Abstract
Steroidogenic factor-1 (SF-1/Ad4BP/NR5A1) plays a major role in regulating steroidogenic enzymes. We have previously shown that SF-1 inhibits aldosterone synthase (CYP11B2) reporter gene activity. Herein, we used the H295R/TR/SF-1 adrenal cells that increase SF-1 in a doxycycline-dependent fashion. Cells were incubated with or without doxycycline to induce SF-1 and then treated with angiotensin II (Ang II). Aldosterone was measured by immunoassay. SF-1 mRNA was silenced by small interfering RNA (siRNA) by Nucleofector technology. mRNA levels were measured by real-time RT-PCR. Ang II treatment without doxycycline increased aldosterone production by 11.3-fold and CYP11B2 mRNA by 116-fold. Doxycycline treatment increased SF-1 mRNA levels by 3.7-fold and inhibited Ang II-induced aldosterone by 84%. Doxycycline treatment inhibited Ang II-stimulated CYP11B2 mRNA levels by 86%. Doxycycline decreased basal CYP11B2 promoter activity by 68%. Doxycycline inhibited Ang II stimulation by 85%. Ang II increased CYP21 mRNA expression by 4.6-fold, whereas doxycycline inhibited induction by 69%. In contrast, doxycycline treatment increased CYP11B1 mRNA by 1.7-fold in basal cells and increased Ang II induction by 3.6-fold. SF-1-specific siRNA significantly reduced SF-1 mRNA expression as compared with cells treated with control siRNA. SF-1 siRNA reversed doxycycline stimulation of CYP B1 and its inhibition of CYP11B2. However, in H295R/TR/SF-1 cells without doxycycline treatment, both CYP11B1 and CYP11B2 mRNAs were significantly decreased, suggesting that both enzymes require a minimal level of SF-1 for basal expression. In summary, SF-1 overexpression dramatically inhibited CYP11B2 expression and decreased aldosterone production. The opposing effects of SF-1 on CYP11B1 and CYP11B2 suggest that the regulation of SF-1 activity may play a role that determines the relative ability to produce mineralocorticoid and glucocorticoid.
In adrenocortical cells high versus low levels of SF-1 differentially regulate the expression of aldosterone synthase and aldosterone production.
The orphan nuclear hormone receptor steroidogenic factor-1 (SF-1/Ad4BP/NR5A1) plays pivotal roles in the development and function of the hypothalamic-pituitary-adrenal and -gonadal axes (1,2,3). Knockout mice lacking SF-1 have adrenal and gonadal agenesis, impaired gonadotropin expression, and structural abnormalities of the ventromedial hypothalamic nucleus (4,5). SF-1 mutations in the human population have been detected and are associated with XY sex reversal, with and without adrenal deficiency (6,7). SF-1 modulates the transcription of many genes involved in steroidogenesis and reproduction, including cytochrome P450 steroid hydroxylases and steroidogenic acute regulatory protein (1,8). SF-1 transcripts and protein are found in many adult tissues including adrenocortical cells, testicular Leydig cells, and ovarian follicular cells (9,10). SF-1 is also present in other tissues, including the skin, spleen, and brain and may play a role in the expression of certain steroid hydroxylases (11).
The primary mechanism of SF-1 action is through its binding to regulatory elements that are variations of an AGGTCA motif, either PyCAAGGPyC or PuPuAGGTCA (12,13,14,15). Most studies have focused primarily on the ability of SF-1 to enhance gene transcription. However, the ability of several nuclear hormone receptors to activate some while repressing other gene targets has been well documented (16,17,18). We have previously shown that SF-1 represses reporter gene activity related to aldosterone synthase (CYP11B2) but activates transcriptional activity of the CYP11B2 isozyme 11β-hydroxylase (CYP11B1) (19,20). Herein, we tested the hypothesis that elevating SF-1 alters adrenal cell differentiation by inhibiting CYP11B2 expression and aldosterone production. We used a recently developed adrenal cortical cell line (H295R/TR/SF-1) that overexpresses SF-1 in a doxycycline-inducible fashion (21). Increasing SF-1 dosage inhibited angiotensin II (Ang II)-stimulated aldosterone production and CYP11B2 expression. This study indicates that SF-1 may play a role in defining the ability of adrenal cells to produce mineralocorticoids vs. glucocorticoids and adrenal androgens.
Materials and Methods
Cell culture and treatments
The SF-1-inducible cell line H295R/TR/SF-1 was cultured in DMEM/F-12 (Invitrogen, Carlsbad, CA) medium supplemented with 2% NuSerum (BD Biosciences, San Jose, CA), 1% ITS Plus (BD Biosciences), and antibiotics (Invitrogen, Carlsbad, CA). This cell line was generated by methods published previously (21). In brief, H295R cells were transfected with the expression plasmid (pcDNA6/TR) encoding the tet repressor and the pcDNA4/TO/SF-1 plasmid, where the human SF-1 cDNA had been inserted. Antibiotic selection with blasticidin (5 μg/ml) and zeocin (100 μg/ml) was used to isolate clones overexpressing SF-1 in a doxycycline-inducible fashion. The cells were subcultured and treated with or without doxycycline (1 μg/ml) for 72 h and Ang II (10 nm) (Sigma-Aldrich, St. Louis, MO) for 24 h. Ang II treatment for transient transfection assay was 6 h.
Steroid hormone assays
The culture medium was collected and subjected to steroid measurement. Medium aldosterone levels were determined by a commercial RIA kit (aldosterone Coat-A-Count) purchased from Siemens (New York, NY) following the manufacturer’s guideline. The assay was read in a γ-counter (DPC γ12) purchased from Diagnostic Products Corp. (Los Angeles, CA). Medium cortisol level was measured by enzyme immunoassay kit (Diagnostic Systems Laboratories Inc., Webster, TX). The well washing was accomplished using an ELX50 automatic microplate washer (Bio-Tek Instruments Inc., Winooski, VT). The absorbance of the solution was read using an ELX800 microplate reader (Bio-Tek Instruments). Protein assay was conducted using the Micro PCA kit (Pierce Biotechnology, Inc., Rockford, IL) following the manufacturer’s instructions. The steroid concentration per well was normalized by its relative protein quantity.
Transient transfection assays
The 5′-flanking DNA from the human CYP11B2 and CYP11B1 gene has been previously described (22). Transient transfections in H295R/TR/SF-1 cells were performed as described (23). In brief, on d 0, 200,000 H295R/TR/SF-1 cells per well were plated in 24-well Costar dishes in a volume of 1.0 ml complete DMEM supplemented with 2% NuSerum (BD Biosciences), 1% ITS Plus (BD Biosciences), antibiotics (Invitrogen), and doxycycline (1.0 μg/ml). The cells were then incubated for 72 h at 37 C. On d 4, cells were transfected with a mixture of plasmid DNA consisting of CYP11B1-pGL3 (or CYP11B2-pGL3, pGL3 Basic) (1.0 μg/well), using the transfection reagent Transfast (Promega, Madison, WI) for 6 h. To normalize luciferase activity, cells were cotransfected with pSV-β-galactosidase plasmid (50 ng/well) (Promega). After recovery of the cells for 20–24 h, cells were treated with Ang II (10 nm) (Sigma-Aldrich) for 6 h before being lysed and assayed for luciferase activity (Promega) and β-galactosidase activity (Tropix, Bedford, MA) using a Fluostar Optima Microplate Luminometer (BMG Labtech, Inc., Durham, NC). Relative level of transactivation was calculated by dividing luciferase units by β-galactosidase units.
Real-time quantitative PCR
Total RNA isolation was conducted by using an RNeasy Mini Kit (QIAGEN, Valencia, CA). The purity and integrity of the RNA were assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA), and its quantity was determined by NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE). RNA (2 μg) was reverse transcribed using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) and incubated at 25 C for 10 min and 37 C for 2 h. The synthesized cDNA was diluted 1:10 and stored at −20 C.
The primer and probe sets for human StAR, side-chain cleavage (CYP11A1), 3β-hydroxysteroid dehydrogenase-2 (HSD3B2), 21-hydroxylase (CYP21), CYP11B1, CYP11B2 were designed using Primer Express 3.0 (Applied Biosystems) and purchased from Integrated DNA Technologies (Coralville, IA) as published previously (24). The sequences of the primers and probes are listed in Table 1. PCR were performed using the ABI 7500 Fast Real-Time PCR System (Applied Biosystems) with a total volume of 20 μl per reaction following the reaction parameters recommended by the manufacturer, which includes denaturation at 95 C for 20 sec followed by amplification for 40 cycles (3 sec at 95 C and 30 sec at 60 C, fluorescence measurement). For each reaction, the 20-μl total volume consisted of 10 μl TaqMan Fast Universal PCR Master Mix (2×) (Applied Biosystems), 900 nm of each primer, 400 nm of the probe, and 5 μl of each first-strand cDNA sample. 18s rRNA was detected and quantified using the TaqMan Ribosomal RNA Control Reagents (Vic Probe) (Applied Biosystems). Each reaction included 10 μl TaqMan PCR Master Mix (2×) (Applied Biosystems), 100 nm probe, and 50 nm primers. Negative controls contained water instead of first-strand cDNA. Quantitative normalization of cDNA in each tissue-derived sample was performed using expression of 18s rRNA as an internal control. The generated cycle threshold (Ct) value of each gene was normalized by its respecting Ct value of 18s rRNA (ΔCt). Then each gene was further normalized using the average ΔCt value of the normal adult adrenal (ΔΔCt). The final fold expression changes were calculated using the equation 2−ΔΔCt (25).
Table 1.
Sequences of primers and probes used for quantification of gene expression by real-time RT-PCR
| Gene | Sequence (5′–3′) |
|---|---|
| StAR | |
| Forward primer | ATGAGTAAAGTGGTCCCAGATG |
| Reverse primer | ACCTTGATCTCCTTGACATTGG |
| Probe | ATCCGGCTGGAGGTCGTGGTGGACGATC |
| CYP11A1 | |
| Forward primer | GAGATGGCACGCAACCTGAAG |
| Reverse primer | CTTAGTGTCTCCTTGATGCTGGC |
| Probe | CGATCTGCCGCGCAGCCAAGACCTCTGAT |
| HSD3B2 | |
| Forward primer | GCGGCTAATGGGTGGAATCTA |
| Reverse primer | CATTGTTGTTCAGGGCCTCAT |
| Probe | TGATACCTTGGTACACTTGTGCGTTAAGACCCA |
| CYP21 | |
| Forward primer | ACCTCAGTTTCTCCTTTATTGC |
| Reverse primer | AGAGCCAGGGTCCTTCAC |
| Probe | CGCGATCAGGAAGCCTTCTCTGCCAGCGAGATC |
| CYP11B1 | |
| Forward primer | GGCAGAGGCAGAGATGCTG |
| Reverse primer | TCTTGGGTTAGTGTCTCCACCTG |
| Probe | TGCTGCACCATGTGCTGAAACACCT |
| CYP11B2 | |
| Forward primer | GGCAGAGGCAGAGATGCTG |
| Reverse primer | CTTGAGTTAGTGTCTCCACCAGGA |
| Probe | CTGCACCACGTGCTGAAGCACT |
SF-1 knockdown
The expression of SF-1 was silenced in H295R/TR/SF-1 cells by RNA interference using the Nucleofector technology (Amaxa Biosystems, Gaithersburg, MD) following the manufacturer’s protocol. Cells were transfected with 10 nm SF-1-specific Stealth small interfering RNA (siRNA) or with a control siRNA oligo (medium GC; Invitrogen) and seeded in six-well culture plates in duplicate at the density of 1 × 106 cells per well in 2 ml complete medium. Cells were treated with or without doxycycline (0.25 μg/ml) for 24 h and harvested 48 h later for total RNA isolation and real-time quantitative RT-PCR. Each experiment was repeated three times.
Statistical analyses
Data were analyzed and compared with control values (mean of basal samples) using the Mann-Whitney rank sum test with the SigmaStat 3.0 software package (SPSS, Chicago, IL). Results were considered significantly different when P value was ≤0.05.
Results
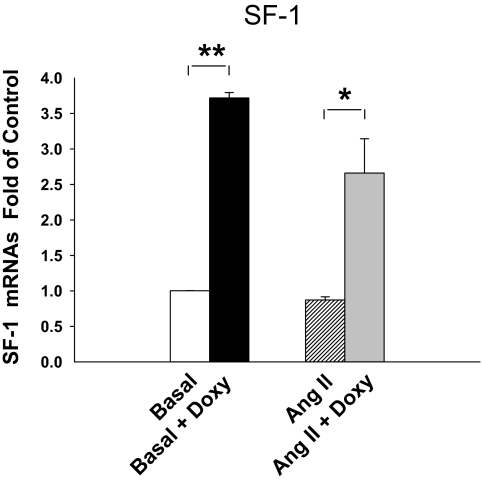
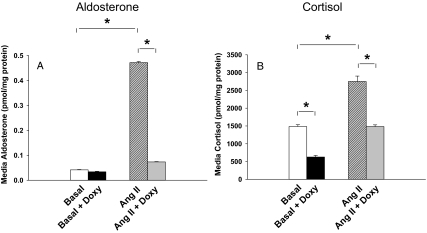
As a model to define the role of SF-1 in adrenal cell differentiation, we used the H295R/TR/SF-1 cell line, which increased expression of SF-1 in a tetracycline/doxycycline-dependent manner. The H295R/TR/SF-1 cell originated from the human adrenocortical H295R cell, which has been widely used to study aldosterone, cortisol, and adrenal androgen production (21,26,27,28). Double transfection of tet repressor and SF-1 coding sequence made this model increase expression of SF-1 after treatment with doxycycline. Doxycycline significantly increased SF-1 mRNA levels in both the basal and Ang II treatment group H295R/TR/SF-1 (3.1- to 3.7-fold, respectively) (Fig. 1). Doxycycline treatment had no effect on the parental H295R/TR cell expression of SF-1 (data not shown). In H295R/TR/SF-1 cells not treated with doxycycline, Ang II stimulated aldosterone production by 11.3-fold (P < 0.01) (Fig. 2A). However, doxycycline induction of SF-1 expression inhibited Ang II-stimulated aldosterone production by 84% (P < 0.01) (Fig. 2A). We next examined effects of doxycycline on cortisol biosynthesis. Without doxycycline treatment, Ang II increased cortisol production by 1.9-fold (P < 0.01) (Fig. 2B). Doxycycline treatment decreased both basal (58%, P < 0.01) and Ang II-stimulated (46%, P < 0.01) cortisol levels (Fig. 2B).
Figure 1.
Doxycycline (Doxy) regulation of SF-1 mRNA level in the H295R/TR/SF-1 cell line. SF-1 mRNA expression was normalized by basal SF-1 mRNA levels in the H295R/TR/SF-1 cell line with and without Ang II (10 nm) or doxycycline (0.25 μg/ml) treatment. The cells were treated with or without doxycycline for 72 h and then with or without Ang II for 24 h. Real-time quantitative RT-PCR was used to measure SF-1 mRNA expression. Values represent the mean ± se from three independent experiments each run in triplicate. *, P < 0.05; **, P < 0.01.
Figure 2.
Effects of elevated SF-1 on aldosterone and cortisol production. H295R/TR/SF-1 cells were incubated with doxycycline (Doxy; 0.25 μg/ml) for 72 h. Cells were then treated with or without Ang II (10 nm) for 24 h. Aldosterone (A) and cortisol (B) levels were then measured in the experimental medium. Values represent the mean ± se from three independent experiments each ran in triplicate. *, P < 0.01.
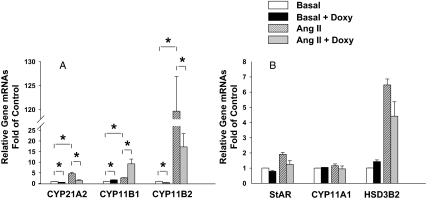
To better define the mechanisms of SF-1 action. we examined expression of the mRNAs encoding steroidogenic enzymes. Ang II treatment increased CYP11B2 mRNA levels by 116-fold (P < 0.01) (Fig. 3A). Doxycycline treatment decreased both basal CYP11B2 (63%) (P < 0.01) and Ang II-stimulated CYP11B2 (86%) (P < 0.01) levels (Fig. 3A). The action of doxycycline appeared related to induction of SF-1, because doxycycline treatment of the parental H295R/TR cell did not influence Ang II induction of CYP11B2 mRNA (data not shown).
Figure 3.
SF-1 effects on steroidogenic enzyme mRNA expression. H295R/TR/SF-1 cells were treated with doxycycline (Doxy; 0.25 μg/ml) for 72 h followed by treatment with or without Ang II (10 nm) for 24 h. Real-time quantitative RT-PCR was used to measure CYP11B1 (A), CYP11B2 (A), CYP21 (A), CYP11A1 (B), HSD3B2 (B), and StAR (B) mRNA expression. Values represent the mean ± se from three independent experiments each run in triplicate. *, P < 0.05; **, P < 0.01.
To determine whether the SF-1 inhibitory effects on CYP11B2 mRNA expression were selective, we examined expression of its isozyme, CYP11B1. Without doxycycline treatment, Ang II stimulated CYP11B1 mRNA levels by 2.6-fold (P < 0.01) (Fig. 3A). As opposed to the inhibitory effects of doxycycline on CYP11B2, doxycycline treatment increased basal cell expression of CYP11B1 mRNA by 1.7-fold (P < 0.01) and increased Ang II-induction of CYP11B1 by 3.6-fold (P < 0.01) (Fig. 3A).
To better define the mechanism of SF-1 repression of cortisol production, we examined all enzymes involved in cortisol biosynthesis. Among these genes, only CYP21 mRNA was inhibited by doxycycline. CYP21 mRNA was increased by 4.5-fold (P < 0.01) after Ang II treatment (Fig. 3A). The mRNA levels of StAR, CYP11A1, and HSD3B2 were not affected by SF-1 overexpression (Fig. 3B). However, doxycycline treatment decreased CYP21 mRNA by 46% (P < 0.01) in basal cells and decreased Ang II induction of CYP21 by 69% (P < 0.01) (Fig. 3A).
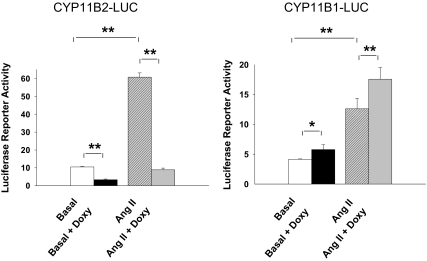
To determine the molecular mechanism leading to an increase in CYP11B1 and inhibition of CYP11B2, we used promoter/reporter constructs to examine gene transcription in the H295R/TR/SF-1 cells. Without doxycycline treatment, Ang II treatment stimulated CYP11B2 reporter activity by 5.8-fold (P < 0.01) (Fig. 4A). Doxycycline-induced SF-1 expression decreased basal CYP11B2 promoter activity by 68% (P < 0.01) and Ang II-stimulated CYP11B2 reporter activity by 85% (P < 0.01) (Fig. 4A). Ang II increased basal CYP11B1 reporter activity by 3-fold (P < 0.01) (Fig. 4B). However, unlike the effects of doxycycline on CYP11B2, doxycycline increased CYP11B1 reporter activity by 1.6-fold (P < 0.05) and Ang II-stimulated CYP11B1 reporter activity by 1.8-fold (P < 0.01) (Fig. 4B).
Figure 4.
Effects of elevated SF-1 on CYP11B1 or CYP11B2 promoter activity H295R/TR/SF-1 cells were incubated with doxycycline (Doxy; 0.25 μg/ml) for 72 h. The cells were then transfected with luciferase reporter vectors containing the 5′-flanking region of CYP11B1 and CYP11B2 gene. After recovery, cells were treated with Ang II (10 nm) for 6 h followed by measurement of luciferase reporter activity. Values represent the mean ± se from three independent experiments each run in triplicate. *, P < 0.05; **, P < 0.01.
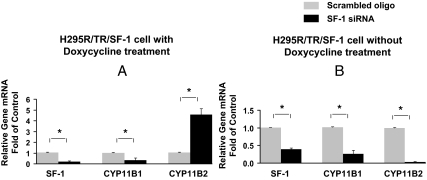
To better evaluate the role of low vs. high SF-1 expression on adrenal cell differentiation, we used SF-1-specific siRNA to silence its expression. We compared its effects on the expression of CYP11B1 and CYP11B2 in H295R/TR/SF-1 cells. The SF-1-specific siRNA significantly reduced SF-1 mRNA as compared with cells treated with control siRNA (Fig. 5). In doxycycline-treated cells, introduction of siRNA for SF-1 significantly decreased CYP11B1 but increased CYP11B2 (Fig. 5A). However, in H295R/TR/SF-1 cells without doxycycline treatment, both CYP11B1 and CYP11B2 mRNAs were decreased along with decreased SF-1 expression (Fig. 5B). These data suggest that a threshold level of SF-1 is needed to allow expression of steroidogenic enzymes including CYP11B2 but that an elevation of SF-1 leads to differential activation of some genes (e.g. CYP11B1) and repression of others (e.g. CYP11B2).
Figure 5.
Effects of SF-1 siRNA on SF-1, CYP11B1, and CYP11B2 expression. Both endogenous and transgene SF-1 expression were silenced in H295R/TR/SF-1 cells by RNA interference using Nucleofector technology. Cells were transfected with 10 nm SF-1-specific Stealth siRNA or with a control siRNA oligo (scrambled oligo) and allowed to recover for 48 h. During the final 24 h, cells were treated with or without doxycycline (0.25 μg/ml) and then harvested for total RNA isolation and real-time quantitative RT-PCR. Data points represent the mean ± se from three independent experiment. *, P < 0.01.
Discussion
The zone-specific expression of CYP11B2 and CYP11B1 are responsible for the adrenal glomerulosa production of aldosterone and fasciculata expression of cortisol. However, the molecular mechanisms that cause the differential expression of these two enzymes remain poorly defined. Herein, data are presented that suggest that SF-1 may play an important role in regulating the ability of adrenal cells to produce aldosterone and maintain CYP11B2 expression.
Five years ago, Bassett et al. (19) found that cotransfection of vectors encoding SF-1 increases CYP11B1 but inhibits CYP11B2 promoter activity. Using this approach, we demonstrated that SF-1 inhibited basal CYP11B2 promoter activity as well as activation by Ang II and the nuclear hormone receptor NURR1 (29). However, these studies focused on the transient transfection of SF-1 and its effects on CYP11B2 and CYP11B1 reporter constructs. Due to the low transfection efficiency of the H295R adrenal cell model, the effects of SF-1 on the expression of endogenous CYP11B2/CYP11B1 or the production of steroid hormones were not examined.
To better define the role of SF-1 in adrenal cell differentiation, Chung and colleagues (30) cloned populations of H295R cells that expressed a truncated SF-1 transgene without the ligand binding or AF2 domain. These cells had impaired steroidogenesis, suggesting wide effects of the mutant SF-1, particularly on basal expression levels of steroidogenic enzymes. However, it should be noted that this approach required clonal expansion of H295R cells with the SF-1 transgene. The experimental comparisons were then made with the wild-type H295R original cell population. Because the H295R cells are known to modify differentiation with time in culture, there is concern that the cloning procedure itself might have been responsible for the decreased steroidogenesis. In addition, no studies were conducted to define the effects of the intact SF-1 transgene on steroidogenesis or gene expression.
The approach in the current study was based on a doxycycline-regulated SF-1 expression system (termed H295R/TR/SF-1). The advantage of this approach was that the H295R/TR/SF-1 cell model acted as the control cell population. Thus, any alterations in cell expression of steroidogenic enzymes or steroid production that occurred as a result of cell cloning were not confused with effects on SF-1 action. This cell line enabled us to investigate SF-1 effects on endogenous steroidogenic gene expression levels and thereby extended the previous study (19). Moreover, we also compared the influence of SF-1 on steroid production through its effect on steroidogenic genes. In agreement with our previous transient transfection study, we found that elevation of SF-1 stimulated endogenous mRNA levels for CYP11B1 but inhibited CYP11B2 (19).
Another advantage of the H295R/TR/SF-1 cell model was the ability to examine effects on steroid hormone production. The H295R cell produces both aldosterone and cortisol both of which can be increased by treatment with Ang II (31). The ability of Ang II to stimulate cortisol has also been seen in freshly isolated human adrenal cells (32,33). Enhanced expression of SF-1 led to a suppression of basal and Ang II-stimulated aldosterone production. Interestingly, elevated CYP11B1 was not associated with increased cortisol production; rather, basal and Ang II-stimulated cortisol levels were decreased. Our experiments suggest that these results can be explained by the suppression of CYP21, which is critical for both aldosterone and cortisol production and in agreement with a previous report (21). It is likely that the combined inhibitory effects of SF-1 on both CYP21 and CYP11B2 explains the more potent inhibition of aldosterone production. It is worth noting that SF-1 did not inhibit StAR or CYP11A1 transcript levels, which helps explain how SF-1 elevation actually raised production of adrenal androgens (21). Taken together, these data suggest that SF-1 has differing roles in regulating the enzymes needed for aldosterone, cortisol, and adrenal androgen production.
An important issue that remains to be defined is the mechanism by which SF-1 activity could be independently regulated between the adrenal zones. There are several potential mechanisms that could regulate SF-1 activity within the adrenal gland. SF-1 activity could be influenced by its protein expression level. There is, however no evidence that SF-1 expression varies between the zones of the adrenal gland (34). Alternatively, there are several mechanisms for the posttranslational regulation of SF-1 activity that could regulate zonal activity of SF-1. First, there is considerable evidence that SF-1 is inhibited by the orphan nuclear receptor DAX-1 (NR0B1). DAX-1 inhibits the ability of SF-1 to stimulate transcription of several steroidogenic enzymes (35,36,37). In addition, DAX-1 is expressed in a zonal manner in mouse adrenal gland with levels in the glomerulosa much higher than seen in the fasciculata (38,39,40). The zonal expression of DAX-1, however, does not occur in the human adrenal gland (41). SF-1 can also be regulated by its state of sumoylation or phosphorylation (42,43). Due to the difficulty associated with isolation of the zones of the adrenal gland, no studies have examined the relative state of SF-1 phosphorylation or sumoylation within the zones. However, we have previously shown that ubc9 and PIAS1, key enzymes involved in sumoylation, are higher in the zona glomerulosa, suggesting that this mechanism could play a role within the adrenal gland (44). Taken together, these studies suggest that the posttranslational regulation of SF-1 is likely to play an important role in determining its activity, and the role of these regulatory mechanisms in adrenal zonation warrants further studies.
To obtain a better understanding of the role of SF-1 in adrenal cell differentiation, we conducted siRNA experiments to silence endogenous and transgene SF-1 expression. It is interesting that SF-1 had a biphasic role in the regulation of CYP11B2 expression. If endogenous levels of SF-1 were silenced in the H295R/TR/SF-1 cell model, there was a repression of both CYP11B1 and CYP11B2 as well as the other major steroidogenic enzymes including CYP11A1, HSD3B2, CYP21, CYP17, and StAR (data not shown). In contrast, damped elevation of transgene SF-1 mRNA in H295R/TR/SF-1 cells (after doxycycline treatment) inhibited CYP11B1 but increased CYP11B2 expression. This phenomenon suggests that relative expression (or transcriptional activity) of SF-1 in adrenal cells may play an important role in defining its steroidogenic phenotype. It is well established that SF-1 expression and transcriptional activity are both subject to regulatory control. Thus, it could be proposed that the cellular level of SF-1 may act as a key regulatory site where there is selective activation of some genes and repression of others.
Taken together, these data further support the concept that SF-1 can be a selective regulator of adrenocortical steroidogenic enzymes. The question arises as to alternative transcription factors that regulate genes such as CYP21 and CYP11B2. We and others have demonstrated a role for the NGFI-B family in the regulation of adrenal cell expression of CYP11B2 (45), HSD3B2 (28), and CYP21 (45). In addition, we have shown a zonal expression pattern for NGFI-B and NURR1 (29,46). NGFI-B is higher in the glomerulosa and fasciculata although almost absent from the zona reticularis. Because this factor acts as a potent regulator of HSD3B2 expression, it appears to play an important role in its expression in the outer zones and loss of expression within the reticularis (45,46). A similar role has been suggested for NURR1, which is higher in the glomerulosa where it likely plays a role in the expression of target genes that include CYP11B2.
In summary, the selective production of steroids from the three zones of the human adrenal gland relates to the zonal expression of key steroidogenic enzymes. The reasons or causes for zonal expression of the enzymes are not well defined. Herein, we demonstrate that elevation of SF-1 blocks the expression of CYP11B2 and aldosterone production. The potential for differential regulation of SF-1 activity within the adrenal gland may be one of the mechanisms controlling the zone-specific expression of the steroidogenic enzymes.
Acknowledgments
We thank Rebecca Key for editorial assistance.
Footnotes
This work was supported by a grant from the National Institutes of Health (DK43140 and DK069950 to W.E.R.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online October 30, 2008
Abbreviations: Ang II, Angiotensin II; Ct, cycle threshold; SF-1, steroidogenic factor-1; siRNA, small interfering RNA.
References
- Parker KL, Rice DA, Lala DS, Ikeda Y, Luo X, Wong M, Bakke M, Zhao L, Frigeri C, Hanley NA, Stallings N, Schimmer BP 2002 Steroidogenic factor 1: an essential mediator of endocrine development. Recent Prog Horm Res 57:19–36 [DOI] [PubMed] [Google Scholar]
- Val P, Lefrancois-Martinez AM, Veyssiere G, Martinez A 2003 SF-1 a key player in the development and differentiation of steroidogenic tissues. Nucl Recept 1:8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer GD, Ingraham HA 1999 Steroidogenic factor-1: its role in endocrine organ development and differentiation. Front Neuroendocrinol 20:199–223 [DOI] [PubMed] [Google Scholar]
- Luo X, Ikeda Y, Parker KL 1994 A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481–490 [DOI] [PubMed] [Google Scholar]
- Sadovsky Y, Crawford PA, Woodson KG, Polish JA, Clements MA, Tourtellotte LM, Simburger K, Milbrandt J 1995 Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc Natl Acad Sci USA 92:10939–10943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler B, Lin L, Ferraz-de-Souza B, Wieacker P, Heidemann P, Schroder V, Biebermann H, Schnabel D, Gruters A, Achermann JC 2008 Five novel mutations in steroidogenic factor 1 (SF1, NR5A1) in 46,XY patients with severe underandrogenization but without adrenal insufficiency. Hum Mutat 29:59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa RV, Domenice S, Bingham NC, Billerbeck AE, Rainey WE, Parker KL, Mendonca BB 2004 A microdeletion in the ligand binding domain of human steroidogenic factor 1 causes XY sex reversal without adrenal insufficiency. J Clin Endocrinol Metab 89:1767–1772 [DOI] [PubMed] [Google Scholar]
- Cartier D, Jegou S, Parmentier F, Lihrmann I, Louiset E, Kuhn JM, Bastard C, Plouin PF, Godin M, Vaudry H, Lefebvre H 2005 Expression profile of serotonin4 (5-HT4) receptors in adrenocortical aldosterone-producing adenomas. Eur J Endocrinol 153:939–947 [DOI] [PubMed] [Google Scholar]
- Lala DS, Rice DA, Parker KL 1992 Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol Endocrinol 6:1249–1258 [DOI] [PubMed] [Google Scholar]
- Morohashi K, Honda S, Inomata Y, Handa H, Omura T 1992 A common trans-acting factor, Ad4-binding protein, to the promoters of steroidogenic P-450s. J Biol Chem 267:17913–17919 [PubMed] [Google Scholar]
- Ikeda Y, Shen WH, Ingraham HA, Parker KL 1994 Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol 8:654–662 [DOI] [PubMed] [Google Scholar]
- Zhang P, Mellon SH 1996 The orphan nuclear receptor steroidogenic factor-1 regulates the cyclic adenosine 3′,5′-monophosphate-mediated transcriptional activation of rat cytochrome P450c17 (17α-hydroxylase/c17-20 lyase). Mol Endocrinol 10:147–158 [DOI] [PubMed] [Google Scholar]
- Burris TP, Guo W, Le T, McCabe ER 1995 Identification of a putative steroidogenic factor-1 response element in the DAX-1 promoter. Biochem Biophys Res Commun 214:576–581 [DOI] [PubMed] [Google Scholar]
- Watanabe K, Clarke TR, Lane AH, Wang X, Donahoe PK 2000 Endogenous expression of Mullerian inhibiting substance in early postnatal rat Sertoli cells requires multiple steroidogenic factor-1 and GATA-4-binding sites. Proc Natl Acad Sci USA 97:1624–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Schimmer BP 1995 Transcriptional regulation of the genes encoding the cytochrome P-450 steroid hydroxylases. Vitam Horm 51:339–370 [DOI] [PubMed] [Google Scholar]
- Newton R, Holden NS 2007 Separating transrepression and transactivation: a distressing divorce for the glucocorticoid receptor? Mol Pharmacol 72:799–809 [DOI] [PubMed] [Google Scholar]
- Cvoro A, Tatomer D, Tee MK, Zogovic T, Harris HA, Leitman DC 2008 Selective estrogen receptor-β agonists repress transcription of proinflammatory genes. J Immunol 180:630–636 [DOI] [PubMed] [Google Scholar]
- Straus DS, Glass CK 2007 Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol 28:551–558 [DOI] [PubMed] [Google Scholar]
- Bassett MH, Zhang Y, Clyne C, White PC, Rainey WE 2002 Differential regulation of aldosterone synthase and 11β-hydroxylase transcription by steroidogenic factor-1. J Mol Endocrinol 28:125–135 [DOI] [PubMed] [Google Scholar]
- Wang XL, Bassett M, Zhang Y, Yin S, Clyne C, White PC, Rainey WE 2000 Transcriptional regulation of human 11β-hydroxylase (hCYP11B1). Endocrinology 141:3587–3594 [DOI] [PubMed] [Google Scholar]
- Doghman M, Karpova T, Rodrigues GA, Arhatte M, De Moura J, Cavalli LR, Virolle V, Barbry P, Zambetti GP, Figueiredo BC, Heckert LL, Lalli E 2007 Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol Endocrinol 21:2968–2987 [DOI] [PubMed] [Google Scholar]
- Bassett MH, Zhang Y, White PC, Rainey WE 2000 Regulation of human CYP11B2 and CYP11B1: comparing the role of the common CRE/Ad1 element. Endocr Res 26:941–951 [DOI] [PubMed] [Google Scholar]
- Pezzi V, Mathis JM, Rainey WE, Carr BR 2003 Profiling transcript levels for steroidogenic enzymes in fetal tissues. J Steroid Biochem Mol Biol 87:181–189 [DOI] [PubMed] [Google Scholar]
- Bassett MH, Mayhew B, Rehman K, White PC, Mantero F, Arnaldi G, Stewart PM, Bujalska I, Rainey WE 2005 Expression profiles for steroidogenic enzymes in adrenocortical disease. J Clin Endocrinol Metab 90:5446–5455 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Saner-Amigh K, Mayhew BA, Mantero F, Schiavi F, White PC, Rao CV, Rainey WE 2006 Elevated expression of luteinizing hormone receptor in aldosterone-producing adenomas. J Clin Endocrinol Metab 91:1136–1142 [DOI] [PubMed] [Google Scholar]
- Shibata H, Kobayashi S, Kurihara I, Suda N, Yokota K, Murai A, Ikeda Y, Saito I, Rainey WE, Saruta T 2004 COUP-TF and transcriptional co-regulators in adrenal steroidogenesis. Endocr Res 30:795–801 [DOI] [PubMed] [Google Scholar]
- Bassett MH, Suzuki T, Sasano H, De Vries CJ, Jimenez PT, Carr BR, Rainey WE 2004 The orphan nuclear receptor NGFIB regulates transcription of 3β-hydroxysteroid dehydrogenase. Implications for the control of adrenal functional zonation. J Biol Chem 279:37622–37630 [DOI] [PubMed] [Google Scholar]
- Bassett MH, Suzuki T, Sasano H, White PC, Rainey WE 2004 The orphan nuclear receptors NURR1 and NGFIB regulate adrenal aldosterone production. Mol Endocrinol 18:279–290 [DOI] [PubMed] [Google Scholar]
- Li LA, Chang YC, Wang CJ, Tsai FY, Jong SB, Chung BC 2004 Steroidogenic factor 1 differentially regulates basal and inducible steroidogenic gene expression and steroid synthesis in human adrenocortical H295R cells. J Steroid Biochem Mol Biol 91:11–20 [DOI] [PubMed] [Google Scholar]
- Rainey WE, Saner K, Schimmer BP 2004 Adrenocortical cell lines. Mol Cell Endocrinol 228:23–38 [DOI] [PubMed] [Google Scholar]
- Lebrethon MC, Jaillard C, Defayes G, Begeot M, Saez JM 1994 Human cultured adrenal fasciculata-reticularis cells are targets for angiotensin-II: effects on cytochrome P450 cholesterol side-chain cleavage, cytochrome P450 17α-hydroxylase, and 3β-hydroxysteroid-dehydrogenase messenger ribonucleic acid and proteins and on steroidogenic responsiveness to corticotropin and angiotensin-II. J Clin Endocrinol Metab 78:1212–1219 [DOI] [PubMed] [Google Scholar]
- Ouali R, LeBrethon MC, Saez JM 1993 Identification and characterization of angiotensin-II receptor subtypes in cultured bovine and human adrenal fasciculata cells and PC12W cells. Endocrinology 133:2766–2772 [DOI] [PubMed] [Google Scholar]
- Ortlepp JR, Hanrath P, Mevissen V, Kiel G, Borggrefe M, Hoffmann R 2002 Variants of the CYP11B2 gene predict response to therapy with candesartan. Eur J Pharmacol 445:151–152 [DOI] [PubMed] [Google Scholar]
- Lalli E, Melner MH, Stocco DM, Sassone-Corsi P 1998 DAX-1 blocks steroid production at multiple levels. Endocrinology 139:4237–4243 [DOI] [PubMed] [Google Scholar]
- Ragazzon B, Lefrancois-Martinez AM, Val P, Sahut-Barnola I, Tournaire C, Chambon C, Gachancard-Bouya JL, Begue RJ, Veyssiere G, Martinez A 2006 Adrenocorticotropin-dependent changes in SF-1/DAX-1 ratio influence steroidogenic genes expression in a novel model of glucocorticoid-producing adrenocortical cell lines derived from targeted tumorigenesis. Endocrinology 147:1805–1818 [DOI] [PubMed] [Google Scholar]
- Nakamoto M, Wang DS, Suzuki A, Matsuda M, Nagahama Y, Shibata N 2007 Dax1 suppresses P450arom expression in medaka ovarian follicles. Mol Reprod Dev 74:1239–1246 [DOI] [PubMed] [Google Scholar]
- Mukai T, Kusaka M, Kawabe K, Goto K, Nawata H, Fujieda K, Morohashi K 2002 Sexually dimorphic expression of Dax-1 in the adrenal cortex. Genes Cells 7:717–729 [DOI] [PubMed] [Google Scholar]
- Bae DS, Schaefer ML, Partan BW, Muglia L 1996 Characterization of the mouse DAX-1 gene reveals evolutionary conservation of a unique amino-terminal motif and widespread expression in mouse tissue. Endocrinology 137:3921–3927 [DOI] [PubMed] [Google Scholar]
- Bastida CM, Cremades A, Castells MT, Lopez-Contreras AJ, Lopez-Garcia C, Sanchez-Mas J, Penafiel R 2007 Sexual dimorphism of ornithine decarboxylase in the mouse adrenal: influence of polyamine deprivation on catecholamine and corticoid levels. Am J Physiol Endocrinol Metab 292:E1010–E1017 [DOI] [PubMed] [Google Scholar]
- Shibata H, Ikeda Y, Mukai T, Morohashi K, Kurihara I, Ando T, Suzuki T, Kobayashi S, Murai M, Saito I, Saruta T 2001 Expression profiles of COUP-TF, DAX-1, and SF-1 in the human adrenal gland and adrenocortical tumors: possible implications in steroidogenesis. Mol Genet Metab 74:206–216 [DOI] [PubMed] [Google Scholar]
- Hammer GD, Krylova I, Zhang Y, Darimont BD, Simpson K, Weigel NL, Ingraham HA 1999 Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: integration of hormone signaling in reproduction and stress. Mol Cell 3:521–526 [DOI] [PubMed] [Google Scholar]
- Komatsu T, Mizusaki H, Mukai T, Ogawa H, Baba D, Shirakawa M, Hatakeyama S, Nakayama KI, Yamamoto H, Kikuchi A, Morohashi K 2004 Small ubiquitin-like modifier 1 (SUMO-1) modification of the synergy control motif of Ad4 binding protein/steroidogenic factor 1 (Ad4BP/SF-1) regulates synergistic transcription between Ad4BP/SF-1 and Sox9. Mol Endocrinol 18:2451–2462 [DOI] [PubMed] [Google Scholar]
- Kurihara I, Shibata H, Kobayashi S, Suda N, Ikeda Y, Yokota K, Murai A, Saito I, Rainey WE, Saruta T 2005 Ubc9 and protein inhibitor of activated STAT 1 activate chicken ovalbumin upstream promoter-transcription factor I-mediated human CYP11B2 gene transcription. J Biol Chem 280:6721–6730 [DOI] [PubMed] [Google Scholar]
- Bassett MH, White PC, Rainey WE 2004 A role for the NGFI-B family in adrenal zonation and adrenocortical disease. Endocr Res 30:567–574 [DOI] [PubMed] [Google Scholar]
- Lu L, Suzuki T, Yoshikawa Y, Murakami O, Miki Y, Moriya T, Bassett MH, Rainey WE, Hayashi Y, Sasano H 2004 Nur-related factor 1 and nerve growth factor-induced clone B in human adrenal cortex and its disorders. J Clin Endocrinol Metab 89:4113–4118 [DOI] [PubMed] [Google Scholar]