkison C, Volpi E, Wolf SE, Jiang J, Rosenblatt JI, Urban RJ 2006 Androgen therapy induces muscle protein anabolism in older women. J Clin Endocrinol Metab 91:3844–3849 [DOI] [PubMed] [Google Scholar]