Abstract
Progesterone (P) is required for normal mammary gland development, and is implicated in the etiology of mammary cancer in rodents and humans. We analyzed mammary gland developmental responses to P and estrogen (E) in two strains of mice (BALB/c and C57BL/6) that exhibit differences in ductal development at sexual maturity and alveologenesis during pregnancy. C57BL/6 mice exhibited reduced proliferative and morphological responses to P. Analysis of known mediators of sidebranching and alveologenesis revealed that reduced P-induced expression of P receptor isoform B and receptor activator of nuclear factor-κB ligand (RANKL), as well as altered expression and regulation of cyclin D1, CCAAT/enhancer binding protein β, and the downstream effectors of RANKL, nuclear Id2 and p21, contribute significantly to the reduced P responsiveness of the C57BL/6 mammary gland. In contrast, E responsiveness was greater in C57BL/6 than in BALB/c glands. E may play a compensatory role in C57BL/6 alveologenesis through its effect on the induction and activation of signal transducer and activator of transcription 5a, a known regulator of RANKL. These observations suggest that in human populations with heterogeneous genetic backgrounds, individuals may respond differentially to the same hormone. Thus, genetic diversity may have a role in determining the effects of P in normal mammary development and tumorigenesis.
Reduced progesterone-induced expression of progesterone receptor and RANKL, altered expression and regulation of C/EBPβ, and of the downstream effectors of RANKL, nuclear Id2 and p21, contribute significantly to the reduced progesterone-responsiveness of the C57BL/6 mammary gland compared to the BALB/c gland.
Progesterone (P) plays an important role in normal mammary gland development in rodents and humans (1,2,3), and is implicated in the etiology of breast cancer (4,5,6,7). P acts through binding to the progesterone receptor (PR), which exists as two isoforms, PRA and PRB, which are functionally distinct transcriptional regulators (8,9,10). The mouse mammary gland is used extensively to study the role of P and PR in normal development and in mammary cancer. Many studies of P action and PR expression have been performed using BALB/c mice (1,11,12,13,14,15,16,17,18,19). PR gene deletion experiments performed in a C57BL/6 × 129SV genetic background (20,21,22) show that PRB is essential for alveologenesis, whereas the specific function(s) of PRA is not well defined.
Mouse strain-specific differences in mammary gland development (23), response to hormones, and susceptibility to carcinogenesis have been reported (24). C57BL/6 mice exhibit reduced P-induced sidebranching and alveologenesis, and reduced susceptibility to carcinogen- and medroxyprogesterone acetate-induced tumorigenesis, compared with BALB/c mice (25,26). Analysis of P action in mammary glands of different mouse strains may provide important information about mechanism(s) of P action in normal development and carcinogenesis. Therefore, we analyzed mammary gland responses to P or estrogen (E), alone or in combination (E plus P), in wild-type BALB/c and C57BL/6 mice. Because PR expression is restricted to the epithelial compartment of the mammary gland (11,27), we focused our analysis on P actions in the epithelium.
C57BL/6 mice displayed a hypoplastic ductal phenotype in virgin glands, delayed alveolar development during pregnancy, and were notably less sensitive to P-induced ductal sidebranching and alveologenesis. C57BL/6 mammary epithelium exhibited altered expression and regulation of downstream effectors of P signaling, including reduced receptor activator of nuclear factor-κB ligand (RANKL) and CCAAT/enhancer binding protein β (C/EBPβ) protein expression, reduced nuclear localization of Id2, failure to reduce nuclear p21 protein levels, and reduced induction of PRB. There also were significant differences in nuclear cyclin D1 (D1) expression between the two strains. These differences within the epithelium are largely responsible for the reduced P sensitivity in C57BL/6 mice. In contrast, C57BL/6 mice were more sensitive than BALB/c mice to the developmental effects of E. We hypothesize that E plays a compensatory role in C57BL/6 alveologenesis through its effect on the induction and activation of signal transducer and activator of transcription (Stat) 5a, a known regulator of RANKL.
Materials and Methods
Animals
BALB/c and C57BL/6 mice were purchased from Harlan (Indianapolis, IN) and The Jackson Laboratory (Bar Harbor, ME), respectively. One week after ovariectomy (OVX), adult virgin mice received daily sc injections for 3, 5, or 10 d with saline control, 17-β-estradiol (E2; 1 μg), P (1 mg), or E2 plus P (1 μg E2 plus 1 mg P). For all experiments, E treatment was performed with E2. Two hours before being killed, mice were injected with 5-bromo-2′-deoxyuridine (BrdU) (70 μg/g body weight). Mammary glands were fixed and processed as whole mounts (28) or paraffin embedded for immunohistochemistry (11).
Cyclin D1−/− (D1−/−) mice of C57BL/6 × 129SV genetic background (Dr. P. Sicinski, Dana Farber Cancer Institute, Boston, MA) were backcrossed to BALB/c mice for six generations to generate D1−/− mice in a BALB/c background. All animal experimentation was conducted according to standards approved by the All University Committee on Animal Use and Care at Michigan State University.
Immunofluorescence
PRA, PRB, Stat5a, and Id2 were detected using anti-PRA (1:50; hPRa7), anti-PRB (1:50; hPRa6) (Neomarkers, Fremont, CA), anti-Stat5a (1:300; BD Biosciences, San Jose, CA), or anti-Id2 (1:50; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) primary antibodies, followed by appropriate secondary antibodies conjugated to Alexa 488 (Molecular Probes, Inc., Eugene, OR) (11). Double labeling of PRA with BrdU, D1, or RANKL was as described previously (11) with anti-BrdU antibody (kit from Amersham Biosciences Inc., Piscataway, NJ), anti-D1 antibody (1:100; Biosource, Camarillo, CA), or anti-RANKL (1: 100; BioLegend Co., San Diego, CA), using appropriate secondary antibodies conjugated to Alexa 488 or Alexa 546. Nuclei were counterstained with either 4′, 6-diamidino-2-phenylindole, dilactate (DAPI) or TOPRO-3 Iodide (Molecular Probes). Sections were visualized and images captured using a Zeiss Pascal laser scanning confocal microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY) or a Nikon inverted epifluorescence microscope (Mager Scientific, Dexter, MI) with MetaMorph software (Molecular Devices Corp., Downingtown, PA).
Immunoblots
Whole mammary glands were homogenized, and cytoplasmic extracts were prepared as previously described (29). Nuclear extracts were prepared from the nuclear pellet of the cytoplasmic extract, and immunoblots were then performed as previously described (30). Detections were performed with anti-RANKL (1:1000), anti-Id2 (1:1000), anti-C/EBPβ (1:1000 dilution), anti-p21 (1:1000), anti-β-actin (1:3000) (all from Santa Cruz Biotechnology), or anti-Sm (1:3000; The Binding Site, San Diego, CA) using appropriate secondary antibodies conjugated to horseradish peroxidase and Western Lightning Chemiluminescence Reagent Plus (PerkinElmer, Waltham, MA). Blots were quantitated with National Institutes of Health ImageJ software (Rasband WS. ImageJ; National Institutes of Health, Bethesda, MD. Available from: http://rsb.info.nih.gov/ij/). The 33-kDa Sm nuclear antigen (31) was detected to evaluate the quality of cellular fractionation. The abundances of RANKL, Id2, C/EBPβ, and p21 were normalized to that of β-actin, which is ubiquitous in the nuclei and cytoplasm of cells (32).
Quantitative RT-PCR
Whole mammary gland total RNA was extracted using the RNeasy Lipid Tissue kit from QIAGEN, Inc. (Valencia, CA). cDNA was produced by reverse transcriptase with random hexamer primers and RT-PCR, and quantitation of murine Wnt-4 (assay identification no. Mm00437341_m1) or 18S RNAs (assay identification no. Hs99999901_s1) was performed as previously described (33).
Quantitation and statistical analyses
BrdU, PRA, estrogen receptor (ER) α, or D1 was quantitated for the number of positive luminal epithelial cell nuclei from captured images using MetaMorph software as previously described (1). Fluorescence intensity was quantitated as previously described (33). A minimum of three mice per treatment group and a minimum of 1000 cells in three independent sections per mouse were analyzed for all experiments. Results are expressed as mean ± sem, and differences are considered significant at P < 0.05 using the Student’s t test or ANOVA, as appropriate.
Results
C57BL/6 mouse mammary glands exhibit a delay in sidebranching compared with BALB/c mice
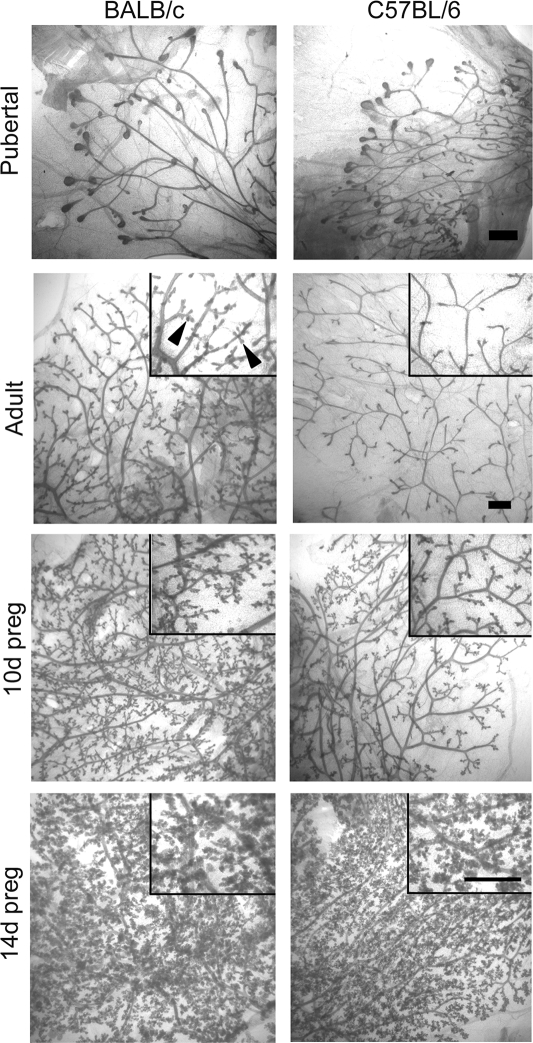
Ductal development, the degree of ductal branching, and end bud number and size were similar between the two strains in 6-wk-old pubertal glands (Fig. 1). The adult BALB/c mammary gland contained well-arborized ducts with sidebranching, whereas the C57BL/6 gland contained a simple ductal network with little sidebranching. During pregnancy, sidebranching and alveologenesis were delayed in the C57BL/6 mouse. However, by 14 d the two strains were indistinguishable.
Figure 1.
Mammary gland development in BALB/c and C57BL/6 mice. Mammary gland whole mounts were prepared from 6-wk-old immature, 20-wk-old adult, and 10 and 14 d pregnant (preg) BALB/c and C57BL/6 mice as described in Materials and Methods. Lower magnification images with higher magnification insets are shown for adult and pregnant mammary glands. Black arrowheads indicate sidebranching in the adult BALB/c mouse. Scale bar, 1 mm.
Differences in hormone-induced proliferation and morphology between strains
We considered that delayed sidebranching and alveologenesis in the C57BL/6 mammary gland might be due to differential responsiveness to P. Because the rate of mammary gland development during early pregnancy was a major difference between the two strains, we examined the effect of E and/or P doses commonly used to induce pregnancy-like alveologenesis in ovariectomized adult mice (1). Since proliferation is required for ductal sidebranching and alveologenesis, proliferative responses of the epithelium were analyzed.
Response to E
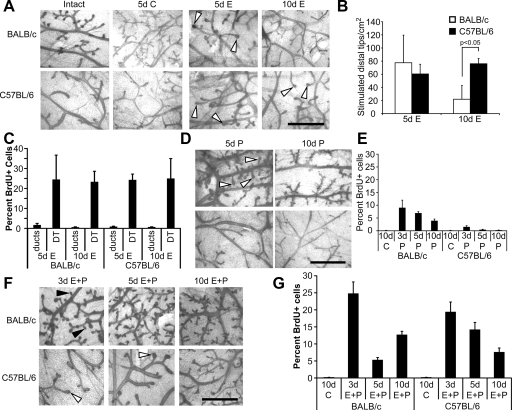
In both strains, E treatment caused enlargement of the distal tips of ducts and ductal dilation that was maximal after 5 d and decreased by 10 d (Fig. 2A). Notably, C57BL/6 mice maintained more enlarged distal tip structures after 10 d treatment (Fig. 2B). E treatment produced a significant increase in epithelial cell proliferation in both strains that was maximal after 5 d and was specifically localized to distal tips (Fig. 2C). Although the percentage of proliferating cells in enlarged distal tips was similar in C57BL/6 and BALB/c mice after 10 d, there were significantly fewer enlarged distal tips in the BALB/c mammary gland (Fig. 2, A and B).
Figure 2.
Effects of E or P treatment on morphology and proliferation. Panels A, D, and F, Representative mammary gland whole mounts from intact and ovariectomized 3, 5, and 10 d control (C)-, E-, P-, or E plus P-treated BALB/c and C57BL/6 mice. White arrowheads indicate enlargement of distal tips of ducts (panels A and F), sidebranches (panel D), and black arrowheads indicate side branches (panel F). Scale bar, 1 mm. B, Quantitation of enlarged distal tips of ducts. The number of enlarged distal tips (area > 0.003 mm2, which represents the area of an unstimulated duct end) per cm2 for ovariectomized adult BALB/c and C57BL/6 mice treated for 5 or 10 d with E. After 10 d E, the number of enlarged distal tips in the BALB/c was less than in the C57BL/6 (P < 0.05). Panels C, E, and G, Quantitation of proliferating luminal epithelial cells in ducts and distal tips (DT) after E (panel C), P (panel E), or E plus P (panel G) treatment as described in Materials and Methods. The values represent the mean ± sem.
Response to P
P treatment produced sidebranching and alveologenesis in BALB/c mammary glands that was maximal after 10 d treatment (Fig. 2D). The percentage of proliferating cells was increased in ducts after 3 d, and in ducts, sidebranches, and alveoli after 5 and 10 d (Fig. 2E). P treatment of C57BL/6 mice produced neither sidebranching nor alveologenesis, and produced minimal proliferation, that was restricted to ducts.
Response to E plus P
In BALB/C mice, E plus P treatment produced extensive sidebranching and alveologenesis that was maximal by 10 d (Fig. 2F). Even after 10 d treatment, very little sidebranching or alveologenesis was observed in C57BL/6 mice. The main response was enlarged duct ends, similar to that observed after E treatment alone (Fig. 2F).
A high percentage of proliferating cells was present in both ducts and sidebranches in E plus P-treated BALB/c glands. Proliferation was maximal after 3 d, decreased after 5 d, and was increased again after 10 d (Fig. 2G). In C57BL/6 glands, maximal proliferation was also observed after 3 d, was maintained at a higher level after 5 d, and decreased after 10 d. Proliferation was localized to ducts and enlarged distal tips, similar to the response to E alone.
PRA regulation
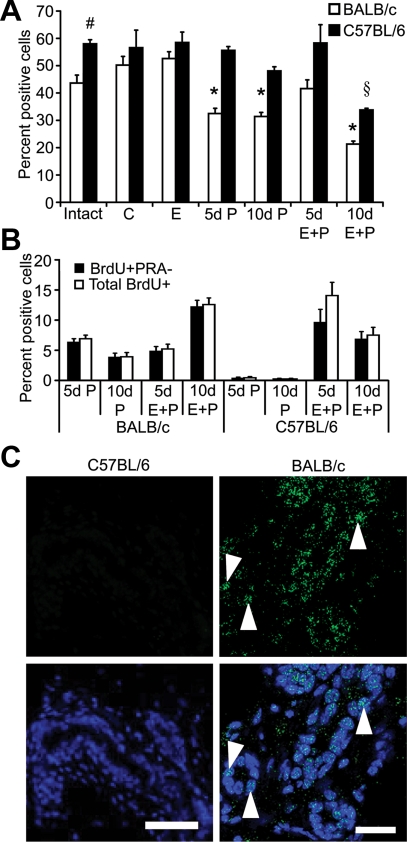
PRA, expressed in a subset of luminal epithelial cells, is the predominant isoform in adult virgin mammary glands (11), and is thought to mediate the initial stages of P-induced sidebranching (1,25). In C57BL/6 glands there were significantly more PRA positive (PRA+) cells (Fig. 3A). After OVX or E treatment, there was no difference in the percentage of PRA+ cells between the two strains. P or E plus P treatment for 5 or 10 d decreased the percentage of PRA+ cells in BALB/c glands. In C57BL/6 glands, PRA+ cells were significantly decreased only after 10 d E plus P treatment. There was no difference in the intensity of PRA staining between the two strains under any treatment (data not shown). The majority of proliferating cells in P or E plus P-treated BALB/c glands were PRA negative (PRA−) (Fig. 3B), suggesting that the decrease of PRA+ cells in BALB/c glands was due to dilution of PRA+ cells by proliferation of PRA− cells. Significant proliferation in C57BL/6 glands occurred only after E plus P treatment, and there were significantly fewer PRA− proliferating cells.
Figure 3.
Hormonal regulation of PRA and PRB expression. PRA- or PRB-expressing cells were detected by immunofluorescence as described in Materials and Methods. Panel A, Quantitation of the PRA+ luminal epithelial cells in adult intact or ovariectomized BALB/c and C57BL/6 mice treated for 5 or 10 d with control (C), E, P, or E plus P. *, PRA+ cells were less abundant in 5 and 10 d P and 10 d E plus P-treated glands than in intact, control, E, and 5 d E plus P-treated glands in BALB/c mice (P < 0.05); #, PRA+ cells were more abundant in intact C57BL/6 than in intact BALB/c glands (P < 0.05); §, PRA+ cells were less abundant in 10 d E plus P-treated C57BL/6 glands than in all other C57BL/6 treatments (P < 0.05). Panel B, Quantitation of BrdU+ and BrdU+/PRA− luminal epithelial cells. Dual-immunofluorescent detection of PRA and BrdU in glands of ovariectomized adult BALB/c and C57BL/6 mice treated for 5 or 10 d with P or E plus P. The values (A and B) represent the mean ± sem. Panel C, Immunofluorescent detection of PRB. PRB staining (green nuclei, white arrowheads) in 10 d E plus P-treated C57BL/6 and BALB/c mammary glands. Nuclei (blue) were counterstained with DAPI. Scale bar, 25 μm.
PRB regulation
Induction of PRB expression occurs in response to P or E plus P treatment of ovariectomized adult BALB/c mice and is correlated with alveolar development (1). In C57BL/6 mammary glands, no PRB expression was detected after either 10 d P or E plus P treatment compared with BALB/c glands (Fig. 3C). The lack of PRB expression in C57BL/6 glands corresponded with a lack of alveologenesis (Fig. 2F).
Regulation of Wnt-4 expression
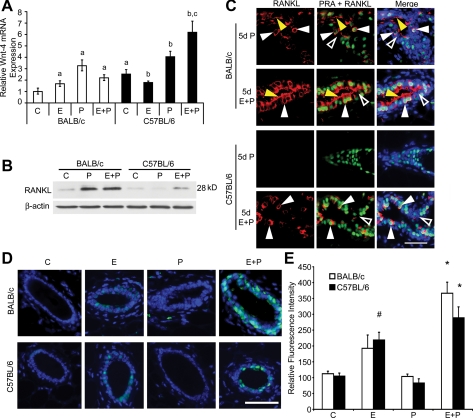
Because most proliferating cells in BALB/c mammary glands were PRA−, this indicated a P-induced paracrine mechanism, as previously suggested (21,34). Wnt-4 is one such paracrine mediator of P-induced sidebranching and proliferation in mammary glands (35). In BALB/c glands, Wnt-4 was most highly induced by P treatment, and also significantly increased by E or E plus P treatment (Fig. 4A). In C57BL/6 glands, Wnt-4 expression was also significantly increased by P or E plus P treatment. Although the fold change induced by P in BALB/c glands was 2-fold greater than that induced by P in C57BL/6 glands, basal levels were significantly higher in ovariectomized, control-treated C57BL/6 glands. Wnt-4 levels were comparable in both BALB/c and C57BL/6 glands after P treatment but were greater in C57BL/6 glands after E plus P treatment.
Figure 4.
Expression and hormonal regulation of Wnt-4, RANKL, and Stat5a. Panel A, RT-PCR detection of Wnt-4 mRNA expressed as fold change relative to ovariectomized control (C) levels in the BALB/c mammary gland. a, P < 0.05 E, P, or E plus P increased Wnt-4 in the BALB/c gland; ovariectomized C57BL/6 control gland expressed more Wnt-4 than BALB/c control gland. b, P < 0.05 E decreased Wnt-4 in C57BL/6 gland; P and E plus P increased Wnt-4 relative to ovariectomized controls. c, P < 0.05 E plus P increased Wnt-4 in C57BL/6 more than in BALB/c gland. Panel B, Immunoblot analysis of RANKL in ovariectomized adult BALB/c and C57BL/6 glands after 5 d control, P, or E plus P. Cytoplasmic extracts from mammary gland were subjected to SDS-PAGE, and RANKL was detected in immunoblot as described in Materials and Methods. Panel C, Dual-immunofluorescent detection of RANKL (red) and PRA (green) in mammary glands of ovariectomized adult BALB/c and C57BL/6 mice after 5 d with P or E plus P; nuclei (blue) were counterstained with DAPI. White arrowheads indicate PRA+/RANKL+ cells; yellow arrowheads indicate RANKL staining in the ductal lumen associated with the apical plasma membranes of RANKL− cells. Open arrowheads indicate PRA−/RANKL− cells. Scale bar, 25 μm. Panel D, Merged Immunofluorescence images of Stat5a (teal) in mammary gland sections from ovariectomized adult BALB/c and C57BL/6 mice after 3 d control, E, P, or E plus P; nuclei (blue) counterstained with DAPI. Scale bar, 25 μm. Panel E, Quantitation of Stat5a immunofluorescence intensity. Values represent the mean ± sem pixel intensity in nuclear Stat5a-positive cells. *, Stat5a intensity after 3 d E plus P in BALB/c and C57BL/6 mammary glands is greater than controls (P < 0.05); #, Stat5a intensity after 3 d E in C57BL/6 mammary glands is greater than control (P < 0.05).
Regulation of RANKL
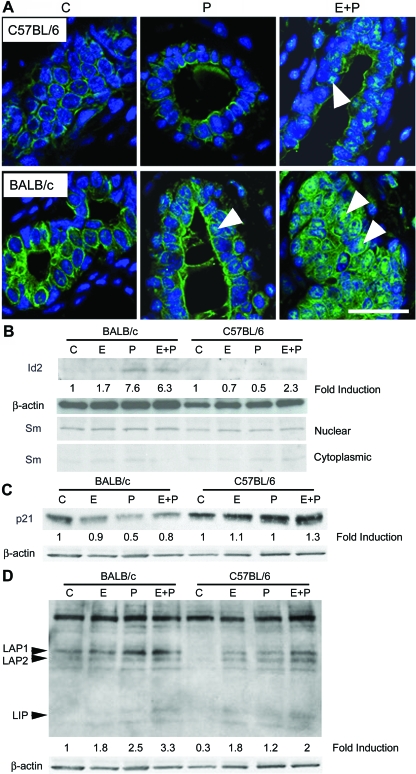
RANKL is another paracrine mediator of P-induced proliferation leading to alveologenesis (21). Little RANKL expression was detected after OVX (Fig. 4B) or E treatment in both mouse strains (data not shown). RANKL expression was induced by P only in BALB/c glands (Fig. 4, B and C), was induced in both strains after 5 d E plus P, but was more strongly induced in BALB/c mice. RANKL was uniformly expressed at the plasma membrane in RANKL-positive cells, and RANKL and PRA were colocalized in the same cells in both strains (Fig. 4C). In addition, RANKL staining was also detected in ductal lumens and associated with the luminal surface of RANKL-negative cells.
Regulation of Stat5a expression and RANKL induction
Stat5a has increased RANKL expression (36), and, in ovariectomized BALB/c mice, E plus P treatment induces activated nuclear Stat5a expression (37). As shown previously, C57BL/6 mice required E in addition to P for RANKL induction. This suggested that E might contribute to RANKL induction through Stat5a activation. E treatment increased nuclear Stat5a in C57BL/6 glands, and there was an overall increase in Stat5a expression after E plus P treatment (Fig. 4, D and E). Similar results were obtained in BALB/c glands. In addition, E plus P treatment induced RANKL colocalization with Stat5a (data not shown).
Regulation of nuclear Id2 and p21 expression
In response to RANKL signaling, Id2 is translocated to the nucleus (38). Nuclear localization of Id2 is required for mammary gland proliferation, and nuclear localization of Id2 is critical for transcriptional down-regulation of cyclin-dependent kinase inhibitor, p21 (38). Therefore, Id2 can promote proliferation by decreasing p21 levels. We considered that reduced RANKL levels in C57BL/6 glands would reduce Id2 nuclear localization, elevate nuclear p21 levels, and reduce P-induced proliferation.
After OVX, cytoplasmic Id2 levels were higher in control-treated BALB/c glands than in C57BL/6 glands, and treatment with P or E plus P for 5 or 10 d increased Id2 nuclear localization (Fig. 5A). In C57BL/6 glands, only treatment with E plus P increased Id2 nuclear localization. Immunoblots of mammary gland nuclear extracts from BALB/c mice treated for 5 d with P or E plus P showed an approximate 5-fold increase in nuclear Id2 (Fig. 5B). The glands of C57BL/6 mice showed a more modest 2.3-fold increase, only after E plus P treatment.
Figure 5.
Hormonal regulation of nuclear Id2, p21, and C/EBPβ. Panel A, Immunofluorescent detection of Id2 in mammary glands of ovariectomized adult BALB/c and C57BL/ mice after 5 d control (C), P, or E plus P. Nuclear Id2 was detected (green nuclei, white arrowheads); nuclei (blue) were counterstained with To-PRO. Scale bar, 25 μm. Panels B–D, Immunoblot analysis of Id2 (panel B), p21 (panel C), and C/EBPβ (panel D) in nuclear extracts of whole mammary glands from ovariectomized adult BALB/c and C57BL/6 mice after 5 d control, E, P, or E plus P. Nuclear extracts were subjected to SDS-PAGE, and Id2, p21, and C/EBPβ were detected in immunoblots as described in Materials and Methods. The 33-kDa Sm nuclear antigen, detected to evaluate the quality of cellular fractionation, was more abundant in nuclear than cytoplasmic extracts, indicating successful fractionation.
Nuclear p21 levels decreased after E, P, or E plus P treatments in BALB/c glands. In contrast, p21 levels remained elevated after all treatments in C57BL/6 glands (Fig. 5C).
C/EBPβ is required for mammary alveologenesis (39) and is a regulator of Id2 expression (40). Immunoblots showed higher basal C/EBPβ levels in BALB/c than C57BL/6 glands, consistent with their higher levels of Id2 expression (Fig. 5D). Although P and E plus P treatment led to similar increases in nuclear C/EBPβ levels in BALB/c glands, E had a minimal effect. In contrast, E or P both increased nuclear C/EBPβ levels in C57BL/6 glands, with E having a greater effect, and E plus P treatment inducing maximal C/EBPβ expression. Once again, the effects of E were more evident in C57BL/6 than BALB/c mice, and E plus P was more potent than P alone.
Regulation and expression of D1
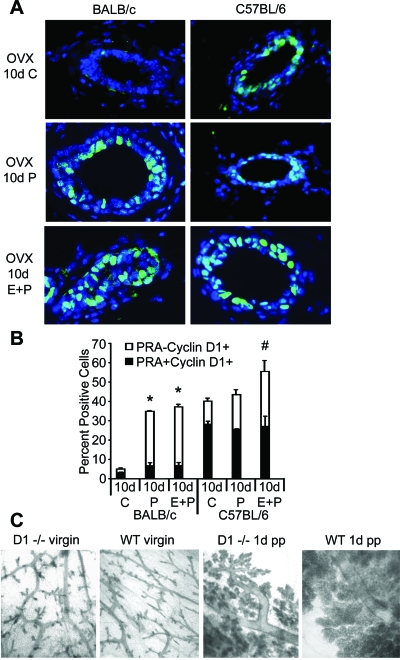
Another mediator of P-induced proliferation is D1, whose expression and nuclear localization increases in response to P treatment (1,41). D1 is also downstream of RANKL signaling in the mammary gland (42), which is hypothesized to increase D1 nuclear localization in PR− cells (21). The lack of P-induced proliferation and reduced expression of RANKL, Id2, and C/EBPβ in C57BL/6 mice suggested that nuclear localization of D1 might also be decreased or absent. Surprisingly, nuclear D1 was present in a significantly higher percentage of cells in ovariectomized control-treated C57BL/6 glands (Fig. 6A); however, a significantly higher percentage of cells coexpressed nuclear D1 and PRA compared with similarly treated BALB/C glands (Fig. 6B). E treatment did not affect nuclear D1 levels in either strain (data not shown). In C57BL/6 glands, nuclear D1 was modestly increased in PRA− cells only after E plus P treatment, coinciding with modest E plus P-induced increases in RANKL (Fig. 6B).
Figure 6.
Hormonal regulation of D1. Panel A, Dual-immunofluorescent detection of PRA and D1 was determined for ovariectomized adult BALB/c and C57BL/6 mice after 10 d control (C), P, or E plus P. Nuclear expression of D1 was detected (green nuclei); nuclei (blue) were counterstained with DAPI. Scale bar, 25 μm. Panel B, Quantitation of D1+ PRA− and PRA+D1+ luminal epithelial cell nuclei. *, Total nuclear D1+ cells after 10 d P and E plus P is greater than in 10 d control in BALB/c glands (P < 0.05); #, total nuclear D1+ cells after 10 d E plus P is greater than after 10 d control or P in C57BL/6 glands (P < 0.05). The values represent the mean ± sem. Panel C, Whole mounts from virgin adult and 1 d postpartum (pp) D1−/− and wild-type (WT) BALB/c mice.
In BALB/c glands, both P and E plus P treatment dramatically increased nuclear D1 expression, indicating P-dependent nuclear localization (Fig. 6A). Increased nuclear D1 expression occurred predominantly in PRA− cells (Fig. 6B). The increase in nuclear D1 was significantly greater than in C57BL/6 glands, corresponding to the greater increase in RANKL in BALB/c glands. These findings also support the hypothesis that RANKL is a paracrine mediator of increased D1 nuclear localization in PR− cells in BALB/c glands. The P-induced increase in D1 coincided with increased RANKL, increased nuclear Id2, and decreased p21, an expression profile favoring proliferation.
D1 is required for alveologenesis during pregnancy based on gene deletion studies in C57BL/6 ×129SV mice (43,44,45). However, P is also essential for alveologenesis (20). Because C57BL/6 glands were less P responsive and expressed high levels of nuclear D1, we sought to determine the relative importance of P and D1 for alveologenesis during pregnancy in BALB/c vs. C57BL/6 glands. Pregnant BALB/c D1−/− mice, generated by backcrossing C57BL/6 × 129SV D1−/− mice with BALB/c mice, exhibit extensive alveolar development, although less than wild-type glands (Fig. 6C). Despite extensive alveolar development, BALB/c D1−/− mice displayed impaired lactation and failed to nurse their pups.
Discussion
P, combined with E and other hormones and growth factors, is required for the extensive sidebranching and alveologenesis that occur during pregnancy. The temporal sequence of changes in proliferation and morphology leading to alveologenesis demonstrates that the development of sidebranches precedes the development of alveoli (1). Previous studies indicate that P promotes ductal sidebranching in the mouse mammary gland through PRA. However, P-induced alveologenesis is mediated through PRB (1,11). P-induced Wnt-4 expression is associated with sidebranching (35), whereas P-induced RANKL and increased nuclear D1 are associated primarily with alveologenesis (1,11,21,44). C57BL/6 mice exhibited delayed sidebranching and alveologenesis during pregnancy compared with BALB/c mice. To understand the basis for this difference, we compared hormonal regulation of Wnt-4, RANKL, Id2, p21, C/EBPβ, PRB, and D1 in C57BL/6 vs. BALB/c mammary glands.
Wnt-4 is proposed to be a paracrine mediator of P-induced proliferation and sidebranching (35). The ectopic expression of Wnt-1 in PR−/− mice leads to precocious sidebranching, suggesting that Wnt expression is critical for sidebranching. We found that overall expression of Wnt-4 was higher in C57BL/6 glands, and that Wnt-4 levels were similar after P treatment in both strains. This indicates that Wnt-4 is not the limiting factor for the development of sidebranches in C57BL/6 glands. It should be noted that Wnt-4 protein levels in mammary glands of either strain could not be determined because Wnt-4 was not detectable by immunoblot or immunohistochemistry using a number of commercially available antibodies (M.D.A., W.W., R.C.S., and S.Z.H., unpublished observations). To our knowledge the effect of P on Wnt-4 protein levels has not been reported for the mouse mammary gland.
RANKL−/− mice in a C57BL/6 ×129SV genetic background have been used to show an essential role for RANKL, another P-induced paracrine factor, in alveolar development (46,47). We showed that RANKL was strongly induced by P or E plus P in BALB/c mammary glands, and was associated with proliferation involved in both sidebranching and alveologenesis. In contrast, in C57BL/6 mammary glands, RANKL was not induced by P and only modestly increased by E plus P. These results extend previous findings to demonstrate that RANKL also plays a role in P-induced sidebranching. Furthermore, the reduced level of RANKL may be a significant contributing factor to determining the extent of sidebranching and alveologenesis in C57BL/6 glands relative to BALB/c glands. It would be informative to examine RANKL−/− mice backcrossed into a BALB/c genetic background for sidebranching defects not observed in the C57BL/6 × 129SV background.
Nuclear localization of Id2, a downstream effector of RANKL signaling, decreases expression of p21, which is important for cell cycle progression leading to proliferation in the mammary gland (38). We showed that Id2 levels are higher, and that nuclear localization of Id2 is increased by P, in BALB/c, but not C57BL/6 glands. Furthermore, nuclear p21 levels are decreased by E, P, or E plus P in BALB/c but not C57BL/6 glands. These results are consistent with a model whereby increased RANKL leads to nuclear Id2 localization and a resultant decrease in nuclear p21 levels. These results also support previous observations that Id2 is an important regulator of sidebranching and alveologenesis in the mammary gland (38,48). We also found that C/EBPβ, a known regulator of Id2 expression (40), is much more highly expressed in BALB/c than C57BL/6 glands. Our finding that C/EBPβ expression is increased by P treatment is interesting in light of previous reports that placed C/EBPβ upstream of PR signaling (34). However, our results are consistent with the increased expression of C/EBPβ observed during the course of murine pregnancy (39), and C/EBPβ has been reported as a target for PRB signaling (9). The potential regulation of PR by C/EBPβ and the converse regulation of C/EBPβ by PR signaling are certainly not mutually exclusive. In fact, such a regulatory circuit could provide a mechanism for enforcing the heightened expression of PRB observed in pregnancy, with signaling through PRB promoting its own expression. Together, these results strongly implicate altered regulation of RANKL, Id2, p21, and C/EBPβ as significant factors responsible for the reduced P response in C57BL/6 mammary glands.
D1 is also considered to be an important mediator of P-induced proliferation (1,11,21). Studies of C57BL/6 D1−/− mice show D1 expression to be critical for alveolar development (43,44,45). In this report, nuclear D1 expression in BALB/c glands was dramatically decreased after OVX but could be increased by P treatment. Nuclear D1 levels were increased predominantly in PRA− cells and were associated with P- or E plus P-induced proliferation, indicating that P likely acts through a paracrine factor to influence D1 localization in neighboring cells. Surprisingly, we found high levels of nuclear D1, even after OVX, in C57BL/6 glands. Notably, nuclear D1 was predominantly expressed in PRA+ cells. Only treatment with E plus P led to an increase in nuclear D1 in PRA− cells, which was associated with E plus P-induced proliferation. As noted earlier, higher nuclear D1 levels in the C57BL/6 mammary gland were also associated with elevated nuclear p21 levels. Mice overexpressing D1, but deficient in Id2, exhibit a defect in mammary epithelial cell proliferation (49). Thus, high D1 levels in the C57BL/6 gland may not be sufficient to induce robust proliferation, when nuclear Id2 levels are low and p21 levels are high.
D1−/− C57BL/6 ×129SV mice are alveologenesis impaired during pregnancy (44,45). However, in this study, D1−/− BALB/c mice exhibited extensive alveologenesis during pregnancy. Interestingly BALB/c D1−/− mice, like C57BL/6 D1−/− mice, were lactation deficient, in agreement with previous reports that D1 also plays a specific role in lactational differentiation (43,44,45). Viewed in the context of reduced responsiveness to P observed in C57BL/6 mice, these results suggest that impaired alveologenesis in D1−/− C57BL/6 mice is likely the result of inherent strain-specific reduced P responsiveness rather than the lack of D1 per se. In addition, these results suggest that D1 is an important, but not essential, mediator of P-induced sidebranching and alveologenesis, and highlight the importance of mouse strain when examining a mammary gland phenotype in genetically modified mice. Because of the inherent reduced P responsiveness, the C57BL/6 strain is not well suited for analysis of P-mediated responses in the mammary gland. In addition, the variable genetic contribution of mixed genetic backgrounds (i.e. C57BL/6 × 129SV) often used for gene deletion studies may yield inconsistent outcomes, thus confounding the interpretation of a phenotype. This problem may be overcome by backcrossing genetically modified mice into a pure genetic background with a well-defined wild-type phenotype.
PRB−/− mice fail to undergo alveologenesis during pregnancy (21). We found that C57BL/6 glands were less responsive to P induction of PRB than BALB/c glands. PRB can be induced in BALB/c glands after 5 or 10 d of P or E plus P treatment; induction of PRB by P occurs subsequent to the development of sidebranches and coincident with alveolar development (1). By contrast, no PRB was detected after the same treatments in C57BL/6 glands. However, at 14 d pregnancy, when alveolar development in BALB/c and C57BL/6 glands is comparable, PRB is abundantly expressed in C57BL/6 glands (M.D.A. and S.Z.H., unpublished observations). Thus, it is likely that the delayed induction of PRB in the C57BL/6 gland is a consequence of the delayed sidebranching response.
We previously hypothesized that the PR− cells that proliferate to form sidebranches are derived from progenitor cells committed to the alveolar luminal cell lineage, and that it is a property of these cells to express PRB and form alveoli. Once PRB expression is induced, P acting through PRB may form a positive regulatory loop to further increase PRB expression and the expansion of alveolar cells (1). In the BALB/c gland, P alone induces approximately 10-fold greater proliferation of PRA− cells after 3 d treatment; this proliferation is sustained after 5 or 10 d treatment and produces sidebranches. This is compared with a feeble and transient response to P treatment in C57BL/6 glands and a lack of sidebranching (Figs. 2E and 4B). We propose that robust sidebranching in the BALB/c gland produces more progenitor cells that contribute to alveolar cells expressing PRB. The lack of sidebranching in C57BL/6 glands results in a lack of alveolar progenitor cells and provides a plausible explanation for the lack of PRB induction in C57BL/6 glands.
In C57BL/6 mice, E in addition to P was critically required for the induction of RANKL. In BALB/c mice, Stat5a has also increased RANKL expression (36), and Stat5a expression colocalizes with RANKL (37). E plus P treatment also increases Stat5a activation and nuclear localization (37). Cotreatment of E plus P with bromocryptine, to block prolactin secretion, only increases cytoplasmic Stat5a, indicating that prolactin is required for activated nuclear Stat5a. Because E increases prolactin levels, we hypothesize that one way that E contributes to alveologenesis in C57BL/6 and BALB/c mice is through increasing prolactin secretion, leading to activation of Stat5a and Stat5a-dependent induction of RANKL. Prolactin is critically required for alveologenesis and lactation in C57BL/6 mice (50), as demonstrated by a permanent lactational defect in prolactin receptor+/− C57BL/6 mice compared with the lactational rescue in second and subsequent pregnancies in prolactin receptor+/− 129SV mice. We hypothesize that E is acting at three levels: 1) to increase PRA expression, thereby promoting P induction of RANKL; 2) acting in concert with P to increase Stat5a levels; and 3) to increase prolactin levels leading to Stat5a activation and subsequent RANKL induction. In C57BL/6 glands, E is required in addition to P because reduced responsiveness to P provides inadequate induction of RANKL. E acts to increase prolactin levels to achieve sufficient levels of Stat5a activation for RANKL induction. Our findings that E and E plus P increased nuclear Stat5a levels in C57BL/6 glands support this hypothesis. We speculate that in C57BL/6 mammary glands during pregnancy, the temporal pattern of increasing E and prolactin levels may determine the temporal pattern of sidebranching and alveologenesis.
Beyond a critical role in C57BL/6 RANKL induction, E displays other differential activities between BALB/c and C57BL/6 glands. Unlike the case with BALB/c glands, E fails to induce any increase in Wnt-4 RNA expression and is capable in the absence of P of substantially up-regulating nuclear C/EBPβ levels. However, E’s role in the regulation of Wnt-4 and C/EBPβ remains to be determined, and further studies are warranted into the detailed mechanism(s) by which E contributes to ductal sidebranching and alveologenesis in C57BL/6 mice.
We noted that the proliferative effect of E was more pronounced in C57BL/6 than BALB/c mice, as evidenced by sustained E-induced enlargement of and proliferation in distal ductal tips. Furthermore, E plus P-treated C57BL/6 glands exhibited enlarged distal tips similar to those seen with E alone, indicating a largely E-dependent response. We have found more ERα-positive cells and higher levels of ERα per cell in C57BL/6 glands than BALB/c glands, consistent with their greater sensitivity to E (M.D.A., and S.Z.H., unpublished observations).
In this report we demonstrate differential sensitivity to P within the epithelium of BALB/c and C57BL/6 mice. We have focused on the epithelium because PRs are exclusively expressed in epithelial cells. Previous studies, comparing sidebranching and hormone responses in different mouse strains, have used transplantation experiments in immune-compromised mice to explore underlying mechanisms, and have concluded that differences are due to host environment, and have implicated the mammary stroma (25,51,52). However, as acknowledged in the most recent studies (25), these experiments do not exclude contributing host factors other than the mammary stroma. Notwithstanding, it is entirely possible that stromal factors also influence differences in epithelial responsiveness, and further studies are warranted to explore the stromal contributions to strain-specific differences.
Of particular significance is the finding that two genetic backgrounds in the same species differ so significantly in E and P responses, indicating that caution is required in generalizing mechanisms of hormone action underlying mammary gland development on the basis of studies in a single mouse strain. These results also suggest that, in human populations with heterogeneous genetic backgrounds, individuals may respond differentially to the same hormone through inherent differences in their regulation of downstream signaling pathways. These differences may apply to hormonal regulation in both the normal breast and in breast cancers.
Footnotes
This work was supported by Breast Cancer and the Environment Research Centers Grant U01 ES/CA 012800 from the National Institute of Environment Health Science and the National Cancer Institute, National Institutes of Health (NIH), Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Environment Health Science or National Cancer Institute, NIH.
Disclosure Statement: The authors have nothing to declare.
First Published Online November 6, 2008
Abbreviations: BrdU, 5-bromo-2′-deoxyuridine; C/EBPβ, CCAAT/enhancer binding protein β; D1, cyclin D1; D1−/−, cyclin D1−/−; DAPI, 4′, 6-diamidino-2-phenylindole, dilactate; E, estrogen; E2, 17-β-estradiol; ER, estrogen receptor; OVX, ovariectomy; P, progesterone; PR, progesterone receptor; PRA−, progesterone receptor A negative; PRA+, progesterone receptor A positive; RANKL, receptor activator of nuclear factor-κB ligand; Stat, signal transducer and activation of transcription.
References
- Aupperlee MD, Haslam SZ 2007 Differential hormonal regulation and function of progesterone receptor isoforms in normal adult mouse mammary gland. Endocrinology 148:2290–2300 [DOI] [PubMed] [Google Scholar]
- Fendrick JL, Raafat AM, Haslam SZ 1998 Mammary gland growth and development from the postnatal period to postmenopause: ovarian steroid receptor ontogeny and regulation in the mouse. J Mammary Gland Biol Neoplasia 3:7–22 [DOI] [PubMed] [Google Scholar]
- Hofseth LJ, Raafat AM, Osuch JR, Pathak DR, Slomski CA, Haslam SZ 1999 Hormone replacement therapy with estrogen or estrogen plus medroxyprogesterone acetate is associated with increased epithelial proliferation in the normal postmenopausal breast. J Clin Endocrinol Metab 84:4559–4565 [DOI] [PubMed] [Google Scholar]
- Graham JD, Yager ML, Hill HD, Byth K, O'Neill GM, Clarke CL 2005 Altered progesterone receptor isoform expression remodels progestin responsiveness of breast cancer cells. Mol Endocrinol 19:2713–2735 [DOI] [PubMed] [Google Scholar]
- Graham JD, Yeates C, Balleine RL, Harvey SS, Milliken JS, Bilous AM, Clarke CL 1996 Progesterone receptor A and B protein expression in human breast cancer. J Steroid Biochem Mol Biol 56:93–98 [DOI] [PubMed] [Google Scholar]
- Hopp TA, Weiss HL, Hilsenbeck SG, Cui Y, Allred DC, Horwitz KB, Fuqua SA 2004 Breast cancer patients with progesterone receptor PR-A-rich tumors have poorer disease-free survival rates. Clin Cancer Res 10:2751–2760 [DOI] [PubMed] [Google Scholar]
- Mote PA, Bartow S, Tran N, Clarke CL 2002 Loss of co-ordinate expression of progesterone receptors A and B is an early event in breast carcinogenesis. Breast Cancer Res Treat 72:163–172 [DOI] [PubMed] [Google Scholar]
- Jacobsen BM, Richer JK, Sartorius CA, Horwitz KB 2003 Expression profiling of human breast cancers and gene regulation by progesterone receptors. J Mammary Gland Biol Neoplasia 8:257–268 [DOI] [PubMed] [Google Scholar]
- Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB 2002 Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem 277:5209–5218 [DOI] [PubMed] [Google Scholar]
- Tung L, Abdel-Hafiz H, Shen T, Harvell DM, Nitao LK, Richer JK, Sartorius CA, Takimoto GS, Horwitz KB 2006 Progesterone receptors (PR)-B and -A regulate transcription by different mechanisms: AF-3 exerts regulatory control over coactivator binding to PR-B. Mol Endocrinol 20:2656–2670 [DOI] [PubMed] [Google Scholar]
- Aupperlee MD, Smith KT, Kariagina A, Haslam SZ 2005 Progesterone receptor isoforms A and B: temporal and spatial differences in expression during murine mammary gland development. Endocrinology 146:3577–3588 [DOI] [PubMed] [Google Scholar]
- Haslam SZ 1988 Acquisition of estrogen-dependent progesterone receptors by normal mouse mammary gland. Ontogeny of mammary progesterone receptors. J Steroid Biochem 31:9–13 [DOI] [PubMed] [Google Scholar]
- Haslam SZ 1988 Progesterone effects on deoxyribonucleic acid synthesis in normal mouse mammary glands. Endocrinology 122:464–470 [DOI] [PubMed] [Google Scholar]
- Haslam SZ 1989 The ontogeny of mouse mammary gland responsiveness to ovarian steroid hormones. Endocrinology 125:2766–2772 [DOI] [PubMed] [Google Scholar]
- Haslam SZ, Counterman LJ, St. John AR 1993 Hormonal basis for acquisition of estrogen-dependent progesterone receptors in the normal mouse mammary gland. Life Sci Adv Steroid Biochem 12:27–34 [Google Scholar]
- Atwood CS, Hovey RC, Glover JP, Chepko G, Ginsburg E, Robison WG, Vonderhaar BK 2000 Progesterone induces side-branching of the ductal epithelium in the mammary glands of peripubertal mice. J Endocrinol 167:39–52 [DOI] [PubMed] [Google Scholar]
- Hovey RC, Trott JF, Ginsburg E, Goldhar A, Sasaki MM, Fountain SJ, Sundararajan K, Vonderhaar BK 2001 Transcriptional and spatiotemporal regulation of prolactin receptor mRNA and cooperativity with progesterone receptor function during ductal branch growth in the mammary gland. Dev Dyn 222:192–205 [DOI] [PubMed] [Google Scholar]
- Satoh K, Hovey RC, Malewski T, Warri A, Goldhar AS, Ginsburg E, Saito K, Lydon JP, Vonderhaar BK 2007 Progesterone enhances branching morphogenesis in the mouse mammary gland by increased expression of Msx2. Oncogene 26:7526–7534 [DOI] [PubMed] [Google Scholar]
- Shyamala G, Schneider W, Schott D 1990 Developmental regulation of murine mammary progesterone receptor gene expression. Endocrinology 126:2882–2889 [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery Jr CA, Shyamala G, Conneely OM, O'Malley BW 1995 Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266–2278 [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM 2003 Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA 100:9744–9749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM 2000 Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science 289:1751–1754 [DOI] [PubMed] [Google Scholar]
- Gardner WU, Strong LC 1935 The normal development of the mammary glands of virgin female mice of ten strains varying in susceptibility to spontaneous neoplasms. Am J Cancer 25:282–290 [Google Scholar]
- Nandi S, Bern HA 1960 Relation between mammary-gland responses to lactogenic hormone combinations and tumor susceptibility in various strains of mice. J Natl Cancer Inst 24:907–931 [PubMed] [Google Scholar]
- Montero Girard G, Vanzulli SI, Cerliani JP, Bottino MC, Bolado J, Vela J, Becu-Villalobos D, Benavides F, Gutkind S, Patel V, Molinolo A, Lanari C 2007 Association of estrogen receptor-α and progesterone receptor A expression with hormonal mammary carcinogenesis: role of the host microenvironment. Breast Cancer Res 9:R22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina D 1974 Mammary tumorigenesis in chemical carcinogen-treated mice. I. Incidence in BALB-c and C57BL mice. J Natl Cancer Inst 53:213–221 [DOI] [PubMed] [Google Scholar]
- Shyamala G, Barcellos-Hoff MH, Toft D, Yang X 1997 In situ localization of progesterone receptors in normal mouse mammary glands: absence of receptors in the connective and adipose stroma and a heterogeneous distribution in the epithelium. J Steroid Biochem Mol Biol 63:251–259 [DOI] [PubMed] [Google Scholar]
- Banerjee MR, Wood BG, Lin FK, Crump LR 1976 Organ culture of whole mammary gland of the mouse. Tissue Culture Association Manual 2:457–462 [Google Scholar]
- Spencer RL, Kalman BA, Cotter CS, Deak T 2000 Discrimination between changes in glucocorticoid receptor expression and activation in rat brain using western blot analysis. Brain Res 868:275–286 [DOI] [PubMed] [Google Scholar]
- Spooner CJ, Sebastian T, Shuman JD, Durairaj S, Guo X, Johnson PF, Schwartz RC 2007 C/EBPβ serine 64, a phosphoacceptor site, has a critical role in LPS-induced IL-6 and MCP-1 transcription. Cytokine 37:119–127 [DOI] [PubMed] [Google Scholar]
- Hinterberger M, Pettersson I, Steitz JA 1983 Isolation of small nuclear ribonucleoproteins containing U1, U2, U4, U5, and U6 RNAs. J Biol Chem 258:2604–2613 [PubMed] [Google Scholar]
- Peters KE, Comings DE 1980 Two-dimensional gel electrophoresis of rat liver nuclear washes, nuclear matrix, and hnRNA proteins. J Cell Biol 86:135–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam SZ, Drolet A, Smith K, Tan M, Aupperlee M 2008 Progestin-regulated luminal cell and myoepithelial cell-specific responses in mammary organoid culture. Endocrinology 149:2098–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagroves TN, Lydon JP, Hovey RC, Vonderhaar BK, Rosen JM 2000 C/EBPβ (CCAAT/enhancer binding protein) controls cell fate determination during mammary gland development. Mol Endocrinol 14:359–368 [DOI] [PubMed] [Google Scholar]
- Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK, McMahon JA, McMahon AP, Weinberg RA 2000 Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev 14:650–654 [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Matsuda M, Hou Z, Bailey JP, Kitazawa R, Herbst MP, Horseman ND 2003 Receptor activator of NF-κB ligand induction via Jak2 and Stat5a in mammary epithelial cells. J Biol Chem 278:46171–46178 [DOI] [PubMed] [Google Scholar]
- Santos SJ, Haslam SZ, Conrad SE 2008 Estrogen and progesterone are critical regulators of Stat5a expression in the mouse mammary gland. Endocrinology 149:329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NS, Kim HJ, Koo BK, Kwon MC, Kim YW, Cho Y, Yokota Y, Penninger JM, Kong YY 2006 Receptor activator of NF-κB ligand regulates the proliferation of mammary epithelial cells via Id2. Mol Cell Biol 26:1002–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagroves TN, Krnacik S, Raught B, Gay J, Burgess-Beusse B, Darlington GJ, Rosen JM 1998 C/EBPβ, but not C/EBPα, is essential for ductal morphogenesis, lobuloalveolar proliferation, and functional differentiation in the mouse mammary gland. Genes Dev 12:1917–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaya K, Mori S, Kimoto H, Shima Y, Tsuji Y, Kurooka H, Akira S, Yokota Y 2005 Regulation of Id2 expression by CCAAT/enhancer binding protein β. Nucleic Acids Res 33:1924–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said TK, Conneely OM, Medina D, O'Malley BW, Lydon JP 1997 Progesterone, in addition to estrogen, induces cyclin D1 expression in the murine mammary epithelial cell, in vivo. Endocrinology 138:3933–3939 [DOI] [PubMed] [Google Scholar]
- Cao Y, Bonizzi G, Seagroves TN, Greten FR, Johnson R, Schmidt EV, Karin M 2001 IKKα provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell 107:763–775 [DOI] [PubMed] [Google Scholar]
- Sicinski P, Donaher JL, Parker SB, Li T, Fazeli A, Gardner H, Haslam SZ, Bronson RT, Elledge SJ, Weinberg RA 1995 Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82:621–630 [DOI] [PubMed] [Google Scholar]
- Sicinski P, Weinberg RA 1997 A specific role for cyclin D1 in mammary gland development. J Mammary Gland Biol Neoplasia 2:335–342 [DOI] [PubMed] [Google Scholar]
- Fantl V, Edwards PA, Steel JH, Vonderhaar BK, Dickson C 1999 Impaired mammary gland development in Cyl-1(−/−) mice during pregnancy and lactation is epithelial cell autonomous. Dev Biol 212:1–11 [DOI] [PubMed] [Google Scholar]
- Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA, Elliott R, Scully S, Voura EB, Lacey DL, Boyle WJ, Khokha R, Penninger JM 2000 The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103:41–50 [DOI] [PubMed] [Google Scholar]
- Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM 1999 OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397:315–323 [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Meyer B, Gruss P, Cui Y, Renou JP, Morgan FV, Smith GH, Reichenstein M, Shani M, Hennighausen L, Robinson GW 2002 Mammary epithelial cells are not able to undergo pregnancy-dependent differentiation in the absence of the helix-loop-helix inhibitor Id2. Mol Endocrinol 16:2892–2901 [DOI] [PubMed] [Google Scholar]
- Mori S, Inoshima K, Shima Y, Schmidt EV, Yokota Y 2003 Forced expression of cyclin D1 does not compensate for Id2 deficiency in the mammary gland. FEBS Lett 551:123–127 [DOI] [PubMed] [Google Scholar]
- Kelly PA, Bachelot A, Kedzia C, Hennighausen L, Ormandy CJ, Kopchick JJ, Binart N 2002 The role of prolactin and growth hormone in mammary gland development. Mol Cell Endocrinol 197:127–131 [DOI] [PubMed] [Google Scholar]
- Naylor MJ, Ormandy CJ 2002 Mouse strain-specific patterns of mammary epithelial ductal side branching are elicited by stromal factors. Dev Dyn 225:100–105 [DOI] [PubMed] [Google Scholar]
- Yant J, Gusterson B, Kamalati T 1998 Induction of strain-specific mouse mammary gland ductal architecture. The Breast 7:269–272 [Google Scholar]