Abstract
The WW domain-containing oxidoreductase (WWOX) gene encodes a 46-kDa tumor suppressor. The Wwox protein contains two N-terminal WW domains that interact with several transcriptional activators containing proline-tyrosine motifs and a central short-chain dehydrogenase/reductase domain that has been suggested to play a role in steroid metabolism. Recently, we have shown that targeted deletion of the Wwox gene in mice leads to postnatal lethality and defects in bone growth. Here, we report that Wwox-deficient mice display impaired steroidogenesis. Mutant homozygous mice are born with gonadal abnormalities, including failure of Leydig cell development in testis and reduced theca cell proliferation in ovary. Furthermore, Wwox−/− mice displayed impaired gene expression of key steroidogenesis enzymes. Affymetrix microarray gene analysis revealed differentially expressed related genes in steroidogenesis in knockout mice testis and ovary as compared with control mice. These results demonstrate the essential requirement for the Wwox tumor suppressor in proper steroidogenesis.
The Wwox tumor suppressor is an essential requirement in proper steroidogenesis.
WW domain-containing oxidoreductase (Wwox) is a 46-kDa protein that contains two N-terminal WW domains that mediate protein-protein interaction, and a central short-chain dehydrogenase/reductase (SDR) domain that has been suggested to play a role in steroid metabolism (1,2). Recently, we reported the generation of a mouse carrying a targeted deletion of the Wwox gene (3). We demonstrated that the loss of both alleles of Wwox resulted in the formation of some juvenile osteosarcomas, whereas the loss of one allele increased the incidence of spontaneous and chemically induced tumors (3,4), indicating that Wwox is a bona fide tumor suppressor. Indeed, WWOX expression is lost or reduced in several types of cancer, including breast, prostate, lung, stomach, and pancreatic carcinomas (5,6). Restoration of WWOX expression in different types of cancer cells lacking expression of endogenous Wwox results in significant growth inhibition and prevents the development of tumors in athymic nude mice (7,8). More recently, Aldaz and colleagues (9) reported the generation of Wwox hypomorphic mice that have a low level of Wwox expression and display more frequent B-cell lymphoma compared with wild-type (WT) mice. Collectively, these results indicate that Wwox has a tumor suppressor function.
The generation of the Wwox-deficient mice had also shed light on Wwox in vivo requirement. Our recent analysis has shown that Wwox-deficient mice die by 3 wk of age due to a severe metabolic defect (10). The analysis of serum chemistry of Wwox knockout (KO) mice at the age of 2 wk revealed that mice suffer from hypoglycemia, hypolipidemia, and hypoproteinemia when compared with WT littermates. Furthermore, Wwox-deficient mice are smaller in size and exhibit an osteopenic phenotype. We found that Wwox physically associates with Runx2, the principal transcriptional regulator of osteoblast differentiation, and functionally suppresses RUNX2 transactivation ability in osteoblasts.
Analysis of the Wwox expression pattern in mouse tissues revealed that Wwox is ubiquitously expressed with prominent expression in hormonally regulated tissues, such as testis, ovary, prostate, and mammary epithelial cells (3,11). Distribution of Wwox expression in normal tissues provided some insight into the potential physiological role of this protein. Further investigation of gonadal tissues in Wwox KO mice indicated an aberrant steroidogenesis both in testis and ovary.
Materials and Methods
Mice
C57Bl/6J × 129/SvJ-F1, F2, F3, F4, and F5 mice (B6-129 F1–F5) were produced in the Ohio State University animal facility. The Wwox offspring were differentiated by genotyping of tail DNA using a PCR-based method (3). Animals were killed; tissues of all organs were removed, fixed in 10% buffered formalin, and examined for histological abnormalities by two pathologists after hematoxylin and eosin staining.
Histology and immunohistochemistry
Tissue from different organs was processed, embedded, and sectioned (4 μm) according to standard methods. Antibodies used for immunohistochemical staining were polyclonal anti-Wwox (dilution 1:8000; kindly provided by Dr. Kay Huebner, Ohio State University, Columbus, OH), and staining was performed as described (12). Rat antimouse Ki67 antibody (dilution 1:500; Dako Corp., Carpinteria, CA) was used as a marker for proliferation, and rat antimouse P450 scc (dilution 1:50; CHEMICON International, Inc., Temecula, CA) as a marker for Leydig cells, and anti-Fsh and anti-Lh (Abcam, Inc., Cambridge, MA) were used to stain pituitary glands. The detection system used was Vectastain Elite (Vector Laboratories, Burlingame, CA). A detailed protocol is available upon request. Pictures were taken using a ×10 or ×40 objective lens; no magnification was used.
Real-time PCR
Total RNA (1 μg) was isolated from the different tissues followed by homogenizing in TRIzol reagent (Invitrogen Corp., Carlsbad CA) according to the manufacturer’s protocol. cDNA was synthesized with oligo-deoxythymidine primers using the SuperScript First-Strand Synthesis Kit (Invitrogen) according to the manufacturer’s protocol. Gene expression was assessed by semiquantitative and quantitative real-time PCR (Cyber green and TaqMan) using primers listed in supplemental data (SI-1), which are published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org. The expression of cyclophilin A or glyceraldehyde-3-phosphate dehydrogenase was used as a control.
Affymetrix analysis
Total RNA from each testis and ovary was hybridized with Affymetrix mouse gene-chip 430 2.0 arrays (Affymetrix, Inc., Santa Clara, CA). Normalization and analysis were performed as described in Ref. 10.
Results
Testicular abnormalities and impaired steroidogenesis in male Wwox−/− mice
Macroscopic examination of the Wwox-deficient mice testis revealed that the testis size is reduced (10), indicating a possible defect in steroid biosynthesis. To define the cause of the testicular hypoplasia, we examined testis histologically. Whereas abundant Leydig cells were readily apparent in the interstitium between the seminiferous tubules of control WT mice, testis of Wwox−/− mice contained very few Leydig cells of smaller size (Fig. 1A). Furthermore, staining of testis sections with anti-Wwox antibody revealed intense staining of Leydig and Sertoli cells in WT mice, whereas in mutant mice Wwox staining was not detected (Fig. 1A, a and c vs. b and d). To verify further the hypoplasia of Leydig cells in mutant testis, we immunostained testis sections with anticytochrome P450 side chain cleavage (ssc) antibody, a marker of Leydig cells, and we observed markedly reduced staining of P450 ssc in mutant testis compared with WT testis (Fig. 1A, f and h vs. e and g). Because it is known that Leydig cells produce testosterone, we measured serum testosterone levels and found that whereas in WT mice testosterone levels were ranging 50–65 pg/ml (13), in KO mice testosterone levels were undetectable.
Figure 1.
Gonadal phenotype in Wwox KO male mice. A, Leydig cell defects. Histology section of testis showing normal Leydig cells in WT testis (a and c) stained with anti-WWOX antibody, whereas the KO testis (b and d) shows sparse interstitium and few Leydig cells and negative staining of Wwox. A positive marker for Leydig cells, anti-P450 scc (e–h), was used to confirm the marked reduction of Leydig cells in KO testis section. Arrows point at Leydig cells. B, Real-time PCR analysis of testis cDNA identifies impaired steroidogenesis in KO testis. Expression of key genes in the steroidogenesis pathway was analyzed. C, Semiquantitative RT-PCR analysis of testis cDNA identifies impaired steroidogenesis in KO testis. Expression of key genes in the steroidogenesis pathway was analyzed. The expression of cyclophilin A was used as a control.
To determine which of the Leydig cell genes encoding testosterone biosynthesis pathway enzymes are altered in the Wwox KO mice, the expression of various markers was analyzed using real-time PCR. We found that Leydig cell markers, including Cyp11a1, Cyp17a1, and Hsd3b6, were down-regulated in KO testis compared with WT and heterozygous (HET) ones (Fig. 1, B and C). We also observed that the expression of thrombospondin 2 and Star, two fetal Leydig cell markers, are reduced in KO testis, indicating a decreased number of fetal Leydig cells in the juvenile-deficient testis. The seminiferous tubules of the KO mice were markedly reduced in size and contained immature germ cells. Based on the fact that KO mice died at juvenile age, we were not able to study the effects of Wwox ablation during spermatogenesis.
Ovarian abnormalities in Wwox−/− female mice
Macroscopic and microscopical evaluation of female reproductive organs of juvenile KO mice revealed that ovaries were reduced in size and that uterus horns were thinner compared with WT littermates (data not shown). Ovaries derived from KO pups contained many primary follicles of normal appearance that were significantly smaller in size compared with WT littermate ovaries (Fig. 2A). Immunohistochemical staining with anti-Wwox antibody showed a prominent expression of Wwox in WT ovary, whereas no staining was observed in KO. Staining with the proliferation marker Ki67 showed reduced expression of Ki67 in theca cells of the KO animals, indicating a decrease in proliferation (Fig. 2A). Because the KO pups die at juvenile age, we did not study the maturation of the primary follicles toward secondary follicles, graafian follicles, and corpora lutea.
Figure 2.
Gonadal phenotype in Wwox KO female mice. A, Histology of the ovary shows multiple follicles at different stages in WT, whereas in KO mice follicles tended to be smaller in size. Staining with anti-WWOX antibody in a and b. c and d demonstrate reduced proliferation in theca cell in KO (d) compared with WT (c) after staining with anti-Ki67 antibody. B, Real-time PCR analysis of ovaries cDNA identifies impaired steroidogenesis in KO ovary. The expression of cyclophilin A was used as a control in B. C, Semiquantitative RT-PCR analysis of ovary cDNA identifies impaired steroidogenesis in KO testis. Expression of key genes in the steroidogenesis pathway was analyzed. The expression of cyclophilin A was used as a control.
Expression of many genes encoding steroid biosynthesis enzymes, including Cyp11a1 and Hsd3b6, were reduced in KO compared with WT and HET ovary (Fig. 2, B and C). In addition, expression of the TGFβ superfamily members, including inhibin and activin, were also reduced in the deficient ovary.
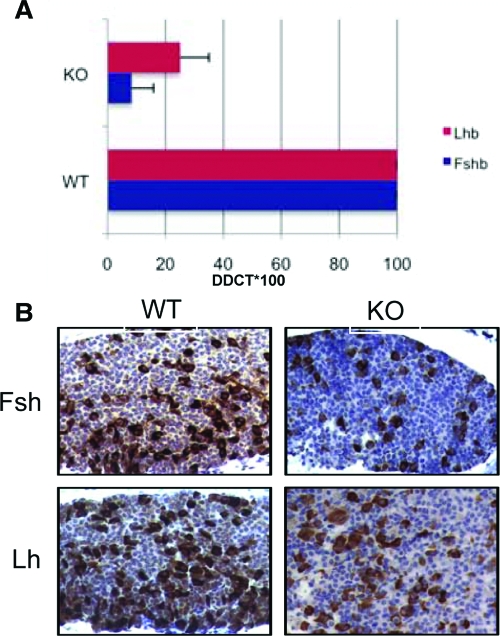
Reduced expression of Fsh and Lh in the pituitary gland of Wwox-deficient mice
Wwox protein level in the pituitary gland, as assessed by immunohistochemistry, is significantly high (3). To addresses whether Wwox may affect upstream targets that control steroidogenic enzymatic pathways, we analyzed the expression of FSH and LH gene expression. We found that Fshb and, to a lesser extent, Lhb expression is down-regulated in pituitary glands of Wwox KO mice as assessed by real-time PCR (Fig. 3A). To further confirm down-regulation of Fsh and Lh expression in the pituitary gland of Wwox KO mice, we performed immunohistochemical staining using anti-Fsh and anti-Lh antibodies. As expected, we observed significant down-regulation of Fsh and, to a lesser extent, Lh protein expression in Wwox KO pituitary compared with WT (Fig. 3B). Both the intensity and number of positive cells stained for Fsh and Lh were less in KO compared with WT. These results may suggest that Wwox may affect upstream targets and, thus, affecting the normal function of gonads.
Figure 3.
Reduced expression of Fshb and Lhb in the pituitary gland of Wwox-null mice. A, Real-time PCR (TaqMan; Applied Biosystems, Foster City, CA) showing down-regulation of Fshb and Lhb in different Wwox KO mice compared with WT mice. B, Immunohistochemical staining of Fsh and Lh in the pituitary gland showing reduced expression in Wwox KO mice.
Gene expression profiling in gonads of Wwox-deficient mice
To investigate thoroughly the in vivo function of Wwox in gonads, we studied the differential expression of mRNAs in the Wwox-deficient mice gonads compared with their WT and HET littermates. mRNAs extracted from ovary and testis were analyzed using mouse Affymetrix microarray gene-chip 430 2.0 arrays. As shown in Table 1, our analysis identified the alteration of 15 key genes involved in steroidogenesis in both tissues, including enzymes of the cytochrome P450 family. These data indicate that Wwox plays a central role in steroid metabolism.
Table 1.
Wwox regulated genes in mouse gonads
| Gene symbol | Description | Fold difference
|
|
|---|---|---|---|
| Testis | Ovary | ||
| Down-regulated in WWOX KO | |||
| Wwox | WW domain-containing oxidoreductase | 0.16 | 0.36 |
| Cyp11a1 | Cytochrome P450, family 11, subfamily a, polypeptide 1 | 0.21 | 0.16 |
| BB144871 | Expressed sequence BB144871 | 0.28 | 0.23 |
| Car3 | Carbonic anhydrase 3 | 0.32 | 0.23 |
| Cyp17a1 | Cytochrome P450, family 17, subfamily a, polypeptide 1 | 0.36 | 0.33 |
| Ren1 | Renin 1 structural | 0.37 | 0.43 |
| Hao3 | Hydroxy acid oxidase (glycolate oxidase) 3 | 0.37 | 0.29 |
| Scd1 | Stearoyl-coenzyme A desaturase 1 | 0.39 | 0.35 |
| Cbr2 | Carbonyl reductase 2 | 0.43 | 0.42 |
| Hp | Haptoglobin | 0.44 | 0.29 |
| Slc38a5 | Solute carrier family 38, member 5 | 0.49 | 0.24 |
| Up-regulated in WWOX KO | |||
| Galntl2 | UDP-N-acetyl-α-d-galactosamine-polypeptide N-acetylgalactosaminyl transferase-like 2 | 2.12 | 2.67 |
| Cyp2e1 | Cytochrome P450, family 2, subfamily e, polypeptide 1 | 2.34 | 3.75 |
| Dlk1 | δ-Like 1 homolog (Drosophila) | 2.38 | 2.35 |
| Inmt | Indolethylamine N-methyltransferase | 3.13 | 2.40 |
Transcriptional profiling using Affymetrix Mouse Genome 430 2.0 array identified 15 differentially expressed genes in the mouse Wwox KO that displayed at least 2-fold expression differences both in testis and in the ovary. Mouse ovary RNA was isolated from three HET and three KO mice and hybridized without pooling. Likewise, testis RNA from four WT, two HET, and six KO mice were also analyzed. For calculating fold differences, WT and HET mice were grouped together and compared with KO. Data have a 90% level of confidence that the false discovery rate was less than 10%.
Overexpression of Wwox in murine Leydig tumor cell line (MLTC)-1 Leydig cells modulates levels of steroidogenic enzymes
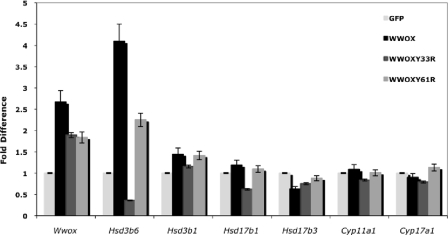
Because the absence of Wwox expression leads to deregulation of many genes encoding steroid biosynthesis enzymes in vivo, we next addressed whether Wwox expression affects the levels of these enzymes in vitro. Transient expression of Wwox in MLTC-1 mouse Leydig cells led to up-regulation of Hsd3b6 and, to a lesser extent, Hsd3b1 and Hsd17b1 expression as assessed by real-time PCR (Fig. 4). Interestingly, expression of WwoxY33R mutant [containing a WW1 domain mutations that abrogates Wwox interaction with PPxY motifs (5,14)] causes down-regulation of these enzymes. No changes were observed in mRNA expression of Hsd17b3, Cyp11a1, and Cyp17a1 after overexpression of Wwox or WwoxY61R (a WW2 domain mutation) in vitro (Fig. 4). These results suggest that the first WW domain of Wwox is indispensable for Wwox function in regulation of steroid enzymes.
Figure 4.
Wwox expression modulates steroidogenic gene expression in MLTC-1 Leydig cells in vitro. MLTC-1 mouse Leydig cells transiently expressing Wwox as indicated were harvested, and total RNA was extracted. Results show real-time PCR analysis of steroidogenic-related gene expression (as indicated) after the different transient expression relative to green fluorescent protein (GFP) expression. Results represent fold difference relative to expression of glyceraldehyde-3-phosphate dehydrogenase.
Discussion
Mice lacking Wwox die approximately 3 wk after birth, and display defects in growth rate and in bone and steroid metabolism. Wwox-deficient mice display growth retardation of key organs that weighed less in KO pups compared with WT littermates (10). Interestingly, we observed that other organs such as adrenal and the pituitary gland of KO pups were heavier compared with WT littermates. Macroscopic and histological examination of these glands showed no significant microscopical lesions (3,10). The complexity of the mutant phenotype, together with early postnatal lethality, supports multiple roles for Wwox in vivo and suggest that Wwox functions may not be compensated for by redundant functions of related proteins of the WW domain-containing proteins or proteins containing the SDR domain.
This steroidogenesis defect we observed in Wwox-deficient mice is contributed by several factors. First, the high expression of Wwox in Leydig and Sertoli cells, as well as in the ovarian follicles suggest that Wwox is required for proper gonadal function. Second, the failure of Leydig cell formation and very low levels of serum testosterone in the testis of KO mice and the reduced proliferation of theca cells and smaller primary follicles in ovary of KO mice suggest that Wwox is directly involved in steroidogenesis pathway. Third, our Affymetrix analysis identified 15 differentially expressed genes in the Wwox KO gonads (Table 1), suggesting that the absence of Wwox alters the expression of steroidogenesis-related genes. Our data also suggest that the FSH (Fshb) and, to a lesser extent, the LH (Lhb) expression is down-regulated in pituitary glands of Wwox KO mice (Fig. 3). These data suggest that Wwox may affect upstream targets that control steroidogenic enzymatic pathways. No changes were observed in mRNA expression of estrogen, progesterone, and androgen receptors in KO mice (data not shown).
Interestingly, Wwox KO mice show a number of similarities to the LH-β-deficient mice (15). Ma et al. (15) have demonstrated that targeted disruption of LH-β-subunit leads to prominent Leydig cell hypoplasia, defects in expression of genes encoding steroid biosynthesis pathway enzymes, and reduced testosterone levels. Wwox KO mice may also share some phenotypical changes similar to mice lacking the FSH-β subunit or FSH receptor (reviewed in Refs. 16 and 17). Because our mice die before sexual maturity, we did not study spermatogenesis and maturations of primary follicles to compare our KO mice with these various animal models. Recently, Wwox hypomorphic mice were generated and found to be viable (9) in contrast to the postnatal lethality we observed in the Wwox-null mice (12). Intriguingly, testis from Wwox hypomorphic males had high numbers of atrophic seminiferous tubules and reduced fertility when compared with WT counterparts (9). These results suggest that total loss of Wwox leads to the failure of Leydig cell development and reduced testosterone levels, whereas reduced expression of Wwox leads to testicular atrophy. Altogether, these data indicate the indispensable role of Wwox in steroidogenesis.
Wwox protein contains two WW domains predicted to mediate protein-protein interaction. Indeed, the first WW domain of Wwox belongs to class I of WW domains (18,19,20). We have previously shown that Wwox binds the PPxY motifs of several transcription factors, sequesters them in the cytoplasm, and reduces their transactivation abilities (19,21,22,23). Here, we show that the first WW domain of Wwox is also important for proper steroid-related gene expression. It is possible that Wwox, via its first WW domain, associates with key transcription factor(s) that regulates steroid enzymes. Work by Chang et al. (24) demonstrated that 17β-estradiol and androgen activate Wwox, via phosphorylation on tyrosine (Y) 33, in different cell lines, and that this activation positively correlates with cancerous progression of the prostate and breast to a premetastatic state. Nevertheless, we cannot exclude that other domains of Wwox also contribute to Wwox function in steroid metabolism. For example, Wwox also possesses a typical SDR domain that has been predicted to play a role in steroid metabolism (1). The exact contribution of each of the Wwox domains toward these phenotypes is still to be determined.
Absent or reduced Wwox expression has been suggested to be associated with prostate carcinoma (25,26). The normal prostate and early-stage prostate cancers depend on androgens for growth and survival, and androgen ablation therapy causes them to regress (27). Our results suggest that the loss of Wwox expression results in reduced levels of testosterone, a condition that may affect prostate normal function and may also contradict with the early role of Wwox during prostate carcinoma. Histological examination of prostate gland in juvenile Wwox-deficient mice did not show any abnormal morphology (3). This may suggest that alternative androgen sources, other than Leydig cells, are adequate for prostate normal morphology. Of note, expression of cytochrome P450 ssc (Cyp11a1) was not affected in the adrenal gland (data not shown), which expresses high levels of Wwox (3). This may suggest that Wwox regulation of testosterone synthesis could be specifically restricted to Leydig cells in the testis. On the other hand, it is possible to speculate that Wwox tumor suppressor function may also be important during tumor progression in prostate cancer, a condition when tumor cells become androgen independent.
In conclusion, our phenotypical analysis of the Wwox KO mice demonstrates an important role of Wwox in fundamental cellular processes, including survival, growth, and steroidogenesis. The defect in steroid and bone metabolism in Wwox KO mice may suggest that both phenotypes are related. Further analysis of mechanisms underlying these phenotypes and contribution of the Wwox different domains toward these phenotypes is to be determined.
Acknowledgments
We thank Dean Marshall and Darshna Bhatt for technical assistance.
Footnotes
This work was supported by Ohio Cancer Research Associates and Kimmel Scholar Award (to R.I.A.), National Cancer Institute grant (to C.M.C.).
Present address for A.d.B.: Department of Pathobiology, Faculty of Veterinary Medicine, University Utrecht, Utrecht 3584CL, The Netherlands.
Disclosure Statement: The authors have nothing to disclose.
First Published Online October 30, 2008
Abbreviations: Fsh, follicle-stimulating hormone; HET, heterozygous; KO, knockout; MLTC, murine Leydig tumor cell line; SDR, short-chain dehydrogenase/reductase; ssc, side chain cleavage; WT, wild type; Wwox, WW domain-containing oxidoreductase.
References
- Bednarek AK, Laflin KJ, Daniel RL, Liao Q, Hawkins KA, Aldaz CM 2000 WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3-24.1, a region frequently affected in breast cancer. Cancer Res 60:2140–2145 [PubMed] [Google Scholar]
- Ried K, Finnis M, Hobson L, Mangelsdorf M, Dayan S, Nancarrow JK, Woollatt E, Kremmidiotis G, Gardner A, Venter D, Baker E, Richards RI 2000 Common chromosomal fragile site FRA16D sequence: identification of the FOR gene spanning FRA16D and homozygous deletions and translocation breakpoints in cancer cells. Hum Mol Genet 9:1651–1663 [DOI] [PubMed] [Google Scholar]
- Aqeilan RI, Trapasso F, Hussain S, Costinean S, Marshall D, Pekarsky Y, Hagan JP, Zanesi N, Kaou M, Stein GS, Lian JB, Croce CM 2007 Targeted deletion of Wwox reveals a tumor suppressor function. Proc Natl Acad Sci USA 104:3949–3954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aqeilan RI, Hagan JP, Aqeilan HA, Pichiorri F, Fong LY, Croce CM 2007 Inactivation of the Wwox gene accelerates forestomach tumor progression in vivo. Cancer Res 67:5606–5610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aqeilan RI, Croce CM 2007 WWOX in biological control and tumorigenesis. J Cell Physiol 212:307–310 [DOI] [PubMed] [Google Scholar]
- Paige AJ, Taylor KJ, Taylor C, Hillier SG, Farrington S, Scott D, Porteous DJ, Smyth JF, Gabra H, Watson JE 2001 WWOX: a candidate tumor suppressor gene involved in multiple tumor types. Proc Natl Acad Sci USA 98:11417–11422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek AK, Keck-Waggoner CL, Daniel RL, Laflin KJ, Bergsagel PL, Kiguchi K, Brenner AJ, Aldaz CM 2001 WWOX, the FRA16D gene, behaves as a suppressor of tumor growth. Cancer Res 61:8068–8073 [PubMed] [Google Scholar]
- Fabbri M, Iliopoulos D, Trapasso F, Aqeilan RI, Cimmino A, Zanesi N, Yendamuri S, Han SY, Amadori D, Huebner K, Croce CM 2005 WWOX gene restoration prevents lung cancer growth in vitro and in vivo. Proc Natl Acad Sci USA 102:15611–15616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludes-Meyers JH, Kil H, Nunez MI, Conti CJ, Parker-Thornburg J, Bedford MT, Aldaz CM 2007 WWOX hypomorphic mice display a higher incidence of B-cell lymphomas and develop testicular atrophy. Genes Chromosomes Cancer 46:1129–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aqeilan RI, Hassan MQ, de Bruin A, Hagan JP, Volinia S, Palumbo T, Hussain S, Lee SH, Gaur T, Stein GS, Lian JB, Croce CM 2008 The WWOX tumor suppressor is essential for post-natal survival and normal bone metabolism. J Biol Chem 283:21629–21639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez MI, Ludes-Meyers J, Aldaz CM 2006 WWOX protein expression in normal human tissues. J Mol Histol 37:115–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aqeilan RI, Kuroki T, Pekarsky Y, Albagha O, Trapasso F, Baffa R, Huebner K, Edmonds P, Croce CM 2004 Loss of WWOX expression in gastric carcinoma. Clin Cancer Res 10:3053–3058 [DOI] [PubMed] [Google Scholar]
- Jeyaraj DA, Grossman G, Petrusz P 2005 Altered bioavailability of testosterone in androgen-binding protein-transgenic mice. Steroids 70:704–714 [DOI] [PubMed] [Google Scholar]
- Chang NS, Hsu LJ, Lin YS, Lai FJ, Sheu HM 2007 WW domain-containing oxidoreductase: a candidate tumor suppressor. Trends Mol Med 13:12–22 [DOI] [PubMed] [Google Scholar]
- Ma X, Dong Y, Matzuk MM, Kumar TR 2004 Targeted disruption of luteinizing hormone β-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci USA 101:17294–17299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairam MR, Krishnamurthy H 2001 The role of follicle-stimulating hormone in spermatogenesis: lessons from knockout animal models. Arch Med Res 32:601–608 [DOI] [PubMed] [Google Scholar]
- Kumar TR 2005 Gonadotropin gene targeting and biological implications. Endocrine 26:227–233 [DOI] [PubMed] [Google Scholar]
- Macias MJ, Wiesner S, Sudol M 2002 WW and SH3 domains, two different scaffolds to recognize proline-rich ligands. FEBS Lett 513:30–37 [DOI] [PubMed] [Google Scholar]
- Aqeilan RI, Pekarsky Y, Herrero JJ, Palamarchuk A, Letofsky J, Druck T, Trapasso F, Han SY, Melino G, Huebner K, Croce CM 2004 Functional association between Wwox tumor suppressor protein and p73, a p53 homolog. Proc Natl Acad Sci USA 101:4401–4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludes-Meyers JH, Kil H, Bednarek AK, Drake J, Bedford MT, Aldaz CM 2004 WWOX binds the specific proline-rich ligand PPXY: identification of candidate interacting proteins. Oncogene 23:5049–5055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aqeilan RI, Palamarchuk A, Weigel RJ, Herrero JJ, Pekarsky Y, Croce CM 2004 Physical and functional interactions between the Wwox tumor suppressor protein and the AP-2γ transcription factor. Cancer Res 64:8256–8261 [DOI] [PubMed] [Google Scholar]
- Aqeilan RI, Donati V, Palamarchuk A, Trapasso F, Kaou M, Pekarsky Y, Sudol M, Croce CM 2005 WW domain-containing proteins, WWOX and YAP, compete for interaction with ErbB-4 and modulate its transcriptional function. Cancer Res 65:6764–6772 [DOI] [PubMed] [Google Scholar]
- Gaudio E, Palamarchuk A, Palumbo T, Trapasso F, Pekarsky Y, Croce CM, Aqeilan RI 2006 Physical association with WWOX suppresses c-Jun transcriptional activity. Cancer Res 66:11585–11589 [DOI] [PubMed] [Google Scholar]
- Chang NS, Schultz L, Hsu LJ, Lewis J, Su M, Sze CI 2005 17β-Estradiol upregulates and activates WOX1/WWOXv1 and WOX2/WWOXv2 in vitro: potential role in cancerous progression of breast and prostate to a premetastatic state in vivo. Oncogene 24:714–723 [DOI] [PubMed] [Google Scholar]
- Watson JE, Doggett NA, Albertson DG, Andaya A, Chinnaiyan A, van Dekken H, Ginzinger D, Ha C, James K, Kamkar S, Kowbel D, Pinkel D, Schmitt L, Simko JP, Volik S, Weinberg VK, Paris PL, Collins C 2004 Integration of high-resolution array comparative genomic hybridization analysis of chromosome 16q with expression array data refines common regions of loss at 16q23-qter and identifies underlying candidate tumor suppressor genes in prostate cancer. Oncogene 23:3487–3494 [DOI] [PubMed] [Google Scholar]
- Qin HR, Iliopoulos D, Semba S, Fabbri M, Druck T, Volinia S, Croce CM, Morrison CD, Klein RD, Huebner K 2006 A role for the WWOX gene in prostate cancer. Cancer Res 66:6477–6481 [DOI] [PubMed] [Google Scholar]
- Feldman BJ, Feldman D 2001 The development of androgen-independent prostate cancer. Nat Rev Cancer 1:34–45 [DOI] [PubMed] [Google Scholar]