Abstract
Peripheral administration of glucagon-like peptide (GLP)-1 reduces food intake in animals and humans, but the sites and mechanism of this effect and its physiological significance are not yet clear. To investigate these issues, we prepared rats with chronic catheters and infused GLP-1 (0.2 ml/min; 2.5 or 5.0 min) during the first spontaneous dark-phase meals. Infusions were remotely triggered 2–3 min after meal onset. Hepatic portal vein (HPV) infusion of 1.0 or 3.0 (but not 0.33) nmol/kg GLP-1 reduced the size of the ongoing meal compared with vehicle without affecting the subsequent intermeal interval, the size of subsequent meals, or cumulative food intake. In double-cannulated rats, HPV and vena cava infusions of 1.0 nmol/kg GLP-1 reduced meal size similarly. HPV GLP-1 infusions of 1.0 nmol/kg GLP-1 also reduced meal size similarly in rats with subdiaphragmatic vagal deafferentations and in sham-operated rats. Finally, HPV and ip infusions of 10 nmol/kg GLP-1 reduced meal size similarly in sham-operated rats, but only HPV GLP-1 reduced meal size in subdiaphragmatic vagal deafferentation rats. These data indicate that peripherally infused GLP-1 acutely and specifically reduces the size of ongoing meals in rats and that the satiating effect of ip, but not iv, GLP-1 requires vagal afferent signaling. The findings suggest that iv GLP-1 infusions do not inhibit eating via hepatic portal or hepatic GLP-1 receptors but may act directly on the brain.
Intrameal hepatic portal and intraperitoneal (IP) infusions of GLP-1 reduce meal size in rats, but only IP GLP-1 requires vagal afferent signaling for this effect.
Glucagon-like peptide-1 (7–6)-amide (GLP) is a gut hormone synthesized and released in response to intraluminal nutrient stimuli by the L cells of the distal ileum and colon and a brain neurotransmitter synthesized and released by neurons in the nucleus tractus solitarii (NTS). GLP-1 appears to act at a number of sites to elicit a variety of physiological or pharmacological effects (1). Both peripheral and central GLP-1 have been implicated in the control of eating (2,3,4). However, rather little is known about the physiological significance, sites of action, or neural mechanisms of GLP-1’s actions on eating.
GLP-1 released from the intestinal L cells enters the systemic circulation rapidly via the intestinal capillaries draining into the hepatic portal vein (HPV) (5) and more slowly via the lymph (6). GLP-1 is quickly degraded by dipeptidyl peptidase IV (DPP-IV) in the intestinal capillaries and in the liver, so that after release from intestinal L cells, the highest GLP-1 concentrations occur in the intestinal submucosal extracellular space, intermediate levels are found in the HPV, and comparatively low concentrations occur in the systemic circulation (5). Unfortunately, as yet there are only few data regarding prandial concentrations of GLP-1 in the systemic circulation and none in the brain. GLP-1 may act at a variety of sites to inhibit eating, as GLP-1 receptors are expressed in several loci that have been implicated in the control of eating, including the arcuate (Arc) and paraventricular (PVN) nuclei of the hypothalamus (7,8), as well as in several peripheral sites, including the pancreatic islets (9), most of the gastrointestinal tract (1), and vagal afferent fibers terminating in the HPV (10,11).
In humans, oral loads of carbohydrates or fats increased systemic plasma GLP-1 levels within 5–15 min (1,12,13), and iv infusion of physiological doses of GLP-1 during meals were sufficient to increase the perception of satiety and to reduce food intake (14,15,16). In rats the situation is less clear. Systemic plasma GLP-1 increased within 15 min after intragastric administration of Ensure (Abbott Laboratories, Abbott Park, IL) (6) or triglyceride emulsions (17). However, neither systemic plasma levels of GLP-1 after orally ingested meals nor prandial levels of GLP-1 in the HPV have been reported in rats. Furthermore, as yet there are no published reports that acute GLP-1 antagonism during meals can increase meal size. Thus, whether GLP-1 has a normal physiological satiating action in rats remains uncertain.
Injections of GLP-1 into the lateral, third, or fourth cerebral ventricles or directly into the PVN have also inhibited eating in rats (18,19,20,21), and GLP-1 receptors are expressed in these areas (7,8), suggesting that intestinal GLP-1 might act directly in the brain to elicit satiation. Again, however, it is not known whether, or in what amount, peripheral GLP-1 reaches these receptors or how concentrations after central injections and during meals compare. However, Turton et al. (21) have reported that intracerebroventricular administration of the GLP-1 antagonist exendin (Ex)-9 does increase eating, encouraging the view that GLP-1 may act on central GLP-1 receptors to produce satiation.
Lesion studies have contributed only limited knowledge about the potential site of GLP-1’s satiating actions. Abbott et al. (22) reported that ip injection of 100 nmol/kg GLP-1 did not significantly reduce food intake in rats with bilateral abdominal vagotomy, suggesting that ip injected GLP-1 acts locally in the gut to initiate a neural signal relayed to the brain via vagal afferents to inhibit eating. However, total subdiaphragmatic vagotomy ablates vagal efferents as well as vagal afferents, which is well known to have marked adverse effects on gastrointestinal motility (23). Total vagotomy has also reduced endogenous GLP-1 release (24). Both of these effects might alter the eating-inhibitory effect of peripherally administered GLP-1.
In view of the available data concerning GLP-1’s potential role in satiation, the present experiments had three goals. The first was to test whether brief, remotely controlled intrameal GLP-1 infusions affect meal patterns in spontaneously eating rats. This has not been examined previously, and such tests have face validity as models of voluntary natural eating, and in several instances have revealed phenomena not apparent in tests of scheduled test meals in food-deprived animals (e.g. see Refs. 25 and 26). The second goal was to investigate the site of action of exogenous GLP-1 by comparing the effects of HPV, vena cava (VC), and ip GLP-1 infusions, and the third was to investigate further the role of the vagus nerve in peripheral GLP-1’s eating-inhibitory effect using rats with subdiaphragmatic vagal deafferentations (SDAs) (27).
Materials and Methods
Subjects and housing
Male Sprague Dawley rats [Charles River, Sulzfeld, Germany; body weight (BW) 200–230 g on arrival] were individually housed in a temperature-controlled (21 ± 2 C) colony room with a 12-h dark, 12-h light cycle with the lights off at 1100 h. The rats had ad libitum access to water and ground rat chow (No. 3433; Provimi Kliba NAFAG, Kaiseraugst, Switzerland). Rat chow was left in an open bin for at least 1 d before use to prevent the availability of fresh chow from disrupting spontaneous eating patterns. Rats were adapted to housing conditions for 10 d before onset of the experiment. All procedures were approved by the Canton of Zurich Veterinary Office.
Catheter implantation
Catheters were sterilized in ethylene oxide before use and implanted using sterile techniques. The custom-made headsets described before (26,28) were used except in the SDA tests, in which headsets made from 20 G (0.90 × 40 mm) surgical stainless-steel tubing (Sterican; Braun, Melsungen, Germany) were bent into U shapes. Catheters consisted of 27 cm silicone tubing [Dow Corning, Midland, MI; inner diameter (ID) 0.508 mm, outer diameter (OD) 0.914 mm]. Connections with the headsets were shielded with 2.5-cm pieces of silicone tubing (ID, 1.02 mm, OD, 2.18 mm). The catheters were then led through a folded 2.5 × 3-cm square of polypropylene surgical mesh (Marlex; Bard Implants, Billerica, MA) to improve adhesion to the skin and fascia.
For infection prophylaxis and analgesia, rats were pretreated with 4 mg/kg trimethoprim/20 mg/kg sulfadoxine (Borgal 24%; Intervet/Shering-Plough Animal Health, Kenilworth, NJ) sc a few hours before surgery and anesthetized by ip injection (1.0 ml/kg) of 80 mg/kg ketamine (Ketasol-100; Dr. E. Gräub AG, Bern, Switzerland) and 4.0 mg/kg xylazine (Rompun; Bayer, Leverkusen, Germany). Atropine (0.05 mg/kg; Sintetica, Mendrisio, Switzerland) was injected sc immediately before surgery. Catheter headsets were led sc from 2-cm midline interscapular incisions to puncture wounds 1-cm rostral to the incision and exteriorized. The proximal ends were led sc from the neck to 5-cm midline laparotomies. HPV catheters were inserted into the ileocolic vein, advanced into the HPV (26,28) so that it ended 1- to 2-mm distal to the gastroduodenal vein, and anchored with silk suture (Silkam, 3/0; Braun) and acrylic glue (Braun). In one group of animals, both HPV and VC catheters were implanted during the same surgery. Inferior VC catheters were implanted just rostral to the renal veins, as described by Kaufman (29), and the tips advanced 3–4 cm so that they lay near the junction of the hepatic vein. In some SDA animals, HPV catheters were implanted at SDA surgery, and ip catheters 7 wk later. Intraperitoneal catheters ended in the peritoneal cavity and were anchored on the left side of the abdominal wall with silk sutures.
Skin and muscle were closed with resorbable sutures (3-0 Vicryl; Ethicon, Norderstedt, Germany). Four mg/kg trimethoprim/20 mg/kg sulfadoxine (Borgal 24%) and 5 mg/kg carprofen (Rimadyl; E. Gräub, Bern, Switzerland) were injected sc on the day after surgery. Catheters were flushed regularly with 0.2 ml 0.9% sterile saline and filled with 80 μl heparinized saline (100 IU heparin/ml saline; heparin; Braun) between tests.
SDA surgery
Rats received 4 mg/kg trimethoprim/20 mg/kg sulfadoxine sc and 0.05 mg/kg atropine before surgery, were anesthetized with 5% isoflurane (Attane; Minrad Inc., Buffalo, NY) in oxygen, and anesthesia was maintained with 2–3% isoflurane in 1:1 oxygen:N2O. SDA consists of a transection of the left dorsal vagal rootlets and dorsal (left) esophageal vagal trunks, resulting in complete SDA, while sparing about half of the abdominal vagal efferents (27). The sham procedure consisted of similarly exposing the vagal rootlets and abdominal vagus, but not manipulating them further. Rats were nursed with special diets for 1 wk after surgery (30).
SDAs were verified functionally and histologically (30). The functional test was lack of cholecystokinin (CCK) satiation, which depends on abdominal vagal afferent fibers (31,32,33). Four-hour food-deprived rats were ip injected with 4 μg/kg CCK-8 (Bachem, Bubendorf, Switzerland) or saline according to a cross-over design. In sham-operated rats, CCK-8 reduced 30-min food intake 40–68%. Therefore, the inclusion criterion for SDA rats was a less than 30% reduction in food intake. To test retrograde labeling of vagal motor neurons in the dorsal motor nucleus of the vagus (DMX) and anterograde labeling of vagal afferents in the NTS (32,33,34,35), rats were ip injected with 2 mg Fluoro-Gold (Fluorochrome, Denver, CO) in 1 ml saline. Two days later, the left nodose ganglion was exposed via a ventral midline neck incision, a glass micropipette was inserted into the ganglion nerve using a micromanipulator, 1.5 μl 2% wheat germ agglutinin-horseradish peroxidase (Vector Laboratories, Burlingame, CA) in saline was pressure injected (PicoSpritzer 3; Parker Instrumentation, Fairfield, NJ) over 5–8 min, and the wound was closed. Rats were treated postoperatively as before, 2 d later anesthetized with pentobarbital (Nembutal; Abbott Laboratories) iv, and perfused and processed as before (30). An observer blind to the rat’s surgery and behavioral data counted Fluoro-Gold-labeled neurons in the DMX and examined the horseradish peroxidase labeling in all NTS sections that included the area postrema. Neurons projecting from this region of the DMX are contained in all branches of the abdominal vagus (34). The inclusion criteria for SDA rats were: 1) for anterograde labeling (27,30,35) that the number of labeled cells in the right DMX be less than 3% of the number in the left DMX; 2) for retrograde labeling (27,33,36) that the left DMX contain some retrograde labeling as a positive control; and 3) that neither the left nor the right NTS contain any labeled fibers. Only rats that met all three criteria were included in the analysis.
Test procedures
After recovery from surgery, rats were placed in custom-made, open-topped acrylic infusion cages (37 × 21 × 41 cm) with stainless-steel grid floors. A 60-W red incandescent light bulb provided dim illumination during the dark phase, and a radio music station was played continuously to mask extraneous noise. Ground chow was available ad libitum in food cups accessible via a 5-cm tunnel, 5 cm above the cage floor. The cups were mounted on electronic balances (Mettler PM 3000; Mettler-Toledo International Inc., Greifensee, Switzerland) interfaced with a computer (Olivetti M 300; Olivetti, Nuremberg, Germany) in an adjacent control room, and a custom-designed program (VZM; Krügel, Munich, Germany) recorded the weights of the food cups every 30 sec. Video cameras (VSS 3440; Philips, Amsterdam, The Netherlands) permitted continuous observation of the rats.
At 0830 h, catheters were connected to infusion pumps (A99; Razel, Stamford, CT) via two segments of polyethylene tubing (0.76 mm ID, 1.22 mm OD; Portex, Hythe, UK). The lower segment of tubing was sheathed with a stainless-steel spring fixed to a swivel joint 45 cm above the cage floor, thus allowing the rats to move freely. The infusion pumps were operated by remote control from the control room. Infusions (0.2 ml/min) started 2–3 min after the onset of the first spontaneous nocturnal meal and lasted for 5 min unless stated otherwise. If a meal was under way at dark onset (1100 h), the infusion was done during the next meal. The criteria for meal onset were a 0.2-g decrease in the food cup weight and visual verification of eating. Catheters were detached at 1500 h, and rats were weighed and food cups refilled between 1700 and 1900 h.
Within-subjects cross-over designs were used in each experiment. GLP-1 (7–37)-amide (Bachem) was dissolved in PBS (Life Technologies, Inc., Basel, Switzerland) with 1% BSA (Sigma, Buchs, Switzerland); control infusions were vehicle. Infusions were given in random order on consecutive days. Pilot experiments (data not shown) were performed to determine GLP-1 doses that were near threshold and moderately supra-threshold, and to ensure that infusion of such doses did not have carryover effects on subsequent days’ eating under our conditions.
Catheter patency was tested after experiments by infusing 0.8 ml/kg of a mixture of ketamine (26 mg/kg) and xylazine (0.9 mg/kg). Rats that did not loose muscle tone completely within 1 min were excluded from analysis.
Satiating effect of HPV GLP-1
To test whether intrameal HPV infusion of GLP-1 has an acute satiating effect during the first spontaneous nocturnal meal, rats (n = 14, BW 343-403 g at test onset) received doses of 0 (vehicle), 0.33, 1.0, or 3.0 nmol/kg BW GLP-1, prepared as described previously.
Comparison of HPV and VC GLP-1
To test whether GLP-1 acts in the liver to inhibit eating during the first spontaneous nocturnal meal, double-cannulated rats (n = 16, BW 280-402 g at test onset) received HPV or VC infusions of 0 or 1.0 nmol/kg BW GLP-1.
Effect of SDA
The necessity of vagal afferent signaling for the eating-inhibitory effects of HPV and ip GLP-1 was tested. First, seven sham-operated and nine SDA rats (BW 306-418 g at test onset) received HPV infusions of 0, 0.25, 0.5, or 1.0 nmol/kg BW GLP-1 during the first spontaneous meal of the dark phase. The GLP-1 doses were modified slightly from those used in the first tests in an attempt to obtain a graded dose-response relation, as described in Results. Seven weeks later, six sham-operated and nine SDA rats (BW 422-603 g at test onset) were equipped with ip catheters and, after recovery, received ip or HPV infusions of 0 or 10 nmol/kg BW GLP-1 (2.5 min infusions at 0.2 ml/min by each route in random order on 4 consecutive days). The larger dose was selected on the basis of pilot tests and the literature (22,37), which suggested that GLP-1 doses sufficient to inhibit eating when infused iv would not suffice when administered ip.
Data analysis
Meals were defined as food removals of 0.2 g or more, with the interval between removals 15 min or less. Sizes and durations of spontaneous meals during the test period, intermeal intervals (IMIs) (duration between the end of one meal and beginning of the next), and first-meal satiety ratios (meal size/subsequent IMI) were analyzed with repeated-measures ANOVA (SAS 9.1; SAS Institute Inc., Cary, NC) and Bonferroni-Holm (38) pairwise comparisons. Data sets that did not meet the ANOVA criteria of normality and equal variance were transformed to logarithms for ANOVA. To identify outliers, data were converted to standard scores using the medians and median absolute deviates × 1.48 (which estimates the sd), and values more than 2.57 (i.e. P < 0.01) were excluded. Differences were considered significant when P < 0.05.
Results
Satiating effect of HPV GLP-1
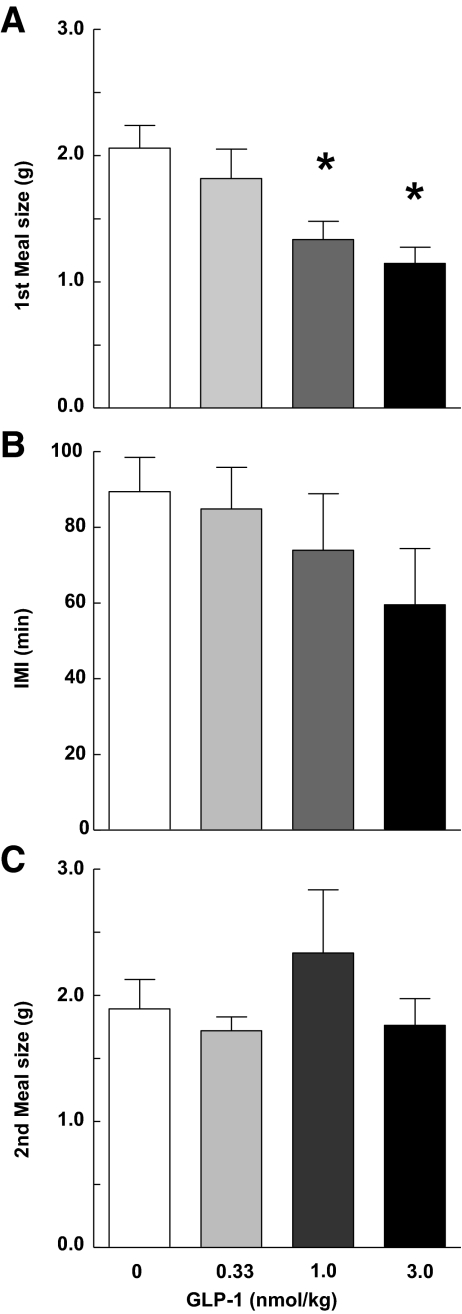
HPV infusions of 1.0 and 3.0, but not 0.33, nmol/kg GLP-1 reduced the size of the first spontaneous dark-phase meal compared with vehicle infusions (F3/39 = 9.19; P < 0.001) (Fig. 1). No significant dose-response relation was detected, i.e. the effects of infusions of 1.0 and 3.0 nmol/kg GLP-1 did not differ reliably (P > 0.05). Meal duration was also reduced by HPV GLP-1 (Table 1). The IMI after the first meal, the corresponding satiety ratio, the size of the second meal, and cumulative food intakes 2, 4, 6, and 22 h after the beginning of the first meal were not significantly affected by any dose of GLP-1. First meal size and meal duration data are from 11 rats that passed the catheter patency test. The number of data points for IMI and second meal size in Fig. 1 is smaller than for first meal size because some rats did not consume a second meal before the infusion pumps were detached.
Figure 1.
A, Intrameal HPV infusions of 1 or 3 nmol/kg GLP-1 reduced the size of the first spontaneous dark-phase meal compared with vehicle infusions (F3/29 = 9.19; P < 0.001). The duration of the after IMI (F3,21 = 2.08; P > 0.05) (B), and the second meal size (F3.22 = 1.55; P > 0.05) (C) were not significantly affected by GLP-1 infusions. Values are means ± sem of 11 (A) or 10 (B and C) rats. *, P < 0.05 vs. zero dose, Bonferroni-Holm test after significant ANOVA.
Table 1.
Effects of intrameal HPV GLP-1 infusions during the first spontaneous meal of the dark on meal duration, satiety ratio, and cumulative food intake
| GLP-1 dose (nmol/kg)
|
||||
|---|---|---|---|---|
| 0 | 0.33 | 1.0 | 3.0 | |
| Meal duration (min) | 9 ± 1.1 | 8 ± 1.3 | 5 ± 0.5a | 4 ± 0.4a |
| Satiety ratio (min/g) | 47.9 ± 6.2 | 55.6 ± 6.0 | 66.2 ± 9.7 | 51.6 ± 14.0 |
| Cumulative food intake (g) | ||||
| 2 h | 3.0 ± 0.2 | 3.6 ± 0.3 | 3.2 ± 0.4 | 2.7 ± 0.4 |
| 4 h | 6.7 ± 0.3 | 6.7 ± 0.1 | 6.5 ± 0.3 | 5.6 ± 0.6 |
| 6 h | 10.3 ± 0.5 | 11.1 ± 0.4 | 11.1 ± 0.4 | 10.4 ± 0.9 |
| 22 h | 25.4 ± 0.7 | 23.5 ± 0.3 | 23.7 ± 1.0 | 22.9 ± 1.2 |
Meal durations are means ± sem from 11 rats.
Different from zero value, Bonferroni-Holm test (38) after significant ANOVA (F3/22 = 5.47; P < 0.01).
Comparison of HPV and VC GLP-1
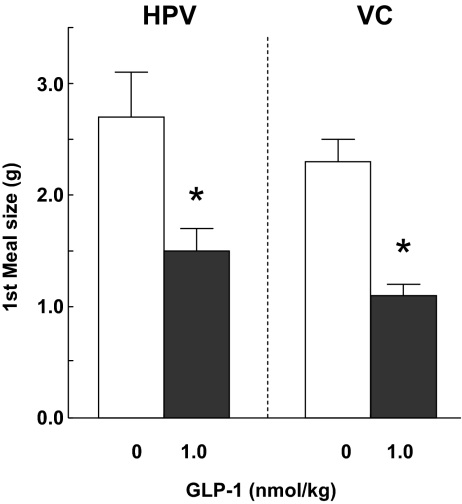
Both HPV and VC infusions of 1 nmol/kg GLP-1 reduced the size of the first spontaneous dark-phase meal, with no significant difference between the effects of HPV and VC infusion detected (Fig. 2). The ANOVA results for the main effects of GLP-1, route of administration, and their interactions were: F1/22 = 46.34, P < 0.0001; and F1/22 = 1.59 and 1.68, P > 0.05, respectively. Neither HPV nor VC GLP-1 infusion significantly affected other spontaneous eating parameters or cumulative food intake (data not shown). Data are from 12 rats that passed the catheter patency test.
Figure 2.
Intrameal VC and HPV infusions of 1 nmol/kg GLP-1 reduced the size of the first spontaneous dark-phase meal similarly. Values are means ± sem from 12 double-cannulated rats. *, P < 0.05 vs. zero dose, Bonferroni-Holm test after significant ANOVA.
Effect of SDA
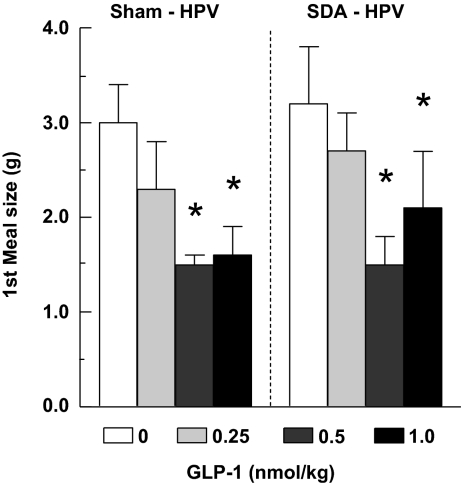
Data from three SDA rats had to be excluded because two failed the HPV catheter patency test and one failed the two criteria for complete SDA. HPV infusions of 0.5 and 1.0, but not 0.25, nmol/kg GLP-1 reduced the size of the first spontaneous dark-phase meal in both sham-operated and SDA rats, and no significant differences in the effects of GLP-1 in the two surgical groups were detected (Fig. 3). The ANOVA results for the main effects of GLP-1, surgical group, and their interaction were: F3/33 = 7.60, P < 0.001; F 1/12 = 0.11, P > 0.05; and F 3/33 = 0.07, P > 0.05, respectively. Other spontaneous eating parameters and cumulative food intake were not affected significantly in either surgical group (data not shown).
Figure 3.
Intrameal HPV infusions of 0.5 or 1.0 nmol/kg GLP-1 reduced the size of the first spontaneous dark-phase meal similarly in rats after SDA (n = 7) or sham surgery (n = 7). *, P < 0.05 vs. zero dose, Bonferroni-Holm test after significant ANOVA.
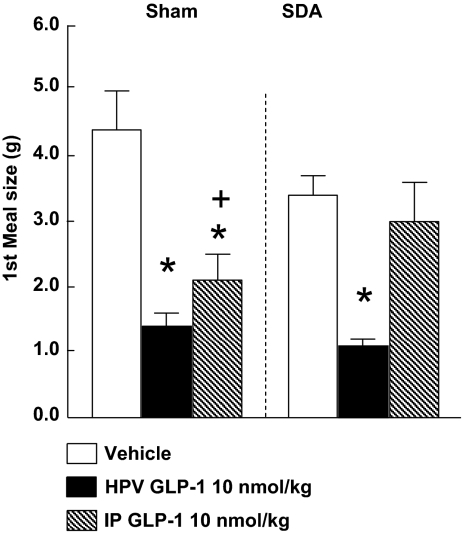
HPV infusions of 10 nmol/kg GLP-1 also reduced the size of the first spontaneous dark-phase meal in both sham-operated and SDA rats (Fig 3). In contrast, ip infusions of 10 nmol/kg GLP-1 reduced first spontaneous dark meal size in sham-operated rats, but not in SDA rats, with the difference in GLP-1’s effects significantly different between the two surgical groups (Fig. 4). The ANOVA results for the main effects of GLP-1, surgical group, and their interaction were: F2/18 = 20.53, P < 0.0001; F1/13 = 0.04, P > 0.05; and F2/18 = 4.24, P < 0.05, respectively. Again, other spontaneous eating parameters and cumulative food intake were not affected significantly in either surgical group (data not shown).
Figure 4.
Intrameal ip infusion of 10 nmol/kg GLP-1 reduced the size of the first spontaneous dark-phase meal in sham-operated rats (n = 6), but not in rats with SDA (n = 9). In contrast, HPV infusions of this dose of GLP-1 similarly reduced meal size in both surgical groups. *, P < 0.05 vs. vehicle; +, P < 0.05 (vehicle − ip GLP-1) difference in sham-operated rats vs. (vehicle − ip GLP-1) difference in SDA rats; Bonferroni-Holm tests after significant ANOVA.
Discussion
Here, we tested for the first time the effects of intrameal peripheral infusions of GLP-1 during spontaneous meals in rats. Our data demonstrate that brief, intrameal peripheral infusions of GLP-1 selectively reduce the size of ongoing meals in rats. In addition, HPV and VC GLP-1 infusions reduced meal size similarly, suggesting that GLP-1’s eating-inhibitory effect does not originate in the hepatic portal area or in the liver. Finally, SDA eliminated the eating-inhibitory effect of ip, but not of HPV, infusions of GLP-1, indicating that circulating GLP-1 does not require vagal afferent signaling to inhibit eating and that different sites and mechanisms mediate the satiating actions of ip and HPV GLP-1.
Independent of whether infused HPV, VC, or ip, intrameal administration of 0.5–10 nmol/kg GLP-1 potently and selectively reduced the size of the ongoing meal without affecting the size of subsequent meals or the duration of the subsequent IMIs and, therefore, meal frequency. Cumulative food intake was also unaffected. These results extend earlier reports of reduced food intake in response to ip (22,37) and iv (39,40) GLP-1 by showing that GLP-1 can reduce the size of ongoing meals, i.e. elicit satiation.
Our GLP-1 infusion procedure was designed to model only the initial secretion of GLP-1 during meals, without considering continued postprandial secretion. Intrameal plasma levels of GLP-1 have not been reported, and we were technically unable to match the plasma levels of GLP-1 after either HPV or VC infusions to the levels during meals. Therefore, whether the satiating effects of GLP-1 we report here represent a physiological action is uncertain. Nevertheless, it is interesting to note that the threshold HPV GLP-1 dose under our conditions, which appeared to be just under 0.5 nmol/kg, is small in comparison to doses used in most studies of peripheral GLP-1’s acute effect on food intake (2,3,4,18,21,22,40,41). However, Chelikani et al. (39) reported that the threshold dose for a reduction of 1 h cumulative food intake (their first measurement) during continuous intrajugular GLP-1 infusion was between 5 and 17 pmol/kg · min, or about 0.3–1 nmol/kg, which is similar to our results.
It has also been proposed that GLP-1 might act as an “across-meal satiating signal” (42). Because we did not test infusions of GLP-1 that continued during the IMI, our experiments did not address this hypothesis, and there are few relevant data in the literature. Intramuscular injections of the long-lasting GLP-1 agonist Ex-4 had a selective effect on meal size in a 6-h test in rhesus monkeys (42). On the other hand, Chelikani et al. (39) reported that 3 h intrajugular infusions of GLP-1 reduced meal frequency in addition to meal size. However, in this study all of the effective doses reduced meal size before they affected meal frequency. Thus, further work on this hypothesis is clearly warranted.
SDA attenuated the satiating effect of ip GLP-1 but did not affect the satiating effect of HPV GLP-1. This indicates that the former, but not the latter, effect depends on abdominal vagal afferent signaling, and suggests that GLP-1 inhibits eating after HPV and ip administration by activating at least partly different mechanisms. The failure of conservatively verified SDA to attenuate the satiating effect of HPV GLP-1 across a wide dose range (0.5–10 nmol/kg) strongly indicates that vagal afferent signaling is not involved in this action of GLP-1. In contrast, our finding that SDA blocked the satiating effect of ip infused GLP-1 confirms and extends previous reports that the eating-inhibitory effects of ip GLP-1 in rats (22) or Ex-4 in mice (43) were reduced by complete subdiaphragmatic vagotomy or by sc capsaicin administration, respectively. However, both these procedures are less selective than SDA: systemic capsaicin causes degeneration of vagal and nonvagal small-diameter unmyelinated sensory neurons (44); and total subdiaphragmatic vagotomy destroys vagal efferents in addition to afferents and, therefore, leads to substantial side effects (23) due to disturbances in gastrointestinal motility and secretion. Moreover, vagal afferents and efferents have been implicated in the meal-related release of endogenous GLP-1 (24), suggesting that complete subdiaphragmatic vagotomy could change the meal-induced release of endogenous GLP-1 in a way that may influence the effects of exogenous GLP-1. In contrast, SDA surgery has few side effects because it leaves about 50% of the vagal efferents intact. The animals are able to eat solid chow in normal amounts, although their eating rate is sometimes slightly reduced, they have similar spontaneous meal sizes as intact or sham-operated rats, and they maintain nearly normal BWs (495 ± 10 and 510 ± 21 g for SDA and sham rats at the end of the experiments).
The failure of ip GLP-1 to reduce meal size in SDA rats demonstrates that ip administered GLP-1 requires intact vagal afferents to inhibit eating and, together with the observation that HPV GLP-1 still reduced meal size in SDA rats, strongly suggests that it acts on GLP-1 receptors near the site of release in the intestines to do so. Endogenous GLP-1 released from the L cells diffuses into the lamina propria and enters the lymph or capillaries. DPP-IV is expressed on the capillary walls and immediately begins to degrade absorbed GLP-1 (45); indeed, this process is so efficient that only 25% of the absorbed GLP-1 is estimated to reach the liver intact (1,5). However, before absorption into the capillaries and the onset of degradation, endogenous GLP-1 should be present in higher concentrations (5) and could act on GLP-1 receptors on vagal afferent endings in the lamina propria (46,47) to trigger vagal afferent signaling and satiation. Therefore, ip GLP-1 may mimic a physiological paracrine action of GLP-1 released from the intestines during meals. Our results suggest that ip GLP-1 reaches these intestinal receptors, which is not obvious because ip administered substances are usually supposed to be absorbed into more superficial intestinal capillaries rather than the deeper lamina propria. Thus, evaluation of the hypothesis that ip administered GLP-1 models a physiological paracrine action of endogenous GLP-1 requires further investigations. Particularly useful would be tests of local administration of the GLP-1 receptor antagonist Ex 9-39, as has been done with the CCK receptor antagonist devazepide in rats (48).
Our HPV infusions were designed to model the meal-induced release of endogenous GLP-1 into the HPV (1,5) and to target GLP-1 receptors in the hepatic portal area or liver (1,10,11). However, the similar satiating effects of HPV and VC GLP-1 infusions suggest that GLP-1 did not act on these receptors to reduce meal size. At first glance, the similar satiating effects of HPV and VC GLP-1 infusions are surprising because only about 50% of GLP-1 entering the liver is supposed to reach the general circulation because of efficient DPP-IV-mediated GLP-1 degradation in the liver (1,5). On the other hand, it appears to be consistent with the lack of a clear dose relationship for the satiating effect of GLP-1 in our experiments. In some other studies, the eating-inhibitory effects of peripheral GLP-1 or Ex-4 were dose related (39,42). We can only speculate about the reasons for this discrepancy. Because these other studies used continuous GLP-1 infusions (39) or im Ex-4 injections (42), it is possible that longer-term GLP-1 receptor activation is necessary to reveal a dose-dependent effect.
The similar satiating effects of HPV and VC GLP-1 infusions, together with the fact that the effect of HPV GLP-1 infusions did not depend on vagal afferent signaling, suggest that iv infused GLP-1 reduces meal size by acting in the brain. Alternatively, it is possible that spinal visceral afferents in the hepatic portal area contributed to the satiating actions of HPV- or VC- infused GLP-1, although to our knowledge spinal visceral afferents have not been directly implicated in any actions of GLP-1. Consistent with the possibility of a central action, circulating GLP-1 apparently passes the blood-brain barrier by simple diffusion both in circumventricular organs (49) and elsewhere (50). Previously, GLP-1 was suggested to act in the Arc nucleus to reduce food intake (41), whereas more recent findings implicate the PVN (19) and the caudal brainstem (18) in the eating-inhibitory effect of GLP-1. Thus, further studies are necessary to determine where in the brain circulating GLP-1 acts to reduce meal size.
The relationship between GLP-1’s satiating action and its other putative physiological actions remain unclear. HPV GLP-1 infusion has increased hepatic branch vagal afferent activity (51), and activation of hepatic portal GLP-1 receptors appears to contribute to the effect of GLP-1 on peripheral glucose handling (11) and to the synergistic effect of GLP-1 and glucose on pancreatic insulin release (10). Therefore, our findings suggesting that hepatic and hepatic portal GLP-1 receptors are not involved in the satiating effect of GLP-1 dissociate GLP-1’s satiating effect from these metabolic effects. A similar dissociation between GLP-1’s central effects on metabolism and eating was recently reported by Sandoval et al. (19), who demonstrated that injection of GLP-1 into the hypothalamic Arc nucleus affected glucose metabolism and insulin release, whereas injection of GLP-1 into the PVN reduced food intake.
Endogenous GLP-1 is also considered to be a major contributor to the “ileal brake,” i.e. to the mechanism through which nutrients in the distal part of the small intestine slow gastric emptying (52). In line with this physiological function, iv GLP-1 (39) and ip Ex-4 (43) administration has inhibited gastric emptying, and intrajugular infusion of the GLP-1 receptor antagonist Ex (9-39) increased intestinal motility, indicating inhibition by endogenous GLP-1, in both rats and humans (52,53). Both intrajugular infusion (39) and intracerebroventricular injection (54) of GLP-1 have inhibited eating in rats sham feeding with open gastric cannulas, suggesting that GLP-1 does not require the presence of food in the stomach to inhibit eating. More recently, Strubbe et al. (55) reported that increased endogenous GLP-1 after intragastric administration of GLP-1 secretagogues also inhibited sham feeding and that infusion of a GLP-1 antibody reduced the satiating effect of GLP-1, but not its effect on gastric emptying. Together, these findings suggest that the satiating effect of peripheral GLP-1 that we report here does not depend on an inhibition of gastric emptying or on the accumulation of ingested food in the stomach. However, this does not preclude the possibility that GLP-1 might synergize with signals related to gastrointestinal motility to control eating.
In sum, the major findings of this study were that: 1) peripherally infused GLP-1 specifically reduces spontaneous meal size in rats; 2) the effect of ip, but not HPV GLP-1 requires vagal afferent signaling; and 3) iv infusions of GLP-1 do not inhibit eating via hepatic portal or hepatic GLP-1 receptors but may act directly on the brain. Whether the satiating effect of ip or iv administered GLP-1 reflects a physiological satiating action of endogenous GLP-1 merits further studies.
Acknowledgments
We thank Dr. Lori Asarian and Isabelle Baumgartner for helping with the statistics and experiments, respectively.
Footnotes
This work was funded by National Institutes of Health Grant DK-060735 (to W.L. and N.G.) and the Danone Institute Germany.
Disclosure Statement: The authors have nothing to disclose.
First Published Online October 23, 2008
Abbreviations: Arc, Arcuate; BW, body weight; CCK, cholecystokinin; DMX, dorsal motor nucleus of the vagus; DPP-IV, dipeptidyl peptidase IV; Ex, exendin; GLP, glucagon-like peptide; HPV, hepatic portal vein; ID, inner diameter; IMI, intermeal interval; NTS, nucleus tractus solitarii; OD, outer diameter; PVN, paraventricular nucleus; SDA, subdiaphragmatic vagal deafferentation; VC, vena cava.
References
- Holst JJ 2007 The physiology of glucagon-like peptide 1. Physiol Rev 87:1409–1439 [DOI] [PubMed] [Google Scholar]
- Cummings DE, Overduin J 2007 Gastrointestinal regulation of food intake. J Clin Invest 117:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz TA, Geary N 2008 Gastrointestinal factors in appetite and food intake—animal research. In: Harris RBS, Mattes RD, eds. Appetite and food intake: behavioral and physiological considerations. Boca Raton, FL: CRC Press; 163–186 [Google Scholar]
- Strader AD, Woods SC 2005 Gastrointestinal hormones and food intake. Gastroenterology 128:175–191 [DOI] [PubMed] [Google Scholar]
- Holst JJ, Deacon CF 2005 Glucagon-like peptide-1 mediates the therapeutic actions of DPP-IV inhibitors. Diabetologia 48:612–615 [DOI] [PubMed] [Google Scholar]
- D'Alessio D, Lu W, Sun W, Zheng S, Yang Q, Seeley R, Woods SC, Tso P 2007 Fasting and postprandial concentrations of GLP-1 in intestinal lymph and portal plasma: evidence for selective release of GLP-1 in the lymph system. Am J Physiol 293:R2163–R2169 [DOI] [PubMed] [Google Scholar]
- Göke R, Larsen PJ, Mikkelsen JD, Sheikh SP 1995 Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci 7:2294–2300 [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Shughrue P 1999 Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 403:261–280 [DOI] [PubMed] [Google Scholar]
- Gromada J, Holst JJ, Rorsman P 1998 Cellular regulation of islet hormone secretion by the incretin hormone glucagon-like peptide 1. Pflügers Arch 435:583–594 [DOI] [PubMed] [Google Scholar]
- Balkan B, Li X 2000 Portal GLP-1 administration in rats augments the insulin response to glucose via neuronal mechanisms. Am J Physiol 279:R1449–R1454 [DOI] [PubMed] [Google Scholar]
- Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, Ellis KS, Woods SC, Seeley RJ, Herman JP, D'Alessio DA 2007 Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology 148:4965–4973 [DOI] [PubMed] [Google Scholar]
- De Leon DD, Crutchlow MF, Ham JY, Stoffers DA 2006 Role of glucagon-like peptide-1 in the pathogenesis and treatment of diabetes mellitus. Int J Biochem Cell Biol 38:845–859 [DOI] [PubMed] [Google Scholar]
- Vilsboll T, Agerso H, Krarup T, Holst JJ 2003 Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects. J Clin Endocrinol Metab 88:220–224 [DOI] [PubMed] [Google Scholar]
- Beglinger C, Degen L 2006 Gastrointestinal satiety signals in humans—physiologic roles for GLP-1 and PYY? Physiol Behav 89:460–464 [DOI] [PubMed] [Google Scholar]
- Flint A, Raben A, Astrup A, Holst JJ 1998 Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 101:515–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzwiller JP, Göke B, Drewe J, Hildebrand P, Ketterer S, Handschin D, Winterhalder R, Conen D, Beglinger C 1999 Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut 44:81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iritani N, Sugimoto T, Fukuda H, Komiya M, Ikeda H 1999 Oral triacylglycerols regulate plasma glucagon-like peptide-1(7-36) and insulin levels in normal and especially in obese rats. J Nutr 129:46–50 [DOI] [PubMed] [Google Scholar]
- Hayes MR, Skibicka KP, Grill HJ 2008 Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology 149:4059–4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval DA, Bagnol D, Woods SC, D'Alessio DA, Seeley RJ 2008 Arcuate GLP-1 receptors regulate glucose homeostasis but not food intake. Diabetes 57:2046–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang-Christensen M, Larsen PJ, Göke R, Fink-Jensen A, Jessop DS, Möller M, Sheikh SP 1996 Central administration of GLP-1-(7-36) amide inhibits food and water intake in rats. Am J Physiol 271:R848–R856 [DOI] [PubMed] [Google Scholar]
- Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR 1996 A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379:69–72 [DOI] [PubMed] [Google Scholar]
- Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR 2005 The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res 1044:127–131 [DOI] [PubMed] [Google Scholar]
- Kraly FS, Jerome C, Smith GP 1986 Specific postoperative syndromes after total and selective vagotomies in the rat. Appetite 7:1–17 [DOI] [PubMed] [Google Scholar]
- Rocca AS, Brubaker PL 1999 Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology 140:1687–1694 [DOI] [PubMed] [Google Scholar]
- Geary N, Le Sauter J, Noh U 1993 Glucagon acts in the liver to control spontaneous meal size in rats. Am J Physiol 264:R116–R122 [DOI] [PubMed] [Google Scholar]
- Surina-Baumgartner DM, Langhans W, Geary N 1995 Hepatic portal insulin antibody infusion increases, but insulin does not alter, spontaneous meal size in rats. Am J Physiol 269:R978–R982 [DOI] [PubMed] [Google Scholar]
- Norgren R, Smith GP 1994 A method for selective section of vagal afferent or efferent axons in the rat. Am J Physiol 267:R1136–R1141 [DOI] [PubMed] [Google Scholar]
- Silberbauer CJ, Surina-Baumgartner DM, Arnold M, Langhans W 2000 Prandial lactate infusion inhibits spontaneous feeding in rats. Am J Physiol Regul Integr Comp Physiol 278:R646–R653 [DOI] [PubMed] [Google Scholar]
- Kaufman S 1980 Chronic, nonocclusive, and maintenance-free central venous cannula in the rat. Am J Physiol 239:R123–R125 [DOI] [PubMed] [Google Scholar]
- Arnold M, Mura A, Langhans W, Geary N 2006 Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci 26:11052–11060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ 1997 Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am J Physiol 272:R1245–R1251 [DOI] [PubMed] [Google Scholar]
- Smith GP, Jerome C, Norgren R 1985 Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol 249:R638–R641 [DOI] [PubMed] [Google Scholar]
- Walls EK, Phillips RJ, Wang FB, Holst MC, Powley TL 1995 Suppression of meal size by intestinal nutrients is eliminated by celiac vagal deafferentation. Am J Physiol 269:R1410–R1419 [DOI] [PubMed] [Google Scholar]
- Norgren R, Smith GP 1988 Central distribution of subdiaphragmatic vagal branches in the rat. J Comp Neurol 273:207–223 [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Baronowsky EA, Powley TL 2000 Regenerating vagal afferents reinnervate gastrointestinal tract smooth muscle of the rat. J Comp Neurol 421:325–346 [PubMed] [Google Scholar]
- Hamilton RB, Norgren R 1984 Central projections of gustatory nerves in the rat. J Comp Neurol 222:560–577 [DOI] [PubMed] [Google Scholar]
- Williams DL, Baskin DG, Schwartz MW 2006 Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes 55:3387–3393 [DOI] [PubMed] [Google Scholar]
- Holm S 1979 A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70 [Google Scholar]
- Chelikani PK, Haver AC, Reidelberger RD 2005 Intravenous infusion of glucagon-like peptide-1 potently inhibits food intake, sham feeding, and gastric emptying in rats. Am J Physiol 288:R1695–R1706 [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Fledelius C, Knudsen LB, Tang-Christensen M 2001 Systemic administration of the long-acting GLP-1 derivative NN2211 induces lasting and reversible weight loss in both normal and obese rats. Diabetes 50:2530–2539 [DOI] [PubMed] [Google Scholar]
- Tang-Christensen M, Vrang N, Larsen PJ 1998 Glucagon-like peptide 1(7-36) amide’s central inhibition of feeding and peripheral inhibition of drinking are abolished by neonatal monosodium glutamate treatment. Diabetes 47:530–537 [DOI] [PubMed] [Google Scholar]
- Scott KA, Moran TH 2007 The GLP-1 agonist exendin-4 reduces food intake in nonhuman primates through changes in meal size. Am J Physiol 293:R983–R987 [DOI] [PubMed] [Google Scholar]
- Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL 2005 Peripheral exendin-4 and peptide YY(3–36) synergistically reduce food intake through different mechanisms in mice. Endocrinology 146:3748–3756 [DOI] [PubMed] [Google Scholar]
- Gamse R 1982 Capsaicin and nociception in the rat and mouse. Possible role of substance P. Naunyn Schmiedebergs Arch Pharmacol 320:205–216 [DOI] [PubMed] [Google Scholar]
- Hansen L, Deacon CF, Orskov C, Holst JJ 1999 Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology 140:5356–5363 [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Blackshaw LA, Brookes SJ, Grundy D 2004 Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil 16(Suppl 1):28–33 [DOI] [PubMed] [Google Scholar]
- Nakagawa A, Satake H, Nakabayashi H, Nishizawa M, Furuya K, Nakano S, Kigoshi T, Nakayama K, Uchida K 2004 Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci 110:36–43 [DOI] [PubMed] [Google Scholar]
- Cox JE 1998 Cholecystokinin satiety involves CCKA receptors perfused by the superior pancreaticoduodenal artery. Am J Physiol 274(5 Pt 2):R1390–R1396 [DOI] [PubMed] [Google Scholar]
- Orskov C, Poulsen SS, Möller M, Holst JJ 1996 Glucagon-like peptide I receptors in the subfornical organ and the area postrema are accessible to circulating glucagon-like peptide I. Diabetes 45:832–835 [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V, Pan W 2002 Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci 18:7–14 [DOI] [PubMed] [Google Scholar]
- Nishizawa M, Nakabayashi H, Uchida K, Nakagawa A, Niijima A 1996 The hepatic vagal nerve is receptive to incretin hormone glucagon-like peptide-1, but not to glucose-dependent insulinotropic polypeptide, in the portal vein. J Auton Nerv Syst 61:149–154 [DOI] [PubMed] [Google Scholar]
- Holst JJ 1997 Enteroglucagon. Annu Rev Physiol 59:257–271 [DOI] [PubMed] [Google Scholar]
- Tolessa T, Gutniak M, Holst JJ, Efendic S, Hellström PM 1998 Glucagon-like peptide-1 retards gastric emptying and small bowel transit in the rat: effect mediated through central or enteric nervous mechanisms. Dig Dis Sci 43:2284–2290 [DOI] [PubMed] [Google Scholar]
- Asarian L, Corp ES, Hrupka B, Geary N 1998 Intracerebroventricular glucagon-like peptide-1 (7-36) amide inhibits sham feeding in rats without eliciting satiety. Physiol Behav 64:367–372 [DOI] [PubMed] [Google Scholar]
- Strubbe JH, Heinsbroek ACM, D'Alessio D, van Dijk G 2008 Involvement of peripheral GLP-1 in satiety, glycemic control and gastric motility. Appetite 51:403 (Abstract) [Google Scholar]