Abstract
Sheep exposed to testosterone (T) during early to midgestation exhibit reproductive defects that include hypergonadotropism, functional hyperandrogenism, polycystic ovaries, and anovulatory infertility, perturbations similar to those observed in women with polycystic ovary syndrome. Obesity increases the severity of the phenotype in women with polycystic ovary syndrome. To determine whether prepubertal weight gain would exaggerate the reproductive disruptions in prenatal T-treated sheep, pregnant sheep were injected with 100 mg T propionate (∼1.2 mg/kg) im twice weekly, from d 30–90 of gestation. Beginning about 14 wk after birth, a subset of control and prenatal T-treated females were overfed to increase body weight to 25% above that of controls. Twice-weekly progesterone measurements found no differences in timing of puberty, but overfed prenatal T-treated females stopped cycling earlier. Detailed characterization of periovulatory hormonal dynamics after estrous synchronization with prostaglandin F2α found 100% of controls, 71% of overfed controls, 43% of prenatal T-treated, and 14% of overfed prenatal T-treated females had definable LH surges. Only one of seven overfed prenatal T-treated female vs. 100% of control, 100% of overfed control, and seven of eight prenatal T-treated females exhibited a luteal progesterone increase. Assessment of LH pulse characteristics during the anestrous season found both overfeeding and prenatal T excess increased LH pulse frequency without an interaction between these two variables. These findings agree with the increased prevalence of anovulation observed in obese women with polycystic ovary syndrome and indicate that excess postnatal weight gain amplifies reproductive disruptions caused by prenatal T excess.
Exposure of sheep to excess testosterone in utero disrupts reproductive cyclicity, with postpubertal excess weight gain amplifying the severity of this adult reproductive phenotype.
Polycystic ovarian syndrome (PCOS) is the most common cause of anovulatory infertility in women (1,2) and also is emerging as a risk factor for diabetes and heart disease in women (3,4,5). Because affected women manifest central adiposity, hypertension, hyperlipidemia, and pancreatic β-cell dysfunction, PCOS also is inextricably related to the metabolic syndrome (6). The onset of PCOS becomes evident during adolescence, with affected girls manifesting hirsutism, oligo- or amenorrhea, and hyperinsulinemia from insulin resistance (7,8). In 2003, the Rotterdam consensus defined PCOS to include at least two of the following three features: 1) clinical or biochemical hyperandrogenism, 2) oligo-anovulation, and 3) polycystic ovaries excluding other endocrinopathies (9). Categorizing PCOS by these different phenotypes has clarified that women with hyperandrogenism and oligo-anovulation have the most severe PCOS phenotype based upon the degree of hyperandrogenism and insulin resistance (10), with obesity exaggerating the severity of the PCOS phenotype (11,12).
Although peripubertal onset and familial clustering suggest a heritable etiology for PCOS (13,14), emerging data implicate a role for excess steroid exposure during fetal and/or prepubertal life in the developmental origin of PCOS (15,16). The prevalence of PCOS in women with classical congenital adrenal hyperplasia and with congenital adrenal virilizing tumors (17,18) provide additional support that the intrauterine steroid milieu may be a contributing factor. Several animal models have documented the ability of experimentally-induced prenatal testosterone (T) excess to produce a permanent PCOS-like phenotype, with prenatal T-treated monkeys and sheep, like PCOS patients, manifesting anovulatory infertility, compensatory hyperinsulinemia, LH excess, neuroendocrine feedback defects, functional hyperandrogenism, polycystic ovaries, and insulin resistance (reviewed in Ref. 19).
In addition to recapitulating many features of the PCOS phenotype, the fundamental neuroendocrine mechanisms regulating cyclicity and the patterns of ovarian follicular growth and selection are similar between sheep and humans (20,21). The duration of the follicular phase is shorter (∼2 d) in sheep, although the neuroendocrine sequence leading up to the gonadotropin surge is similar to that of humans. As in humans and other mammals, elevated levels of progesterone (P4) characterize the 2-wk luteal phase of sheep. With P4 plummeting during transition to the follicular phase, the rising concentrations of estradiol (E2) culminate in a preovulatory E2 rise leading to the generation of the LH surge, which is then followed by ovulation 24 h later. Unlike humans, sheep are seasonal breeders having reproductive cycles for 6 months each year; the rest of the time, they remain acyclic due to heightened seasonal sensitivity to E2 negative feedback (21).
Achieving puberty at the normal age of about 28 wk, prenatal T-treated sheep manifest neuroendocrine and reproductive cycle disruptions during both prepubertal and postpubertal life (19,22,23). Prenatal T-treated sheep show progressive reproductive dysfunction, with some becoming anovulatory in the first year of life (24,25,26). There is also considerable variability in estrous cycles in those females that cycle, ranging from normal intervals to oligo-anovulation (24,25,26). These differences in reproductive function, combined with genetic variability, implicate epigenetic changes during prepubertal and postnatal life in the ontogeny of the PCOS phenotype, mediated through interactions between genes and their environment and ultimately causing hereditary modifications independent of changes in the nucleotide sequence. Because obesity-related ovulatory dysfunction in PCOS women (11,12) might also occur in prenatal T-treated female sheep with a similar PCOS-like phenotype, we investigated whether excess weight gain during postnatal life amplifies the reproductive disruptions previously established in our prenatal T-treated sheep model of PCOS, thereby increasing the prevalence of anovulation.
Materials and Methods
Breeding, prenatal treatment, and maintenance
Details of breeder ewe maintenance, breeding, and lambing have been described in detail elsewhere (25,26). Briefly, T treatment consisted of twice-weekly injections of 100 mg T propionate (∼1.2 mg/kg; Sigma-Aldrich Corp., St. Louis, MO) in cottonseed oil (2 ml) from d 30–90 of gestation (term = 147 d). Mean (±sem) weights of control and T-injected females just before breeding were 78.0 ± 1.7 and 80.8 ± 1.7 kg, respectively. Body condition score, based on the level of muscle and fat deposition around the vertebrae in the loin area for the control and T-injected females were 2.9 ± 0.1 and 3.0 ± 0.1, respectively, based on the scale of 1, emaciated; 2, thin; 3, average; 4, fat; and 5, obese. The concentrations of T achieved in maternal circulation and fetal blood are in the range noted in adult males and male fetuses, respectively (27) (Padmanabhan, V., and D. A. Abbott, unpublished data). Control sheep did not receive vehicle because no differences have been observed in cycle characteristics between offspring of control ewes that did or did not receive vehicle (26).
At about 2 months of age, all lambs resulting from this breeding (17 control and 24 prenatal T-treated) were weaned and maintained outdoors at the Sheep Research Facility (Ann Arbor, MI; 42°, 18′N). Before weaning, subsets of control and prenatal T-treated lambs were assigned, based on date of birth (lambing occurred within a 32-d period), to either the control (n = 6), prenatal T-treated (T, n = 8), overfed control (OFC, n = 7), or overfed prenatal T-treated (OFT, n = 7) treatment groups (the remaining four control and nine T animals were used in other studies). Twin females were divided between the regular-fed and overfed groups so that only one lamb from each dam was included in a given treatment group. Lambs were weighed 24 h after birth, weekly for 39 wk, and less frequently (every 2–4 wk) thereafter. All procedures were approved by the University Animal Care and Use Committee at the University of Michigan.
Diet
All feed provided for these studies were purchased in bulk and dispensed between the regular-fed and overfed females to prevent potential confounding effects from differences in feeds. Starting 6 wk before the expected date of delivery and continuing until the time of delivery, pregnant ewes were group-fed 0.5 kg shelled corn, 2 kg alfalfa hay, and 250 mg aureomycin crumbles (chlortetracycline) per ewe per day. Lactating ewes were provided a ration of 1 kg shelled corn and 2–2.5 kg of alfalfa hay per ewe per day. Until 3 months of age, all lambs had ad libitum access to commercial feed pellets (Shur-Gain, Elma, NY; contains 18% crude protein) and alfalfa hay. All lambs and ewes were provided with water and minerals ad libitum and were treated regularly with anthelminthics to minimize parasitic infection. Beginning at 14 wk of age, females assigned to the overfed group were maintained and group-fed separately. The daily diet of the regular-fed (normal weight gain) females, on a per lamb basis, consisted of 1.4 lb corn, 1.4 lb hay, and 0.03 lb supplement (36% crude protein). The daily diet of overfed females consisted of 1.7 lb corn, 0.03 lb supplement, and 1.6 lb hay initially and then ad libitum. The diet for the regular-fed females was designed to achieve optimal growth without excess fat deposition, whereas the weight gain of overfed females was designed to achieve a body weight about 25% above that of normal weight gain females. To compensate for increased energy demands as the lambs grew and to meet additional energy demands during inclement weather, the ration was increased in regular-fed and overfed females by the same percentage.
Effect of excess weight gain on cycle dynamics
Twice-weekly blood samples were obtained during the first breeding season (July to May) for measurement of P4 to determine onset of puberty and duration and end of the first breeding season.
Effect of excess weight gain on periovulatory dynamics
For detailed characterization of cycle dynamics, six control, seven OFC, eight T, and seven OFT females were included. After achieving puberty midway through the first breeding season, circulating patterns of gonadotropins, E2, and luteal P4 concentrations were characterized after synchronization of estrus with two 20-mg injections of prostaglandin F2α (PGF2α, Lutalyse; Pfizer Animal Health, Kalamazoo, MI) administered 11 d apart. Starting immediately after the second PGF2α injection, blood samples were obtained every 2 h for 120 h to characterize the preovulatory changes in LH, FSH, and E2 patterns. This was followed by collection of daily blood samples for 19 d for measurement of P4 to confirm the presence or absence of functional corpora lutea.
Effect of excessive weight gain on seasonal sensitivity to E2 negative feedback
To determine whether overfeeding (overweight) amplifies the disruptive effects of prenatal T on LH responsiveness to E2 negative feedback, blood samples were obtained every 20 min for 12 h during the anestrous season after the first breeding season, at which time sheep are naturally sensitive to E2 negative feedback.
RIAs
A well-validated assay (28) was used to measure circulating concentrations of LH. Sensitivity of the LH assay averaged 0.5 ± 0.1 ng/ml. The intraassay coefficients of variations (CV), based on four quality control pools measuring 3.4 ± 0.1, 7.5 ± 0.2, 12.9 ± 0.3, and 22.9 ± 0.6 ng/ml, averaged 6.9, 5.4, 6.5, and 4.0%, respectively. Interassay CV based on the same quality control pools were 6.1, 7.3, 4.8, and 6.2%, respectively. Circulating concentrations of FSH were measured in duplicate using a validated RIA (29). Sensitivity of the FSH assay averaged 0.6 ± 0.1 ng/ml. The intraassay CV based on two quality control pools measuring 3.8 ± 0.2 and 12.0 ± 0.4 ng/ml were 7.4 and 8.5%, respectively. Interassay CV based on the same two quality control pools were 10.3 and 13.4%, respectively. Circulating concentrations of E2 were measured in duplicate (300 μl plasma) using a validated assay first developed by Butcher et al. (30) and modified by Tortonese et al. (31). Assay sensitivity averaged 0.8 ± 0.1 pg/ml (mean ± sem, n = 14 assays). Intraassay CV based on two quality control pools measuring 1.6 ± 0. 1 and 4.5 ± 0.2 pg/ml averaged 10.5 ± 1.3 and 11.9 ± 1.9%, respectively. The interassay CV for the same quality control pools were 17.6 and 9.3%, respectively. Plasma concentrations of P4 were measured in duplicate using a validated (32) commercial RIA kit (Coat-A-Count P4; Siemens Medical Solutions/Diagnostic, Los Angeles, CA). Sensitivity of this assay was 0.06 ± 0.02 ng/ml. The intraassay CV based on two quality control pools measuring 1.6 ± 0.03 and 14.3 ± 0.2 ng/ml were 8.0 ± 1.3 and 3.8 ± 1.2%, respectively. The interassay CV for the same quality control pools were 9.3 and 5.4%, respectively.
Statistical analyses
Weight data collected before 14 wk (when animals assigned to regular and overfed groups received similar ration) and after 14 wk (when the overfed group was provided increased ration) were analyzed separately. A linear random-effect mixed model was used to capture the weight growth curves and test differences among groups (control vs. T groups before 14 wk and control, OFC, T, and OFT groups after 14 wk), assuming that the growth curve of each group followed a linear or quadratic function. Random effects were modeled to account for correlations between weight measurements from the same animal. Time of puberty, duration and end of the first breeding season, as well as peak serum P4 levels and cycle duration of the progestogenic cycles were analyzed by ANOVA.
All proportional data (i.e. percentages of animals responding to PGF2α and having an LH surge) were compared using Fisher’s exact test. For determining cycle attributes, all values below assay sensitivity were assigned the detection limit of the assay. LH- and primary FSH-surge baselines were determined by averaging the lowest point during the upslope of the primary surge and the preceding nine and five points, respectively. To determine the onset of primary LH/FSH surges, the following criteria were applied: 1) increase of circulating LH and FSH baseline by two times assay sensitivity and 2) peak concentration of LH/FSH exceeding at least twice the baseline concentrations of circulating LH/FSH. The end of the surge was defined as the time when peripheral LH/FSH levels fell below the established criteria for surge onset. Duration of luteal P4 increase was defined as the time after PGF2α administration when daily P4 levels fell and then rose above 0.5 ng/ml and subsequently decreased below less than 0.5 ng/ml. Time relationships, such as interval between PGF2α to peak E2, to primary LH/ FSH surge (onset and peak), and to secondary FSH surge (onset and time to peak); interval between time of peak E2 and LH/FSH surges; and interval between primary and secondary FSH surges, peak E2 achieved, and gonadotropin surge characteristics (peak height, total, and duration) were analyzed using preestablished comparisons of means (control vs. OFC, T vs. OFT, and OFC vs. OFT) with post hoc tests.
LH data from infrequent blood samples collected during anestrus was subjected to pulse analysis using the Cluster algorithm (33). The Cluster algorithm identifies pulses using criteria that define a pulse such that the peak of the pulse differs significantly from both the preceding and following nadirs according to two-sample t tests. For analysis with Cluster, the minimum number of data points in a peak and nadir were set at 2 and 2, respectively. The t-statistic values used to identify a significant increase from preceding nadir and a decrease to following nadir were both 2.0. The amplitude was calculated as the difference between the pulse peak value and preceding nadir value. All Cluster-identified peaks with LH increases greater than two times the assay sensitivity from the preceding nadir were considered as pulses for further statistical analyses. LH pulse characteristics (mean and frequency) were analyzed by ANOVA. For all other LH pulse attributes (interpulse interval, amplitude, and pulse width), variance analysis of repeated measures was used to test the effect of treatment, obesity, and the interaction between the two factors. Appropriate transformations (square root for frequency, log for all else) to account for heterogeneity of variance were applied before analyses. All analyses were carried out using SAS for Windows release 9.1.3 (SAS Institute Inc., Cary, NC).
Results
Growth rates
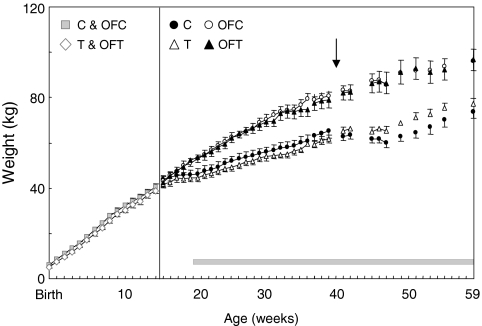
The growth curves of the T-treated and control animals before wk 14 are both linear and are parallel to each other. From birth to wk 14, on average, the control animals were slightly heavier (approximately 1.3 kg, P = 0.043) than the T-treated animals. This difference did not change through wk 14 (P = 0.95). (Fig. 1). Weights of control and T-treated females at 14 wk were similar, with T females catching up to the weight of controls (P = 0.96). The growth curves after 14 wk for all four groups (control, OFC, T, and OFT) followed quadratic functions (P < 0.0001). The growth curves of the regular and overfed females differed (P < 0.001), but the prenatal T-treated and control groups within each weight gain group did not (P = 0.95). The overfed group gained weight faster than the regular fed group (overfed, 2.5 kg/wk; regular, 1.0 kg/wk; P < 0.0001). The rate of weight gain slowed in all animals as they aged, slowing sooner in regular vs. overfed animals (P < 0.0001) but becoming similar by 39 wk (both groups gaining at ∼0.5 kg/wk, P = 0.80). At 39 wk of age, the mean weight of overfed animals was 18 kg heavier than regular-fed animals (P < 0.0001), representing an approximately 30% increase over the body weight of regular-fed animals.
Figure 1.
Changes in body weight (kilograms) for control (C), OFC, T, and OFT females from birth to 59 wk of age. Beginning at 14 wk of age, a subset of control and T animals were overfed (OFC and OFT). Weights were taken at weekly intervals up to 39 wk and at 2- to 4-wk intervals thereafter. The vertical black line indicates when overfeeding began. The horizontal gray bar indicates the period of twice-weekly P4 sampling. The arrow indicates the time when a detailed cycle characterization was undertaken.
Puberty and reproductive cyclicity
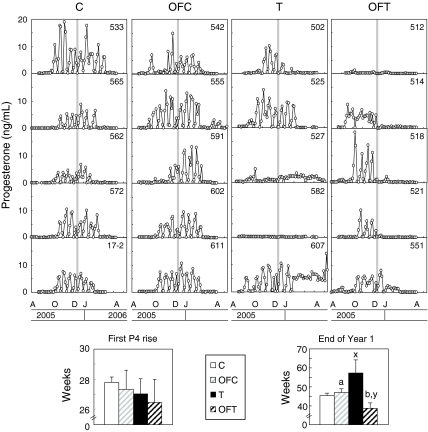
Figure 2 (top panel) shows progestogenic cycles of five control, five OFC, five T, and five OFT females during the first breeding season. There were no differences in the age of puberty (first P4 rise; Fig 2, bottom left) among the four groups. During the first year of reproductive life, cycles ceased earlier (P < 0.05) in OFT (December 10 ± 21.8 d) than OFC (February 23 ± 11.4 d) and also between OFT and T (April 25 ± 44.4 d). Cycles ceased on February 4 ± 5.9 d for controls, which was later than the OFT group, thereby shortening the duration (defined by the start and end of P4 cycles) of the first breeding season in OFT females. Mean peak P4 concentrations tended to be lower (P = 0.09) in OFT (5.1 ± 0.9 ng/ml) than OFC (7.2 ± 0.7 ng/ml) or control (7.1 ± 0.8 ng/ml) but similar to that of T females (5.0 ± 1.4 ng/ml).
Figure 2.
Plasma P4 profiles, first P4 rise (age at puberty; mean ± sem), and end of the first breeding season for control (C), OFC, T, and OFT females. Biweekly P4 levels were measured starting a few weeks before the expected time of puberty and ending a few weeks after the expected end of the breeding season. The vertical gray line indicates when sampling began during the periovulatory dynamics assessment. Bar graphs show first P4 rise (puberty) and end of breeding season. Bars with different letters (a vs. b and x vs. y) differed; P < 0.05.
Cycle dynamics
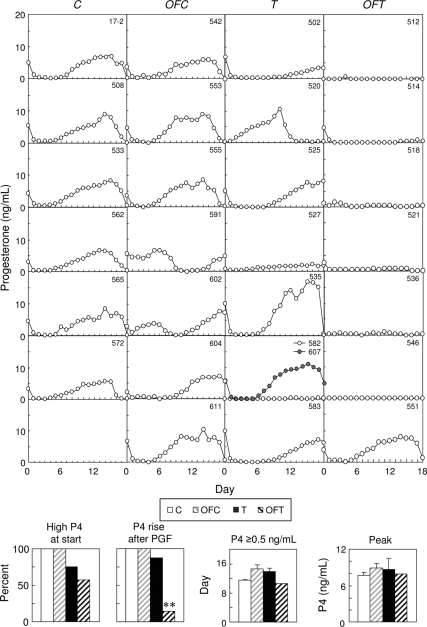
Elevated P4 levels were present in all control females at the time of the second PGF2α injection (Fig. 3, first point), with serum P4 levels dropping to less than 0.5 ng/ml (Fig. 3, second point) and confirming luteolysis. Although P4 concentrations were also high in all OFC females at the time of the second PGF2α injection, only five of the seven females showed a decrease in serum P4 concentrations to less than 0.5 ng/ml. Six of the eight T females and four of seven OFT also had elevated P4, which decreased appropriately.
Figure 3.
Circulating patterns of daily P4 in control (C), OFC, T, and OFT females after induction of luteolyis with PGF2α. Bar graphs show percentage of females with high P4 levels at the second PGF2α injection, percentage of females that exhibited a rise in P4 after PGF2α, duration of luteal P4 secretion, and mean peak levels of P4. Asterisks indicate significant treatment differences: *, P < 0.05; **, P < 0.01.
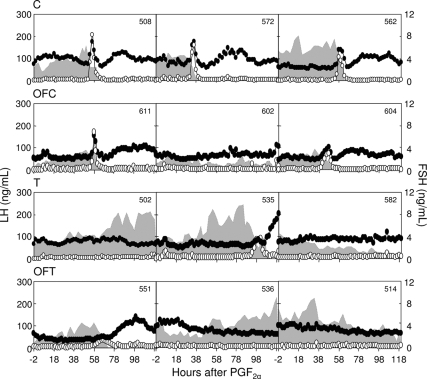
Circulating patterns of LH, FSH, and E2 after the second PGF2α injection from three control, three OFC, three T, and three OFT females are shown in Fig 4. As expected, all control and five of seven OFC females manifested expected changes in periovulatory hormonal dynamics (preovulatory E2 rise, presence of primary gonadotropin surges, and secondary FSH surge) after estrous synchronization with PGF2α. In contrast, T and OFT females showed varying periovulatory disruptions, with only three T and one OFT having LH surges, on the basis of criteria defined above (summary data are shown in Table 1). Comparison of control and OFC groups found no differences in the timing or magnitude of the preovulatory E2 rise or the LH/FSH surge dynamics (Table 1). On the other hand, T females that had definable LH surges showed a delayed E2 rise (P < 0.05) and delayed LH surges (P = 0.009). Only one OFT female had an LH surge, which was abnormal and lasted for 34 h (twice as long as control and OFC). There were no differences in secondary FSH surge dynamics.
Figure 4.
Circulating patterns of LH (white circles), FSH (black circles), and E2 (shaded areas) in control, OFC, T, and OFT females after induction of luteolyis with PGF2α.
Table 1.
Summary data showing timing relationships and hormonal dynamics of E2, LH, and FSH after induction of luteolysis with PGF2α for control, OFC, T, and OFT females
| Number of animals with LH surges
|
||||
|---|---|---|---|---|
| Control, n = 6 of 6 | OFC, n = 5 of 7 | T, n = 3 of 8 | OFT, n = 1 of 7 | |
| Timing relationships | ||||
| PGF2α to E2 peak (h) | 56.6 ± 6.6 | 48.8 ± 4.4 | 90.7 ± 13.1e | 48 |
| PGF2α to LH surge start (h) | 54.0 ± 6.3 | 49.2 ± 3.5 | 89.3 ± 13.4 | 54 |
| PGF2α to LH surge peak (h) | 59.0 ± 6.6 | 55.2 ± 3.5 | 98.0 ± 11.7f | 68 |
| E2 peak to LH surge peak (h) | 3.0 ± 0.7 | 6.4 ± 1.6 | 7.3 ± 5.5 | 20 |
| E2 peak to secondary FSH surge start (h) | 21.3 ± 1.7 | 22.8 ± 2.6 | 14 | 28 |
| LH surge peak to secondary FSH surge peak (h) | 18.0 ± 1.6 | 16.4 ± 2.1 | 10 | 8 |
| Hormonal dynamics | ||||
| E2 peak (pg/ml) | 7.3 ± 1.1 | 5.6 ± 1.1 | 10.9 ± 1.7 | 6.2 |
| LH surge peak (ng/ml) | 140.0 ± 17.2 | 132.3 ± 30.9 | 88.4 ± 38.5 | 34.7 |
| LH surge duration (h) | 15.3 ± 1.8 | 15.2 ± 1.5 | 18,16a | 34 |
| Primary FSH surge peak (ng/ml) | 6.2 ± 0.3 | 4.8 ± 0.5 | 3.4,4.7b | 2.0 |
| Primary FSH surge duration (h) | 9.3 ± 0.8 | 8.4 ± 0.4 | 10,12b | 10 |
| Secondary FSH surge peak (ng/ml) | 5.0 ± 0.4 | 4.0 ± 0.2 | 8.2c | 5.58 |
| Secondary FSH surge duration (h) | 38.0 ± 4.2 | 31.3 ± 3.7 | 34c | 44d |
One of the three surges occurred at 106 h, and hence LH surge duration could not be calculated.
Although an increase in FSH was evident corresponding to LH peak in the third animal, it did not meet criteria of surge definition.
Because the primary surge was very delayed, start and peak of secondary FSH could not be calculated.
Underestimated because secondary FSH was still high at end of sampling.
, P < 0.05;
, P < 0.01.
Luteal patterns of P4 during daily sampling are presented in Fig. 3. All control females had consistent luteal P4 increases during the 19-d sampling period. All OFC females also exhibited luteal P4 increases, despite one failing to synchronize (no. 591) and one having a short luteal phase (no. 602). All but one T female (no. 582) showed P4 increases, with two (nos. 502 and 527) having subluteal responses (below mean ± 2 sd of controls). In contrast, only one of the seven OFT females exhibited a P4 increase during this sampling period. Although there were no differences in percentage of females showing a P4 increase among control, OFC, and T females (Fig. 3, bottom), there was a significant reduction in the percentage of OFT animals showing a P4 increase (P < 0.05). There were no differences in peak P4 levels among the female groups.
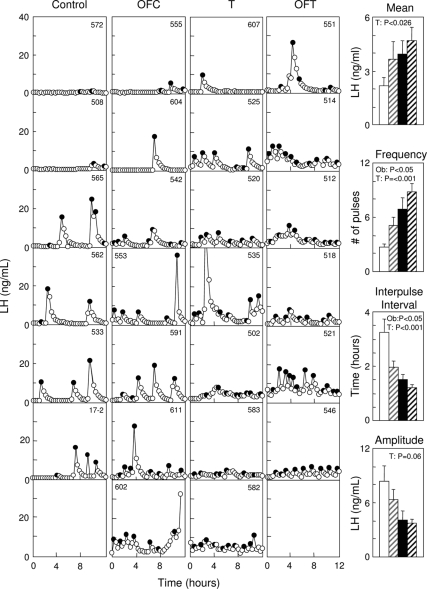
Effect of obesity on LH pulse dynamics
Circulating patterns of LH in control, OFC, T, and OFT females during the first anestrous season are shown in Fig. 5 (left panel), with summary statistics for pulse variables in bar graphs (right). On average, T females had about 68% higher mean circulating LH concentrations than untreated animals. Analyses of mean circulating LH levels over the 12-h sampling period found a significant prenatal T treatment effect (P = 0.026) without an effect of overfeeding (P = 0.14) or an interaction between prenatal treatment and overfeeding (P = 0.58). In terms of LH pulse frequency, on an average, the T females had approximately four more pulses than the untreated controls, whereas overfed animals had approximately two more pulses than the regular-fed animals. Analyses of LH pulse frequency revealed significant prenatal T treatment (P = 0.0002) and weight gain (P = 0.020) effects, without an interaction between these two variables (P = 0.80).
Figure 5.
LH pulse patterns during the nonbreeding season of control (C), OFC, T, and OFT females are shown on the left. Black circles depict LH pulses identified by the Cluster algorithm. Bar graphs show mean ± sem circulating levels of LH, LH pulse frequency (number of pulses/12 h), interpulse interval, and pulse amplitude (note one T and one OFT died before the anestrus study). White bar, control; gray striped bar, OFC; black bar, T; black striped bar, OFT. Ob, over-fed.
There was no significant interaction between overfeeding and prenatal T treatment for any pulse variables (pulse width, amplitude, or interpulse interval). The LH pulses of prenatal T-treated females had shorter pulse width (P = 0.011) and interpulse interval (P = 0.0005) vs. untreated controls. The overfed females also had shorter interpulse intervals compared with the normal-fed females (P = 0.015). The average interpulse interval for the prenatal T-treated and overfed animals were approximately 63 and 74%, respectively, of the average interpulse interval of corresponding controls.
Discussion
The overall objective of this study was to determine whether excess weight gain exaggerates the reproductive defects previously observed in prenatal T-treated sheep, a model that simulates the reproductive phenotype of women with PCOS (1,2,19,22,23,24,26). Its findings confirm such a hypothesis and are relevant to the effects of postnatal weight gain on pubertal onset, periovulatory hormonal changes, neuroendocrine feedback, severity of PCOS phenotype, and public healthcare issues as it relates to obesity and environmental exposures.
Puberty
It is well established in both humans and animals that body mass index plays a key role in timing of puberty, with higher body mass index associated with earlier pubertal onset (34,35) and increased adiposity in children contributing to early reproductive development in U.S. girls (36,37). Importantly, regulation of prepubertal gonadotropin secretion differs between sheep and primates. On one hand, primate development is characterized by an extended juvenile period of steroid-independent hypogonadotropism (38), which in sheep is proposed to occur during prenatal life (39,40). On the other hand, gonadotropin secretion during postnatal life in prepubertal sheep is steroid dependent (and therefore subject to inhibition by ovarian steroids), whereas in monkeys, a similar period of sensitivity to steroids occurs between menarche (outward sign of puberty) and first ovulation, typically occurring several months later. Irrespective of these differences in timing of steroid suppression of gonadotropins, our findings that overweight sheep entered puberty at the same time as controls should be interpreted with caution because maintaining sheep under a natural photoperiod enables one to detect pubertal delay, rather than its advancement, because this species depends on photoperiodic cues for pubertal onset (41).
Periovulatory hormonal changes
With an increasing prevalence of obese reproductive-aged women with infertility (42,43), it is crucial to prospectively investigate the biological mechanisms by which obesity impairs fecundity. One postulate is that obesity disrupts the regulatory mechanisms governing the hypothalamo-pituitary-gonadal axis (44,45,46). Our findings show that postnatal obesity alone has no impact on the characteristics of periovulatory gonadotropin surges in these relatively young animals, although obesity may impair fecundity in other ways. Most notably, being overweight has an adverse effect on human reproduction that is independent of PCOS, with increasing body mass index in women undergoing assisted reproduction decreasing their fecundity in a linear manner, presumably from altered endometrial receptivity (47). Equally important, our findings also show that postnatal obesity obliterated LH surges in 86% (six of seven) of OFT females vs. 38% (three of eight) of T females, thereby reducing luteal P4 rises to only 14% (one of seven) of OFT vs. 75% (six of eight) of T females (with other T females exhibiting a luteal P4 rise without a defined LH surge, perhaps from a subtle LH increase during or after the 120-h sampling interval). These additional data suggest that postnatal excess weight gain worsens the deleterious effects of prenatal T on cyclicity, corresponding with early cessation of the breeding season in the former females.
The biological basis for this early cessation of cycles in OFT females is unclear. The mechanisms underlying the early cessation in OFT females likely differs from what normally occurs with transition to anestrus, which is the result of reduction in LH pulse frequency from increased sensitivity to E2 negative feedback (48). The finding that both overfeeding and prenatal T excess increases LH pulse frequency suggests that the mechanisms responsible for early cessation of cyclicity in OFT but not OFC or T females involve other factors. One possibility is that postnatal amplification of adiposity-dependent insulin resistance and prenatal T excess may predispose such females to ovulatory dysfunction, establishing an association between hyperinsulinemia and the mechanisms of hyperandrogenic anovulation, as postulated from studies in both prenatal T-treated adult female rhesus monkeys (49) and PCOS women (10). We have previously shown prenatal T excess leads to insulin resistance (50), whereas other studies have found overfeeding to induce obesity leads also to insulin resistance in sheep (51). The overfed animals used in this study also manifest hyperinsulinemia (Padmanabhan, V., unpublished).
Neuroendocrine response
In female sheep, like other seasonal breeders, the seasonal onset and cessation of the estrous cycle represents altered E2 negative feedback (48). Physiological concentrations of circulating E2 markedly suppress LH pulse frequency only during the nonbreeding season, thus reducing gonadotropin stimulation and ovarian cyclicity (52). Consequently, changes in LH pulse frequency during the nonbreeding season serve as surrogates of hypothalamic sensitivity to E2 negative feedback. These data demonstrate that exposure to excess T in utero resulted in a significant increase in LH pulse frequency during the anestrous season, an effect also observed in overfed females. The presence of increased pulse frequency is more in line with reduced hypothalamic responsiveness to E2 feedback, a phenomenon proven to exist in T-treated females but not yet established in overfed females. Although it is theoretically possible that increased LH pulse frequency in overfed females resulted from decreased circulating E2 levels, estrogen as well as androgen production generally is enhanced in the presence of increased adiposity (53,54,55) so that the hypothalamus is more likely to be exposed to increased, rather than decreased, circulating estrogen levels.
Because obesity (51,56) and prenatal T excess (50,57) promote insulin resistance, insulin, as an important regulator of hypothalamic GnRH release in mammals, could cross the blood-brain barrier via active transport or passively via the circumventricular region (58,59,60) to bind to its own receptors in the brain (61) and enhance hypothalamic GnRH pulse frequency. In support of this, neuron-specific knockout of insulin receptor in mice causes a 10–15% increase in body weight while decreasing fertility (62).
Relevance to PCOS
Like the prenatally T-treated sheep of this study, prenatal T-treated monkeys (49) and PCOS women (10) have a propensity toward ovulatory dysfunction in the presence of increased adiposity. Specifically, anovulatory PCOS patients have a greater body mass index than their ovulatory sisters, despite both siblings having ovarian hyperandrogenism (63), whereas weight loss in obese PCOS patients induces ovulation and reverses anovulatory infertility (64,65). As a putative mechanism for neuroendocrine dysfunction in PCOS women, hyperandrogenism from LH hypersecretion and/or hyperinsulinemia from adiposity-dependent insulin resistance may stimulate sufficient androgen excess to reduce hypothalamic sensitivity to steroid negative feedback on LH (66,67). In support of this, reduced hypothalamic sensitivity to steroid negative feedback in PCOS women is restored by the administration of flutamide, an androgen receptor blocker (68). Our findings in young OFT sheep also support this hypothesis because independent, stimulatory effects of T treatment and excess weight gain enhance LH pulsatility, agreeing with increased LH pulsatility in both PCOS girls (69,70) and obese normoandrogenic adolescents (71).
Conversely, an inverse relationship between LH and body mass index in adult PCOS women appears to occur via a pituitary, rather than a hypothalamic, mechanism (72) and is further enhanced by increased clearance of LH from changes in its isoform composition (73). It is possible that neuroendocrine disruption changes over time as PCOS women age, particularly if the onset of excess weight gain relative to organization of neural networks regulating hypothalamic steroid negative feedback is important. In other words, if neuroendocrine development is incomplete before puberty, then excess weight gain during prepubertal life (as in this study) might perturb neuronal organization, causing reduced steroid feedback on LH secretion, a phenomenon that does not occur in later life as neuronal plasticity abates. Alternatively, a shift from the positive relationship between LH and leptin levels to a negative one as overweight PCOS patients become obese (74) raises the possibility that overweight prenatal T-treated sheep with increased LH pulsatility have not yet acquired sufficient body weight to attenuate LH secretion through leptin resistance.
Public health impact
Regardless of mechanisms, the inappropriate programming of the reproductive system by fetal exposure to excess steroids and consequent impact on adult well-being is a major health concern. The female fetus may be exposed to several environmental steroids, particularly if its mother unknowingly conceives while using oral contraceptives, uses anabolic steroids during pregnancy, or handles environmental compounds with estrogenic (e.g. polychlorinated biphenyls) (75) or androgenic (e.g. effluents from paper mills, concentrated animal feeding operations, 11-ketotestosterone etc.) (76,77) activity. A major concern regarding such prenatal exposures to environmental steroids is that much of the reprogramming that occurs during fetal life may go unrecognized until adulthood (78). Even more importantly, clinical manifestation of such prenatal reprogramming may be profoundly influenced by the postnatal environment. The findings from our study agree with this premise and the recent proposal of a two-step process (79) whereby an early perturbation from in utero T treatment resets the reproductive trajectory, whereas a later-onset metabolic abnormality influences the severity of the adult reproductive phenotype. In this regard, the interplay between epigenetic and genetic interaction would undoubtedly be a key factor in determining individual susceptibility to a wide range of diseases. With its dramatically increasing prevalence over the last 30 yr, childhood obesity is now an epidemic (80,81), likely providing metabolic abnormalities that amplify preceding prenatal insults. Alarmingly, as more adolescents become obese, the severity of PCOS in young women may worsen (10,82), demanding improved understanding of developmental reprogramming of the reproductive axis and its susceptibility through postnatal environmental changes to long-term adult disease.
Acknowledgments
We are grateful to Douglas Doop for generation of experimental lambs, expert animal care, and facility management; Wen Ye for help with statistical analyses; and Mohan Manikkam, James S. Lee, Olga Astapova, and Pamela Olton for assistance with prenatal steroid treatment, animal experimentation, and/or performance of RIAs.
Footnotes
This work was supported by United States Public Health Service Grant P01-HD44232 to V.P.
Disclosure Summary: Authors have nothing to disclose.
First Published Online October 30, 2008
Abbreviations: CV, Coefficients of variation; E2, estradiol; OFC, overfed control; OFT, overfed prenatal T-treated; P4, progesterone; PCOS, polycystic ovarian syndrome; PGF2α, prostaglandin F2α; T, testosterone.
References
- Franks S 1995 Polycystic ovary syndrome. N Engl J Med [Erratum (1996) 334:668] 333:853–861 [DOI] [PubMed] [Google Scholar]
- Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R 1998 Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab 83:3078–3082 [DOI] [PubMed] [Google Scholar]
- Hoffman LK, Ehrmann DA 2008 Cardiometabolic features of polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab 4:215–222 [DOI] [PubMed] [Google Scholar]
- Kousta E, Franks S 2007 Long-term health consequences of polycystic ovary syndrome. Kovacs G, Norman R, eds. Polycystic ovary syndrome. Cambridge, UK: Cambridge University Press [Google Scholar]
- Cho LW, Atkin SL 2007 Cardiovascular risk in women with polycystic ovary syndrome. Minerva Endocrinol 32:263–273 [PubMed] [Google Scholar]
- Apridonidze T, Essah PA, Iuorno MJ, Nestler JE 2005 Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab 90:1929–1935 [DOI] [PubMed] [Google Scholar]
- Coviello AD, Legro RS, Dunaif A 2006 Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab 91:492–497 [DOI] [PubMed] [Google Scholar]
- Fruzzetti F, Perini D, Lazzarini V, Parrini D, Genazzani AR 13 August 2008 Adolescent girls with polycystic ovary syndrome showing different phenotypes have a different metabolic profile associated with increasing androgen levels. Fertil Steril 10.1016/j.fertnstert.2008.06.004 [DOI] [PubMed] [Google Scholar]
- The Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group 2004 Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19:41–47 [DOI] [PubMed] [Google Scholar]
- Welt CK, Gudmundsson JA, Arason G, Adams J, Palsdottir H, Gudlaugsdottir G, Ingadottir G, Crowley WF 2006 Characterizing discrete subsets of polycystic ovary syndrome as defined by the Rotterdam criteria: the impact of weight on phenotype and metabolic features. J Clin Endocrinol Metab 91:4842–4848 [DOI] [PubMed] [Google Scholar]
- Barber TM, McCarthy MI, Wass JA, Franks S 2006 Obesity and polycystic ovary syndrome. Clin Endocrinol (Oxf) 65:137–145 [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E 2007 Role of obesity and adiposity in polycystic ovary syndrome. Int J Obes (Lond) 31(Suppl 2):S8–S13; discussion S31–S32 [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Piperi C, Spina J, Argyrakopoulou G, Papanastasiou L, Bergiele A, Panidis D 2006 Polycystic ovary syndrome: the influence of environmental and genetic factors. Hormones (Athens) 5:17–34 [DOI] [PubMed] [Google Scholar]
- Crosignani PG, Nicolosi AE 2001 Polycystic ovarian disease: heritability and heterogeneity. Hum Reprod Update 7:3–7 [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Schramm RD, Abbott DH 2005 Early origins of polycystic ovary syndrome. Reprod Fertil Dev 17:349–360 [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Abbott DH, Padmanabhan V 2007 Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord 8:127–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes RB, Rosenfield R, Ehrmann DA, Cara JF, Cuttler L, Levitsky LL, Rosenthal IM 1994 Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders: evidence for perinatal masculinization of neuroendocrine function in women. J Clin Endocrinol Metab 79:1328–1333 [DOI] [PubMed] [Google Scholar]
- Stikkelbroeck NM, Hermus AR, Braat DD, Otten BJ 2003 Fertility in women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Obstet Gynecol Surv 58:275–284 [DOI] [PubMed] [Google Scholar]
- Abbott DH, Dumesic DA, Levine JE, Dunaif A, Padmanabhan V 2006 Animal models and fetal programming of PCOS. In: Azziz R, Nestler JE, Dewailly D, eds. Contemporary endocrinology: androgen excess disorders in women: polycystic ovary syndrome and other disorders. 2nd ed. Totowa, NJ: Humana Press; 259–272 [Google Scholar]
- McNeilly AS 1991 The ovarian follicle and fertility. J Steroid Biochem Mol Biol 40:29–33 [DOI] [PubMed] [Google Scholar]
- Goodman RL 1994 Neuroendocrine control of the ovine estrous cycle. In: Knobil E, Neill JD, eds. The physiology of reproduction. Vol 2, 2nd ed. New York: Raven Press; 659–709 [Google Scholar]
- Padmanabhan V, Manikkam M, Recabarren S, Foster D 2006 Prenatal testosterone excess programs reproductive and metabolic dysfunction in the female. Mol Cell Endocrinol 246:165–174 [DOI] [PubMed] [Google Scholar]
- Foster DL, Jackson LM, Padmanabhan V 2007 Novel concepts about normal sexual differentiation of reproductive neuroendocrine function and the developmental origins of female reproductive dysfunction: the sheep model. Soc Reprod Fertil Suppl 64:83–107 [DOI] [PubMed] [Google Scholar]
- Birch RA, Padmanabhan V, Foster DL, Unsworth WP, Robinson JE 2003 Prenatal programming of reproductive neuroendocrine function: fetal androgen exposure produces progressive disruption of reproductive cycles in sheep. Endocrinology 144:1426–1434 [DOI] [PubMed] [Google Scholar]
- Manikkam M, Steckler TL, Welch KB, Inskeep EK, Padmanabhan V 2006 Fetal programming: prenatal testosterone treatment leads to follicular persistence/luteal defects. Partial restoration of ovarian function by cyclic progesterone treatment. Endocrinology 147:1997–2007 [DOI] [PubMed] [Google Scholar]
- Veiga-Lopez A, Ye W, Phillips DJ, Herkimer C, Knight PG, Padmanabhan V 2008 Developmental programming: deficits in reproductive hormone dynamics and ovulatory outcomes in prenatal, testosterone-treated sheep. Biol Reprod 78:636–647 [DOI] [PubMed] [Google Scholar]
- Wood RI, Ebling FJ, I'Anson H, Bucholtz DC, Yellon SM, Foster DL 1991 Prenatal androgens time neuroendocrine sexual maturation. Endocrinology 128:2457–2468 [DOI] [PubMed] [Google Scholar]
- Niswender GD, Reichert LE, Midgley AR, Nalbandov AV 1969 Radioimmunoassay for bovine and ovine luteinizing hormone. Endocrinology 84:1166–1173 [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, McFadden K, Mauger DT, Karsch FJ, Midgley AR Jr 1997 Neuroendocrine control of follicle-stimulating hormone (FSH) secretion. I. Direct evidence for separate episodic and basal components of FSH secretion. Endocrinology 138:424–432. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW 1974 Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17β throughout the 4-day estrous cycle of the rat. Endocrinology 94:1704–1708 [DOI] [PubMed] [Google Scholar]
- Tortonese DJ, Lewis PE, Papkoff H, Inskeep EK 1990 Roles of the dominant follicle and the pattern of oestradiol in induction of preovulatory surges of LH and FSH in prepubertal heifers by pulsatile low doses of LH. J Reprod Fertil 90:127–135 [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Evans NP, Dahl GE, McFadden KL, Mauger DT, Karsch FJ 1995 Evidence for short or ultrashort loop negative feedback of GnRH secretion. Neuroendocrinology 62:248–258 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Johnson ML 1986 Cluster analysis: a simple versatile, and robust algorithm for endocrine pulse detection. Am J Physiol 250:E486–E493 [DOI] [PubMed] [Google Scholar]
- Frisch RE, McArthur JW 1974 Menstrual cycles: fatness as a determinant of minimum weight for height necessary for their maintenance and onset. Science 185:949–951 [DOI] [PubMed] [Google Scholar]
- Garn SM, Haskell JA 1959 Fat and growth during childhood. Science 130:1711–1712 [DOI] [PubMed] [Google Scholar]
- Kaplowitz PB, Slora EJ, Wasserman RC, Pedlow SE, Herman-Giddens ME 2001 Earlier onset of puberty in girls: relation to increased body mass index and race. Pediatrics 108:347–353 [DOI] [PubMed] [Google Scholar]
- Lee JM, Appugliese D, Kaciroti N, Corwyn RF, Bradley RH, Lumeng JC 2007 Weight status in young girls and the onset of puberty. Pediatrics [Erratum (2007) 120:251] 119:e624–e630 [DOI] [PubMed] [Google Scholar]
- Plant TM 1994 Puberty in primates. In: Knobil E, Neill JD, eds. The physiology of reproduction. Vol 2, 2nd ed. New York: Raven Press; 452–485 [Google Scholar]
- Foster DL 1994 Puberty in the sheep. In: Knobil E, Neill JD, eds. The physiology of reproduction. Vol 2, 2nd ed. New York: Raven Press; 411–451 [Google Scholar]
- Foster DL, Ebling FJP, Ryan KD, Yellon SM 1989 Mechanisms timing puberty: a comparative approach. In: Delemarre-van de Wall HA, Plant TM, Van Reees GP, Schoemaker J, eds. Control of the onset of puberty III. Amsterdam: Elsevier; 227–249 [Google Scholar]
- Foster DL, Ebling FJ, Claypool LE 1988 Timing of puberty by photoperiod. Reprod Nutr Dev 28:349–364 [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Johnson CL 2002 Prevalence and trends in obesity among US adults, 1999–2000. JAMA 288:1723–1727 [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Flegal KM 2003 Epidemiologic trends in overweight and obesity. Endocrinol Metab Clin North Am 32:741–760 [DOI] [PubMed] [Google Scholar]
- Pralong FP, Castillo E, Raposinho PD, Aubert ML, Gaillard RC 2002 Obesity and the reproductive axis. Ann Endocrinol 63:129–134 [PubMed] [Google Scholar]
- Pasquali R, Patton L, Gambineri A 2007 Obesity and infertility. Curr Opin Endocrinol Diabetes Obes 14:482–487 [DOI] [PubMed] [Google Scholar]
- Haslam DW, James WP 2005 Obesity. Lancet 366:1197–1209 [DOI] [PubMed] [Google Scholar]
- Wang JX, Davies M, Norman RJ 2000 Body mass and probability of pregnancy during assisted reproduction treatment: retrospective study. BMJ 321:1320–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsch FJ, Bittman EL, Foster DL, Goodman RL, Legan SJ, Robinson JE 1984 Neuroendocrine basis of seasonal reproduction. Recent Prog Horm Res 40:185–232 [DOI] [PubMed] [Google Scholar]
- Abbott DH, Dumesic DA, Eisner JR, Colman RJ, Kemnitz JW 1998 Insights into the development of PCOS from studies of prenatally androgenized female rhesus monkeys. Trends Endocrinol Metab 9:62–67 [DOI] [PubMed] [Google Scholar]
- Recabarren SE, Padmanabhan V, Codner E, Lobos A, Durán C, Vidal M, Foster DL, Sir-Petermann T 2005 Postnatal developmental consequences of altered insulin sensitivity in female sheep treated prenatally with testosterone. Am J Physiol Endocrinol Metab 289:E801–E806 [DOI] [PubMed] [Google Scholar]
- Bergman EN, Reulein SS, Corlett RE 1989 Effects of obesity on insulin sensitivity and responsiveness in sheep. Am J Physiol 257(5 Pt 1):E772–E781 [DOI] [PubMed] [Google Scholar]
- Karsch FJ, Dahl GE, Evans NP, Manning JM, Mayfield KP, Moenter SM, Foster DL 1993 Seasonal changes in gonadotropin-releasing hormone secretion in the ewe: alteration in response to the negative feedback action of estradiol. Biol Reprod 49:1377–1383 [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Matteri RL 1993 Obesity affects circulating estradiol levels of premenopausal women receiving leuprolide acetate depot. Int J Fertil 38:139–146 [PubMed] [Google Scholar]
- Zhang YW, Stern B, Rebar RW 1984 Endocrine comparison of obese menstruating and amenorrheic women. J Clin Endocrinol Metab 58:1077–1083 [DOI] [PubMed] [Google Scholar]
- Pasquali R 2006 Obesity and androgens: facts and perspectives. Fertil Steril 85:1319–1340 [DOI] [PubMed] [Google Scholar]
- Metwally M, Ledger WL, Li TC 2008 Reproductive endocrinology and clinical aspects of obesity in women. Ann NY Acad Sci 1127:140–146 [DOI] [PubMed] [Google Scholar]
- Eisner JR, Dumesic DA, Kemnitz JW, Abbott DH 2000 Timing of prenatal androgen excess determines differential impairment in insulin secretion and action in adult female rhesus monkeys. J Clin Endocrinol Metab 85:1206–1210 [DOI] [PubMed] [Google Scholar]
- Banks WA 2004 The source of cerebral insulin. Eur J Pharmacol 490:5–12 [DOI] [PubMed] [Google Scholar]
- Woods SC, Seeley RJ, Baskin DG, Schwartz MW 2003 Insulin and the blood-brain barrier. Curr Pharm Des 9:795–800 [DOI] [PubMed] [Google Scholar]
- Plata-Salamán CR 1991 Insulin in the cerebrospinal fluid. Neurosci Biobehav Rev 15:243–258 [DOI] [PubMed] [Google Scholar]
- Plum L, Schubert M, Brüning JC 2005 The role of insulin receptor signaling in the brain. Trends Endocrinol Metab 16:59–65 [DOI] [PubMed] [Google Scholar]
- Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR 2000 Role of brain insulin receptor in control of body weight and reproduction. Science 289:2122–2125 [DOI] [PubMed] [Google Scholar]
- Legro RS, Bentley-Lewis R, Driscoll D, Wang SC, Dunaif A 2002 Insulin resistance in the sisters of women with polycystic ovary syndrome: association with hyperandrogenemia rather than menstrual irregularity. J Clin Endocrinol Metab 87:2128–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AM, Thornley B, Tomlinson L, Galletley C, Norman RJ 1998 Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod 13:1502–1505 [DOI] [PubMed] [Google Scholar]
- Kiddy DS, Hamilton-Fairley D, Bush A, Short F, Anyaoku V, Reed MJ, Franks S 1992 Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 36:105–111 [DOI] [PubMed] [Google Scholar]
- Pastor CL, Griffin-Korf ML, Aloi JA, Evans WS, Marshall JC 1998 Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab 83:582–590 [DOI] [PubMed] [Google Scholar]
- Eagleson CA, Bellows AB, Hu K, Gingrich MB, Marshall JC 2003 Obese patients with polycystic ovary syndrome: evidence that metformin does not restore sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by ovarian steroids. J Clin Endocrinol Metab 88:5158–5162 [DOI] [PubMed] [Google Scholar]
- Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, Marshall JC 2000 Polycystic ovary syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab 85:4047–4052 [DOI] [PubMed] [Google Scholar]
- Marshall JC 2008 Obesity in adolescent girls: is excess androgen the real bad actor? J Clin Endocrinol Metab 91:393–395 [DOI] [PubMed] [Google Scholar]
- Marshall JC, Presidential plenary and clinical investigator award lecture: GnRH pulses—obesity, hyperandrogenemia, and the etiology of PCOS. Program of the 90th Annual Meeting of The Endocrine Society, San Francisco, CA, 2008 p 13 (Abstract L1-1) [Google Scholar]
- Yoo RY, Dewan A, Basu R, Newfield R, Gottschalk M, Chang RJ 2006 Increased luteinizing hormone pulse frequency in obese oligomenorrheic girls with no evidence of hyperandrogenism. Fertil Steril 85:1049–1056 [DOI] [PubMed] [Google Scholar]
- Pagán YL, Srouji SS, Jimenez Y, Emerson A, Gill S, Hall JE 2006 Inverse relationship between luteinizing hormone and body mass index in polycystic ovarian syndrome: investigation of hypothalamic and pituitary contributions. J Clin Endocrinol Metab 91:1309–1316 [DOI] [PubMed] [Google Scholar]
- Srouji SS, Pagán YL, D'Amato F, Dabela A, Jimenez Y, Supko JG, Hall JE 2007 Pharmacokinetic factors contribute to the inverse relationship between luteinizing hormone and body mass index in polycystic ovarian syndrome. J Clin Endocrinol Metab 92:1347–1352 [DOI] [PubMed] [Google Scholar]
- LaZovic G, Radivojevic U, Milicevic S, Spremovic S 2007 Influence of adiposity on leptin, LH and androgen levels in lean, overweight and obese PCOS patients. Int J Fertil Womens Med 52:82–88 [PubMed] [Google Scholar]
- Dickerson SM, Gore AC 2007 Estrogenic environmental endocrine-disrupting chemical effects on reproductive neuroendocrine function and dysfunction across the life cycle. Rev Endocr Metab Disord 8:143–159 [DOI] [PubMed] [Google Scholar]
- Gray LE Jr., Wilson VS, Stoker T, Lambright C, Furr J, Noriega N, Howdeshell K, Ankley GT, Guillette L 2006 Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals. Int J Androl 29:96–104 [DOI] [PubMed] [Google Scholar]
- Uzumcu M, Zachow R 2007 Developmental exposure to environmental endocrine disruptors: consequences within the ovary and on female reproductive function. Reprod Toxicol 23:337–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V 2007 Environment and origin of disease. Rev Endocr Metab Disord 8:67–69 [DOI] [PubMed] [Google Scholar]
- Tang WY, Newbold R, Mardilovich K, Jefferson W, Cheng RY, Medvedovic M, Ho SM 2008 Persistent hypomethylation in the promoter of nucleosomal binding protein 1 (Nsbp1) correlates with overexpression of Nsbp1 in mouse uteri neonatally exposed to diethylstilbestrol or genistein. Endocrinology 149:5922–5931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig DS 2007 Childhood obesity: the shape of things to come. N Engl J Med 357:2325–2327 [DOI] [PubMed] [Google Scholar]
- James WP 2008 The epidemiology of obesity: the size of the problem. J Intern Med 263:336–352 [DOI] [PubMed] [Google Scholar]
- Yildiz BO, Knochenhauer ES, Azziz R 2008 Impact of obesity on the risk for polycystic ovary syndrome. J Clin Endocrinol Metab 93:162–168 [DOI] [PMC free article] [PubMed] [Google Scholar]