Abstract
S-glutathionylation is a physiological, reversible protein modification of cysteine residues with glutathione in response to mild oxidative stress. Because the key cell growth regulator signal transducer and activator of transcription (STAT) 3 is particularly susceptible to redox regulation, we hypothesized that oxidative modification of cysteine residues of STAT3 by S-glutathionylation may occur. Herein, we show that the cysteine residues of STAT3 are modified by a thiol-alkylating agent and are the targets of S-glutathionylation. STAT3 protein thiol reactivity was reversibly attenuated with concomitant increase in the S-glutathionylation of STAT3 upon treatment of human HepG2 hepatoma cells with pyrrolidine dithiocarbamate, glutathione disulfide, or diamide. Under these conditions there was a marked reduction in IL-6-dependent STAT3 signaling, including decreased STAT3 tyrosine phosphorylation, loss in nuclear accumulation of STAT3, and impaired expression of target genes, such as fibrinogen-γ. In a cell-free system, diamide induced glutathionylation of STAT3, which was decreased upon addition of glutaredoxin (GRX)-1, a deglutathionylation enzyme, or the reducing agent, dithiothreitol. Glutathionylated STAT3 was a poor Janus protein tyrosine kinase 2 substrate in vitro, and it exhibited low DNA-binding activity. Cellular GRX-1 activity was inhibited by diamide and pyrrolidine dithiocarbamate treatment; however, ectopic expression of GRX-1 was accompanied by a modest increase in phosphorylation, nuclear translocation, and DNA-binding ability of STAT3 in response to IL-6. These results are the first to show S-glutathionylation of STAT3, a modification that may exert regulatory function in STAT3 signaling.
Reversible S-glutathionylation of STAT3 regulates its activity as transcription factor.
Members of the IL-6 family of proinflammatory cytokines exert pleiotropic biological actions, including direct effects on proliferation, differentiation, survival, and apoptosis via activation of a latent pool of the DNA-binding transcription factor signal transducer and activator of transcription (STAT) 3. This activation is initiated by oligomerization of the liganded IL-6 receptor with gp130 and recruitment of Janus protein tyrosine kinases (JAKs), which, in turn, promotes STAT3 tyrosine phosphorylation on Tyr705, and binding and/or retention of STAT3 dimers to the promoter of target genes (reviewed in Ref. 1). Moreover, phosphorylation on Ser727 is indispensable for maximal transcriptional activity of STAT3 (2), which also requires its association with transcriptional coactivators upon IL-6 stimulation (3,4,5,6). Hepatocyte-specific ablation of STAT3 severely attenuates the induction of a subset of acute phase proteins [e.g. haptoglobin, fibrinogen-γ (FBG)] involved in the regulation of systemic inflammation (7). A transition from inflammation to proliferation and remodeling is required for normal wound healing after injury. However, pathological persistent activation of STAT3 contributes to fibrosis (8), and the development of liver and inflammatory bowel diseases (9,10). Of interest, STAT3 is believed to participate not only in early stages of carcinogenesis but also in driving malignant progression in vivo (11,12). Experimental approaches aimed at the deactivation of STAT3 activity have resulted in inhibition of growth and metastasis of human hepatocellular carcinoma cells, and increase in their chemo-sensitivity (13,14,15,16). Together, these studies suggest that IL-6/gp130/STAT3 signaling may play a role in the pathogenesis of a number of inflammatory diseases and in tumor development, thus raising the possibility of a selective therapeutic intervention that targets this pathway (17).
Several lines of evidence suggest that STAT3 activation is subject to redox regulation. For example, reactive oxygen species (ROS) trigger tyrosine phosphorylation and nuclear translocation of STAT3 in human lymphocytes without cytokine stimulation (18). Oxidized low-density lipoprotein also up-regulates the DNA binding activity of STAT3 in a ROS-dependent manner (19). Increase in STAT3 transcriptional activity in rat liver after heat shock and reperfusion stress are other examples (20). However, pretreatment of macrophages with TNF-α or lipopolysaccharide, two inducers of oxidative stress, was found to attenuate the subsequent IL-6-induced phosphorylation of STAT3 (21). These results suggest that the mechanism(s) underlying ROS regulated STAT3 signaling is complex and poorly defined.
The susceptibility of reactive cysteines in proteins to ROS and nitrosative stress render them vulnerable to oxidative modification. In most cases, low-molecular mass thiols such as glutathione (GSH) can form mixed disulfides with oxidized cysteine forms in protein (22). This process, known as S-glutathionylation, has emerged as a redox-sensitive posttranslational modification that can modulate enzymatic activities, protein folding and functions, and protein-protein interactions (23,24,25,26). Protein S-glutathionylation is readily reversible via the action of deglutathionylation enzymes [e.g. glutaredoxin (GRX), thioredoxin] or increases in the cellular GSH to glutathione disulfide (GSSG) ratio (27). Of interest, pyrrolidine dithiocarbamate (PDTC) decreases the GSH to GSSG ratio in several cell types (28,29), including the human HepG2 hepatoma cells (30). Our recent study provides evidence for an increased level of GSH-bound proteins in PDTC-treated HepG2 cells (31).
PDTC blocks IL-6 signaling in hepatocytes by inhibiting STAT3 recruitment to the gp130/JAK heterocomplex, thus preventing subsequent nuclear translocation and transcriptional activation of target genes by STAT3 (32). However, the mechanism used by PDTC and other cellular oxidants to modulate STAT3 function remains poorly defined. In the present study, we investigated the possibility that protein S-glutathionylation exerts biological effects in STAT3 activation and signaling, and found that this posttranslational modification represents a mechanism of regulation of STAT3 activity in cells.
Materials and Methods
Cell culture, transient transfection, and reporter assays
Human HepG2 hepatocarcinoma cells (American Type Culture Collection, Manassas, VA) were grown in α-MEM medium supplemented with 10% fetal bovine serum, 1 mm pyruvate, 2 mm l-glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin at 37 C in a humidified 5% CO2 incubator. Full-length cDNA encoding human STAT3 (pCMV6-XL4; OriGene Technologies, Inc., Rockville, MD) was subcloned in-frame into N-terminal p3xFLAG-CMV (Sigma-Aldrich Corp., St. Louis, MO) to generate the Flag-STAT3 fusion construct. Cells were transiently transfected with Flag-STAT3, human GRX-1 (pReceiver-M15; GeneCopoeia, Inc., Germantown, MD), and pcDNA3.1 (Invitrogen Corp., Carlsbad, CA) plasmids in 35-mm plates with 2 μg DNA/plate using Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer’s protocol. For reporter assays, cells were seeded in 35-mm dishes at 60–70% confluency and grown for 24 h. For each dish, 1 μg STAT3 reporter plasmid (pGAS-TA-Luc; Clontech Laboratories, Inc., Mountain View, CA) and 125 ng pCMVβ-gal vector (Clontech Laboratories) were mixed in OPTI-MEM I (Invitrogen) with the expression vectors for Flag-STAT3, GRX-1, and/or pcDNA3.1. The cells were incubated for 48 h after transfection and then were serum starved for 4 h before the addition of vehicle or recombinant human IL-6 (20 ng/ml; Sigma-Aldrich) for 6 h. Cells were harvested, and the relative luciferase reporter assays were performed as previously described (33). These experiments were repeated two times in triplicate.
Chromatin immunoprecipitation (ChIP) assay
HepG2 cells were transiently transfected with Flag-STAT3 together with pcDNA3.1 or GRX-1 plasmids in 100-mm plates with 12 μg DNA per plate using Lipofectamine 2000. The cells were treated 2 d later without or with 20 ng/ml IL-6 for 30 min. ChIP assays were then performed essentially as described by Honda et al. (34). Briefly, cells were cross-linked with 1% formaldehyde at room temperature for 10 min. After harvesting cells with sodium dodecyl sulfate lysis buffer, DNA was sheared to 200–1000 bp by sonication, and the cell lysates were precleared by centrifugation. Agarose-bound M2 Flag antibody (Sigma-Aldrich) was used to immunoprecipitate the protein-DNA complexes, whereas anti-STAT3 (sc-7179; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was used to recover DNA complexes containing the endogenous STAT3. In addition, precleared lysates were also incubated with murine IgG as a control for potential nonspecific coprecipitations caused by transfection. DNA was quantified by real-time PCR using promoter-specific primers for HP, FGG, JUNB, and DNAJB4 (Table 1). Each experiment was repeated three times.
Table 1.
Promoter-specific primer sequences used for ChIP assays
| Gene | Promoter-specific primers |
|---|---|
| HP | 5′-CCTTACCAGGGCCAAAGTTTG-3′ (forward) |
| 5′-TCCCCCTTCTCCATTTCGTA-3′ (reverse) | |
| FGG | 5′-AGTCTATCCTTAAGATGGGTCCTGC-3′ (forward) |
| 5′-TTCTTTTGGTAATTCAGGTGATGG-3′ (reverse) | |
| JUNB | 5′-CTGGCCAGAAGGACAGATGC-3′ (forward) |
| 5′-GGAAGAGGTCCCAGCGTGA-3′ (reverse) | |
| DNAJB4 | 5′-TCGTGGAGGAGGAGAAAACATAG-3′ (forward) |
| 5′-CCCAGCTGCCTCTAGTCAAAGT-3′ (reverse) |
Immunoprecipitation
Cells were lysed in radioimmune precipitation (RIPA) buffer containing 20 mm Tris HCl (pH 8.0), 150 mm NaCl, 1% (wt/vol) Nonidet P-40, 1 mm EDTA, 10% glycerol, 100 mm NaF, 1 mm Na3VO4, and protease cocktail inhibitor set I (Calbiochem, San Diego, CA) for 30 min on ice with occasional vortexing. Equal amounts of proteins from the clarified cell lysates were incubated overnight at 4 C with rotation in the presence of 2 μg STAT3 antibody (Santa Cruz Biotechnology). The immune complexes were collected by addition of protein A/G agarose (Millipore Corp., Billerica, MA), washed extensively, and eluted in 1.5× Laemmli sample buffer. In some instances, cell lysates were incubated with anti-Flag M2 affinity gel.
Western blot analysis
Cells were lysed and processed for Western blotting as previously described (32). Primary antibodies generated against STAT3, ERK1/2, and BRG1 (Santa Cruz Biotechnology), GRX-1 (AbFrontier Co., Ltd., Seoul, Korea), Tyr705-phosphorylated STAT3 (pY-STAT3; Cell Signaling Technology, Inc., Danvers, MA), and protein-conjugated GSH (ViroGen Corp., Watertown, MA) were used. The bands were visualized by enhanced chemiluminescence (GE Healthcare Bio-Sciences Corp., Piscataway, NJ/Amersham Biosciences Inc., Piscataway, NJ) and quantified using SigmaScan imaging software (Jandel Scientific, San Rafael, CA).
Labeling of protein reactive thiols in cellular extracts
Cells were lysed in RIPA buffer supplemented with 200 μm maleimidobutyrylbiocytin (MBB) (EMD Chemicals Inc.-Calbiochem, San Diego, CA), an irreversible thiol-specific biotinylating agent, for 30 min on ice, followed by the addition of an excess of l-cysteine (5 mm) to quench the reaction. An aliquot of the clarified lysates was subjected to STAT3 immunoprecipitation and Western blotting. Polyvinylidene difluoride membranes (Invitrogen) were blocked with 1% polyvinylpyrrolidone (Sigma-Aldrich) in Tris-buffered saline with 0.1% (wt/vol) Tween 20 and incubated with horseradish peroxidase (HRP)-conjugated streptavidin (Vector Laboratories, Burlingame, CA) for the detection of biotin-labeled protein thiols. A second aliquot was incubated with CaptAvidin-agarose beads (Invitrogen) for 1 h at 4 C to immobilize thiol-biotinylated proteins, which were then eluted with 50 mm NaHCO3 (pH 10.0). After neutralization, STAT3 immunoprecipitation and Western blot analysis were performed as indicated previously.
Modified version of the biotin switch assay
The biotin switch assay was performed as described previously by Jaffrey and Snyder (35) with modifications. Details are provided in the supplemental methods, which are published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org.
Cell-free glutathionylation/deglutathionylation assay
Protein S-glutathionylation and deglutathionylation reactions were performed according to the protocol of Caplan et al. (24) and are detailed in the supplemental methods.
GRX activity
GRX activity was assayed according to the protocol of Gan and Wells (36). Details are provided in the supplemental methods.
DNA-binding activity of S-glutathionylated STAT3
Nuclear extracts from HepG2 cells were prepared using the NE-PER extraction kit (Pierce; Thermo Fisher Scientific Inc., Waltham, MA) and quantified using the BCA assay kit (Pierce). In vitro S-glutathionylation reaction and quantitation of DNA-binding activity were performed according to the protocol of Pineda-Molina et al. (37) and are detailed in the supplemental methods.
In vitro phosphorylation of STAT3 by recombinant JAK2
STAT3 immunoprecipitates from clarified HepG2 cell lysates were treated with 2 mm GSH and 1 mm diamide in degassed buffer A for 1 h at room temperature to induce protein S-glutathionylation. The immune pellets were washed, and the tyrosine phosphorylation reaction was then initiated in the presence of 50 ng recombinant active human JAK2 (United States Biological, Swampscott, MA) in phosphorylation buffer [50 mm HEPES (pH 7.6), 0.1 m NaCl, 0.1% Triton X-100, 6.25 mm MnCl2, and 100 μm ATP] for 10 min at 30 C (38). The reaction was quenched by the addition of 30 mm EDTA. The immune pellets were washed, and the proteins eluted for pY-STAT3 and STAT3 immunoblotting.
Indirect immunofluorescence analysis
For the evaluation of STAT3 nuclear translocation, HepG2 cells were seeded on coverslips and then serum starved for 4 h before a 2-h pretreatment with 50 μm PDTC, followed by stimulation with 20 ng/ml IL-6 for an additional 30 min. Cells were fixed with ice-cold methanol, blocked, and probed with a polyclonal antibody against STAT3 (1:50 dilution; Santa Cruz Biotechnology), and goat antirabbit secondary antibody conjugated with AlexaFluor 488 (1:1000 dilution; Molecular Probes, Inc., Eugene, OR). Nuclei were counterstained with TO-PRO-3 (Molecular Probes) before mounting, and fluorescence images were acquired using a ×63 oil-immersion objective lens on a Zeiss LSM-410 inverted confocal microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY).
Results
Influence of PDTC and diamide on IL-6-mediated STAT3 function
As a first step in characterizing the effect of PDTC and diamide on STAT3 function, we assayed pY-STAT3 levels in response to IL-6 challenge. An inhibition of IL-6-stimulated pY-STAT3 immunoreactivity was observed in HepG2 cells pretreated with either PDTC or diamide (Fig. 1A). The action of diamide was rapid and markedly attenuated the kinetics of STAT3 phosphorylation by IL-6 (supplemental Fig. 1, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). Consistent with the reversible nature of oxidized cysteine forms on proteins, the removal of diamide from the incubation medium led to a time-dependent recovery of IL-6 signaling, as early as 5 min after diamide wash and near full recovery at 15 min (Fig. 1B, lane 4 vs. 1). The blots exhibited equivalent loading of ERK1/2 protein levels in all samples (bottom panels), therefore validating the STAT3 phosphorylation data.
Figure 1.
Reversible reduction in IL-6-induced STAT3 signaling in response to mild oxidative stress. A, HepG2 cells were serum starved for 3–4 h and then left untreated or treated with PDTC (100 μm, 2 h) or diamide (500 μm, 30 min), followed by stimulation with IL-6 (20 ng/ml) for 20 min at 37 C. B, Cells were pretreated with diamide (500 μm) for 30 min, washed, and incubated with fresh medium for 0, 5, 15, and 45 min before the addition of IL-6 for 15 min. A and B, Cells were lysed and analyzed by Western blot with an antibody raised against pY-STAT3 (top panels). ERK1/2 was used as a loading control (lower panels). The OD of the pY-STAT3 band in untreated, IL-6-stimulated cells was arbitrarily given the value of 1.0. The numbers given under the different panels [relative units (Rel. Units)] represent the relative intensities of the protein bands from two independent experiments. The positions of pY-STAT3 and ERK1/2 are indicated on the left and that of the molecular mass markers (in kDa) are shown on the right. C, PDTC decreases nuclear translocation of STAT3 in HepG2 cells. Cells were preincubated with vehicle (veh.) or 50 μm PDTC for 2 h, then treated with 20 ng/ml IL-6 for 20 min. Cells were fixed and probed with STAT3 antibody to detect subcellular localization of STAT3. Nuclei were visualized by staining with Topro. Confocal images in the bottom panels are overlay of STAT3 and Topro images. The results are representative to three independent experiments. D, Effect of PDTC on FBG protein levels of HepG2 cells stimulated with IL-6. HepG2 cells were left untreated or treated with PDTC for 2 h before the addition of IL-6 (20 ng/ml) or IL-1β (20 ng/ml) for 24 h. Cells were lysed and analyzed by immunoblotting for expression of FBG (top panel), pY-STAT3 (middle panel), and ERK1/2 (lower panel) as a loading control. Similar results were obtained in two independent experiments.
Nuclear retention and transcriptional activity of STAT3 are greatly enhanced by JAK-mediated STAT3 tyrosine phosphorylation (1). Because PDTC attenuates pY-STAT3 levels, confocal microscopy studies were performed to examine intracellular localization of STAT3 in HepG2 cells in response to PDTC treatment. Figure 1C shows that STAT3 immunoreactivity, which was diffuse both in the cytoplasmic and nuclear compartments under basal conditions, accumulated to great extent in the nucleus of IL-6-stimulated cells and that the presence of PDTC blocked this process. Impairment in IL-6-mediated nuclear accumulation of STAT3 was also observed after diamide treatment (data not shown). These results imply that PDTC or diamide could impair STAT3 function.
Consistent with its ability to impair nuclear accumulation of STAT3, PDTC caused a 50% inhibition of FBG protein expression after a 24-h stimulation with IL-6 (Fig. 1D). Exposure of HepG2 cells to IL-1β alone failed to elicit STAT3 tyrosine phosphorylation and subsequent expression of FBG, in accordance with reports suggesting that IL-6, but not IL-1β, induces production of type II acute phase proteins, such as FBG (39) (Fig. 1D, lane 1 vs. 3).
STAT3 protein contains cysteine residues that are targets for S-glutathionylation
Because of its redox sensitivity, we investigated the incorporation of a thiol-alkylating agent into the accessible cysteines on the surface of native STAT3. The irreversible thiol-biotinylating agent MBB was used to modify reactive thiols on proteins present in cell lysates as described in Materials and Methods. Therefore, we examined incorporation of biotin into STAT3 in the presence of PDTC and oxidants such as diamide and GSSG in HepG2 cells. The Western blot in Fig. 2A shows the results obtained in STAT3 immunoprecipitates. Modification of STAT3 protein with biotinylated maleimide occurred in untreated cells, and it decreased with PDTC, diamide, or GSSG (∼35–55% reduction), suggesting that STAT3 contains reactive thiols in cells under physiological conditions and that cysteine oxidation on STAT3 could occur in response to moderate stress. The blots exhibited equivalent STAT3 protein levels in all samples (Fig. 2A, bottom panel). In a second set of experiments, thiol-biotinylated samples were enriched by CaptAvidin-agarose chromatography, and Western blot analysis was performed to detect the CaptAvidin-bound STAT3. Consistent with the difference in STAT3 thiol reactivity, a much lower amount of CaptAvidin-bound STAT3 was observed with PDTC when compared with untreated controls (Fig. 2B).
Figure 2.
STAT3 contains cysteine residues that are targets for oxidant-mediated S-glutathionylation. A and B, Serum-starved HepG2 cells were left untreated or incubated either with PDTC (50 μm, 2 h), GSH disulfide (GSSG, 20 mm, 30 min) or diamide (500 μm, 30 min), after which cells were homogeneized in RIPA buffer supplemented with the thiol biotinylating agent, MBB (200 μm), for 30 min on ice. A, After quenching the alkylation reaction with excess l-cysteine, the clarified cell lysates were subjected to immunoprecipitation with STAT3 antibody and immunoblotted with streptavidin-conjugated HRP (top panel) and STAT3 (lower panel) as a loading control. B, Alternatively, the clarified cell lysates were incubated with CaptAvidin-linked agarose for 1 h at 4 C, and the immobilized proteins were eluted with biotin, followed by Western blot analysis with anti-STAT3. The band intensity of thiol-biotinylated STAT3 in untreated cells was arbitrarily given the value of 1.0. Results are representative of two separate experiments with similar results. C, Reversible oxidation of STAT3 cysteine residues by PDTC. HepG2 cells were treated without or with PDTC for 2 h. Cell lysates were subjected to biotin switch assay, followed by affinity precipitation with streptavidin-agarose and then immunoblot analysis with anti-STAT3 antibody. Bands were detected only in PDTC-treated cells and when biotin-HPDP was included in the assay. D, HepG2 cells were left untreated or incubated with PDTC, GSSG, or diamide as indicated in A. STAT3 immunoprecipitates were separated by SDS-PAGE under nonreducing conditions, and analyzed by Western blot with a monoclonal antibody against protein-bound GSH (αSSG, top panel) and STAT3 (lower panel) as a loading control. The S-glutathionylated STAT3 band intensity in untreated cells was arbitrarily given the value of 1.0. Rel. Units, Relative units; veh, vehicle.
The biotin switch assay has been developed for the detection of reversible protein S-nitrosylation (35). To demonstrate the reversible STAT3 modification in cells, we used a modified version of the biotin switch assay, whereby the alkylating agent methyl methanethiosulfonate was replaced by N-ethyl maleimide, and the reductant dithiothreitol (DTT) was used as a substitute for ascorbate. In brief, the alkylation reaction was performed first to “inactivate” oxidant-unreactive cysteines on proteins from HepG2 cell extracts, followed by DTT treatment to release labile disulfide linkages, and incubation with the reversible thiol-biotinylating agent, N-[6-(biotinamido)hexyl]-3′-(2′-pyridyldithio)propionamide (biotin-HPDP). The extracts were gel filtered to remove HPDP before streptavidin affinity chromatography and specific immunoblotting to detect bound STAT3. When HepG2 cells were treated with PDTC, STAT3 was clearly detected among the proteins that were reversibly S-thiolated (Fig. 2C).
In a complementary approach, STAT3 immunoprecipitates from control and oxidant-treated cells were separated by SDS-PAGE under nonreducing conditions, and the resulting Western blots were probed with an antibody that detect S-glutathionylated protein thiols (40). Figure 2D shows that immunoprecipitated STAT3 was recognized by GSH antibodies. The data indicate that small amounts of STAT3 exist in a thiolated state in serum-starved HepG2 cells, and that STAT3 glutathionylation signal increased in response to PDTC, diamide, and GSSG. The blots exhibited equivalent STAT3 protein levels in all samples (Fig. 2D, bottom panel).
Influence of in vitro glutathionylation on STAT3 phosphorylation and function
For testing the possibility of S-glutathionylation of STAT3 in vitro, STAT3 immunoprecipitates were treated with diamide and GSH, and analyzed by Western blot with anti-GSH antibodies. As anticipated, glutathionylated STAT3 level increased after diamide/GSH treatment compared with that in untreated samples (top panel of Fig. 3A, lane 2 vs. 1). Rapid dethiolation occurred when Escherichia coli GRX-1 was added to STAT3 immunoprecipitates that were washed after the diamide/GSH treatment (Fig. 3A, lane 3 vs. 4). Exposure of the diamide-treated immunoprecipitates to DTT completely eliminated the glutathionylation signal, consistent with reversible S-thiolation of STAT3 in vitro (Fig. 3A, lane 5 vs. 4).
Figure 3.
In vitro S-glutathionylation inhibits JAK2-mediated STAT3 phosphorylation and its DNA-binding activity. A, STAT3 is an in vitro target of glutathionylation/deglutathionylation reaction. Anti-STAT3 immunoprecipitates from control HepG2 cells were left untreated (lane 1) or treated with diamide (1 mm)/2 mm GSH for 1 h at room temperature (lanes 2–5), after which vehicle (veh.), recombinant E. coli GRX-1 or DTT was added for 30 min. The immune pellets were resolved by SDS-PAGE under nonreducing conditions, and analyzed by Western blot with antibodies raised against αSSG (top panel) and STAT3 as a loading control (bottom panel). B, STAT3 immunoprecipitates were left untreated or treated with 1 mm diamide/GSH as described under Materials and Methods. Tyrosine phosphorylation was then performed using recombinant active JAK2 for 10 min at 30 C. The proteins in the immune pellets were analyzed by Western blot using antibodies against pY-STAT3 (upper panel) and total STAT3 (lower panel). The reversibility of the inhibitory effect of diamide was shown by using 2 mm DTT before the JAK2-mediated phosphorylation step. The numbers given under the panel [relative units (Rel. Units)] represent the quantitated ratios of pY-STAT3 to total STAT3 relative to that of untreated vehicle control. Results are representative of two independent experiments. C, Cells were treated in the absence or the presence of IL-6 (20 ng/ml) for 30 min. Nuclear extracts were prepared, and DNA binding activity of STAT3 was determined as described under Materials and Methods. Nuclear extracts were incubated with agarose-bound oligonucleotide probe containing the wild-type (wt) GAS consensus sequence (lanes 1–2) or the same probe mutated (mut) at the GAS site (lanes 3–4). Bound STAT3 was resolved by SDS-PAGE, and analyzed by Western blot with anti-pY-STAT3. An identical result was obtained in an additional experiment. D, The nuclear extracts of IL-6-stimulated cells were treated without or with 3 mm GSSG for 1 h at 37 C to induce in vitro S-glutathionylation. STAT3 immobilized with agarose-bound wild-type oligonucleotide probe was analyzed by Western blot using anti-pY-STAT3. The numbers given in the panel (Rel. Units) represent the intensities of pY-STAT3 protein bands relative to that of control IL-6-stimulated cells. Results are representative of four independent observations from two separate experiments with similar results.
Because the extent of STAT3 glutathionylation increased after the addition of oxidants, we examined the effect of this posttranslational modification on STAT3 function. First, immunoprecipitates of STAT3 protein, treated or not with diamide/GSH, were washed and then incubated with recombinant active JAK2 (Fig. 3B). In the presence of JAK2, STAT3 protein exhibited an increased level of phosphorylation at tyrosine 705, a prerequisite for STAT3 function (1). Furthermore, the levels of pY-STAT3 were significantly abated in response to diamide treatment but were recovered after treating the immunoprecipitates with DTT before JAK2-mediated phosphorylation (Fig. 3B, lane 5 vs. 4). This is consistent with dethiolation of STAT3 in the diamide-treated samples. The blots exhibited equivalent STAT3 protein levels in all samples (Fig. 3B, bottom panel).
A DNA affinity immunoprecipitation assay was then used to assess the binding of S-thiolated STAT3 to the agarose-conjugated consensus or mutated recognition sequences. The assay combines affinity step and Western blot analysis to detect the DNA-bound STAT3. Because phosphorylation of tyrosine 705 enhances dimerization and binding of STAT3 to DNA, immunoblotting was performed with antibody against phospho-STAT3. This procedure was applied initially with nuclear extracts prepared from control and IL-6-stimulated HepG2 cells. In the absence of IL-6, no DNA-bound pY-STAT3 was apparent, whereas a strong and reproducible increase in the level of binding of phosphorylated STAT3 to its target DNA was observed upon IL-6 stimulation (Fig. 3C, lane 2 vs. 1). The oligonucleotides with mutated recognition sequence bound poorly with pY-STAT3 (Fig. 3C, compare lane 3 vs. 2). Specific immunoblotting to detect the DNA-bound total STAT3 yielded similar results (data not shown). In the next series of experiments, nuclear extracts from IL-6-treated cells were exposed to 3 mm GSSG to promote glutathionylation of proteins, and DNA binding reactions were performed. S-thiolation by GSSG reduced STAT3-specific DNA binding by 50% (Fig. 3D). Together, these results strongly suggest that S-thiolation inhibits STAT3 function.
GRX activity is reduced by oxidants
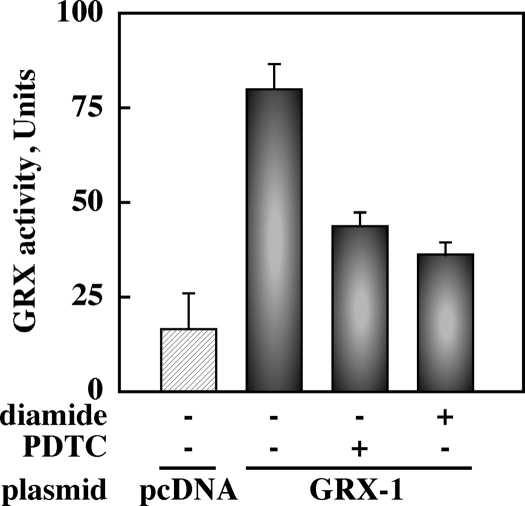
Because the impairment in dethiolation reaction may contribute to higher steady-state levels of protein glutathionylation after oxidative stress, we transiently transfected HepG2 cells with a plasmid encoding the thioltransferase GRX-1 and subjected them to PDTC or diamide before assessing GRX-1 activity in cell extracts. GRX is one of several cytosolic GSH-dependent redox enzymes responsible for protein dethiolation (41). Addition of PDTC or diamide resulted in 57 and 69% decrease in GRX-1 activity, respectively (Fig. 4). These results imply that oxidation of reactive cysteines in the catalytic center of GRX-1 could abrogate its activity, which constitutes a regulatory mechanism for protein S-glutathionylation after oxidative stress.
Figure 4.
GRX activity is reduced by oxidants. HepG2 cells transiently transfected with pcDNA vector (hatched bar) and GRX-1 plasmid (filled bars) were left untreated or incubated with PDTC (50 μm, 2 h) or diamide (500 μm, 30 min). GRX activity was measured in cell lysates as described in Materials and Methods. Bars are means ± sd of a single experiment performed in triplicate. Results are representative of two separate experiments with similar results.
Effect of ectopic expression of GRX-1 on IL-6-induced STAT3 signaling
Because enhanced GRX expression may reduce the extent of protein glutathionylation and, thus, regulate cellular functions (42), we performed transient cotransfection of HepG2 cells with plasmids encoding GRX-1 and Flag-tagged STAT3, and exposed them to IL-6 for various periods of time. First, STAT3 immunoprecipitates prepared from the MBB-treated cell lysates were resolved by SDS-PAGE, followed by Western blot analysis using streptavidin-linked HRP. This procedure showed that the levels of biotin incorporation in cysteines on STAT3 protein were not influenced by GRX-1 overexpression (Fig. 5A, upper panel). Reprobing the membranes indicated constitutive interaction between transfected GRX-1 and Flag-tagged STAT3 (Fig. 5A, bottom panel).
Figure 5.
Ectopic expression of GRX-1 enhances IL-6-induced STAT3 signaling. Cells were transiently transfected with Flag-STAT3 together with pcDNA vector or GRX-1 plasmid. A, Forty-eight hours later, cells were homogeneized in RIPA buffer containing MBB, and anti-Flag immunoprecipitates were analyzed by Western blot using HRP-conjugated streptavidin (Strep.) (top panel) or anti-STAT3 (second panel) and anti-GRX-1 (bottom panel) antibodies. B, Serum-starved transfected cells were left untreated or incubated with diamide (500 μm) for 30 min, followed by the addition of IL-6 (20 ng/ml) for 20 min. The anti-Flag immunoprecipitates were analyzed by Western blotting for the detection of pY-STAT3 (top panel), and Flag-STAT3 (second panel). Immunoblot analysis showed detection of GRX only in clarified lysates of GRX-1-transfected cells (third panel). Endogenous ERK 1/2 was detected at comparable levels in each lane (bottom panel). The pY-STAT3 band intensity in Flag-STAT3-transfected cells stimulated with IL-6 was arbitrarily given the value of 1.0. No pY-STAT3 signal was detected in the absence of IL-6 (data not shown). IP, Immunoprecipitation; IB, immunoblots. C, The levels of Flag-STAT3 in the nuclear (Nuc) (second panel) and cytosolic (Cyt) (third panel) fractions of serum-starved cells exposed to IL-6 (20 ng/ml) for 30 min were assessed by Western blot analysis. The membranes were reprobed with BRG-1 (top panel) and ERK 1/2 (bottom panel) antibodies to demonstrate equal protein loading in each lane. D, Nuclear Flag-STAT3 band intensities were normalized to cellular pool (nuclear + cytosolic) of Flag-STAT3 protein. The dot plot represents the individual measurements that were derived from two separate experiments, each performed in duplicate dishes. Rel. Units, Relative units; veh., vehicle.
Next, IL-6-induced tyrosine phosphorylation of Flag-STAT3 from control and diamide-treated cells was assessed in Flag immunoprecipitates. When compared with pcDNA-transfected cells, cells overexpressing GRX-1 showed a small but reproducible 30% increase in Flag-STAT3 tyrosine phosphorylation by IL-6. Ectopic expression of GRX-1 did not confer protection against diamide (Fig. 5B, upper panel, lanes 6 and 7 vs. 4 and 5). We propose that thiol oxidation of Flag-STAT3 by diamide mediates functional impairment in STAT3 phosphorylation, in agreement with the in vitro phosphorylation data (Fig. 3B).
Because phosphorylated STAT3 translocates and functions in nuclei, it was of interest to examine the subcellular localization of Flag-STAT3. Control and GRX-1-transfected HepG2 cells were subjected to IL-6 stimulation, and the cytosolic and nuclear fractions were separated, followed by Western blot analysis using anti-Flag antibody. The data in Fig. 5C (second panel) show that the level of nuclear Flag-STAT3 increased several fold by IL-6. The blots exhibited equivalent levels of the nuclear marker BRG1 (Fig. 5C, top panel). Ectopic expression of GRX-1 was accompanied by a small but statistically significant 30% increase in nuclear Flag-STAT3 levels (Fig. 5D, dot plots).
We then attempted to evaluate the effect of GRX-1 on in vivo interactions between STAT3 and specific DNA regions using the ChIP method. ChIP assays showed that Flag-STAT3 was recruited onto the promoter region of known STAT3 targets (e.g. FGG, HP, and JUNB) in HepG2 cells upon IL-6 stimulation but failed to bind to the DNAJB4 promoter region in an IL-6-dependent manner (Fig. 6A and supplemental Fig. S2). Ectopic expression of GRX-1 elicited only a modest increase in Flag-STAT3 binding when compared with GRX-1 nontransfected cells. When chromatin samples were immunoprecipitated with a control murine IgG, a complete absence of Flag-STAT3 recruitment onto FGG promoter region was observed (Fig. 6A). A second series of experiments was undertaken to test whether the binding properties of Flag-STAT3 onto the promoter region of the genes under study were comparable to those of endogenous STAT3 after IL-6 stimulation. The results clearly show that the pattern of STAT3 binding in nontransfected cells was similar to that of Flag-STAT3 in transfected cells (supplemental Fig. S3).
Figure 6.
Effect of GRX-1 overexpression on STAT3 DNA binding and induction of target genes. A, ChIP assays using agarose-conjugated Flag antibody (M2-Ag) were performed in HepG2 cells transfected with Flag-STAT3 plasmid together with empty vector (pcDNA) or GRX-1 plasmid, and then left untreated or stimulated with IL-6 for 30 min. The results are expressed as percentage of immunoprecipitated (IP) DNA to total DNA input (input). Similar results were obtained in three independent experiments. B, HepG2 cells were transiently cotransfected with pGAS-TA-Luc reporter plasmid, pCMVSport-β-galactosidase expression plasmid, and Flag-tagged STAT3 together with either pcDNA empty vector or GRX-1 plasmid. Twenty-four hours later, the cells were serum starved for 4 h and then pretreated with vehicle (veh.) (open bars) or 20 ng/ml IL-6 (filled bars) for 6 h. Cell extracts were analyzed for luciferase activity and normalized for β-galactosidase. All values are expressed relative to that of Flag-STAT3-expressing cells without IL-6. Results are expressed as means ± sd of a single experiment performed in triplicate dishes. Results are representative of two separate experiments with similar results.
To analyze the functional roles of GRX-1 on STAT3-mediated gene expression, a reporter construct containing pSTAT3-TA-Luc was transfected into HepG2 cells along with plasmids encoding Flag-STAT3 and GRX-1. The promoter activity level was stimulated approximately 2.8-fold upon IL-6 treatment in the presence or absence of GRX-1 overexpression (Fig. 6B). Together, these data suggest that the ectopic expression of GRX-1 was accompanied by a marginal increase in IL-6-mediated STAT3 functions.
Effect of GRX-1 knockdown on IL-6 signaling
To assess independently the role of GRX-1 on STAT3 activation cascade, we knocked down GRX1 by silencing RNA in HepG2 cells, and found that down-regulation of GRX-1 did not elicit any significant difference in terms of STAT3 phosphorylation and nuclear translocation in response to IL-6 (data not shown).
Discussion
In the present study, we demonstrated that STAT3 is a novel target for protein S-glutathionylation. Cell treatment with diamide, oxidized GSH, or PDTC elicited an increase in STAT3 S-glutathionylation, which was accompanied by reduction in IL-6-induced tyrosine phosphorylation of this transcription factor, with subsequent impairment in the retention of STAT3 to the nucleus and transcriptional activation of target genes. This posttranslational modification was reversible, and STAT3 dethiolation was catalyzed by GRX-1 in vitro. Glutathionylation of STAT3 in a cell-free system made this protein a poor JAK2 substrate and abrogated STAT3-specific DNA binding. Several lines of evidence also show that ectopic expression or suppression of GRX-1 had only minimal effects on IL-6 responsiveness.
Protein S-glutathionylation has been proposed to occur via two distinct pathways, one that involves the initial oxidation of a reduced protein cysteinyl residue to a sulfenic acid, which may then react with GSH to form a mixed disulfide (43). The second pathway involves the oxidative modification of GSH to form GSSG, which subsequently interacts with a reduced protein thiol to yield a protein-mixed disulfide (41). We show here that a small but detectable S-glutathionylation of STAT3 occurs in intact cells under basal conditions, and that the exogenous oxidant diamide elicits significant increase in STAT3 S-glutathionylation as well as coincident changes in phosphorylation, and cellular redistribution and function of STAT3 in response to IL-6. Diamide reacts with physiological levels of GSH to form GSSG (44), thereby making it likely that STAT3 may be S-glutathionylated by a thiol-disulfide exchange mechanism. However, diamide can also form a conjugate with proteins harboring reactive free thiols, enabling the protein-S•diamide adduct to react with GSH to promote ROS-independent glutathionylation (45). Whether the relative reactivity of cysteines at the surface of STAT3 protein allows for formation of the STAT3-S•diamide adduct is unclear.
We report for the first time that PDTC elicits STAT3 S-glutathionylation. In agreement with the diamide data, it is proposed that PDTC may promote protein S-thiolation by lowering the GSH to GSSG ratio. In mammalian cells, GSH is present in millimolar concentrations at about 100-fold excess over GSSG. By favoring an oxidative equilibrium, PDTC alters GSH homeostasis through increasing levels of GSSG at the expense of GSH (28,29,30). Many components of the nuclear factor-κB (NF-κB) activity are regulated by S-glutathionylation, leading to decreased nuclear translocation and DNA binding activity of p50 and p65 subunits (37,46,47,48). It is interesting to speculate that the known inhibitory effects of PDTC on NF-κB-induced production of proinflammatory gene products stems from S-glutathionylation of specific elements of the NF-κB pathway. Similarly, the fact that PDTC elicits impairment in nuclear translocation and transcriptional activity of STAT3 in response to IL-6 (32) may be the result of S-glutathionylation.
The steady-state level of oxidatively modified proteins, via formation of mixed disulfides, is controlled by glutathionylation/deglutathionylation processes. Although glutathionylation occurs by nonenzymatic mechanisms, there is a family of enzymes whose function catalyzes the reduction of mixed disulfides of proteins. Notably, GRX and thioredoxin possess a redox active dithiol catalytic center, whereas sulfiredoxin contains a reactive monothiol in its catalytic domain (41). Therefore, one may speculate that oxidative modification of these conserved cysteine residues will result in a marked loss in their deglutathionylation function. In the present study, we demonstrate that GRX-1 activity was inhibited after cell treatment with PDTC or diamide, indicating the possible role of this enzyme in influencing STAT3 glutathionylation levels. GRX has been recognized as a critical component in deglutathionylation of proteins back to their reduced states. However, depending on the cellular context and differences in experimental conditions, GRX-1 has been shown to protect against apoptosis (49,50) or act as proapoptotic mediator via caspase-3 activation (51). Oxidative stress associated with high glucose was found to induce GRX in rat retinal glial cells, with concomitant NF-κB activation (48). In our hands, ectopic expression of GRX-1 in HepG2 cells does not offer protection against oxidant-induced S-glutathionylation of STAT3. The simplest explanation for these results is the susceptibility of GRX to acute oxidative inhibition (Fig. 4) (52). Our results indicate that GRX overexpression promoted modest up-regulation of IL-6/STAT3 cascade, whereas knockdown of GRX by small interference RNA had no adverse effects in IL-6-induced STAT3 signaling, demonstrating that GRX may exert minimal function on STAT3, at least in HepG2 cells. This conclusion is supported by a recent study showing that the protection against cellular damage elicited by various oxidants may occur via GRX-1 dependent and -independent mechanisms, and that GRX-1 may exert its function on specific target proteins (53). This study and our work do not contradict the role of GRX in the reduction of protein S-glutathionylation in other experimental models. Because of the participation of sulfiredoxin in the reversal of protein glutathionylation after oxidative stress (54), one should consider the possibility that the dynamic redox regulation of STAT3 involves this enzyme or other family members of the protein thiol-disulfide oxidoreductases.
Human STAT3 possesses a number of cysteines in the C-terminal region, which encompasses the unique tyrosine phosphorylation site (Tyr-705) located near the Src homology 2 domain involved in recognition of phosphorylated tyrosine, thereby allowing STAT3 to homodimerize in response to IL-6. In our hands, S-thiolation of STAT3 in vitro caused the protein to become a poor substrate for recombinant JAK2, which was readily reversed via dethiolation of STAT3 in the presence of DTT. Therefore, it appears that this posttranslational modification hampers the access of the Tyr-705 region to the STAT3 recognition site on JAK2. STAT3 also possesses a cluster of cysteines in the DNA binding domain, whose modification may down-regulate the transcriptional potency of STAT3 in response to diverse stimuli. There are studies showing impairment in DNA binding activity of a multitude of transcription factors upon glutathionylation of redox-reactive cysteines (37,55,56,57). In the current study, we report that S-thiolation reduces the DNA binding ability of pY-STAT3 in cell-free extracts. It is possible that essential transcriptional coactivators of STAT3 may also be the targets of dynamic redox control through S-glutathionylation. Further studies are required to test this hypothesis.
A number of questions still remain with regard to the molecular aspects of STAT3 glutathionylation. It is unclear at the present whether there is a single cysteine that is particularly reactive for glutathionylation. Moreover, enhanced glutathionylation by oxidants may mean that more STAT3 molecules are glutathionylated, and/or the degree of glutathionylation of single STAT3 molecules may be higher. Upon inspection of the three-dimensional structure of STAT3 bound to DNA (Protein Data Bank entry code 1BG1), we could not locate putative reactive cysteines in the surrounding of Tyr705, whose glutathionylation might have blocked phosphorylation. It is tempting to speculate that inactivation of STAT3 through glutathionylation may occur via modification of several reactive cysteines on the protein.
In summary, our results shed light on a novel aspect of redox-regulated STAT3 signaling by demonstrating that this protein is a S-glutathionylated target, whose modification elicits direct inhibitory effects on STAT3 activation and function. Persistent activation of STAT3 initiates oncogenic events, which could be amenable to pharmacological manipulation. The ability of GSH disulfide mimetics, such as NOV-002, to promote S-glutathionylation of cellular proteins has resulted in increased efficacy and improved tolerance to chemotherapy (58). Although S-thiolation may transiently prevent activation of STAT3 upon cellular stress, this modification may also confer protection from irreversible oxidation of reactive cysteines on STAT3. To date, no point mutations in cysteine residues of STAT3 have been shown in human cancers (http://www.sanger.ac.uk/genetics/CGP/cosmic/), which could allow STAT3 to escape redox regulation. Collectively, these data support the conclusion that S-glutathionylation can alter the functional properties of STAT3, and may have therapeutic potential in the context of inflammation and unregulated proliferation.
Supplementary Material
Acknowledgments
We thank Fred. E. Indig, Research Resources Branch at National Institute on Aging, for his expert assistance with the confocal studies.
Footnotes
This research was supported entirely by the Intramural Research Program of the National Institutes of Health, National Institute on Aging. A portion of that support was through a Research and Development contract with MedStar Research Institute.
Disclosure Statement: The authors have nothing to disclose.
First Published Online November 6, 2008
Abbreviations: Biotin-HPDP, N-[6-biotinamido) hexyl]-3′-(2′-pyridyldithio) propionamide; ChIP, chromatin immunoprecipitation; DTT, dithiothreitol; FBG, fibrinogen-γ; GRX, glutaredoxin; GSH, glutathione; GSSG, glutathione disulfide; HRP, horseradish peroxidase; JAK, Janus protein tyrosine kinase; MBB, maleimidobutyrylbiocytin; NF-κB, nuclear factor-κB; PDTC, pyrrolidine dithiocarbamate; pY-STAT3, Tyr705-phosphorylated signal transducer and activator of transcription 3; RIPA, radioimmune precipitation; ROS, reactive oxygen species; STAT, signal transducer and activator of transcription.
References
- Kishimoto T 2005 Interleukin-6: from basic science to medicine—40 years in immunology. Annu Rev Immunol 23:1–21 [DOI] [PubMed] [Google Scholar]
- Fu AK, Fu WY, Ng AK, Chien WW, Ng YP, Wang JH, Ip NY 2004 Cyclin-dependent kinase 5 phosphorylates signal transducer and activator of transcription 3 and regulates its transcriptional activity. Proc Natl Acad Sci USA 101:6728–6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuringa JJ, Schepers H, Vellenga E, Kruijer W 2001 Ser727-dependent transcriptional activation by association of p300 with STAT3 upon IL-6 stimulation. FEBS Lett 495:71–76 [DOI] [PubMed] [Google Scholar]
- Kortylewski M, Feld F, Krüger KD, Bahrenberg G, Roth RA, Joost HG, Heinrich PC, Behrmann I, Barthel A 2003 Akt modulates STAT3-mediated gene expression through a FKHR (FOXO1a)-dependent mechanism. J Biol Chem 278:5242–5249 [DOI] [PubMed] [Google Scholar]
- Lufei C, Koh TH, Uchida T, Cao X 2007 Pin1 is required for the Ser727 phosphorylation-dependent Stat3 activity. Oncogene 26:7656–7664 [DOI] [PubMed] [Google Scholar]
- Kwon MC, Koo BK, Moon JS, Kim YY, Park KC, Kim NS, Kwon MY, Kong MP, Yoon KJ, Im SK, Ghim J, Han YM, Jang SK, Shong M, Kong YY 2008 Crif1 is a novel transcriptional coactivator of STAT3. EMBO J 27:642–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonzi T, Maritano D, Gorgoni B, Rizzuto G, Libert C, Poli V 2001 Essential role of STAT3 in the control of the acute-phase response as revealed by inducible gene activation in the liver. Mol Cell Biol 21:1621–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diegelmann RF, Evans MC 2004 Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 9:283–289 [DOI] [PubMed] [Google Scholar]
- Demetris AJ, Lunz 3rd JG, Specht S, Nozaki I 2006 Biliary wound healing, ductular reactions, and IL-6/gp130 signaling in the development of liver disease. World J Gastroenterol 12:3512–3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyama K, Sata M, Rose-John S 2006 Interleukin-6 trans-signaling in inflammatory bowel disease. Cytokine Growth Factor Rev 17:451–461 [DOI] [PubMed] [Google Scholar]
- Hodge DR, Hurt EM, Farrar WL 2005 The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer 41:2502–2512 [DOI] [PubMed] [Google Scholar]
- Huang HF, Murphy TF, Shu P, Barton AB, Barton BE 2005 Stable expression of constitutively activated STAT3 in benign prostatic epithelial cells changes their phenotype to that resembling malignant cells. Mol Cancer 4:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WC, Ye SL, Sun RX, Liu YK, Tang ZY, Kim Y, Karras JG, Zhang H 2006 Inhibition of growth and metastasis of human hepatocellular carcinoma by antisense oligonucleotide targeting signal transducer and activator of transcription 3. Clin Cancer Res 12:7140–7148 [DOI] [PubMed] [Google Scholar]
- Choudhari SR, Khan MA, Harris G, Picker D, Jacob GS, Block T, Shailubhai K 2007 Deactivation of Akt and STAT3 signaling promotes apoptosis, inhibits proliferation, and enhances the sensitivity of hepatocellular carcinoma cells to an anticancer agent, Atiprimod. Mol Cancer Ther 6:112–121 [DOI] [PubMed] [Google Scholar]
- Lau CK, Yang ZF, Lam SP, Lam CT, Ngai P, Tam KH, Poon RT, Fan ST 2007 Inhibition of Stat3 activity by YC-1 enhances chemo-sensitivity in hepatocellular carcinoma. Cancer Biol Ther 6:1900–1907 [DOI] [PubMed] [Google Scholar]
- Sun X, Zhang J, Wang L, Tian Z 2008 Growth inhibition of human hepatocellular carcinoma cells by blocking STAT3 activation with decoy-ODN. Cancer Lett 262:201–213 [DOI] [PubMed] [Google Scholar]
- Siddiquee K, Zhang S, Guida WC, Blaskovich MA, Greedy B, Lawrence HR, Yip ML, Jove R, McLaughlin MM, Lawrence NJ, Sebti SM, Turkson J 2007 Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci USA 104:7391–7396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo M, Conde M, El Bekay R, Martín-Nieto J, Camacho MJ, Monteseirín J, Conde J, Bedoya FJ, Sobrino F 1999 Oxidative stress triggers STAT3 tyrosine phosphorylation and nuclear translocation in human lymphocytes. J Biol Chem 274:17580–17586 [DOI] [PubMed] [Google Scholar]
- Mazière C, Alimardani G, Dantin F, Dubois F, Conte MA, Mazière JC 1999 Oxidized LDL activates STAT1 and STAT3 transcription factors: possible involvement of reactive oxygen species. FEBS Lett 448:49–52 [DOI] [PubMed] [Google Scholar]
- Tacchini L, Fusar-Poli D, Sironi M, Mantovani A, Bernelli-Zazzera A 2003 Activation of signal transducer and activator of transcription 3 in rat liver after heat shock and reperfusion stress. Int J Biochem Cell Biol 35:316–323 [DOI] [PubMed] [Google Scholar]
- Bode JG, Schweigart J, Kehrmann J, Ehlting C, Schaper F, Heinrich PC, Häussinger D 2003 TNF-α induces tyrosine phosphorylation and recruitment of the Src homology protein-tyrosine phosphatase 2 to the gp130 signal-transducing subunit of the IL-6 receptor complex. J Immunol 171:257–266 [DOI] [PubMed] [Google Scholar]
- Klatt P, Lamas S 2000 Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem 267:4928–4944 [DOI] [PubMed] [Google Scholar]
- Cross JV, Templeton DJ 2004 Oxidative stress inhibits MEKK1 by site-specific glutathionylation in the ATP-binding domain. Biochem J 381(Pt 3):675–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan JF, Filipenko NR, Fitzpatrick SL, Waisman DM 2004 Regulation of annexin A2 by reversible glutathionylation. J Biol Chem 279:7740–7750 [DOI] [PubMed] [Google Scholar]
- Kil IS, Park JW 2005 Regulation of mitochondrial NADP+-dependent isocitrate dehydrogenase activity by glutathionylation. J Biol Chem 280:10846–10854 [DOI] [PubMed] [Google Scholar]
- Clavreul N, Adachi T, Pimental DR, Ido Y, Schöneich C, Cohen RA 2006 S-glutathiolation by peroxynitrite of p21ras at cysteine-118 mediates its direct activation and downstream signaling in endothelial cells. FASEB J 20:518–520 [DOI] [PubMed] [Google Scholar]
- Ghezzi P, Bonetto V, Fratelli M 2005 Thiol-disulfide balance: from the concept of oxidative stress to that of redox regulation. Antioxid Redox Signal 7:964–972 [DOI] [PubMed] [Google Scholar]
- Haddad JJ, Olver RE, Land SC 2000 Antioxidant/pro-oxidant equilibrium regulates HIF-1α and NF-κB redox sensitivity. Evidence for inhibition by glutathione oxidation in alveolar epithelial cells. J Biol Chem 275:21130–21139 [DOI] [PubMed] [Google Scholar]
- Thompson JS, Asmis R, Glass J, Liu H, Wilson C, Nelson B, Brown SA, Stromberg AJ 2006 P53 status influences regulation of HSPs and ribosomal proteins by PDTC and radiation. Biochem Biophys Res Commun 343:435–442 [DOI] [PubMed] [Google Scholar]
- Wild AC, Mulcahy RT 1999 Pyrrolidine dithiocarbamate up-regulates the expression of the genes encoding the catalytic and regulatory subunits of γ-glutamylcysteine synthetase and increases intracellular glutathione levels. Biochem J 338(Pt 3):659–665 [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xie Y, Bernier M, Wainer IW 22 June 2008 Determination of free and protein-bound glutathione in HepG2 cells using capillary electrophoresis with laser-induced fluorescence detection. J Chromatogr A, Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HJ, Zhu TN, Xie Y, Fan J, Kole S, Saxena S, Bernier M 2006 Pyrrolidine dithiocarbamate inhibits interleukin-6 signaling through impaired STAT3 activation and association with transcriptional coactivators in hepatocytes. J Biol Chem 281:31369–31379 [DOI] [PubMed] [Google Scholar]
- Zhu TN, He HJ, Kole S, D'Souza T, Agarwal R, Morin PJ, Bernier M 2007 Filamin A-mediated down-regulation of the exchange factor Ras-GRF1 correlates with decreased matrix metalloproteinase-9 expression in human melanoma cells. J Biol Chem 282:14816–14826 [DOI] [PubMed] [Google Scholar]
- Honda H, Pazin MJ, D'Souza T, Ji H, Morin PJ 2007 Regulation of the CLDN3 gene in ovarian cancer cells. Cancer Biol Ther 6:1733–1742 [DOI] [PubMed] [Google Scholar]
- Jaffrey SR, Snyder SH 2001 The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE 2001:PL1 [DOI] [PubMed] [Google Scholar]
- Gan ZR, Wells WW 1986 Purification and properties of thioltransferase. J Biol Chem 261:996–1001 [PubMed] [Google Scholar]
- Pineda-Molina E, Klatt P, Vázquez J, Marina A, García de Lacoba M, Pérez-Sala D, Lamas S 2001 Glutathionylation of the p50 subunit of NF-κB: a mechanism for redox-induced inhibition of DNA binding. Biochemistry 40:14134–14142 [DOI] [PubMed] [Google Scholar]
- Argetsinger LS, Campbell GS, Yang X, Witthuhn BA, Silvennoinen O, Ihle JN, Carter-Su C 1993 Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell 74:237–244 [DOI] [PubMed] [Google Scholar]
- Moshage H 1997 Cytokines and the hepatic acute phase response. J Pathol 181:257–266 [DOI] [PubMed] [Google Scholar]
- Craghill J, Cronshaw AD, Harding JJ 2004 The identification of a reaction site of glutathione mixed-disulphide formation on γS-crystallin in human lens. Biochem J 379(Pt 3):595–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallogly MM, Mieyal JJ 2007 Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr Opin Pharmacol 7:381–391 [DOI] [PubMed] [Google Scholar]
- Shelton MD, Chock PB, Mieyal JJ 2005 Glutaredoxin: role in reversible protein S-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid Redox Signal 7:348–366 [DOI] [PubMed] [Google Scholar]
- Johansson M, Lundberg M 2007 Glutathionylation of β-actin via a cysteinyl sulfenic acid intermediary. BMC Biochem 8:26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosower NS, Kosower EM 1987 Formation of disulfides with diamide. Methods Enzymol 143:264–270 [DOI] [PubMed] [Google Scholar]
- Di Simplicio P, Cacace MG, Lusini L, Giannerini F, Giustarini D, Rossi R 1998 Role of protein -SH groups in redox homeostasis—the erythrocyte as a model system. Arch Biochem Biophys 355:145–152 [DOI] [PubMed] [Google Scholar]
- Reynaert NL, van der Vliet A, Guala AS, McGovern T, Hristova M, Pantano C, Heintz NH, Heim J, Ho YS, Matthews DE, Wouters EF, Janssen-Heininger YM 2006 Dynamic redox control of NF-κB through glutaredoxin-regulated S-glutathionylation of inhibitory κB kinase β. Proc Natl Acad Sci USA 103:13086–13091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthy AK, Savinova OV, Ho JQ, Wang VY, Vu D, Ghosh G 2006 The 20S proteasome processes NF-κB1 p105 into p50 in a translation-independent manner. EMBO J 25:1945–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton MD, Kern TS, Mieyal JJ 2007 Glutaredoxin regulates nuclear factor κB and intercellular adhesion molecule in Müller cells: model of diabetic retinopathy. J Biol Chem 282:12467–12474 [DOI] [PubMed] [Google Scholar]
- Daily D, Vlamis-Gardikas A, Offen D, Mittelman L, Melamed E, Holmgren A, Barzilai A 2001 Glutaredoxin protects cerebellar granule neurons from dopamine-induced apoptosis by dual activation of the ras-phosphoinositide 3-kinase and jun n-terminal kinase pathways. J Biol Chem 276:21618–21626 [DOI] [PubMed] [Google Scholar]
- Murata H, Ihara Y, Nakamura H, Yodoi J, Sumikawa K, Kondo T 2003 Glutaredoxin exerts an antiapoptotic effect by regulating the redox state of Akt. J Biol Chem 278:50226–50233 [DOI] [PubMed] [Google Scholar]
- Pan S, Berk BC 2007 Glutathiolation regulates tumor necrosis factor-α-induced caspase-3 cleavage and apoptosis: key role for glutaredoxin in the death pathway. Circ Res 100:213–219 [DOI] [PubMed] [Google Scholar]
- Hashemy SI, Johansson C, Berndt C, Lillig CH, Holmgren A 2007 Oxidation and S-nitrosylation of cysteines in human cytosolic and mitochondrial glutaredoxins: effects on structure and activity. J Biol Chem 282:14428–14436 [DOI] [PubMed] [Google Scholar]
- Ho YS, Xiong Y, Ho DS, Gao J, Chua BH, Pai H, Mieyal JJ 2007 Targeted disruption of the glutaredoxin 1 gene does not sensitize adult mice to tissue injury induced by ischemia/reperfusion and hyperoxia. Free Radic Biol Med 43:1299–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay VJ, Townsend DM, Morris TE, Fraser JP, He L, Tew KD 2006 A novel role for human sulfiredoxin in the reversal of glutathionylation. Cancer Res 66:6800–6806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt P, Molina EP, De Lacoba MG, Padilla CA, Martinez-Galesteo E, Barcena JA, Lamas S 1999 Redox regulation of c-Jun DNA binding by reversible S-glutathiolation. FASEB J 13:1481–1490 [DOI] [PubMed] [Google Scholar]
- Cao X, Kambe F, Lu X, Kobayashi N, Ohmori S, Seo H 2005 Glutathionylation of two cysteine residues in paired domain regulates DNA binding activity of Pax-8. J Biol Chem 280:25901–25906 [DOI] [PubMed] [Google Scholar]
- Velu CS, Niture SK, Doneanu CE, Pattabiraman N, Srivenugopal KS 2007 Human p53 is inhibited by glutathionylation of cysteines present in the proximal DNA-binding domain during oxidative stress. Biochemistry 46:7765–7780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend DM, He L, Hutchens S, Garrett TE, Pazoles CJ, Tew KD 2008 NOV-002, a glutathione disulfide mimetic, as a modulator of cellular redox balance. Cancer Res 68:2870–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.