Abstract
Glucagon-like peptide-1 (GLP-1) diminishes postmeal glucose excursions by enhancing insulin secretion via activation of the β-cell GLP-1 receptor (Glp1r). GLP-1 may also control glucose levels through mechanisms that are independent of this incretin effect. The hyperinsulinemic-euglycemic clamp (insulin clamp) and exercise were used to examine the incretin-independent glucoregulatory properties of the Glp1r because both perturbations stimulate glucose flux independent of insulin secretion. Chow-fed mice with a functional disruption of the Glp1r (Glp1r−/−) were compared with wild-type littermates (Glp1r+/+). Studies were performed on 5-h-fasted mice implanted with arterial and venous catheters for sampling and infusions, respectively. During insulin clamps, [3-3H]glucose and 2[14C]deoxyglucose were used to determine whole-body glucose turnover and glucose metabolic index (Rg), an indicator of glucose uptake. Rg in sedentary and treadmill exercised mice was determined using 2[3H]deoxyglucose. Glp1r−/− mice exhibited increased glucose disappearance, muscle Rg, and muscle glycogen levels during insulin clamps. This was not associated with enhanced muscle insulin signaling. Glp1r−/− mice exhibited impaired suppression of endogenous glucose production and hepatic glycogen accumulation during insulin clamps. This was associated with impaired liver insulin signaling. Glp1r−/− mice became significantly hyperglycemic during exercise. Muscle Rg was normal in exercised Glp1r−/− mice, suggesting that hyperglycemia resulted from an added drive to stimulate glucose production. Muscle AMP-activated protein kinase phosphorylation was higher in exercised Glp1r−/− mice. This was associated with increased relative exercise intensity and decreased exercise endurance. In conclusion, these results show that the endogenous Glp1r regulates hepatic and muscle glucose flux independent of its ability to enhance insulin secretion.
During increased glucose flux, the glucagon-like peptide-1 receptor regulates endogenous glucose production and muscle glucose uptake independent of its ability to stimulate insulin secretion.
The ingestion of food stimulates the secretion of glucagon-like peptide (GLP)-1 and glucose-dependent insulinotropic polypeptide (GIP) from intestinal enteroendocrine cells. These hormones mediate the increased secretion of insulin in response to oral vs. iv glucose delivery, a phenomenon known as the incretin effect (1,2,3). This occurs via activation of specific G protein-coupled receptors for GLP-1 (Glp1r) and GIP (Gipr) expressed not only in pancreatic islets but also lungs, kidneys, heart, adipose tissue, and the central and peripheral nervous system (4,5,6,7). GLP-1, but not GIP, also inhibits glucagon secretion and delays gastric emptying (8,9,10,11), thereby diminishing glucose excursions after a meal.
Since its discovery, it has been suggested that GLP-1 exerts glucoregulatory properties that are independent of its ability to modulate pancreatic secretions and gastric emptying. In type 1 diabetic patients, simultaneous peripheral GLP-1 infusion and enteral glucose delivery resulted in increased glucose disappearance, even as arterial insulin and glucose levels were clamped (12). Subsequent studies further suggested that GLP-1 enhances glucose utilization and suppresses hepatic glucose production when insulin and glucagon levels are experimentally controlled (13,14,15,16,17,18,19). However, other studies suggest that any glucoregulatory effects of GLP-1 are solely due to its ability to regulate the secretion of pancreatic hormones (20,21,22,23,24,25,26). Thus, the notion that GLP-1 can directly regulate glucose production and utilization independent of its effects on the pancreas remains disputed.
The aim of the present studies was to determine whether the endogenous GLP-1 receptor regulates hepatic glucose production and muscle glucose uptake (MGU) independent of its ability to enhance insulin secretion. To this end, the hyperinsulinemic-euglycemic clamp (insulin clamp) and exercise were used to stimulate glucose flux in mice lacking Glp1r expression. Glp1r knockout (Glp1r−/−) mice exhibit oral glucose intolerance and impaired insulin secretion, but basal glucose metabolism is otherwise normal (27,28). During an insulin clamp, glucose flux is stimulated by experimentally controlled hyperinsulinemia. Conversely, with exercise, glucose flux is stimulated via insulin-independent mechanisms (29). Thus, these two metabolic perturbations were used to reveal glucoregulatory properties of the Glp1r that are both insulin dependent and insulin independent.
Materials and Methods
Mouse maintenance and genotyping
All procedures performed were approved by the Vanderbilt University Animal Care and Use Committee. At 3 wk of age, wild-type (Glp1r+/+) and Glp1r knockout (Glp1r−/−) littermates on the C57BL/6 background were separated by sex and were placed on a standard chow diet (Purina 5001; Purina Mills, St. Louis, MO). Genotyping was performed by PCR on genomic DNA obtained from tail biopsies. All experiments were performed on mice at about 4 months of age. Body composition was determined on 5-h-fasted mice using an mq10 nuclear magnetic resonance analyzer (Bruker Optics, The Woodlands, TX). Mice were maintained on a standard light-dark cycle (0600–1800 h light).
Surgical procedures
Catheters were implanted in the left common carotid artery and right jugular vein for sampling and infusions, respectively, as previously described (30,31), except surgeries were performed under inhaled anesthesia (VetEquip, Pleasanton, CA). Animals were individually housed after surgery and allowed to recover for 5–7 d, during which time body weight was recorded daily. Mice whose weight did not return to within 10% of presurgery weight were excluded.
Hyperinsulinemic-euglycemic clamps
After 5 d of recovery, insulin clamps were performed on 5-h-fasted mice (30,31,32). A 5-μCi bolus of [3-3H]glucose was given at t = −90 min before insulin infusion, followed by a 0.05-μCi · min−1 infusion for 90 min. Blood samples were obtained via the arterial catheter (30,31,32). Basal glucose-specific activity was determined from blood samples at t = −15 and −5 min. Fasting insulin and nonesterified fatty acid (NEFA) levels were determined from blood samples taken at t = −5 min. The clamp was begun at t = 0 min with a continuous infusion of human insulin (4 mU · kg−1 · min−1; Humulin R; Eli Lilly, Indianapolis, IN). The [3-3H]glucose infusion was increased to 0.15 μCi · min−1 for the remainder of the experiment. Euglycemia (∼150–160 mg · dl−1) was maintained by measuring blood glucose every 10 min starting at t = 0 min and infusing 50% dextrose as necessary. Mice received saline-washed erythrocytes from donors throughout the clamp (5–6 μl · min−1) to prevent a fall of greater than 5% hematocrit. A 12-μCi bolus of 2[14C]deoxyglucose (DG) was given at t = 120 min. Blood samples (80–240 μl) were taken every 10 min from t = 80 to 135 min and processed to determine plasma [3-3H]glucose and 2[14C]DG. Clamp insulin and NEFA was determined at t = 120 min. At t = 135 min, mice were anesthetized with sodium pentobarbital. The soleus, gastrocnemius, superficial vastus lateralis (SVL), liver, diaphragm, heart, and brain were excised, immediately frozen, and stored at −80 C until analyzed. In a separate set of experiments, liver and gastrocnemius from 5-h-fasted mice were excised for determination of basal glycogen content.
Exercise experiments
After 5 d of recovery, mice were acclimated to treadmill running with a single 10-min exercise bout (15.5 m · min−1, 0% grade). Exercise experiments were performed 2 d after this acclimation trial. All exercise experiments were performed on 5-h-fasted mice. One hour before the exercise bout, mice were placed in the treadmill for acclimation. At t = 0 min, an arterial sample (100 μl) was taken for the measurement of baseline blood glucose, hematocrit, and plasma insulin and NEFAs. Mice either remained sedentary or ran on the treadmill for 30 min at 16 m · min−1, 0% grade. This work intensity is about 75% of maximal oxygen consumption in mice (33). Mice were encouraged to run with the use of an electric grid placed at the back end of the treadmill (1.5 mA, 200 msec pulses, 4 Hz). At t = 5 min, a 12-μCi bolus of 2[3H]DG was administered via the jugular vein catheter. At t = 7, 10, 15, and 20 min, arterial samples (∼50 μl) were taken to determine blood glucose and plasma 2[3H]DG. At t = 30 min, an arterial sample (150 μl) was taken for the measurement of blood glucose; hematocrit; and plasma insulin, NEFAs, and 2[3H]DG. Mice were then anesthetized and tissues were excised and stored as in clamp experiments.
Exercise stress and exercise endurance testing
Whole-body O2 consumption (VO2) was measured in mice placed in an enclosed treadmill using an Oxymax Deluxe system (Columbus Instruments, Columbus, OH) with an airflow rate of 1 liter · min−1. For exercise stress testing, mice were placed in the enclosed treadmill and allowed to acclimate for at least 30 min. Resting VO2 was determined as the average of measurements over the 10 min before the beginning of the stress test. Mice then began running at 10 m · min−1, 0% grade, and the speed was increased by 4 m · min−1 every 3 min until exhaustion. Mice were encouraged to run with the use of an electric grid placed at the back end of the treadmill (1.5 mA, 200 msec pulses, 4 Hz). Mice were defined as exhausted when they spent more than 5 continuous seconds on the electric grid. Maximal VO2 (VO2,max) was achieved when VO2 no longer increased despite an increase in work rate.
Endurance capacity was determined at least 5 d after maximal testing. Mice were placed in the enclosed treadmill to acclimate and resting VO2 was determined as before. Mice were run at 20 m · min−1 until exhausted, with exhaustion defined as before. Exercise VO2 was determined as the average of measurements over the 10 min before exhaustion. Postexercise VO2 was determined as the average of measurements over the 10 min immediately after exhaustion. Respiratory exchange ratio (RER) was calculated as CO2 production/VO2.
Processing of plasma and tissue samples
Insulin levels were determined by ELISA (Linco, St. Charles, MO). NEFAs were measured spectrophotometrically by an enzymatic colorimetric assay (Wako NEFA HR(2) kit; Wako Chemicals, Richmond, VA). Plasma [3-3H]glucose and 2[14C]DG (insulin clamps) or plasma 2[3H]DG (exercise) and tissue 2[14C]DG-6-phosphate 2[14C]DGP or tissue 2[3H]DG-6-phosphate (2[3H]DGP) radioactivity were determined as previously described (31,32). The accumulation of 2[14C]DGP or 2[3H]DGP was normalized to tissue weight. Liver and gastrocnemius glycogen was determined by the method of Chan and Exton (34).
Protein immunoblots
Liver and muscle (gastrocnemius) tissue (20–40 mg) was homogenized in 10 μl · mg−1 tissue extraction buffer [50 mm Tris, 1 mm EDTA, 1 mm EGTA, 10% glycerol, 1% Triton X-100 (pH 7.5)] supplemented with protease (Pierce, Rockford, IL) and phosphatase (Sigma, St. Louis, MO) inhibitor cocktails. Homogenates were centrifuged (20 min, 4500 × g, 4 C), pellets were discarded, and supernatants were retained for protein determination. Protein content was determined using a BCA protein assay kit (Bio-Rad, Hercules, CA). Whole-cell (20–100 μg) extracts were separated on 10% Bis-Tris SDS-PAGE gels (Invitrogen, Carlsbad, CA), followed by electrophoretic transfer to polyvinylidene fluoride membranes. Primary antibodies were incubated with the membranes overnight at 4 C. Secondary antibodies were incubated at room temperature for 1 h. Imaging and densitometry were performed using the Odyssey imaging system (Li-COR, Lincoln, NE). Antibodies for Akt, phosphorylated Akt (Ser473), glycogen synthase kinase (GSK)-3β, phosphorylated GSK-3β (Ser9), total (α1/α2) AMP-activated protein kinase (AMPK), and phosphorylated AMPK (Thr172) were from Cell Signaling (Beverly, MA). Antibody for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was from Abcam (Berkeley, CA).
Calculations
Whole-body glucose appearance (Ra) and disappearance (Rd) were determined using Steele nonsteady-state equations (35,36). Endogenous glucose production (endoRa) was determined by subtracting the glucose infusion rate (GIR) from total glucose appearance. Glucose metabolic index (Rg) and glucose clearance (Kg) were calculated as previously described (37,38) and were normalized to values obtained in the brain.
Statistical analysis
Data are presented as means ± sem. Differences between groups were determined by one-way ANOVA followed by Tukey’s post hoc tests or by two-tailed t test as appropriate. The significance level was P < 0.05.
Results
Glp1r−/− mice exhibit enhanced insulin-stimulated MGU but impaired suppression of endoRa
Baseline characteristics in 5-h-fasted mice undergoing insulin clamps are shown in Table 1. Total, fat, and muscle mass was not different between genotypes. There were no differences in fasting glucose, insulin, and NEFA levels among genotypes.
Table 1.
Basal (5 h fasted) and insulin clamp characteristics
| Glp1r+/+ | Glp1r−/− | |
|---|---|---|
| n (male/female) | 12 (6/6) | 11 (5/6) |
| Weight (g) | 24 ± 1 | 23 ± 1 |
| Lean mass (g) | 19 ± 1 | 17 ± 1 |
| Fat mass (g) | 2.7 ± 0.3 | 2.5 ± 0.1 |
| Arterial glucose (mg · dl−1) | ||
| Basal | 143 ± 5 | 145 ± 8 |
| Insulin clamp | 156 ± 3 | 158 ± 3 |
| Insulin (ng · ml−1) | ||
| Basal | 0.8 ± 0.1 | 0.6 ± 0.2 |
| Insulin clamp | 3.4 ± 0.4 | 2.9 ± 0.3 |
| NEFA (mm) | ||
| Basal | 1.1 ± 0.1 | 1.2 ± 0.1 |
| Insulin clamp | 0.6 ± 0.1 | 0.6 ± 0.2 |
| GIR (mg · kg−1 · min−1) | 41 ± 3 | 53 ± 5a |
| Insulin sensitivity index (mg · ml) · (ng · kg · min)−1 | 14 ± 2 | 21 ± 4a |
Basal values represent averages from samples taken at t = −15 and −5 min. Insulin clamp values and GIR represent averages from samples taken at t = 80–120 min. Insulin sensitivity index is calculated as the ratio of GIR to clamp insulin levels.
P < 0.05 vs. Glp1r+/+.
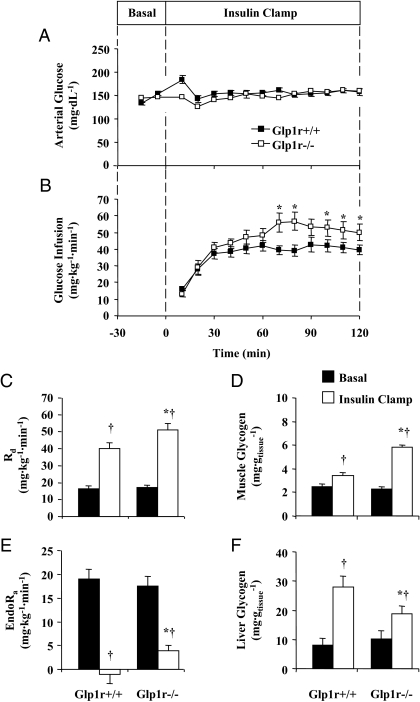
Insulin action was assessed in conscious, unrestrained mice via the insulin clamp. This approach was used to determine the role of the Glp1r in the regulation of hepatic glucose production (endo Ra) and MGU under controlled hyperinsulinemic conditions. Arterial glucose was clamped at similar levels (∼150 mg · dl−1) in Glp1r+/+ and Glp1r−/− mice (Fig. 1A). The GIR necessary to maintain euglycemia was significantly higher in Glp1r−/− mice (Fig. 1B and Table 1). Clamp insulin and NEFA levels were not different between genotypes (Table 1). Normalizing the GIR to clamp insulin levels, an index of whole-body insulin sensitivity, shows that disruption of Glp1r expression results in an approximately 1.5-fold increase in insulin sensitivity (Table 1). This was due to an effect on insulin-stimulated Rd, which was enhanced in Glp1r−/− mice (Fig. 1C). Paralleling this, muscle glycogen accumulation was significantly greater in Glp1r−/− mice (Fig. 1D). In contrast to the effects on Rd, suppression of endoRa was significantly impaired in Glp1r−/− mice (Fig. 1E). This was associated with decreased glycogen accumulation in the liver (Fig. 1F).
Figure 1.
Insulin clamp experiments in 5-h-fasted mice. Arterial glucose (A) and GIR (B) during insulin clamps are presented for Glp1r+/+ (black squares) and Glp1r−/− (white squares) mice. Rd (C), muscle (gastrocnemius) glycogen (D), endoRa (E), and liver glycogen (F) from 5-h-fasted (basal, black bars) mice and mice from clamp experiments (insulin clamp, white bars) are presented. Data are mean ± sem for 11–12 mice/genotype. *, P < 0.05 vs. Glp1r+/+; †, P < 0.05 vs. basal within the same genotype.
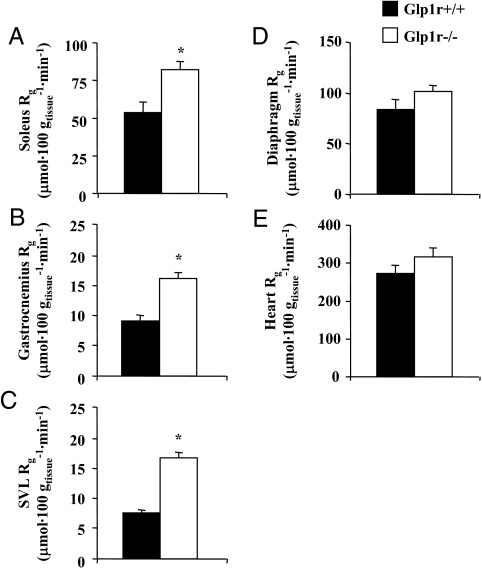
Rg was measured to assess the effect of disrupting Glp1r expression on glucose uptake in specific tissues. As shown in Fig. 2, A–C, Rg in hindlimb skeletal muscles was significantly elevated in Glp1r−/− mice. Rg in the diaphragm and heart was not significantly different between genotypes (Fig. 2, D and E). Taken together, these results show that disruption of Glp1r expression results in enhanced insulin-stimulated MGU and muscle glycogen accumulation but impaired suppression of endogenous glucose production and hepatic glycogen accumulation.
Figure 2.
Rg after insulin clamp experiments in 5-h-fasted mice. Rg in soleus (A), gastrocnemius (B), SVL (C), diaphragm (D), and heart (E) are presented for Glp1r+/+ (black bars) and Glp1r−/− (white bars) mice. Data are mean ± sem for 11–12 mice/genotype. *, P < 0.05 vs. Glp1r+/+.
Disruption of Glp1r expression does not enhance muscle insulin signaling but does impair hepatic insulin signaling
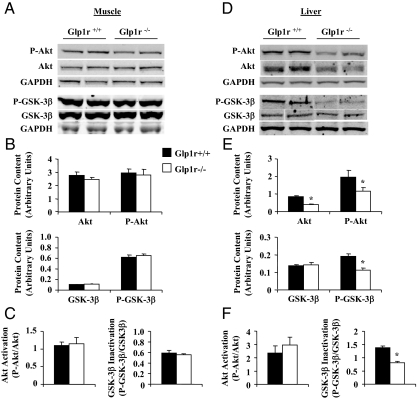
To determine whether increased muscle Rg in Glp1r−/− mice was due to enhanced insulin action, activation of insulin signaling proteins was assessed (Fig. 3). Disruption of Glp1r expression did not increase levels of phosphorylated or total Akt in muscle (Fig. 3B). Thus, activation of muscle Akt, defined as the ratio of phosphorylated to total Akt, was not different between genotypes (Fig. 3C). Phosphorylation of muscle GSK-3β, which inactivates this enzyme and promotes glycogen synthesis, was also not affected by the lack of Glp1r expression (Fig. 3, B and C). These results show that increased muscle Rg and muscle glycogen accumulation in Glp1r−/− mice is not associated with enhanced insulin signaling.
Figure 3.
Immunoblots for insulin signaling proteins after insulin clamp experiments in 5-h-fasted mice. Representative immunoblots from gastrocnemius muscle (A) and liver (D) extracts are shown. Protein content and protein activation is presented for Glp1r+/+ (black bars) and Glp1r−/− (white bars) mice. Protein content of Ser473-phosphorylated Akt (P-Akt), total Akt, Ser9-phosphorylated GSK-3β (P-GSK-3β), and total GSK-3β (GSK-3β) is shown from muscle (B) and liver (E) extracts. Protein content is normalized to the GAPDH loading control. Akt activation (P-Akt/Akt) and GSK-3β inactivation (P-GSK-3β/GSK-3β) are also shown from muscle (C) and liver (F) extracts. Inactivation of GSK-3β promotes glycogen synthesis. Data are mean ± sem for 11–12 mice/genotype. *, P < 0.05 vs. Glp1r+/+.
In contrast with muscle phenotypes, the inability of insulin to completely suppress endoRa in Glp1r−/− mice does correlate with impaired hepatic insulin signaling. Although Akt activation was not different between genotypes, levels of phosphorylated and total Akt were decreased in Glp1r−/− mice (Fig. 3, E and F). Furthermore, decreased hepatic glycogen accumulation in Glp1r−/− mice was associated with decreased phosphorylation of GSK-3β (Fig. 3, E and F).
Glp1r−/− mice exhibit hyperglycemia during exercise even as MGU is normal
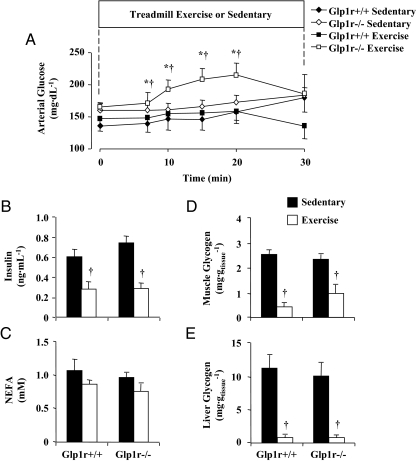
Exercise stimulates glucose flux in the absence of an increase in insulin levels. This physiological intervention was used to further characterize the glucoregulatory properties of the Glp1r that are independent of its ability to stimulate insulin secretion. During moderate exercise, increased glucose use by the working muscle is typically matched by an increase in hepatic glucose production (29). The consequence of this is that arterial glucose levels do not vary appreciably during exercise, as was observed in Glp1r+/+ mice (Fig. 4A). Such was not the case in Glp1r−/− mice, which exhibited exercise-induced hyperglycemia (Fig. 4A). This could not be attributed to an effect on the dynamics of insulin because both genotypes showed a similar decrease in the levels of this hormone (Fig. 4B). Plasma NEFA levels were not different between the genotypes in sedentary or exercised mice (Fig. 4C). Similarly, muscle and liver glycogen levels were not different between genotypes in either sedentary or exercised mice (Fig. 4, D and E).
Figure 4.
Exercise experiments in 5-h-fasted mice. Arterial glucose (A) levels are presented for Glp1r+/+ (black diamonds and squares) and Glp1r−/− (white diamonds and squares) mice that remained sedentary (diamonds) or underwent treadmill exercise (squares) for 30 min. Insulin (B), NEFA (C), muscle (gastrocnemius) glycogen (D), and liver glycogen (E) levels in sedentary (black bars) and exercised (white bars) Glp1r+/+ and Glp1r−/− mice are shown. Data are mean ± sem for 14–16 mice/genotype. *, P < 0.05 vs. Glp1r+/+ exercise; †, P < 0.05 vs. Glp1r−/− sedentary.
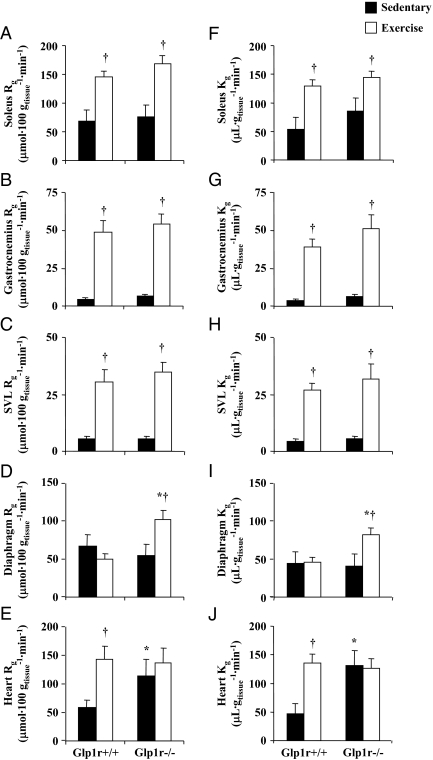
To assess whether exercise-induced hyperglycemia in Glp1r−/− mice was due to a defect in MGU, Rg was measured in various muscle types (Fig. 5). Rg in hindlimb skeletal muscles was not different between genotypes in sedentary mice and was equally increased in response to exercise (Fig. 5, A–C). Whereas Rg in the diaphragm did not increase with exercise in Glp1r+/+ mice, it was significantly increased in Glp1r−/− mice (Fig. 5D). Conversely, Rg in the heart was stimulated by exercise in Glp1r+/+ mice but was unaffected in Glp1r−/− mice (Fig. 5E). This likely is due to the fact that sedentary Rg in the heart was elevated in Glp1r−/− mice, such that exercise did not increase it further. In summary, hyperglycemia during exercise in Glp1r−/− mice was not due to an impairment in MGU because exercise-induced Rg was not different or even elevated compared with Glp1r+/+ mice.
Figure 5.
Rg and Kg in 5-h-fasted sedentary (black bars) and exercised (white bars) Glp1r+/+ and Glp1r−/− mice. Rg in soleus, gastrocnemius, SVL, diaphragm, and heart (A–E) is shown. Kg is shown for the same tissues (F–J). Data are mean ± sem for 14–16 mice/genotype. *, P < 0.05 vs. Glp1r+/+ within the same experimental condition; †, P < 0.05 vs. sedentary within the same genotype.
Measurement of Rg is dependent on glucose concentration (37). Because glucose levels were higher in Glp1r−/− mice during exercise, this could inflate values of Rg. Kg is an index of glucose uptake that is independent of glucose concentration (37). As shown in Fig. 5, F–J, sedentary and exercise-stimulated values of Kg recapitulated the results obtained via measurement of Rg. Thus, Kg in hindlimb muscles was not different between genotypes in sedentary and exercised mice (Fig. 5, F–H). Furthermore, exercise-stimulated Kg in the diaphragm and sedentary Kg in the heart were elevated in Glp1r−/− mice (Fig. 5, I and J). Taken together, the results of exercise studies show that exercise-induced hyperglycemia in Glp1r−/− mice is not due to an impairment in MGU. This suggests an inappropriate increase in hepatic glucose production beyond the requirements of the working muscle.
Disruption of Glp1r expression enhances phosphorylation of AMP-activated protein kinase in the muscle during exercise
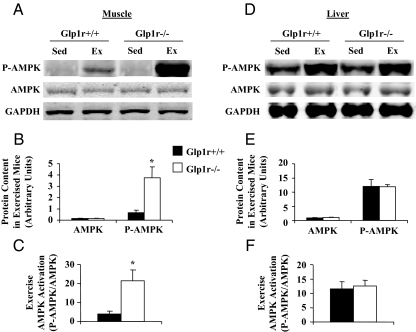
Activation of the AMPK is proposed to be involved in the stimulation glucose uptake during exercise (39,40,41). Interestingly, although MGU during exercise did not differ between genotypes, muscle AMPK activation was significantly enhanced in Glp1r−/− mice (Fig. 6, A–C). This was only evident in the muscle, as activation of AMPK in the liver was not different between the genotypes (Fig. 6, D–F).
Figure 6.
Immunoblots for AMPK in 5-h-fasted sedentary and exercised Glp1r. Representative immunoblots are shown from gastrocnemius muscle (A) and liver (D) extracts. Protein content and protein activation is presented for Glp1r+/+ (black bars) and Glp1r−/− (white bars) mice. Protein content of Thr172-phosphorylated AMPK (P-AMPK), and α1/α2 AMPK (AMPK) is shown from muscle (B) and liver (E) extracts from exercised mice. Protein content is normalized to the GAPDH loading control. AMPK activation (P-AMPK/AMPK) is also shown from muscle (C) and liver (F) extracts from exercised mice. Data are mean ± sem for eight to 10 mice/genotype. *, P < 0.05 vs. Glp1r+/+.
Glp1r−/− mice exercise at a relatively higher intensity and have impaired exercise endurance
High intensity exercise is characterized by increased arterial glucose levels and enhanced skeletal muscle AMPK activation (29,42,43). To assess whether Glp1r−/− mice exercise at different relative intensities for a given treadmill speed, exercise stress and exercise endurance tests were performed. Capacity for maximum intensity exercise was not different between the two genotypes, as noted by the running rates during an exercise stress test. However, VO2,max was significantly higher in Glp1r−/− mice (Table 2). During an exercise endurance test, mice from both genotypes ran at the same absolute speed (20 m · min−1). As shown in Table 2, this work intensity corresponded to about 78% of VO2,max in Glp1r+/+ mice but about 88% of VO2,max in Glp1r−/− mice. This shows that at a given absolute work intensity, Glp1r−/− mice exercise at a higher relative intensity. This was associated with a decrease in endurance capacity, measured as time to exhaustion during treadmill exercise at 20 m · min−1 (Table 2).
Table 2.
Results from exercise stress and exercise endurance tests
| Glp1r+/+ | Glp1r−/− | |
|---|---|---|
| n (male/female) | 14 (9/5) | 17 (10/7) |
| Maximum running rate (m · min−1) | 36.3 ± 1.3 | 34.0 ± 1.0 |
| VO2,max (ml · kg−1 · min−1) | 132 ± 4 | 138 ± 2 |
| Endurance time (min) | 120 ± 13 | 89 ± 9a |
| VO2 (ml · kg−1 · min−1) | ||
| Rest | 90 ± 5 | 90 ± 4 |
| Exercise | 103 ± 3 | 121 ± 4a |
| After exercise | 61 ± 5 | 66 ± 3 |
| RER | ||
| Rest | 0.75 ± 0.04 | 0.78 ± 0.01 |
| Exercise | 0.77 ± 0.03 | 0.83 ± 0.02 |
| After exercise | 0.70 ± 0.02 | 0.73 ± 0.01 |
Maximum running rate and VO2,max were determined from exercise stress tests. Endurance time VO2 and RER were determined from exercise endurance tests.
P < 0.05 vs. Glp1r+/+.
Discussion
The role of the Glp1r to enhance insulin secretion in response to nutrient intake has been clearly established. It was thus assumed that this incretin action mediated through the β-cell was solely responsible for any effects of Glp1r activation on hepatic glucose production and MGU. Whereas it has been proposed that the Glp1r can regulate glucose production and utilization independent of its effect on pancreatic hormone secretion (13,14,15,16,17,18,19), this idea remains controversial (20,21,22,23,24,25,26). In the present study, insulin clamps and exercise were used to stimulate glucose flux under conditions in which circulating insulin levels were experimentally controlled (insulin clamps) or decreased (exercise). This approach was taken to assess the potential role of the Glp1r to regulate MGU independent of its ability to stimulate endogenous insulin secretion. Given its role in mediating the incretin effect, it was surprising to observe that Glp1r−/− mice exhibited enhanced skeletal muscle glucose uptake during an insulin clamp. Conversely, suppression of hepatic glucose production was impaired in these mice. These effects were paralleled by increased muscle glycogen accumulation and decreased hepatic glycogen accumulation. In response to exercise, Glp1r−/− mice became hyperglycemic. This was not due to a defect in glucose uptake as MGU was normal or increased in Glp1r−/− mice. Taken together, these results demonstrate that, under conditions of increased glucose flux, the endogenous Glp1r is a regulator of glucose production and MGU independent of its ability to stimulate endogenous insulin secretion.
We have previously shown that disruption of both the Glp1r and the Gipr in double-incretin receptor knockout (DIRKO) mice preserves insulin action in response to chronic high-fat feeding, a dietary intervention that precipitates insulin resistance in C57BL/6 mice (32). DIRKO mice are also protected from high-fat diet-induced obesity due to increased energy expenditure (32,44). Disruption of the Gipr increases fat oxidation and decreases adiposity in genetic and dietary rodent models of obesity (45,46,47). This results in improved glucose tolerance and insulin sensitivity (45,46,47,48). Thus, the enhanced insulin action in DIRKO mice is likely due, at least in part, to the improved metabolic profile resulting from the loss of Gipr expression. In the present studies, we show that fat mass and muscle mass is normal in Glp1r−/− mice. Therefore, the phenotypes observed in Glp1r−/− mice are not secondary to an effect on body composition.
Our findings from insulin clamp and exercise studies reveal a novel role for the Glp1r in the regulation of hepatic glucose production and MGU during conditions of increased glucose flux. A direct effect of GLP-1 on the liver and muscle is not likely because neither tissue expresses the Glp1r. Recent evidence indicates that Glp1r action in the brain, particularly in the hypothalamus, can acutely regulate glucose production and use. Knauf et al. (49) showed that glucose requirements during a hyperinsulinemic-hyperglycemic clamp were higher in Glp1r−/− mice and mice receiving an intracerebroventricular infusion of the Glp1r antagonist exendin (9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39). Furthermore, hepatic glycogen levels were lower, whereas muscle glycogen levels were higher, in Glp1r−/− mice and mice receiving intracerebroventricular infusions of exendin (9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39). A similar phenotype was observed in the present studies using Glp1r−/− mice and was associated with impaired suppression of endoRa but enhanced MGU. The effect of central Glp1rs to regulate hepatic and muscle insulin action appears to be limited to receptors in the arcuate nucleus of the hypothalamus. Infusion of GLP-1 into the arcuate nucleus, but not the paraventricular nucleus, has recently been shown to enhance suppression of endoRa but impair stimulation of Rd during insulin clamps in rats (50). This is in agreement with the present studies, which show that disruption of Glp1r expression impairs suppression of endoRa but enhances stimulation of Rd.
Enhanced insulin-stimulated MGU and muscle glycogen accumulation in Glp1r−/− mice was not associated with increased activation of insulin signaling proteins. This is also consistent with the findings of Knauf et al. (49), who showed that increased muscle glycogen accumulation via inhibition of central Glp1rs does not require expression of the insulin receptor in muscle. This effect of central Glp1r action did require innervation of the muscle because severing of the sciatic nerve blunted muscle glycogen accumulation (49). Contrasting the effects observed in the muscle, the hepatic phenotypes observed in Glp1r−/− mice were associated with an effect on insulin signaling. Thus, the impaired ability of insulin to suppress endoRa in Glp1r−/− mice correlated with decreased levels of total and phosphorylated Akt. Furthermore, decreased hepatic glycogen storage in these mice was associated with decreased phosphorylation of GSK-3β.
We next addressed whether the Glp1r regulates MGU in the absence of an increase in insulin levels. Exercise is a useful tool to address this question because it stimulates MGU via insulin-independent mechanisms. Whereas arterial glucose levels did not vary appreciably during exercise in Glp1r+/+ mice, Glp1r−/− mice exhibited exercise-induced hyperglycemia. This was not due to an impairment in MGU, which was normal in hindlimb muscles and even elevated in the diaphragm. These findings suggest that exercise-induced hyperglycemia in Glp1r−/− mice results from an inappropriate increase in hepatic glucose production. During moderate exercise, hepatic glucose production typically increases to match glucose use by the contracting muscle (29). The phenotype observed in Glp1r−/− mice suggests an added drive to further increase glucose production beyond what the working muscle demands. This is similar to what was observed in the insulin clamp studies, in which the disruption of the Glp1r resulted in an inappropriate increase in endoRa for the prevailing hyperinsulinemia. These results suggest that the endogenous Glp1r normally inhibits hepatic glucose production in response to increased glucose flux. Because activation of the Glp1r normally occurs concurrent with increased insulin (e.g. after a meal), then a role for the Glp1r in the regulation of hepatic glucose production during insulin clamps makes physiologic sense. Such a role for the Glp1r during exercise is less clear because exercise is characterized by an increase in hepatic glucose production, and activation of Glp1rs would counter this hepatic response. Venous levels of GLP-1 increase with exercise (51), raising the possibility that activation of the Glp1r is involved in the normal response to exercise.
Glucagon is a major driving force behind the increase in endoRa during exercise (52). Because activation of the Glp1r suppresses glucagon secretion (8,9,11), it is possible that increased hepatic glucose production in Glp1r−/− mice during insulin clamps and exercise results from increased glucagon secretion. Previous studies have shown normal fasting glucagon levels, as well as normal suppression of glucagon by oral glucose loading, in Glp1r−/− mice (27,53,54). However, these measurements of plasma glucagon were made from cardiac blood samples. Because glucose clearance occurs in the liver, portal vein glucagon levels would more accurately represent changes in the secretion of this hormone. Thus, it is still possible that increased glucagon secretion plays a role in the hepatic phenotypes observed in the present studies.
Activation of muscle AMPK has been implicated as necessary for the increase in MGU during exercise (39,40,41). Interestingly, phosphorylation of muscle AMPK, a marker of activation, was significantly greater in Glp1r−/− mice during exercise, even as MGU was not different from that in Glp1r+/+ mice. This suggests that activation of AMPK only partially mediates the increase in MGU during exercise. Indeed, studies have shown normal contraction-induced stimulation of MGU in the absence of functional increases in AMPK activity (55,56,57,58). Activation of muscle AMPK increases with exercise intensity (42,43). Results from maximal and endurance exercise tests performed in the present studies suggest that for the same absolute speed, Glp1r−/− mice exercise at a greater intensity than Glp1r+/+ mice. Thus, at a speed of 20 m · min−1, the speed used in endurance tests in the present studies, Glp1r+/+ mice were exercising at about 78% of their VO2,max, whereas Glp1r−/− mice were exercising at about 88% of their VO2,max. This was associated with lower endurance time in Glp1r−/− mice. Furthermore, Glp1r−/− mice exhibited increased glucose uptake into the diaphragm, suggesting increased breathing and effort. Taken together, these results suggest that the metabolic response to exercise in mice lacking Glp1r expression resembles that of high intensity exercise. Interestingly, one of the metabolic features of high intensity exercise is an increase in glucose production that exceeds the increase in glucose use, leading to a rise in circulating glucose levels (29).
The Glp1r also exerts regulatory control on cardiovascular function (59). Activation of central Glp1rs increases heart rate and blood pressure (60,61). Recent studies also demonstrate a protective role for the Glp1r against cardiac injury and heart failure (62,63,64,65,66,67,68). Disruption of Glp1r expression results in defects in cardiac morphology and function, including increased left ventricular thickness and impaired left ventricular contractility (69). These cardiac phenotypes are often associated with a preference for glucose over fatty acid oxidation in the heart (70,71,72). In support of this, sedentary cardiac glucose uptake was increased in Glp1r−/− mice in the present studies. It is thus possible that these phenotypes associated with impaired cardiac function explain the decreased exercise capacity in Glp1r−/− mice.
In conclusion, the present studies show that the Glp1r regulates glucose production and use independent of its ability to stimulate insulin secretion. The findings from the insulin clamp studies suggest an important role for the Glp1r in the proper disposal of meal-derived glucose. By enhancing hepatic insulin action at the expense of MGU, activation of Glp1rs ensures that hepatic glucose clearance is increased and hepatic glycogen stores are replenished. This is crucial for the maintenance of glucose homeostasis during the postabsorptive period. Results from exercise studies suggest that activation of Glp1rs also occurs during exercise. Although endoRa was not directly measured, the fact that glucose levels rose during exercise in Glp1r−/− mice in the absence of a defect in MGU suggests an increase in glucose production exceeding the rate of use. This demonstrates a role for the Glp1r in the regulation of glucose production independent of insulin. Taken together, these results extend the importance of GLP-1 action in regulating glucose homeostasis beyond the pancreas and show it to be a key determinant of glucose fate during times of increased flux.
Acknowledgments
We thank Dr. Owen McGuinness, Carlo Malabanan, and Tasneem Ansari (Vanderbilt Mouse Metabolic Phenotyping Center Metabolic Pathophysiology Core) for body composition and exercise endurance analysis.
Footnotes
This work was supported by National Institutes of Health Grants R01 DK-50277 (to D.H.W.) and U24 DK-59637 (to the Vanderbilt Mouse Metabolic Phenotyping Center) and Juvenile Diabetes Research Foundation Grant 1-2006-796 (to D.J.D.).
Disclosure Statement: All of the authors have nothing to disclose.
First Published Online November 13, 2008
Abbreviations: AMPK, AMP-activated protein kinase; DG, deoxyglucose; DGP, DG-6-phosphate; DIRKO, double-incretin receptor knockout; endoRa, endogenous glucose production; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GIP, glucose-dependent insulinotropic polypeptide; Gipr, GIP receptor; GIR, glucose infusion rate; GLP, glucagon-like peptide; Glp1r, GLP-1 receptor; GSK, glycogen synthase kinase; Kg, glucose clearance; MGU, muscle glucose uptake; NEFA, nonesterified fatty acid; Rd, glucose disappearance; RER, respiratory exchange ratio; Rg, glucose metabolic index; SVL, superficial vastus lateralis; VO2, O2 consumption; VO2,max, maximal VO2.
References
- McIntyre N, Holdsworth CD, Turner DS 1964 New interpretation of oral glucose tolerance. Lancet 2:20–21 [DOI] [PubMed] [Google Scholar]
- Dupre J, Ross SA, Watson D, Brown JC 1973 Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab 37:826–828 [DOI] [PubMed] [Google Scholar]
- Kreymann B, Williams G, Ghatei MA, Bloom SR 1987 Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet 2:1300–1304 [DOI] [PubMed] [Google Scholar]
- Thorens B 1992 Expression cloning of the pancreatic β cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci USA 89:8641–8645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos RV, Lee YC, Drucker DJ 1994 Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology 134:2156–2164 [DOI] [PubMed] [Google Scholar]
- Bullock BP, Heller RS, Habener JF 1996 Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology 137:2968–2978 [DOI] [PubMed] [Google Scholar]
- Miyawaki K, Yamada Y, Yano H, Niwa H, Ban N, Ihara Y, Kubota A, Fujimoto S, Kajikawa M, Kuroe A, Tsuda K, Hashimoto H, Yamashita T, Jomori T, Tashiro F, Miyazaki J, Seino Y 1999 Glucose intolerance caused by a defect in the entero-insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc Natl Acad Sci USA 96:14843–14847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu R, Matsuyama T, Namba M, Watanabe N, Itoh H, Kono N, Tarui S 1989 Glucagonostatic and insulinotropic action of glucagonlike peptide I-(7–36)-amide. Diabetes 38:902–905 [DOI] [PubMed] [Google Scholar]
- Dupre J, Behme MT, Hramiak IM, McFarlane P, Williamson MP, Zabel P, McDonald TJ 1995 Glucagon-like peptide I reduces postprandial glycemic excursions in IDDM. Diabetes 44:626–630 [DOI] [PubMed] [Google Scholar]
- Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, Ritzel R, Schmiegel WH 1997 Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol 273:E981–E988 [DOI] [PubMed] [Google Scholar]
- de Heer J, Rasmussen C, Coy DH, Holst JJ 2008 Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia 51:2263–2270 [DOI] [PubMed] [Google Scholar]
- Gutniak M, Orskov C, Holst JJ, Ahren B, Efendic S 1992 Antidiabetogenic effect of glucagon-like peptide-1 (7–36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med 326:1316–1322 [DOI] [PubMed] [Google Scholar]
- D'Alessio DA, Kahn SE, Leusner CR, Ensinck JW 1994 Glucagon-like peptide 1 enhances glucose tolerance both by stimulation of insulin release and by increasing insulin-independent glucose disposal. J Clin Invest 93:2263–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessio DA, Prigeon RL, Ensinck JW 1995 Enteral enhancement of glucose disposition by both insulin-dependent and insulin-independent processes. A physiological role of glucagon-like peptide I. Diabetes 44:1433–1437 [DOI] [PubMed] [Google Scholar]
- Shalev A, Ninnis R, Keller U 1998 Effects of glucagon-like peptide 1 (7–36 amide) on glucose kinetics during somatostatin-induced suppression of insulin secretion in healthy men. Horm Res 49:221–225 [DOI] [PubMed] [Google Scholar]
- Meneilly GS, McIntosh CH, Pederson RA, Habener JF, Gingerich R, Egan JM, Finegood DT, Elahi D 2001 Effect of glucagon-like peptide 1 on non-insulin-mediated glucose uptake in the elderly patient with diabetes. Diabetes Care 24:1951–1956 [DOI] [PubMed] [Google Scholar]
- Egan JM, Meneilly GS, Habener JF, Elahi D 2002 Glucagon-like peptide-1 augments insulin-mediated glucose uptake in the obese state. J Clin Endocrinol Metab 87:3768–3773 [DOI] [PubMed] [Google Scholar]
- Prigeon RL, Quddusi S, Paty B, D'Alessio DA 2003 Suppression of glucose production by GLP-1 independent of islet hormones: a novel extrapancreatic effect. Am J Physiol Endocrinol Metab 285:E701–E707 [DOI] [PubMed] [Google Scholar]
- Azuma K, Radikova Z, Mancino J, Toledo FG, Thomas E, Kangani C, Dalla Man C, Cobelli C, Holst JJ, Deacon CF, He Y, Ligueros-Saylan M, Serra D, Foley JE, Kelley DE 2008 Measurements of islet function and glucose metabolism with the dipeptidyl peptidase 4 inhibitor vildagliptin in patients with type 2 diabetes. J Clin Endocrinol Metab 93:459–464 [DOI] [PubMed] [Google Scholar]
- Orskov L, Holst JJ, Moller J, Orskov C, Moller N, Alberti KG, Schmitz O 1996 GLP-1 does not acutely affect insulin sensitivity in healthy man. Diabetologia 39:1227–1232 [DOI] [PubMed] [Google Scholar]
- Toft-Nielson M, Madsbad S, Holst JJ 1996 The effect of glucagon-like peptide I (GLP-I) on glucose elimination in healthy subjects depends on the pancreatic glucoregulatory hormones. Diabetes 45:552–556 [DOI] [PubMed] [Google Scholar]
- Ahren B, Larsson H, Holst JJ 1997 Effects of glucagon-like peptide-1 on islet function and insulin sensitivity in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 82:473–478 [DOI] [PubMed] [Google Scholar]
- Ryan AS, Egan JM, Habener JF, Elahi D 1998 Insulinotropic hormone glucagon-like peptide-1-(7–37) appears not to augment insulin-mediated glucose uptake in young men during euglycemia. J Clin Endocrinol Metab 83:2399–2404 [DOI] [PubMed] [Google Scholar]
- Ahren B, Pacini G 1999 Dose-related effects of GLP-1 on insulin secretion, insulin sensitivity, and glucose effectiveness in mice. Am J Physiol 277:E996–E1004 [DOI] [PubMed] [Google Scholar]
- Vella A, Shah P, Basu R, Basu A, Holst JJ, Rizza RA 2000 Effect of glucagon-like peptide 1(7–36) amide on glucose effectiveness and insulin action in people with type 2 diabetes. Diabetes 49:611–617 [DOI] [PubMed] [Google Scholar]
- Vella A, Shah P, Reed AS, Adkins AS, Basu R, Rizza RA 2002 Lack of effect of exendin-4 and glucagon-like peptide-1-(7,36)-amide on insulin action in non-diabetic humans. Diabetologia 45:1410–1415 [DOI] [PubMed] [Google Scholar]
- Scrocchi LA, Brown TJ, MaClusky N, Brubaker PL, Auerbach AB, Joyner AL, Drucker DJ 1996 Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med 2:1254–1258 [DOI] [PubMed] [Google Scholar]
- Baggio L, Kieffer TJ, Drucker DJ 2000 Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, regulates fasting glycemia and nonenteral glucose clearance in mice. Endocrinology 141:3703–3709 [DOI] [PubMed] [Google Scholar]
- Wasserman DH 1995 Regulation of glucose fluxes during exercise in the postabsorptive state. Annu Rev Physiol 57:191–218 [DOI] [PubMed] [Google Scholar]
- Ayala JE, Bracy DP, McGuinness OP, Wasserman DH 2006 Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 55:390–397 [DOI] [PubMed] [Google Scholar]
- Ayala JE, Bracy DP, Julien BM, Rottman JN, Fueger PT, Wasserman DH 2007 Chronic treatment with sildenafil improves energy balance and insulin action in high fat-fed conscious mice. Diabetes 56:1025–1033 [DOI] [PubMed] [Google Scholar]
- Ayala JE, Bracy DP, Hansotia T, Flock G, Seino Y, Wasserman DH, Drucker DJ 2007 Insulin action in the double incretin receptor knockout mouse. Diabetes [DOI] [PubMed] [Google Scholar]
- Fernando P, Bonen A, Hoffman-Goetz L 1993 Predicting submaximal oxygen consumption during treadmill running in mice. Can J Physiol Pharmacol 71:854–857 [DOI] [PubMed] [Google Scholar]
- Chan TM, Exton JH 1976 A rapid method for the determination of glycogen content and radioactivity in small quantities of tissue or isolated hepatocytes. Anal Biochem 71:96–105 [DOI] [PubMed] [Google Scholar]
- Steele R, Wall JS, De Bodo RC, Altszuler N 1956 Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol 187:15–24 [DOI] [PubMed] [Google Scholar]
- Debodo RC, Steele R, Altszuler N, Dunn A, Bishop JS 1963 On the hormonal regulation of carbohydrate metabolism: studies with C14 glucose. Recent Prog Horm Res 19:445–488 [PubMed] [Google Scholar]
- Kraegen EW, James DE, Jenkins AB, Chisholm DJ 1985 Dose-response curves for in vivo insulin sensitivity in individual tissues in rats. Am J Physiol 248:E353–E362 [DOI] [PubMed] [Google Scholar]
- Fueger PT, Bracy DP, Malabanan CM, Pencek RR, Wasserman DH 2004 Distributed control of glucose uptake by working muscles of conscious mice: roles of transport and phosphorylation. Am J Physiol Endocrinol Metab 286:E77–E84 [DOI] [PubMed] [Google Scholar]
- Koh HJ, Brandauer J, Goodyear LJ 2008 LKB1 and AMPK and the regulation of skeletal muscle metabolism. Curr Opin Clin Nutr Metab Care 11:227–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen SB, Jensen TE, Richter EA 2007 Role of AMPK in skeletal muscle gene adaptation in relation to exercise. Appl Physiol Nutr Metab 32:904–911 [DOI] [PubMed] [Google Scholar]
- Towler MC, Hardie DG 2007 AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res 100:328–341 [DOI] [PubMed] [Google Scholar]
- Chen ZP, Stephens TJ, Murthy S, Canny BJ, Hargreaves M, Witters LA, Kemp BE, McConell GK 2003 Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes 52:2205–2212 [DOI] [PubMed] [Google Scholar]
- Wadley GD, Lee-Young RS, Canny BJ, Wasuntarawat C, Chen ZP, Hargreaves M, Kemp BE, McConell GK 2006 Effect of exercise intensity and hypoxia on skeletal muscle AMPK signaling and substrate metabolism in humans. Am J Physiol Endocrinol Metab 290:E694–E702 [DOI] [PubMed] [Google Scholar]
- Hansotia T, Maida A, Flock G, Yamada Y, Tsukiyama K, Seino Y, Drucker DJ 2007 Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. J Clin Invest 117:143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClean PL, Irwin N, Cassidy RS, Holst JJ, Gault VA, Flatt PR 2007 GIP receptor antagonism reverses obesity, insulin resistance, and associated metabolic disturbances induced in mice by prolonged consumption of high-fat diet. Am J Physiol Endocrinol Metab 293:E1746–E1755 [DOI] [PubMed] [Google Scholar]
- Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, Fujimoto S, Oku A, Tsuda K, Toyokuni S, Hiai H, Mizunoya W, Fushiki T, Holst JJ, Makino M, Tashita A, Kobara Y, Tsubamoto Y, Jinnouchi T, Jomori T, Seino Y 2002 Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med 8:738–742 [DOI] [PubMed] [Google Scholar]
- Gault VA, McClean PL, Cassidy RS, Irwin N, Flatt PR 2007 Chemical gastric inhibitory polypeptide receptor antagonism protects against obesity, insulin resistance, glucose intolerance and associated disturbances in mice fed high-fat and cafeteria diets. Diabetologia 50:1752–1762 [DOI] [PubMed] [Google Scholar]
- Gault VA, Irwin N, Green BD, McCluskey JT, Greer B, Bailey CJ, Harriott P, O'Harte F P, Flatt PR 2005 Chemical ablation of gastric inhibitory polypeptide receptor action by daily (Pro3)GIP administration improves glucose tolerance and ameliorates insulin resistance and abnormalities of islet structure in obesity-related diabetes. Diabetes 54:2436–2446 [DOI] [PubMed] [Google Scholar]
- Knauf C, Cani PD, Perrin C, Iglesias MA, Maury JF, Bernard E, Benhamed F, Gremeaux T, Drucker DJ, Kahn CR, Girard J, Tanti JF, Delzenne NM, Postic C, Burcelin R 2005 Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Invest 115:3554–3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval DA, Bagnol D, Woods SC, D'Alessio DA, Seeley RJ 2008 Arcuate GLP-1 receptors regulate glucose homeostasis but not food intake. Diabetes 57:2046–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins C, Morgan LM, Bloom SR, Robertson MD 2007 Effects of exercise on gut peptides, energy intake and appetite. J Endocrinol 193:251–258 [DOI] [PubMed] [Google Scholar]
- Wasserman DH, O'Doherty RM, Zinker BA 1995 Role of the endocrine pancreas in control of fuel metabolism by the liver during exercise. Int J Obes Relat Metab Disord 19(Suppl 4):S22–S30 [PubMed] [Google Scholar]
- Scrocchi LA, Marshall BA, Cook SM, Brubaker PL, Drucker DJ 1998 Identification of glucagon-like peptide 1 (GLP-1) actions essential for glucose homeostasis in mice with disruption of GLP-1 receptor signaling. Diabetes 47:632–639 [DOI] [PubMed] [Google Scholar]
- Baggio LL, Huang Q, Brown TJ, Drucker DJ 2004 A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes 53:2492–2500 [DOI] [PubMed] [Google Scholar]
- Mu J, Brozinick Jr JT, Valladares O, Bucan M, Birnbaum MJ 2001 A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 7:1085–1094 [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Mourtzakis M, Hillig T, Saltin B, Pilegaard H 2002 Dissociation of AMPK activity and ACCβ phosphorylation in human muscle during prolonged exercise. Biochem Biophys Res Commun 298:309–316 [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF 2004 Knockout of the α2 but not α1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem 279:1070–1079 [DOI] [PubMed] [Google Scholar]
- McConell GK, Lee-Young RS, Chen ZP, Stepto NK, Huynh NN, Stephens TJ, Canny BJ, Kemp BE 2005 Short-term exercise training in humans reduces AMPK signalling during prolonged exercise independent of muscle glycogen. J Physiol 568:665–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ 2006 The biology of incretin hormones. Cell Metab 3:153–165 [DOI] [PubMed] [Google Scholar]
- Barragan JM, Rodriguez RE, Blazquez E 1994 Changes in arterial blood pressure and heart rate induced by glucagon-like peptide-1-(7–36) amide in rats. Am J Physiol 266:E459–E466 [DOI] [PubMed] [Google Scholar]
- Barragan JM, Eng J, Rodriguez R, Blazquez E 1999 Neural contribution to the effect of glucagon-like peptide-1-(7–36) amide on arterial blood pressure in rats. Am J Physiol 277:E784–E791 [DOI] [PubMed] [Google Scholar]
- Nystrom T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahren B, Sjoholm A 2004 Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab 287:E1209–E1215 [DOI] [PubMed] [Google Scholar]
- Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, Stolarski C, Shen YT, Shannon RP 2004 Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation 110:955–961 [DOI] [PubMed] [Google Scholar]
- Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM 2005 Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes 54:146–151 [DOI] [PubMed] [Google Scholar]
- Bose AK, Mocanu MM, Carr RD, Yellon DM 2005 Glucagon like peptide-1 is protective against myocardial ischemia/reperfusion injury when given either as a preconditioning mimetic or at reperfusion in an isolated rat heart model. Cardiovasc Drugs Ther 19:9–11 [DOI] [PubMed] [Google Scholar]
- Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen YT, Shannon RP 2006 Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther 317:1106–1113 [DOI] [PubMed] [Google Scholar]
- Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP 2006 Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail 12:694–699 [DOI] [PubMed] [Google Scholar]
- Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M 2008 Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 117:2340–2350 [DOI] [PubMed] [Google Scholar]
- Gros R, You X, Baggio LL, Kabir MG, Sadi AM, Mungrue IN, Parker TG, Huang Q, Drucker DJ, Husain M 2003 Cardiac function in mice lacking the glucagon-like peptide-1 receptor. Endocrinology 144:2242–2252 [DOI] [PubMed] [Google Scholar]
- Opie LH 1968 Metabolism of the heart in health and disease. I. Am Heart J 76:685–698 [DOI] [PubMed] [Google Scholar]
- Depre C, Vanoverschelde JL, Taegtmeyer H 1999 Glucose for the heart. Circulation 99:578–588 [DOI] [PubMed] [Google Scholar]
- Taylor M, Wallhaus TR, Degrado TR, Russell DC, Stanko P, Nickles RJ, Stone CK 2001 An evaluation of myocardial fatty acid and glucose uptake using PET with [18F]fluoro-6-thia-heptadecanoic acid and [18F]FDG in patients with congestive heart failure. J Nucl Med 42:55–62 [PubMed] [Google Scholar]