Abstract
Although LH is essential for survival and function of the corpus luteum (CL) in higher primates, luteolysis occurs during nonfertile cycles without a discernible decrease in circulating LH levels. Using genome-wide expression analysis, several experiments were performed to examine the processes of luteolysis and rescue of luteal function in monkeys. Induced luteolysis with GnRH receptor antagonist (Cetrorelix) resulted in differential regulation of 3949 genes, whereas replacement with exogenous LH (Cetrorelix plus LH) led to regulation of 4434 genes (1563 down-regulation and 2871 up-regulation). A model system for prostaglandin (PG) F2α-induced luteolysis in the monkey was standardized and demonstrated that PGF2α regulated expression of 2290 genes in the CL. Analysis of the LH-regulated luteal transcriptome revealed that 120 genes were regulated in an antagonistic fashion by PGF2α. Based on the microarray data, 25 genes were selected for validation by real-time RT-PCR analysis, and expression of these genes was also examined in the CL throughout the luteal phase and from monkeys treated with human chorionic gonadotropin (hCG) to mimic early pregnancy. The results indicated changes in expression of genes favorable to PGF2α action during the late to very late luteal phase, and expressions of many of these genes were regulated in an opposite manner by exogenous hCG treatment. Collectively, the findings suggest that curtailment of expression of downstream LH-target genes possibly through PGF2α action on the CL is among the mechanisms underlying cross talk between the luteotropic and luteolytic signaling pathways that result in the cessation of luteal function, but hCG is likely to abrogate the PGF2α-responsive gene expression changes resulting in luteal rescue crucial for the maintenance of early pregnancy.
Results of genome-wide analyses suggest that curtailment of expression of LH target-genes through PGF2α action in corpus luteum involves cross talk between luteotropic and luteolytic signaling pathways.
Corpus luteum (CL), through secretion of progesterone (P4) as well as other hormones/ factors, is absolutely essential for the establishment and, in some species, maintenance of pregnancy in mammals (1,2). In higher primates, although the requirement of circulating LH levels for the development and maintenance of its function during nonfertile cycles is well established, it is rather intriguing that spontaneous luteolysis occurs despite the lack of dramatic changes in circulating LH levels toward the end of the luteal phase (3,4,5). In striking contrast the luteolytic process gets curtailed during early pregnancy by chorionic gonadotropin (CG), the pituitary LH analog, elaborated by the placental trophoblast (6). Although LH and CG bind the same G protein-coupled receptor and activate multiple signal transduction pathways in target tissues (7,8,9), the biochemical and molecular events that govern their tropic actions in the primate CL that is endowed with dual responsibilities viz., timely luteolysis and rescue, remain poorly understood. Recent studies provide compelling evidence for LH/CG-regulated expression of several genes being central to the control of structure and function of the CL (10,11). These and other studies have used indirect approaches such as inhibition of LH secretion to examine gene expression changes in the CL, however, whether LH regulates expression of genes in the CL has not been systematically examined.
The uterus clearly regulates the steroidogenic activity and life span of the CL in infra-primate mammals, but its role in altering luteal function has not been established in primates (12,13). In several nonprimate species, prostaglandin (PG) F2α originating from the uterus has been identified as a chief luteolysin (14,15,16,17). The downstream signaling mechanisms responsible for PGF2α-mediated inhibition of P4 production and cell death have been reported by several studies (18,19,20). An emerging concept supported by these studies is that PGF2α antagonizes the actions of luteotropic hormones. For example, in vitro studies have documented actions of PGF2α via the protein kinase (PK) C pathway to be responsible for antagonizing LH-stimulated P4 secretion in luteal cells (reviewed in Ref. 21). In rodents, PGF2α accomplishes its antisteroidogenic effects by targeting the expression of the same set of genes that are under the control of the luteotropic complex comprising LH and prolactin (PRL), but in an antagonistic fashion (22). Vast circumstantial evidence exists in the literature that supports the hypothesis that PGF2α of an intraovarian source might function as a luteolysin in primates (23,24,25,26,27). The primate ovary has synthesized significant amounts of PGF2α (25,28), but direct evidence for a role of PGF2α in the luteal regression comes from studies performed by Aksel et al. (29), who reported increased concentrations of PGF2α in the ovarian venous blood during the period coinciding with the onset of luteolysis. Moreover, administration of PGF2α or synthetic inhibitors and its analogs have been demonstrated to be luteolytic in humans (30) and monkeys both in pregnant and nonpregnant cycles (31,32). With the findings of the aforementioned studies and our own intuitive thinking about the participation of luteolytic factors in the primate CL regression, we hypothesize that PGF2α might be one of the factors that antagonizes actions of LH downstream of its receptor signaling during spontaneous luteolysis.
To define transcriptional targets of luteotropic and luteolytic factors in the monkey CL, we developed and/ or used a variety of model systems of luteolysis and rescue of luteal function to investigate systematically: 1) the requirement of tropic LH levels, using inhibition of LH secretion and exogenous LH replacement models, with a view to identify genes that are specific targets of LH at the genome level by microarray analysis; 2) a model system for PGF2α-induced luteolysis and determine transcriptome profiling with a view to compare the PGF2α-responsive transcriptome with that of LH-responsive transcriptome; and 3) expression patterns of genes identified as common targets of LH and PGF2α, during different stages of the CL and from the simulated early pregnancy model system. The results of these experiments provide new insight into the processes of luteolysis and rescue at the genome level in the monkey CL.
Materials and Methods
Reagents
The RNeasy mini-purification kit (no. 74104) and GeneChip Rhesus Macaque Genome Array (no. 900656) were procured from QIAGEN GmbH (Hilden, Germany) and Affymetrix, Inc. (Santa Clara, CA), respectively. Oligonucleotide primers were synthesized by Sigma-Genosys (Bangalore, India). DyNAzyme II DNA polymerase (no. F-501L) and DyNAmo HS SYBR Green qPCR kit (no. F410) were purchased from Finnzymes (Espoo, Finland). Restriction enzymes, Moloney murine leukemia virus reverse transcriptase (RT), and 100 bp DNA ladder were obtained from MBI Fermentas GmbH (St. Leon-Rot, Germany). GnRH-receptor (GnRH-R) antagonist [Cetrorelix (CET)] was a kind gift from Asta Medica (Frankfurt, Germany). Recombinant human LH (rhLH) was a gift from Ares Serono (Aubonne, Switzerland). The BioArray High Yield RNA Transcript Labeling kit was obtained from ENZO (Farmingdale, NY). Tiaprost Trometamol (Iliren), a synthetic analog of PGF2α, was procured from Intervet International B.V. (Boxmeer, Holland). The antisera to P4 (GDN no. 337) and estradiol (E2) (GDN no. 244) were kindly provided by Professor G.D. Niswender (Colorado State University, Fort Collins, CO). All other reagents were purchased from Sigma-Aldrich Corp. (St. Louis, MO) or sourced locally.
Animal protocols, blood samples, and CL collection
Experimental protocols in the monkeys described here were approved by the Institutional Animal Ethics Committee of the Indian Institute of Science. The general care and housing of monkeys at the Primate Research Laboratory, Indian Institute of Science, Bangalore, have been described elsewhere (33,34). Adult female bonnet monkeys (Macaca radiata) were monitored daily for the onset of menses, and blood samples through femoral venipuncture were collected daily from d 8–12 of the menstrual cycle for determining the day of onset of E2 surge. Further blood samples were collected either daily or at more frequent intervals until the time of CL retrieval or the onset of menses. In this study, 1 d after day of E2 peak was designated as d 1 of the luteal phase. The CL on the designated day of the luteal phase (see below) was retrieved from the ketamine hydrochloride [15 mg/kg body weight (BW)] and/or pentobarbital sodium (8 mg/kg BW)-anesthetized monkeys subjected to laparotomy under aseptic conditions.
Experiment 1: transcriptome changes during GnRH-R antagonist-induced luteolysis
To induce luteolysis, a single injection of CET at a dose of 150 μg/kg BW on d 7 of the luteal phase was used. For purposes of microarray and real-time RT-PCR analyses, CLs were collected 24 h after CET (150 μg/kg BW) or 5.25% glucose [control (VEH)] sc injections to female monkeys on d 7 of the luteal phase. Blood samples were collected before, and 12 and 24 h after treatments. Both VEH and CET treatments (n = 5 monkeys) were performed in the same monkeys, but on separate occasions, to minimize variation in the expressions (microarray and real-time RT-PCR analyses) observed between VEH and CET treatments. The extirpated CL was transferred to a sterile petri dish, cut into four to five pieces, placed in individual cryovials, and flash frozen in liquid nitrogen before storing at −70 C until analysis.
Experiment 2: effects of rhLH treatment on CL function in CET-treated monkeys and LH-mediated transcriptome changes
On d 7 of the luteal phase, six monkeys were treated with CET (150 μg/kg BW sc), and 24 h later, monkeys were further treated with either PBS (VEH, n = 3 monkeys) or rhLH 20 IU/kg BW (rhLH, n = 3) iv, and CLs were collected at the end of 8 h treatment. In addition, CLs were collected from monkeys (n = 3) that served as VEH for both CET and rhLH treatments, received 5.25% glucose (VEH) on d 7 of the luteal phase plus PBS iv, injection 24 h after VEH treatment. Blood samples were collected at different intervals after CET/VEH plus PBS or rhLH treatments. The CL tissues were processed for microarray and real-time RT-PCR analyses (see below).
Experiment 3: gene expression changes during PGF2α-induced luteolysis
Considering the reported refractoriness of early CL to luteolytic actions of PGF2α in many species (30,35,36,37) and the transient nature of actions of PGF2α after systemic infusion (38,39,40,41), an experimental regimen has been standardized in the laboratory in which injections of Iliren (59 μg/kg BW) administered on d 10 of the luteal phase induced luteolysis in monkeys (42). The dose and day of treatment were chosen based on a pilot study in which administration of doses of Iliren ranging from 10–30 μg/kg BW as well as treatment during d 5–9 of the luteal phase were largely ineffective in decreasing circulating P4 levels (42). In contrast, administration of 59 μg/kg BW im of Iliren on d 10 of the luteal phase caused a significant decrease in serum P4 levels, but the P4 levels rebounded within 12 h of the start of the treatment. In the present study, to ensure that P4 levels did not rebound, three injections of Iliren were administered beginning on d 10 of the luteal phase (0900, 1700, and 0100 h), and CL (n = 3) was collected on d 11 of the luteal phase, i.e. 24 h after initiation of treatment. Similarly, on a separate occasion, the same monkeys received PBS injections (three injections: 0900, 1700, and 0100 h) beginning on d 10 of the luteal phase, and CLs were collected on d 11 of the luteal phase to serve as VEH. Blood samples were collected at different intervals before and after treatment. The extirpated CL was transferred to a sterile petri dish, cut into four to five pieces, placed in individual Cryovials, and flash frozen in liquid nitrogen and stored at −70 C until analysis.
Experiment 4: gene expression changes during different stages of CL development and function
CLs (n = 3 per stage) were collected from monkeys at the early (d 5), mid (d 8), and late (d 14) luteal phase of the menstrual cycle as described previously (34). In addition, CL (n = 3) was collected from monkeys on d 1 menses, a time point when luteolytic events are considered to be initiated. Blood samples were collected on designated days, and on d 1 of menses to determine the steroidogenic status of CL during different stages of development and function. CLs retrieved during different stages of the luteal phase and on d 1 menses were used to compare the expression patterns of some of the selected genes that were differentially regulated after CET and PGF2α treatments, with a view to gain information on the molecular events that regulate CL structure and function during different stages of the luteal phase.
Experiment 5: gene expression changes during simulated early pregnancy
To compare the expression changes in genes identified to be regulated differentially during luteolysis (experiments 1 and 3) or LH rescue of CL function (experiment 2) with that of rescue of CL function that occurs after establishment of pregnancy, a model system that mimics early pregnancy in nonmated cycling monkeys was used (10). In the present study, monkeys were administered exogenous human chorionic gonadotropin (hCG) in incremental doses on d 9–13 of the luteal phase (15, 30, 45, 90, and 180 IU) twice daily im, 0900 and 1700 h, and CL was retrieved on d 14 of the luteal phase. For comparison, CL collected on d 14 of the luteal phase, but without treatment, was used as the untreated VEH. Blood samples were collected before and at different time intervals after initiation of hCG treatment until the end of the experiment.
RNA isolation
Total RNA was isolated from CL tissues harvested from different experiments using TRI Reagent (Ambion, Inc., Austin, TX), followed by a cleanup step using the RNeasy mini-purification kit, and RNA samples that gave A260/A280 approximately 1.8–1.9 in NanoDrop ND-1000 UV-VIS spectrophotometer (NanoDrop Products, Wilmington, DE) and RNA integrity number (RIN) more than eight in the Agilent Bioanalyzer 2100 (Agilent Technologies, Inc., Palo Alto, CA) were used for this study.
Affymetrix oligonucleotide microarray
For genome-wide analysis, the GeneChip Rhesus Macaque Genome Array, a 11-μm array chip design in which each gene or portion of a gene is represented by 11 or 16 oligonucleotides of 25-mer referred to as probe, was used. The gene chip contains 52,024 Macaca mulatta probe sets to monitor expressions of approximately 47,000 transcripts and three rhesus housekeeping/VEH transcripts viz., β-actin, elongation factor-1, and glyceraldehyde-3-phosphate dehydrogenase. The array includes high-quality VEHs for monitoring array hybridization, washing, and staining for reproducible results. In addition, 20× eukaryotic hybridization VEHs comprising biotinylated and fragmented cRNA of bio B, bio C, and bio D from Escherichia coli and cre from P1 bacteriophage were included in eukaryotic expression probe arrays. The chip also includes VEH oligo B2 to provide VEH and alignment signals for the array analysis. Eukaryotic poly A RNA VEHs containing lys, phe, thr, and dap spiked directly into RNA samples before target labeling, and their resultant signal intensities on gene chip arrays serve as indicators of target preparation and the labeling reaction efficiency, independent of RNA sample quality.
Affymetrix microarray protocol
Total RNA (5 μg) was labeled as described in the Affymetrix Gene Chip expression analysis technical manual. Briefly, cDNA was synthesized from total RNA in RT reaction using a T7-(dT)24primer. The purified double-stranded cDNA containing the T7 promoter sequence was used as a template for in vitro transcription-labeling assays in the presence of biotin-labeled ribonucleotides, using the BioArray High Yield RNA Transcript Labeling kit with T7 RNA polymerase as described by the manufacturer. The cRNA was further purified by RNeasy spin column and subjected to fragmentation in buffer containing 40 mm Tris (pH 8.1), 100 mm potassium acetate, and 30 mm magnesium acetate. After fragmentation, targeted cRNAs were combined with VEH oligomers (used for grid alignment during image processing) and VEH cRNA (for bioB, bioC, bioD, and cre) in hybridization buffer, and each target was hybridized to the Macaque Genome Array Chip using protocols described in the Affymetrix expression analysis technical manual. Microarray analysis was performed as a biological replicate, i.e. RNA from individual CL tissue was hybridized to its own chip.
Real-time RT-PCR analysis
To confirm the results from microarray experiments, real-time RT-PCR analysis was performed using the RNA preparations that were used in the microarray experiments. Total RNA was treated with DNase I, and the RT-PCR was performed. Total RNA was reverse transcribed at 42 C for 60 min using Moloney murine leukemia virus RT. The resulting cDNA was diluted, and cDNA (10 ng) was subjected to real-time PCR (qPCR) using the ABI Prism 7900 HT Sequence Detection system with the SDS 2.1 program (Applied Biosystems, Foster City, CA) using Finnzymes DyNAmo HS SYBR Green qPCR kit. The details of primers used along with the annealing temperature and expected product size are provided in supplemental Table 1, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org.
Hormone assays
LH, E2, and P4 concentrations in serum were determined by specific RIA as reported previously (10,43).
Statistical analyses
Data were expressed as mean ± sem. Serum P4 concentrations in each experiment were analyzed by one-way ANOVA, followed by the Newman-Keuls multiple comparison test (PRISM GraphPad, version 4.0; GraphPad Software Inc., San Diego, CA).
Microarray data analysis
Normalized data from the Affymetrix software were analyzed using the GeneSifter microarray data analysis system (VizX Labs, Seattle, WA). This program identifies differentially expressed genes, and establishes the biological significance based on gene ontology classification into the biological process, molecular function, and cellular component. The GeneSifter program also produces z-score reports. A z score is a statistical rating of gene ontologies and indicates whether each gene ontology term occurs more or less frequently than expected. For the ontology report (molecular function), see supplemental Table 2. The differential expression of genes was calculated by averaging the normalized samples and running a pairwise analysis. Statistical significance was determined using the Student’s t test (two tail, paired) with correction factor for false discovery rate (Benjamini and Hochberg) included (44).
The linear regression analysis as well as the F test to assess goodness-of-fit was performed on log2-transformed expression ratios (treated to untreated) obtained from real-time RT-PCR and microarray experiments using the Microsoft Excel Analysis Tool Pak add-in module (Microsoft Corp., Redmond, WA), and a statistically-significant (P < 0.05) correlation between the two methods was indicated. In addition, the analysis of real-time RT-PCR expression data (experiments 4 and 5) was subjected for a t test and one-way ANOVA, followed by the Newman-Keuls multiple comparison tests. A P value of less than 0.05 was considered significant.
Results
Genome-wide identification of LH-regulated genes in the primate CL
To understand gene expression changes mediated by LH in the luteal transcriptome, we used two approaches. First, we characterized the changes occurring in the expression of luteal genes after CET-mediated gonadotropin withdrawal during the midluteal phase by microarray analysis. Second, we used an exogenous LH replacement model to examine expressions of genes regarded as specific targets of LH.
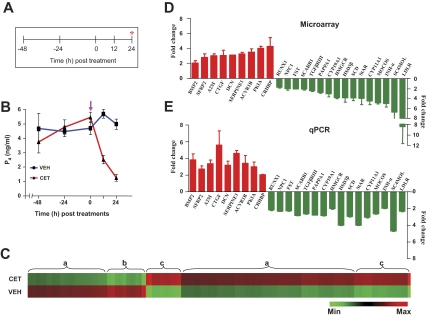
Figure 1A shows the experimental regimen followed for the CET-mediated gonadotropin withdrawal in the monkey. Serum LH levels before CET injection were 1.8 ± 0.2 ng/ml, and the levels became undetectable (<0.19 ng/ml) 12 h after CET treatment. Serum P4 levels before and at different time points after administration of VEH or CET treatment are represented in Fig. 1B, and the levels were 5.46 ± 0.35 ng/ml at 0 h, and decreased precipitously to 2.52 ± 0.29 and 1.25 ± 0.23 ng/ml at 12 and 24 h after CET treatment (P < 0.05), respectively. In contrast, no significant changes in serum P4 levels were observed in monkeys after VEH treatment (Fig. 1B). Microarray analysis was performed on RNA samples isolated from CLs collected from monkeys treated with VEH or CET for 24 h; the hybridization details and individual CEL and CHP files have been deposited at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE8371. Microarray analysis results revealed that inhibition of pituitary LH secretion by CET treatment significantly (P < 0.05) affected the expression of 3949 genes (>2-fold change with Benjamini and Hochberg correction for false discovery rate), of which 2728 and 1221 genes were up-regulated and down-regulated, respectively. For details on the list of differentially expressed transcripts and a pie chart depicting classification of transcripts into ontological categories, see supplemental data, which are published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org, and Fig. 1. The patterns of differential expression of genes with relatively high or low regulation and regions of up-regulation or down-regulation of genes of the transcriptome are represented in Fig. 1C. For validation of microarray data in the three experimental models studied in the present study, and with a view to study the relative expressions in different models of luteolysis and rescue of CL function, 25 genes were chosen for expression analysis studies with the following considerations. Expressions of some of the genes associated with cholesterol biosynthesis and transport, and genes associated with the process of steroidogenesis that have been reported to be under the control of luteotropic factors such as LH, and also some of the genes associated with the remodeling processes during luteolysis and rescue of luteal function during pregnancy were chosen for real-time RT-PCR analysis in all experiments described in the present study.
Figure 1.
Effect of inhibition of pituitary LH secretion on gene expression changes in the monkey CL. A and B depict the experimental protocol and circulating P4 levels at different time points before and after injection of CET (indicated by arrows), a GnRH-R antagonist, 150 μg/kg BW sc on d 7 of the luteal phase in monkeys. The CL was harvested at the end of 24 h treatment. C, A heat map for all the probe sets from VEH and CET treatment groups (n = 5 monkeys per group) that were differentially expressed (>2-fold change; t test, P < 0.05). Each row represents the VEH and treatment group, respectively, and comprises a collection of probe sets corresponding to an individual transcript. Approximate regions of a similar set of differentially expressed genes are grouped and indicated as flower brackets with alphabets. Region a, Set of differentially expressed genes with relatively low regulation compared with b and c; region b, set of differentially expressed genes that is down-regulated; region c, set of differentially expressed genes that is up-regulated. D and E, Microarray and qPCR analyses of fold changes in the expressions of selected genes. More details are provided in the Results section. Max, Maximum; Min, minimum.
The cumulative fold changes in up-regulated genes (BMP2, SERPINE1, A2M, SFRP2, CRHBP, DCN, CTGF, ACVR1B, and PKIA) and down-regulated genes (MOCOS, StAR, LDLR, SCD, HMGCR, SC4MOL, CYP11A1, CYP19A1, PAPPA1, INH-α, TGFβRIII, FST, NPC1, SCARB1, 3βHSD, and RUNX1) are shown in Fig. 1D. These genes were selected in a wide range of fold changes to validate the microarray analysis. Real-time RT-PCR analysis for expression of these selected genes corroborated well with the microarray data, except for the expressions of HMGCR, MOCOS, LDLR, and INH-βA, which were lower in qPCR analysis (see Figs. 1E and 4).
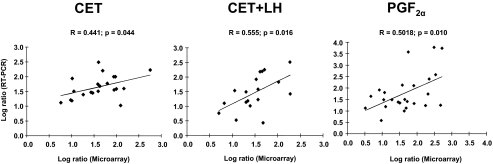
Figure 4.
Correlation analysis between expression ratios obtained from microarray and real-time RT-PCR analyses. Linear regression analysis was performed for selected differentially expressed genes for all three experiments [left panel, experiment 1 (CET); middle panel, experiment 2 (CET + LH); right panel, experiment 3 (PGF2α)] using log2-transformed RT-PCR relative expression values (2−ΔΔCT; y-axis) and mean relative expression values obtained by microarray analysis (x-axis). P value indicates the significance of the correlation as determined by F test. R, correlation coefficient generated for the theoretical line of best fit (represented as solid line in each panel).
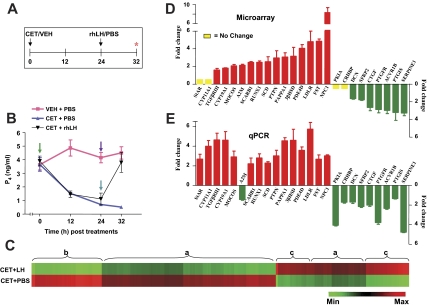
In experiment 2, the experimental regimen for VEH, CET, and PBS or rhLH administrations are represented in Fig. 2A. Serum P4 levels before CET injection were 3.65 ± 0.49 ng/ml, decreased (P < 0.05) to 1.52 ± 0.10 ng/ml, and 0.70 ± 0.11 ng/ml at 12 and 24 h after CET treatment, and the levels remained lower (0.51 ± 0.01) at 32 h, i.e. 8 h after initiation of PBS treatment (Fig. 2B). In monkeys that received CET plus rhLH treatment, serum P4 levels before CET injection were 3.90 ± 0.37, and decreased to 1.46 ± 0.23 and 1.11 ± 0.43 ng/ml at 12 and 24 h after treatment respectively, but the levels rebounded to 3.80 ± 0.73 ng/ml at 8 h after rhLH treatment. Serum P4 levels for monkeys receiving VEH plus PBS treatments are represented in Fig. 2B. Microarray comparison analysis for RNA from the CL of monkeys treated with CET plus PBS and CET plus rhLH, hybridization details, and individual CEL and CHP files have been deposited at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE7827. Microarray analysis results revealed that replacement of exogenous rhLH after inhibition of pituitary LH secretion by CET treatment significantly (P < 0.05) affected the expression of 4434 genes in the CL (>2-fold change with Benjamini and Hochberg correction for false discovery rate), of which 1563 and 2871 genes were up-regulated and down-regulated, respectively. For a complete list of differentially expressed genes and the pie chart depicting ontological classifications, see supplemental data and Fig. 2. The patterns of differentially expressed genes is represented as a heat map comprising regions of high or low regulation of the transcriptome in Fig. 2C. The fold change in the expressions of some of the genes and corresponding qPCR analysis data are shown in Fig. 2, D and E. With the exception of a few genes (discussed below), qPCR analysis results were in agreement with the microarray data (see Fig. 4). Of the 25 genes that were selected for further analysis, differential expression of StAR was not observed by microarray analysis, but more than a 2-fold change in the expression was observed by qPCR analysis. After LH treatment, down-regulation or decreased expressions of CRHBP (∼2-fold) and PKIA (∼4.5-fold) were detectable only in qPCR analysis (Fig. 2, D and E). In addition, the fold changes in the expression of up-regulated genes (except FST, RUNX1, and NPC1) were higher in qPCR analysis (Fig. 2E). On the other hand, expressions of down-regulated genes (except CRHBP and PK1A) showed higher fold changes in microarray analysis compared with qPCR analysis (Fig. 2, D and E).
Figure 2.
Effect of the replacement of LH on gene expression changes in the CL from monkeys pretreated with CET to induce luteolysis. A and B, Experimental protocol and circulating P4 levels at different time points after injections of VEH or CET on d 7 of the luteal phase followed 24 h later by injection of PBS or rhLH (20 IU/kg BW). Arrows indicate VEH/CET and PBS/rhLH treatments. The CL was collected at the end of 32 h VEH or CET treatment, i.e. 8 h after PBS or rhLH treatments (indicated by asterisk). C, A heat map for all the probe sets from CET plus PBS and CET plus LH treatment groups (n = 3 monkeys per group) that were differentially expressed (>2-fold change; t test, P < 0.05). Each group is represented by the row, and each row comprises a collection of probe sets corresponding to an individual transcript. Approximate regions of a similar set of differentially expressed genes are grouped and indicated as flower brackets with alphabets. Region a, Set of differentially expressed genes with relatively low regulation compared with b and c; b, set of differentially expressed genes that is down-regulated; region c, set of differentially expressed genes that is up-regulated. D and E, Microarray and qPCR analyses of fold (mean ± sem) changes in the expressions of selected genes. Max, Maximum; Min, minimum.
The results of analysis of gene expression changes in these two model systems had two outcomes. First, they identified the LH-target genes in the luteal tissue, and second, these results confirmed our initial notion of a larger number of genes being targets of LH, contrary to what was initially thought of as an absence of nuclear actions in the primate CL by other investigators.
Effect of exogenous PGF2α on circulating P4 levels, microarray analysis, and real-time PCR validation of differentially expressed genes in the CL
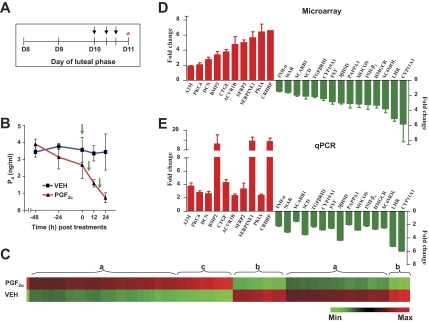
To understand the changes effected on the primate CL by the luteolytic factors, and given the uncertainties in the literature on the effects of PGF2α, the luteolysin conserved across species, on the primate CL, we have used a model system of PGF2α-induced luteolysis in the monkey. A brief outline of the experiment is illustrated in Fig. 3A. Circulating P4 levels were 2.66 ± 0.80 ng/ml before treatment and decreased significantly to reach 0.73 ± 0.28 ng/ml at 24 h after initiation of PGF2αtreatment (P < 0.05; Fig. 3B). A similar VEH treatment regimen did not decrease P4 levels significantly (P > 0.05). Microarray analysis was performed on CLs collected from VEH and PGF2α-treated monkeys. Microarray comparison analysis, hybridization details, and individual CEL and CHP files have been deposited online at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE7971. Microarray analysis results revealed that PGF2α treatment significantly (P < 0.05) affected the expression of 2290 genes in the CL (>2-fold change with Benjamini and Hochberg correction for false discovery rate), of which 1232 and 1058 genes were up-regulated and down-regulated, respectively. For a complete list of differentially expressed genes, see the supplemental data. Genes whose expressions were found to be regulated by PGF2α belonged to diverse functional categories (provided as supplemental Fig. 3, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org), and the patterns of differential expression of genes with relatively high or low regulation and regions of up-regulation or down-regulation of genes of the transcriptome are represented in Fig. 3C. A total of 25 genes was identified for further characterization while considering the fact that the same set of genes or at least majority of selected genes were also differentially expressed in experiments 1 and 2. The fold changes in expression of 25 genes, which are up-regulated (ACVR1B, PKIA, Decorin, PKCδ, SFRP-2, A2M, CTGF, BMP-2, CRHBP, and SERPINE1) and down-regulated (SR-B1, INH-α, TGF-β RIII, INH-βA, SC4MOL, PAPPA1, FST, HMGCR, CYP19A1, MOCOS, StAR, SCD, 3βHSD, LHR, and CYP11A1), are presented in Fig. 3D. Examination of the expressions of the same genes by qPCR analysis agreed well with the microarray data (Figs. 3E and 4). These results indicate that exogenous PGF2α treatment causes luteolysis in the macaque, and for the first time demonstrate PGF2α-mediated global changes in the primate luteal transcriptome.
Figure 3.
Effect of PGF2α treatment on gene expression changes in the monkey CL. A and B, Experimental protocol and circulating P4 levels at different time points before and after injection of VEH or PGF2α. Arrows indicate the time of injections. Asterisk indicates the time of CL collection. E, A heat map for all the probe sets from VEH and PGF2α treatment groups that were differentially expressed (>2-fold change; t test, P < 0.05). Each row represents VEH and treatment group, respectively, and comprises a collection of probe sets corresponding to an individual transcript. C, Approximate regions of a similar set of differentially expressed genes are grouped and indicated as flower brackets with alphabets. Region a, Set of differentially expressed genes with relatively low regulation compared with b and c; region b, set of differentially expressed genes that is down-regulated; region c, set of differentially expressed genes that is up-regulated. D and E, Microarray and qPCR analyses of fold changes (mean ± sem) in the expressions of selected genes. Max, Maximum; Min, minimum.
Identification of genes that are common targets of LH and PGF2α actions
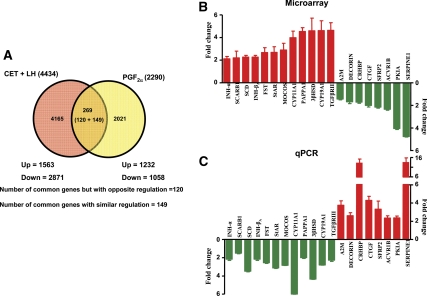
Comparison of microarray analysis data from PGF2α treatment experiment with that of other experiments revealed that a large number of genes appeared to have opposite or similar regulation. Figure 5A shows a Venn diagram representing the total number of differentially expressed genes in the CL retrieved from monkeys treated with CET plus LH (experiment 2) and from monkeys treated with PGF2α (experiment 3). Expression of 269 genes was identified to be common between CET plus LH and PGF2α treatments. Of these differentially expressed genes, 149 genes were identified to show similar regulation, whereas expressions of 120 genes were identified to be regulated in an antagonistic fashion in the two experiments (Fig. 5A). Furthermore, analysis of the few genes regulated by LH and PGF2α in an inverse fashion was examined, and the fold changes in expression of some of the genes belonging to diverse groups are represented in Fig. 5B. Real-time RT-PCR expressions of 20 genes considered to be associated with the control of structure and function of the CL are represented in Fig. 5C. As can be seen from Fig. 5, B and C, expressions of StAR, CYP11A1, 3βHSD, and CYP19A1 were higher after LH treatment, but after PGF2α treatment, expressions of these genes were down-regulated. Expressions of CTGF, CRHBP, and SERPINE 1 were lower after LH treatment, but their expressions were up-regulated after PGF2α treatment.
Figure 5.
A, Venn diagram with details of differentially expressed genes after microarray analysis of the CL from monkeys of CET plus LH replacement (experiment 2) and PGF2α treatments (experiment 3). B and C, qPCR analysis of fold changes (mean ± sem) in the expressions of selected genes from both the experiments (2 and 3).
Gene expression analysis in the CL during different stages and from monkeys treated with hCG to mimic early pregnancy
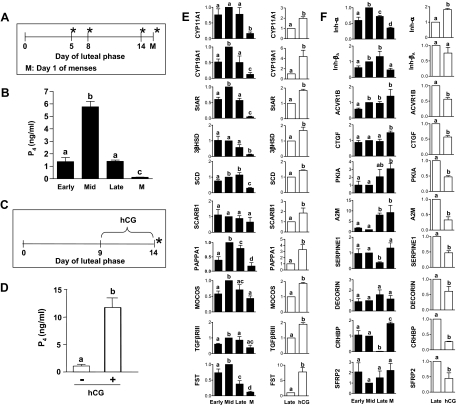
Based on the results of identification of genes differentially expressed in experiments 1–3, the next question we addressed is whether changes in expressions of these common target genes are identifiable in the late or very late stages of the luteal phase of the nonfertile cycles. Figure 6A shows the protocol outline for collection of the CL during different stages of the luteal phase. Serum P4 concentrations on the day of CL collection are represented in Fig. 6B. Expressions of CYP11A1, CYP19A1, StAR, 3βHSD, SCD, FST, and INH-βA were lowest (P < 0.05) in the CL on the day of menses compared with the other stages. Expressions of CYP19A1, StAR, MOCOS, INH-α, and FST tended to be higher in the CL retrieved from monkeys at the midluteal phase (Fig. 6E). The expression changes can be classified into two groups: the first group that showed changes at the late luteal phase, and the second that showed changes at the very late luteal phase (i.e. d 1 menses), suggesting their differential sensitivity to the gonadotropin milieu and, perhaps, the presence of intraovarian luteolysin.
Figure 6.
Expression patterns of genes associated with steroidogenesis and other functional groups in the CL from monkeys during different stages, including the CL from monkeys on d 1 menses and from a simulated early pregnancy model. A–D, Experimental protocols and circulating P4 levels from experiments 4 and 5. The CL collection is indicated by an asterisk. E and F, qPCR analysis of fold changes (mean ± sem) in the expressions of selected genes from both the experiments. The genes identified for analysis are based on the microarray results of experiments 1–3. More details on the analysis are provided in Results.
After identifying the changes in expression of these genes during the late luteal phase that showed a pattern similar to the PGF2α-induced luteolysis model, we asked whether these changes can be reversed or altered during early pregnancy. A brief outline of the hCG treatment performed in monkeys to mimic early pregnancy and schedule of CL collection are depicted in Fig. 6C. As expected, circulating P4 levels were several folds higher in hCG-treated monkeys compared with untreated monkeys (Fig. 6D). The real-time RT-PCR analysis of expression of the selected genes was performed, and the results are presented in Fig. 6, E and F. Expressions of CYP11A1, CYP19A1, StAR, MOCOS, TGFβRIII, SCARB1, INH-α, PAPPA1, and FST were highest (P < 0.05; Fig. 6, E and F), whereas expressions of CRHBP, A2M, CTGF, PK1A, SERPINE1, ACVR1B, and SFRP2 were lower (P < 0.05) in the CL from monkeys treated with hCG (P < 0.05; Fig. 6F).
Interplay between luteotropic and luteolytic factors on differential expression of genes: a comparative analysis of expression of genes
Having characterized the patterns of expressions of genes at the whole genome level imposed by various treatments, spontaneous luteolysis, and in hCG-simulated early pregnancy, the expressions of a set of genes determined by qPCR analysis were compared in all the models studied, and the data are represented in Table 1. As can be seen from Table 1, a discernible opposing action of LH and PGF2α on expressions of genes was observed. The genes whose expressions were altered by PGF2α/LH showed a favorable change toward the PGF2α mode of regulation during the late to very late luteal phase, and these changes were completely reversed by the exogenous hCG treatment. An example of such a type of regulation is described for one of the genes, MOCOS. mRNA expression of MOCOS was down-regulated during the CET-mediated luteolysis by 2.6-fold, but the expression was restored to pretreatment levels upon LH supplementation, suggesting that it is positively regulated by LH. In contrast, the expression of MOCOS was down-regulated by PGF2α treatment, much similar to the change observed in the CET-induced luteolysis model. The qPCR analysis of its expression during spontaneous luteolysis showed 2.3-fold reductions, but hCG treatment increased to its expression observed at the midluteal phase. Similar changes were observed in the expressions of other genes that were positively regulated by LH, and an inverse of this regulation was true for many genes that were negatively regulated by LH.
Table 1.
List of selected genes identified to be common targets of LH and PGF2α in the CL, and qPCR fold changes in their expressions in different models
| Gene | Treatments
|
SL | hCG treatmenta | ||
|---|---|---|---|---|---|
| CET | CET + LH | PGF2 | |||
| INH-A | 1.96 ↓ | 2.12 ↑ | 2.17 ↓ | 2.83 ↓ | 1.82 ↑ |
| SCARB1 | 2.85 ↓ | 2.20 ↑ | 1.49 ↓ | 1.52 ↓ | 1.85 ↑ |
| SCD | 2.94 ↓ | 2.26 ↑ | 3.48 ↓ | 3.37 ↓ | 1.44 ↑ |
| INH-BA | – | 1.80 ↑ | 2.17 ↓ | 2.08 ↓ | 1.32 ↑ |
| FST | 2.27 ↓ | 2.67 ↑ | 2.54 ↓ | 8.57 ↓ | 7.78 ↑ |
| StAR | 4.00 ↓ | 2.68 ↑ | 3.11 ↓ | 22.22 ↓ | 1.84 ↑ |
| MOCOS | 2.63 ↓ | 2.90 ↑ | 2.83 ↓ | 2.32 ↓ | 1.87 ↑ |
| CYP11A1 | 3.03 ↓ | 3.99 ↑ | 5.98 ↓ | 6.12 ↓ | 1.98 ↑ |
| PAPPA1 | 2.77 ↓ | 4.54 ↑ | 2.00 ↓ | 5.55 ↓ | 3.32 ↑ |
| HSD3B | 4.00 ↓ | 4.59 ↑ | 4.34 ↓ | 8.10 ↓ | 1.69 ↑ |
| CYP19A1 | 2.85 ↓ | 4.60 ↑ | 2.80 ↓ | 7.89 ↓ | 4.39 ↑ |
| TGF RIII | 2.63 ↓ | 4.63 ↑ | 2.27 ↓ | 2.63 ↓ | 1.90 ↑ |
| A2 m | 3.36 ↑ | 1.44 ↓ | 3.75 ↑ | 9.22 ↑ | 3.03 ↓ |
| DCN | 3.20 ↑ | 1.69 ↓ | 2.60 ↑ | 1.33 ↑ | 1.39 ↓ |
| CRHBP | 2.04 ↑ | 1.72 ↓ | 13.33 ↑ | 1.80 ↑ | 3.77 ↓ |
| CTGF | 5.60 ↑ | 2.01 ↓ | 4.28 ↑ | 1.50 ↑ | 1.75 ↓ |
| SFRP2 | 2.73 ↑ | 2.15 ↓ | 3.33 ↑ | 2.21 ↑ | 2.23 ↓ |
| ACVR1B | 3.40 ↑ | 2.34 ↓ | 2.34 ↑ | 1.40 ↑ | 1.77 ↓ |
| PKIA | 3.00 ↑ | 4.05 ↓ | 2.36 ↑ | 3.09 ↑ | 2.12 ↓ |
| SERPINE1 | 4.60 ↑ | 4.76 ↓ | 13.65 ↑ | 1.31 ↑ | 2.12 ↓ |
CET, GnRH-R antagonist induced luteolysis; ↓, down-regulation; –, no change; PGF2α, exogenous PGF2α treatment-induced luteolysis; SL, spontaneous luteolysis (d 1 menses); ↑, up-regulation.
To mimic early pregnancy.
Discussion
In higher primates the processes of luteolysis and rescue of luteal function during nonconception and conception cycles, respectively, are not completely understood, in part, due to the lack of systematic information concerning cellular events and molecular players associated with either of these processes. The CG with a faster rate of biosynthesis and continuous secretion by the syncytiotrophoblast cells appears to be the sole factor responsible for rescue of CL function during the conception cycle. It appears then that luteal cells are endowed with the capacity to respond to diametrically opposite situations depending on the absence or presence of conceptus. Several studies have reported that LH/hCG regulates expression of a number of genes in the CL (10,11,45). Genome-wide gene expression by microarray analysis in the present study provides more comprehensive information on the transcriptional changes in the monkey CL after LH deprival and replacement of LH. In the present study, microarray analysis of RNA samples from bonnet monkeys was performed using rhesus monkey genome oligonucleotide array chips. A number of studies using indirect and direct approaches observed no detectable differences in the dynamic range of absolute intensity levels between closely related primate species without the loss of information (46,47). It should be pointed out that the use of the robust multiarray average algorithm is well suited for cross-species microarray analysis because it considers hybridization from perfect match and not mismatch probes. Moreover, the microarray data were further validated by real-time RT-PCR analysis for few differentially regulated genes. The demonstration that expressions of a number of genes were regulated after LH treatment that restored circulating P4 levels in CET-treated monkeys to that of VEH monkeys indicated that LH plays a critical role in the regulation of CL function, and it does so by regulating expression of various genes. Whether an increased P4 level that occurs after LH treatment has a role in the regulation of expression of many of these genes needs to be ascertained. However, our preliminary results of effect of P4 on expressions of some of the genes examined have excluded the possibility for these subsets of genes are direct targets of P4 (Suresh, P., and R.Medhamurthy, unpublished observations). Of the differentially expressed genes associated with steroidogenesis, expression of StAR, considered as critical and one of the indices of functional CLs, was significantly lower after inhibition of pituitary LH secretion in both microarray and qPCR analyses, but with LH treatment, restoration of its expression was observed only by qPCR analysis. A decreased expression was seen after PGF2α treatment and also in CL collected from monkeys on d 1 menses. Interestingly, as observed in another study, coincident with the increased P4 secretion, the phospho form of StAR level was higher within 1–2 h after LH injection in CET-treated monkeys, unaccompanied by changes in messenger levels (Suresh, P., and R. Medhamurthy, manuscript under preparation). The importance of cotranslational modification of StAR as was also observed by Arakane et al. (48) under in vitro conditions suggests additional complexities in the LH-mediated regulation of StAR and P4 biosynthesis. Expressions of genes such as HMGCR, SC4MOL, and SCD5 involved in de novo synthesis of cholesterol decreased after LH withdrawal and restored after LH supplementation, indicating that genes involved in the de novo biosynthesis of cholesterol are dependent on LH for their expressions; a similar gonadotropin regulation of SCD2 expression has been reported in rats (49). The results also suggest, for the first time, that macaque CL has all the components required for de novo biosynthesis of cholesterol and that the cholesterol biosynthetic pathway appears to be regulated by LH. In this study it was observed that expressions of many of the genes (CRHBP, CTGF, SERPINE1, MMPs, and TIMPs) were higher after LH withdrawal, whereas LH supplementation decreased their expressions. Similar to the findings on the expression of CRHBP in the present study, Xu et al. (11) also observed differential expression of this gene, and further, the authors reported the existence of a local CRH/UCN-R-BP system in the macaque CL. The CRHBP levels were lowest during the midluteal phase but increased during the late luteal phase (50). However, the mechanism by which the ligand-receptor activity of CRH may contribute to the maintenance of CL structure and function remains unclear. Another gene that was differentially expressed during LH withdrawal and LH replacement experiments was CTGF, a heparin-binding growth factor belonging to the family of CTGF/cysteine rich 61/nephroblastoma genes (51). CTGF was initially isolated from endothelial cells with proposed roles in wound healing and angiogenesis (52,53,54). Recently, CTGF has been implicated as a factor regulating collagen deposition in fibroblasts (51), and it was identified as a FSH suppressible and TGF-β inducible gene in rat granulosa cells (55). mRNA levels of CTGF in CL tissue were also suppressed in vivo after exogenous hCG treatment both in rats (56) and humans (57). CTGF appears to mediate some of its action by the cAMP-PKA pathway because treatment with (Bu)2cAMP inhibited CTGF expression in human granulosa-lutein cells (58). In addition, Duncan et al. (57) observed that CTGF expression was localized to fibroblast-like and endothelial cells, and the expression in these cells was suppressed indirectly by hCG actions on luteinized granulosa cells. Contrary to its role in angiogenesis, Inoki et al. (59) reported inhibitory action of CTGF to vascular endothelial growth factor-induced angiogenesis both in vitro and in vivo. Considering the aforementioned roles of CTGF, it appears that LH regulates its expression perhaps by interfering with angiogenesis, and its expression might get up-regulated during luteolysis because it may be required during the process of regression of the CL, an event that closely resembles wound healing.
PGs have profound effects on CL function by luteotropic (PGE2) and luteolytic (PGF2α) actions in several nonprimate species, and both luteotropic and luteolytic PGs are synthesized within the CL of nonprimate and primate species (60). Rothchild (61) hypothesized that the final common agent responsible for luteolysis in all mammals might be intraluteal PGs. Several studies performed in higher primates strongly favor the aforementioned hypothesis. Local inhibition of PG biosynthesis by sodium meclofenamate after intraluteal infusion has decreased the function and life span of the CL in the rhesus monkey (62). In addition, exogenous PGs have affected luteal production of P4 in vitro (63) and in vivo (24). Administration of a combination of PGF2α and PGE2 analogs has intercepted/interrupted pregnancy in monkeys (31). It is conceivable that the luteotropin to luteolysin ratio within the primate CL might account for a progressive decrease in luteal function in nonconception cycles. In a more recent study, Bogan et al. (64) performed a comprehensive analysis of expressions (messenger and protein) of genes associated with PGE2 and PGF2α biosynthesis, metabolism and signaling in CL tissues throughout the luteal phase in the rhesus monkey, and indicated the involvement of intraluteal PGF2α synthesis and signaling around the time of luteolysis. In the rat CL, Stocco et al. (22) reported a “yin-yang” relationship between luteotropic (PRL) and luteolytic (PGF2α) factors on the regulation of several genes. In the present study, microarray analysis results from all three experiments did not reveal changes in expressions of genes associated with PG (both luteotropic and luteolytic factors) biosynthesis, metabolism, and receptors (except for changes in expressions of PG synthase and PGE3 receptor genes). However, microarray analysis of CL tissue from PGF2α-treated monkeys revealed that expression of 120 genes was regulated in opposite directions compared with CET-treated monkeys, whose CL function was rescued by LH replacement. Included among those were genes associated with steroidogenesis, tissue remodeling, and signaling pathways, but many were genes with unknown functions. Surprisingly, one of the genes whose expression was altered by PGF2α treatment is LHR. It has been reported that expression of LHR gets inhibited after PGF2α treatment in several species (22,65,66,67,68,69), and in the present study, a decreased expression LHR was observed after PGF2α treatment. It is conceivable that PGF2α treatment may have interfered with certain aspects of LHR signaling, but this remains to be established.
The results of the present studies and of others (64) suggest that CG in the conception cycles may rescue CL function during the late luteal phase by overriding the action of PGF2α. In the present study, the findings that a large number of genes that were differentially expressed after PGF2α treatment were also seen to be regulated by exogenous hCG treatment suggest that CG rescues CL function during the late luteal phase by overriding the intraluteal actions of PGs. Stocco et al. (22) reported that PGF2α treatment decreased the expression of PRL receptors, part of the luteotropic complex in the regulation of luteal function in rodents. However, contrary to the findings in rodents, in a recent study involving monkeys, expression of PRL receptor, both long and short isoforms, increased progressively throughout the luteal phase, but the expressions were lowest in the CL on the day close to the onset of menses (70). In the present study, although PRL receptor expression showed down-regulation after CET treatment, its expression did not change after PGF2α treatment, suggesting a need for more studies to examine the role of PRL receptor signaling during spontaneous luteolysis in higher primates.
Several studies have previously reported that LH/hCG regulates expressions of the number of genes, including the apoptotic genes in the primate CL (11,44,64,71). To further examine events associated with spontaneous luteolysis, we have taken the opportunity of the microarray analysis data generated for different stages of the CL of the rhesus monkey deposited recently in the public domain by Bogan et al. (70) at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE10367. The analysis of microarray data between late midluteal phase and very late luteal phase by GeneSifter software revealed that 2882 genes were differentially regulated (1280 and 1522 up-regulation and down-regulation, respectively). Of the 2882 differentially expressed genes identified, we found that nearly 440 genes were common to the genes differentially expressed after PGF2α treatment-induced luteolysis in our studies, further suggesting that intraluteal PGF2α may function as a luteolysin during spontaneous luteolysis.
In summary, the three sets of experiments describe global gene expression changes in the CL during induced luteolysis and rescue of function. Together with these results and the expression analysis, results of selected genes from experiments involving different stages of the CL and from a simulated early pregnancy model indicate that the curtailment of downstream LH-target genes possibly through PGF2α action are among the mechanisms underlying cross talk between luteotropic and luteolytic signaling pathways that result in the cessation of luteal function in monkeys, and that CG appears to abrogate this PGF2α-responsive gene expression changes resulting in luteal rescue essential for maintenance of pregnancy until such time that the placenta takes over the major burden of P4 secretion.
Supplementary Material
Acknowledgments
We are grateful to Dr. Basavanagowda and the staff of the Primate Research Laboratory for assistance with surgeries.
Footnotes
This work was supported by Indo-US program on Contraception and Reproductive Health Research, Department of Biotechnology, India and National Institutes of Health, Bethesda, Maryland (HD52155).
Disclosure Statement: The authors have nothing to declare.
First Published Online November 6, 2008
Abbreviations: BW, Body weight; CET, Cetrorelix; CG, chorionic gonadotropin; CL, corpus luteum; E2, estradiol; GnRH-R, GnRH-receptor; hCG, human chorionic gonadotropin; P4, progesterone; PG, prostaglandin; PK, protein kinase; PRL, prolactin; rhLH, recombinant human LH; RT, reverse transcriptase; VEH, control.
References
- Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW 2000 Mechanisms controlling the function and life span of the corpus luteum. Physiol Rev 80:1–29 [DOI] [PubMed] [Google Scholar]
- Stouffer RL 2006 Structure, function and regulation of the corpus luteum. In: Knobil E, Neill JD, eds. The physiology of reproduction. New York: Raven Press; 475 [Google Scholar]
- Ellinwood WE, Norman RL, Spies HG 1984 Changing frequency of pulsatile luteinizing hormone and progesterone secretion during the luteal phase of the menstrual cycle of rhesus monkeys. Biol Reprod 31:714–722 [DOI] [PubMed] [Google Scholar]
- Filicori M, Butler JP, Crowley Jr WF 1984 Neuroendocrine regulation of the corpus luteum in the human. Evidence for pulsatile progesterone secretion. J Clin Invest 73:1638–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filicori M, Santoro N, Merriam GR, Crowley Jr WF 1986 Characterization of the physiological pattern of episodic gonadotropin secretion throughout the human menstrual cycle. J Clin Endocrinol Metab 62:1136–1144 [DOI] [PubMed] [Google Scholar]
- Wilks JW, Noble AS 1983 Steroidogenic responsiveness of the monkey corpus luteum to exogenous chorionic gonadotropin. Endocrinology 112:1256–1266 [DOI] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Robker RL, Dajee M, Alliston TN 1998 Molecular mechanisms of ovulation and luteinization. Mol Cell Endocrinol 145:47–54 [DOI] [PubMed] [Google Scholar]
- Richards JS 2001 New signaling pathways for hormones and cyclic adenosine 3′,5′-monophosphate action in endocrine cells. Mol Endocrinol 15:209–218 [DOI] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Ochsner S, Hsieh M, Doyle KH, Falender AE, Lo YK, Sharma SC 2002 Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent Prog Horm Res 57:195–220 [DOI] [PubMed] [Google Scholar]
- Yadav VK, Muraly P, Medhamurthy R 2004 Identification of novel genes regulated by LH in the primate corpus luteum: insight into their regulation during the late luteal phase. Mol Hum Reprod 10:629–639 [DOI] [PubMed] [Google Scholar]
- Xu J, Stouffer RL, Hennebold JD 2005 Discovery of luteinizing hormone (LH) regulated genes in the primate corpus luteum. Mol Hum Reprod 11:151–159 [DOI] [PubMed] [Google Scholar]
- Beling CG, Marcus SL, Markham SM 1970 Functional activity of the corpus luteum following hysterectomy. J Clin Endocrinol Metab 30:30–39 [DOI] [PubMed] [Google Scholar]
- Castracane VD, Moore GT, Shaikh AA 1979 Ovarian function of hysterectomized Macaca fascicularis. Biol Reprod 20:462–472 [DOI] [PubMed] [Google Scholar]
- Scaramuzzi RJ, Baird DT 1976 The oestrous cycle of the ewe after active immunization against prostaglandin F2 α. J Reprod Fertil 46:39–47 [DOI] [PubMed] [Google Scholar]
- Fairclough RJ, Smith JF, McGowan LT 1981 Prolongation of the oestrous cycle in cows and ewes after passive immunization with PGF antibodies. J Reprod Fertil 62:213–219 [DOI] [PubMed] [Google Scholar]
- Flint AP, Sheldrick EL 1985 Continuous infusion of oxytocin prevents induction of uterine oxytocin receptor and blocks luteal regression in cyclic ewes. J Reprod Fertil 75:623–631 [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Yamasaki A, Segi E, Tsuboi K, Aze Y, Nishimura T, Oida H, Yoshida N, Tanaka T, Katsuyama M, Hasumoto K, Murata T, Hirata M, Ushikubi F, Negishi M, Ichikawa A, Narumiya S 1997 Failure of parturition in mice lacking the prostaglandin F receptor. Science 277:681–683 [DOI] [PubMed] [Google Scholar]
- Niswender GD, Nett TM 1988 The corpus luteum and its control. In: Knobil E, Neill JD, eds. The physiology of reproduction. New York: Raven Press; 489 [Google Scholar]
- Yadav VK, Sudhagar RR, Medhamurthy R 2002 Apoptosis during spontaneous and prostaglandin F2 α-induced luteal regression in the buffalo cow (Bubalus bubalis): involvement of mitogen-activated protein kinases. Biol Reprod 67:752–759 [DOI] [PubMed] [Google Scholar]
- Yadav VK, Lakshmi G, Medhamurthy R 2005 Prostaglandin F2 α-mediated activation of apoptotic signaling cascades in the corpus luteum during apoptosis: involvement of caspase-activated DNase. J Biol Chem 280:10357–10367 [DOI] [PubMed] [Google Scholar]
- Goravanahally MP, Sen A, Inskeep EK, Flores JA 2007 PKC epsilon and an increase in intracellular calcium concentration are necessary for PGF2 α to inhibit LH-stimulated progesterone secretion in cultured bovine steroidogenic luteal cells. Reprod Biol Endocrinol 5:37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco C, Callegari E, Gibori G 2001 Opposite effect of prolactin and prostaglandin F(2 α) on the expression of luteal genes as revealed by rat cDNA expression array. Endocrinology 142:4158–4161 [DOI] [PubMed] [Google Scholar]
- Wilks JW, Forbes KK, Norland JF 1972 Synthesis of prostaglandin F2 by the ovary and uterus. J Reprod Med 9:271–276 [PubMed] [Google Scholar]
- Auletta FJ, Kamps DL, Pories S, Bisset J, Gibson M 1984 An intra-corpus luteum site for the luteolytic action of prostaglandin F2 α in the rhesus monkey. Prostaglandins 27:285–298 [DOI] [PubMed] [Google Scholar]
- Johnson MS, Ottobre AC, Ottobre JS 1988 Prostaglandin production by corpora lutea of rhesus monkeys: characterization of incubation conditions and examination of putative regulators. Biol Reprod 39:839–846 [DOI] [PubMed] [Google Scholar]
- Houmard BS, Ottobre JS 1989 Progesterone and prostaglandin production by primate luteal cells collected at various stages of the luteal phase: modulation by calcium ionophore. Biol Reprod 41:401–408 [DOI] [PubMed] [Google Scholar]
- McCracken JA, Custer EE, Lamsa JC 1999 Luteolysis: a neuroendocrine-mediated event. Physiol Rev 79:263–323 [DOI] [PubMed] [Google Scholar]
- Ottander U, Leung CH, Olofsson JI 1999 Functional evidence for divergent receptor activation mechanisms of luteotrophic and luteolytic events in the human corpus luteum. Mol Hum Reprod 5:391–395 [DOI] [PubMed] [Google Scholar]
- Aksel S, Schomberg DW, Hammond CB 1977 Prostaglandin F2 α production by the human ovary. Obstet Gynecol 50:347–350 [PubMed] [Google Scholar]
- Bennegard B, Hahlin M, Wennberg E, Noren H 1991 Local luteolytic effect of prostaglandin F2 α in the human corpus luteum. Fertil Steril 56:1070–1076 [PubMed] [Google Scholar]
- Wilks JW 1983 Pregnancy interception with a combination of prostaglandins: studies in monkeys. Science 221:1407–1409 [DOI] [PubMed] [Google Scholar]
- Zelinski-Wooten MB, Stouffer RL 1990 Intraluteal infusions of prostaglandins of the E, D, I, and A series prevent prostaglandin F2 α-induced, but not spontaneous, luteal regression in rhesus monkeys. Biol Reprod 43:507–516 [DOI] [PubMed] [Google Scholar]
- Srinath BR 1979 Husbandry and breeding of bonnet monkeys (Macaca radiata). In: Anand Kumar TC, ed. Non-human primate models for study of human reproduction: satellite symposium to the 7th congress of the International Primatological Society on relevance of researches on non-human primates to the understanding of human reproduction, Bangalore, January 1979. Basel: Karger; 17–22 [Google Scholar]
- Yadav VK, Medhamurthy R 2006 Dynamic changes in mitogen-activated protein kinase (MAPK) activities in the corpus luteum of the bonnet monkey (Macaca radiata) during development, induced luteolysis, and simulated early pregnancy: a role for p38 MAPK in the regulation of luteal function. Endocrinology 147:2018–2027 [DOI] [PubMed] [Google Scholar]
- Rowson LE, Tervit R, Brand 1972 The use of prostaglandins for synchronization of oestrus in cattle. J Reprod Fertil 29:145 [DOI] [PubMed] [Google Scholar]
- Diehl JR, Day BN 1974 Effect of prostaglandin F2 α on luteal function in swine. J Anim Sci 39:392–396 [DOI] [PubMed] [Google Scholar]
- Summers PM, Wennink CJ, Hodges JK 1985 Cloprostenol-induced luteolysis in the marmoset monkey (Callithrix jacchus). J Reprod Fertil 73:133–138 [DOI] [PubMed] [Google Scholar]
- Jones GC, Wentz AG 1972 The effect of prostaglandin F2 α infusion on corpus luteum function. Am J Obstet Gynecol 114:393–404 [DOI] [PubMed] [Google Scholar]
- LeMaire WJ, Shapiro AG 1972 Prostaglandin F2 α: its effect on the corpus luteum of the menstrual cycle. Prostaglandins 1:259–267 [DOI] [PubMed] [Google Scholar]
- Karim SM, Hillier K 1979 Prostaglandins in the control of animal and human reproduction. Br Med Bull 35:173–180 [DOI] [PubMed] [Google Scholar]
- Wilks JW 1980 Inhibition of the monkey corpus luteum with 15-methyl prostaglandins. Prostaglandins 20:793–805 [DOI] [PubMed] [Google Scholar]
- Yadav VK 2005 Studies on corpus luteum function in primates: new insights into the mechanisms regulating luteolysis and rescue, PhD thesis, Indian Institute of Science, Bangalore, India [Google Scholar]
- Selvaraj N, Medhamurthy R, Ramachandra SG, Sairam MR, Moudgal NR 1996 Assessment of luteal rescue and desensitization of macaque corpus luteum brought about by human chorionic gonadotrophin and de-glycosylated human chorionic gonadotrophin treatment. J Biosci 21:497–510 [Google Scholar]
- Reiner A, Yekutieli D, Benjamini Y 2003 Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics 19:368–375 [DOI] [PubMed] [Google Scholar]
- Sasson R, Rimon E, Dantes A, Cohen T, Shinder V, Land-Bracha A, Amsterdam A 2004 Gonadotrophin-induced gene regulation in human granulosa cells obtained from IVF patients. Modulation of steroidogenic genes, cytoskeletal genes and genes coding for apoptotic signalling and protein kinases. Mol Hum Reprod 10:299–311 [DOI] [PubMed] [Google Scholar]
- Vahey MT, Nau ME, Taubman M, Yalley-Ogunro J, Silvera P, Lewis MG 2003 Patterns of gene expression in peripheral blood mononuclear cells of rhesus macaques infected with SIVmac251 and exhibiting differential rates of disease progression. AIDS Res Hum Retroviruses 19:369–387 [DOI] [PubMed] [Google Scholar]
- Oshlack A, Chabot AE, Smyth GK, Gilad Y 2007 Using DNA microarrays to study gene expression in closely related species. Bioinformatics 23:1235–1242 [DOI] [PubMed] [Google Scholar]
- Arakane F, King SR, Du Y, Kallen CB, Walsh LP, Watari H, Stocco DM, Strauss 3rd JF 1997 Phosphorylation of steroidogenic acute regulatory protein (StAR) modulates its steroidogenic activity. J Biol Chem 272:32656–32662 [DOI] [PubMed] [Google Scholar]
- Moreau C, Froment P, Tosca L, Moreau V, Dupont J 2006 Expression and regulation of the SCD2 desaturase in the rat ovary. Biol Reprod 74:75–87 [DOI] [PubMed] [Google Scholar]
- Xu J, Hennebold JD, Stouffer RL 2006 Dynamic expression and regulation of the corticotropin-releasing hormone/urocortin-receptor-binding protein system in the primate ovary during the menstrual cycle. J Clin Endocrinol Metab 91:1544–1553 [DOI] [PubMed] [Google Scholar]
- Brigstock DR 1999 The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev 20:189–206 [DOI] [PubMed] [Google Scholar]
- Shimo T, Nakanishi T, Nishida T, Asano M, Kanyama M, Kuboki T, Tamatani T, Tezuka K, Takemura M, Matsumura T, Takigawa M 1999 Connective tissue growth factor induces the proliferation, migration, and tube formation of vascular endothelial cells in vitro, and angiogenesis in vivo. J Biochem 126:137–145 [DOI] [PubMed] [Google Scholar]
- Babic AM, Chen CC, Lau LF 1999 Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin αvβ3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol 19:2958–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A, Holmes A, Black CM, Abraham DJ 2003 Connective tissue growth factor gene regulation. Requirements for its induction by transforming growth factor-β 2 in fibroblasts. J Biol Chem 278:13008–13015 [DOI] [PubMed] [Google Scholar]
- Harlow CR, Davidson L, Burns KH, Yan C, Matzuk MM, Hillier SG 2002 FSH and TGF-β superfamily members regulate granulosa cell connective tissue growth factor gene expression in vitro and in vivo. Endocrinology 143:3316–3325 [DOI] [PubMed] [Google Scholar]
- Slee RB, Hillier SG, Largue P, Harlow CR, Miele G, Clinton M 2001 Differentiation-dependent expression of connective tissue growth factor and lysyl oxidase messenger ribonucleic acids in rat granulosa cells. Endocrinology 142:1082–1089 [DOI] [PubMed] [Google Scholar]
- Duncan WC, Hillier SG, Gay E, Bell J, Fraser HM 2005 Connective tissue growth factor expression in the human corpus luteum: paracrine regulation by human chorionic gonadotropin. J Clin Endocrinol Metab 90:5366–5376 [DOI] [PubMed] [Google Scholar]
- Liu J, Kosma VM, Vanttinen T, Hyden-Granskog C, Voutilainen R 2002 Gonadotrophins inhibit the expression of insulin-like growth factor binding protein-related protein-2 mRNA in cultured human granulosa-luteal cells. Mol Hum Reprod 8:136–141 [DOI] [PubMed] [Google Scholar]
- Inoki I, Shiomi T, Hashimoto G, Enomoto H, Nakamura H, Makino K, Ikeda E, Takata S, Kobayashi K, Okada Y 2002 Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. FASEB J 16:219–221 [DOI] [PubMed] [Google Scholar]
- Wiltbank MC, Ottobre JS 2003 Regulation of intraluteal production of prostaglandins. Reprod Biol Endocrinol 1:91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothchild I 1981 The regulation of the mammalian corpus luteum. Recent Prog Horm Res 37:183–298 [DOI] [PubMed] [Google Scholar]
- Sargent EL, Baughman WL, Novy MJ, Stouffer RL 1988 Intraluteal infusion of a prostaglandin synthesis inhibitor, sodium meclofenamate, causes premature luteolysis in rhesus monkeys. Endocrinology 123:2261–2269 [DOI] [PubMed] [Google Scholar]
- Patwardhan VV, Lanthier A 1985 Luteal phase variations in endogenous concentrations of prostaglandins PGE and PGF and in the capacity for their in vitro formation in the human corpus luteum. Prostaglandins 30:91–98 [DOI] [PubMed] [Google Scholar]
- Bogan RL, Murphy MJ, Stouffer RL, Hennebold JD 2008 Prostaglandin synthesis, metabolism, and signaling potential in the rhesus macaque corpus luteum throughout the luteal phase of the menstrual cycle. Endocrinology 149:5861–5871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Canto F, Sierralta W, Kohen P, Muñoz A, Strauss 3rd JF, Devoto L 2007 Features of natural and gonadotropin-releasing hormone antagonist-induced corpus luteum regression and effects of in vivo human chorionic gonadotropin. J Clin Endocrinol Metab 92:4436–4443 [DOI] [PubMed] [Google Scholar]
- Grinwich DL, Hichens M, Behrman HR 1976 Control of the LH receptor by prolactin and prostaglandin F2 α in rat corpora lutea. Biol Reprod 14:212–218 [DOI] [PubMed] [Google Scholar]
- Guy MK, Juengel JL, Tandeski TR, Niswender GD 1995 Steady-state concentrations of mRNA encoding the receptor for luteinizing hormone during the estrous cycle and following prostaglandin F2α treatment of ewes. Endocrine 3:585–589 [DOI] [PubMed] [Google Scholar]
- Bjurulf E, Selstam G 1996 Rat luteinizing hormone receptor messenger ribonucleic acid expression and luteolysis: inhibition by prostaglandin F2 α. Biol Reprod 54:1350–1355 [DOI] [PubMed] [Google Scholar]
- Smith GW, Gentry PC, Roberts RM, Smith MF 1996 Ontogeny and regulation of luteinizing hormone receptor messenger ribonucleic acid within the ovine corpus luteum. Biol Reprod 54:76–83 [DOI] [PubMed] [Google Scholar]
- Bogan RL, Murphy MJ, Stouffer RL, Hennebold JD 2008 Systematic determination of differential gene expression in the primate corpus luteum during the luteal phase of the menstrual cycle. Mol Endocrinol 22:1260–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluffo MC, Young KA, Stouffer RL 2005 Dynamic expression of caspase-2, -3, -8, and -9 proteins and enzyme activity, but not messenger ribonucleic acid, in the monkey corpus luteum during the menstrual cycle. J Clin Endocrinol Metab 90:2327–2335 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.