Abstract
As is typical of other hormone systems, the actions of the thyroid hormones (TH) differ from tissue to tissue depending upon a number of variables. In addition to varying expression levels of TH receptors and transporters, differing patterns of TH metabolism provide a critical mechanism whereby TH action can be individualized in cells depending on the needs of the organism. The iodothyronine deiodinases constitute a family of selenoenzymes that selectively remove iodide from thyroxine and its derivatives, thus activating or inactivating these hormones. Three deiodinases have been identified, and much has been learned regarding the differing structures, catalytic activities, and expression patterns of these proteins. Because of their differing properties, the deiodinases appear to serve varying functions that are important in regulating metabolic processes, TH action during development, and feedback control of the thyroid axis. This review will briefly assess these functional roles and others proposed for the deiodinases and examine some of the current challenges in expanding our knowledge of these important components of the thyroid homeostatic system.
This review assesses the functional roles of TH action and others proposed for the deiodinases and examines current challenges in expanding knowledge of these components of the thyroid homeostatic system.
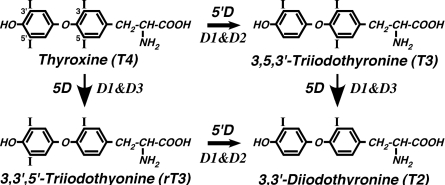
The deiodination of T4, T3, and other iodothyronines is an integral component of thyroid hormone (TH) homeostasis. Catalyzed by three separate enzymes coded from three different genes, deiodination, depending on whether it occurs at the 5- or 5′-position on the iodothyronine molecule, serves to either activate or inactivate this class of compounds (Fig. 1). Thus, along with transport mechanisms that govern the flux of TH into and out of cells (1), the deiodinases function at a prereceptor level to influence both extracellular and intracellular TH levels and TH action.
Figure 1.
Reactions catalyzed by the iodothyronine deiodinases. T4 is a relatively poor substrate for 5′-deiodination by the D1, whereas 5-deiodination by this enzyme most efficiently uses the sulfated derivatives (at the 4′-position) of T4 and T3 as substrates. 5D, 5-Deiodination; 5′D, 5′-deiodination.
Over four decades of research have brought us to the point where a great deal is known regarding the properties of these enzymes, referred to as the types 1, 2, and 3 deiodinases (D1, D2, D3) (2,3,4). In particular, studies using tissue homogenates and cell culture systems, as well as molecular cloning techniques, have defined to a considerable extent the structures, catalytic properties, expression patterns and regulatory mechanisms that pertain to these enzymes in a variety of mammalian and other vertebrate species. Key elements of this information for the human and rodent deiodinases are summarized in Table 1, including phenotypic features of deiodinase-deficient transgenic mice. Much of this information has recently been reviewed in detail elsewhere (2,3,4).
Table 1.
Characteristics of the iodothyronine deiodinases
| Characteristic | D1 | D2 | D3 |
|---|---|---|---|
| Reaction catalyzed | 5 or 5′ | 5′ | 5 |
| Substrate preference | 5: T4S > T3S >> T3, T4 5′: rT3, rT3S > T2S >> T4 | T4 > rT3 | T3 > T4 |
| Km (dithiothreitol as cofactor) | T4S (5): 0.3 μmol/liter | T4 (5′): 1 nmol/liter | T3 (5): 6 nmol/liter |
| rT3 (5′): 0.06 μmol/liter | T4 (5): 37 nmol/liter | ||
| T4 (5′): 2.3 μmol/liter | |||
| Molecular mass (kDa) | 29 | 30 | 32 |
| Selenocysteine | Present | Present | Present |
| Homodimer | Yes | Yes | Yes |
| Chromosomal location (human) | 1p32-p33 | 14q24.3 | 14q32 |
| Location | Liver, kidney, thyroid, pituitary | Pituitary, brain, BAT, thyroid,a heart,a skeletal musclea | Brain, skin, uterus, placenta, fetus |
| Activity in hypothyroidism | ↓, Liver, kidney ↑, Thyroid | ↑, All tissues | ↓, Brain |
| Activity in hyperthyroidism | ?, Liver, kidney ↑, Thyroid | ↓, Most tissues ↑, Thyroida | ↑, Brain |
| Inhibitors | |||
| Propylthiouracil | ++++ | + | +/− |
| Aurothioglucose | ++++ | ++ | ++ |
| Iopanoic acid | +++ | ++++ | +++ |
| Knockout phenotypes | |||
| Principal phenotypic features of deiodinase-deficient mouse | ? Serum T4 and rT3 Normal serum T3 and TSH Enhanced fecal excretion of endogenous iodothyronines | ↑Serum T4 and TSH Normal serum T3 and rT3 Pituitary resistance to T4 Impaired thermogenesis Impaired hearing Mild impairment of neurocognition | Increased perinatal mortality Growth retardation Impaired fertility in females and males Hyperthyroidism in the perinatal period Hypothyroidism in adulthood |
Humans only.
Of particular note are the catalytic actions of the D2, which is directed almost exclusively at the efficient conversion of T4 to T3 by 5′-deiodination (an activating reaction), and the action of the D3, which inactivates T4 and T3 by converting them to relatively inactive lesser iodothyronines (rT3 and 3,3′-diiodothyronine, T2) by 5-deiodination (5). In contrast, the D1 is able to catalyze both 5- and 5′-deiodination and can act on a variety of different iodothyronines, including those that have undergone sulfate conjugation of the hydroxyl group at the 4′-position on the outer ring (5,6).
Another notable feature of the deiodinases is that all are selenoenzymes; they contain the rare amino acid selenocysteine as a key residue at the active site (7). Substitution of cysteine for selenocysteine markedly impairs the catalytic efficiency of the deiodinases and substitution with any other amino acids renders the protein inactive (8,9,10). The presence of selenocysteine has implications beyond catalytic activity, in that the cellular processes for synthesizing selenoproteins are complex and inefficient (11) and may be impaired in some tissues by nutritional selenium deficiency (12,13,14).
The purpose of this brief review is to step back, for a moment, from what is a large body of experimental data and try to provide a perspective of the field in terms of what we have learned about the physiological roles of these enzymes and the challenges we face in trying to define further their function and bring this knowledge into the clinical arena.
What We Know: Concepts Well Established
The deiodinases play essential roles in coordinating TH action during vertebrate development
TH are critical signaling molecules that help to orchestrate the developmental programs of all vertebrate species (15,16). The deiodinases, particularly the D2 and D3, are expressed widely and in a dynamic, coordinated fashion during development, and it is now clear that they are important for regulating the circulating and tissue levels of TH during this period (17). As such, they play a critical role in developmental processes, and this has been demonstrated in several species.
Amphibian metamorphosis is a particularly dramatic example of the importance of TH in development and illustrates the key roles played by the deiodinases (18,19,20). In the absence of TH, metamorphosis of the tadpole into the mature frog does not occur (21). Furthermore, using iopanoic acid to block D2 activity in Rana catesbeiana tadpoles renders T4, but not T3, ineffective in inducing metamorphic changes, demonstrating the importance of this enzyme in tadpole development (22). Additional studies have clearly demonstrated that the diverse expression patterns of the D2 and D3 during tadpole development are exquisitely coordinated to regulate TH action at the local cellular level in a manner that ensures the correct timing of the metamorphic changes taking place in various tissues (20,23,24).
The D3 has also been demonstrated to be critically important for regulating the degree of exposure of the mammalian fetus and neonate to TH (25,26,27). A deficiency of TH during mammalian development, such as occurs with severe iodine deficiency, can lead to life-long and catastrophic neurological impairment and result in the syndrome of cretinism (28,29,30). Yet, almost from the moment of implantation until birth, the mammalian fetus is surrounded by tissues (i.e. the decidua and placenta) that express extraordinarily high levels of D3 and appear to be instrumental in maintaining circulating and tissue levels of fetal TH exceedingly low relative to maternal or adult levels (31,32,33). This has led to the concept that TH levels during development are tightly regulated within narrow limits to avoid either an excess or a deficiency of these compounds and that the D3, along with the D2, is largely responsible for this precise level of control.
That being the case, one might predict that a D3 deficiency would have profound consequences with regard to fertility and developmental processes. Characterization of a transgenic D3-deficient [D3 knockout (D3KO)] mouse demonstrates that this is the case (34,35). Both male and female D3KO mice have impaired fertility such that breeding for the purpose of maintaining a colony of transgenic animals is best accomplished by mating heterozygous parents. There is also significant perinatal mortality of the D3KO pups, and both pups and adults are significantly growth impaired. In addition, the developmental programming of the thyroid axis is markedly perturbed in the D3KO mouse, presumably by the overexposure of the animal to excessive levels of TH in utero and during the first 2 wk of perinatal life. Thus, an initial phase of hyperthyroidism gives way in the latter part of the perinatal period to a moderate state of hypothyroidism that persists into adulthood, where functional abnormalities in the hypothalamus, pituitary gland, and thyroid gland have all been demonstrated (36). Notably, these abnormalities of the thyroid axis resemble those observed in human children born to mothers with poorly controlled hyperthyroidism during pregnancy (37,38) and also rodents administered exceeding high doses of TH transiently in the immediate neonatal period (i.e. neo-T4 syndrome) (39,40,41,42). Although much remains to be learned regarding the exact mechanisms leading to the phenotypic abnormalities in the D3KO mouse, the studies to date clearly demonstrate the proof of principle regarding the importance of the D3 during development.
The deiodinases regulate TH action within selected tissues during both development and in adulthood
The concept that the D2 and D3 provide a means of regulating local concentrations of active TH, thereby individualizing TH action within a subset of cells within a tissue, has been demonstrated by direct functional studies in diverse species and systems. Thus, in an example again taken from the metamorphosing tadpole, Marsh-Armstrong et al. (43) have demonstrated that the inactivating D3 is expressed in the dorsal ciliary marginal zone of the eye where it inactivates TH, thus inhibiting proliferation of these cells and allowing for the asymmetric growth of the retina that is characteristic of the frog. Direct confirmation of their hypothesis employed the use of the deiodinase inhibitor iopanoic acid and the expression of a D3 transgene in elegant studies that assessed the rates of cellular proliferation and anatomic changes in the tadpole retina.
Convincing evidence that the deiodinases play critical roles in modulating development in selective mammalian tissues has also been derived from the study of deiodinase-deficient mouse models. For example, Campos-Barros et al. (44) have demonstrated a striking transient spike in D2 activity in the cochlea of wild-type mice between postnatal d 2 (P2) and P12 of life, resulting in a T3/T4 ratio in this organ at d P8 of 1.76, compared with the corresponding serum hormone ratio of 0.02, a nearly 100-fold enrichment in the T3 content of this organ relative to that of serum. This suggested that a D2-mediated increase in the local T3 content might be critical for cochlear development and hearing. This hypothesis was confirmed when genetically modified mice completely devoid in D2 activity (D2KO mouse) were found to have markedly impaired hearing, a trait that could be rescued by the judiciously timed administration of high doses of T3 via the nursing dam (45). More recently, it has been found that D3 is also expressed in the mouse cochlea starting before the spike in D2 expression, and indeed D3KO mice also have impaired hearing (46). Thus, either premature exposure of the developing cochlea to T3 (due to an absence of D3) or failure to achieve the needed high local concentration of T3 later in development (due to an absence of D2) results in uncoordinated development and impaired hearing. This demonstrates that, as in the tadpole, coordinated expression of the D2 and D3 is required for proper development of selected mammalian processes.
The importance of local regulation of TH levels by the deiodinases is not confined to the developmental period. The D2KO mouse in adulthood has elevated levels of both serum T4 and TSH (with a normal serum T3), suggesting that there is resistance to the feedback effects of T4 at the level of the hypothalamus and/or anterior pituitary (47). This thesis has been confirmed by demonstrating impaired suppression of the TSH level by the administration of T4, but not T3, to the mutant mice (47). A similar pattern of serum TH levels and resistance to T4 has recently been described in the only report to date of what is likely a D2 deficiency in humans, in this case resulting from a deficiency of the selenocysteine insertion sequence-binding protein 2, which results in impaired synthesis of D2 and other selenoproteins (48).
D2 has also been demonstrated to be essential as a local source of T3 in brown adipose tissue (BAT) to facilitate adaptive thermogenesis in the face of cold exposure (49,50). Thus, despite a normal circulating T3 level in the adult D2KO mouse, cold exposure results in hypothermia due to impaired BAT thermogenesis, and the animals survive only by compensatory shivering with consequent acute weight loss (51). The importance of the D2 in energetic pathways has recently been demonstrated further by the report that in mice, the induction of D2 in BAT by bile acids, working through a unique G protein-coupled receptor (TGR5), protects against insulin resistance and diet-induced obesity, a finding with potentially important implications with regard to the treatment of human obesity (52).
A remarkable feature of the deiodinases is that their activity levels are highly regulated by thyroid status via both pre- and posttranslational mechanisms (Table 1) (2,53,54). For the D2 and D3, the effects of hypo- and hyperthyroidism result in alterations in enzyme activities that appear designed to maintain local tissue T3 content as normal as possible in the face of altered serum hormone levels. Thus, under conditions of iodine deficiency and in hypothyroidism, D2 activity is markedly up-regulated (55,56), and this has been demonstrated to increase the proportion of T3 formed locally in D2-expressing tissues and to mitigate decreases in tissue T3 content (57,58). D3 activity is decreased in hypothyroidism presumably to lessen degradation of T4 and T3, although this has not been directly demonstrated (56). Opposite changes in D2 and D3 activity occur in hyperthyroidism (56). In another apparent adaptive response, TSH stimulation of the thyroid gland in primary hypothyroidism results in increased D1 activity in this organ, which may serve to increase T4 to T3 conversion and hence T3 secretion under these circumstances (59). Interestingly, in the setting of Graves’ disease, stimulation of D1 activity by thyroid-stimulating Ig may serve to exacerbate the hyperthyroid state (60,61).
Overexpression of deiodinases can cause human disease
Finally, the potential for overexpression of the D2 or D3 to cause human disease has clearly been demonstrated. Thus, a high level of expression of the D3 in certain vascular tumors can lead to a state of consumptive hypothyroidism wherein the metabolism and inactivation of T4 and T3 by this enzyme outstrip the ability of the thyroid axis to secrete TH and necessitate the administration of high doses of these hormones in order to maintain a euthyroid state (62,63). Alternatively, the overexpression of the D2 in rare cases of metastatic follicular thyroid carcinoma can render a patient thyrotoxic due to enhanced T3 production in malignant cells while taking standard doses of T4 for replacement or suppressive therapy (64,65). Increased expression of the D1 and/or D2 in the thyroid gland in functioning thyroid nodules, Graves’ disease (61,66), and McCune-Albright syndrome (67) also likely results in an enhanced rate of intrathyroidal T4 to T3 conversion and contributes to the increased secretion of T3 observed in these conditions.
What We Think We Know: Concepts Surmised
The success in demonstrating conclusively the roles of the D2 and D3 in the above physiological and pathological situations results from the availability of appropriate model systems that allow the activity of the deiodinases to be selectively altered to assess directly the effects of such manipulations on clearly defined actions of TH (e.g. retinal proliferation in the tadpole, hearing in the mouse, and suppression of TSH). In contrast, many current concepts about the roles of these enzymes remain tentative or incomplete, in part because it has not been possible to bring such stringent experimental criteria to bear on these problems. Examples of these follow.
Conversion of T4 to T3 by the 5′-deiodinases in peripheral tissues and the thyroid gland is an important contributor to systemic T3 production and serum T3
A generally accepted concept is that the D1 and D2 contribute significantly to whole-body T3 production and the serum T3 level in humans and animals (68). Indeed, common experience indicates that the administration of T4 to athyreotic humans results in the production of sufficient quantities of T3 to maintain a clinical state of euthyroidism with normal serum T3 levels. In vivo kinetic studies, as well as direct measurements of the rates of thyroidal T4 and T3 secretion, have led to estimates that in the euthyroid state, as much as 80% of the daily T3 production in humans and 50% in rodents results from 5′-deiodination processes in extrathyroidal tissues (68,69,70). Both the D1 and the D2 appear to contribute to this T3 production with estimates of the contribution by the D2 in humans and rodent ranging from 23–44% as determined by early reports using noncompartmental analysis of in vivo kinetic data (71,72,73). More recent analyses have made use of other techniques. Thus, using direct whole-body steady-state measurements of T3 production, Nguyen et al. (74) have suggested that the D1 and D2 contribute equally to extrathyroidal T3 production in rats. An even greater role for the D2 in humans has recently been suggested by Maia et al. (75). Based on extrapolating deiodination rates observed in a cell culture system to the intact organism, these investigators have suggested that approximately two thirds of the T3 production in humans is derived from the D2 with higher and lower proportions obtained in hypothyroidism and hyperthyroidism, respectively, due to the changes in D1 and D2 activities observed under these conditions.
In addition to the importance of the D1 and D2 in converting T4 to T3 in peripheral tissues, these enzymes are also highly expressed in the thyroid gland (human D1 and D2 and rodent and dog D1 only) (76,77,78). There is evidence that T4 to T3 conversion occurs within this organ, as based on references cited above and more definitively on perfusion studies that demonstrate that thyroidal secretions in dog and human contain a higher ratio of T3 to T4 than that found in thyroglobulin molecules, and that this preferential secretion of T3 can be inhibited by propylthiouracil, which impairs the activity of the D1 (79,80).
Taken together, this substantial body of evidence would lead one to predict that the inability to carry out 5′-deiodination in the thyroid and in extrathyroidal tissues would severely compromise T3 production and result in a decreased serum T3 level. Such is not the case, at least not in the mouse under euthyroid conditions. Thus, the D1KO and the D2KO mouse both manifest a normal serum T3 level (47,81) as, surprisingly, does the combined D1/D2KO animal (82). In this animal, there is no compensatory decrease in D3 activity, nor is the clearance of T3 significantly reduced. The serum TSH levels in the D2-deficient mouse models (D2KO and D1/D2KO) are approximately twice normal but dramatically less than would be encountered in a hypothyroid animal (47). The only source of T3 in the D1/D2KO mouse is that derived directly from the hydrolysis of thyroglobulin, thus demonstrating that under selected circumstances, the thyroid gland itself, under the direction of the hypothalamus and pituitary, is able to supply sufficient T3 to maintain the serum T3 concentration as normal. This ability of the thyroid axis to compensate for the lack of 5′-deiodinase processes is a remarkable display of its adaptive ability. Indeed, within the warm confines of our vivarium, the D1/D2KO mouse maintains surprisingly normal thyroid homeostasis despite the complete inability to convert T4 to T3 in any tissues including the thyroid gland.
Whether such a significant degree of compensation would be observed in humans completely lacking 5′-deiodinase activity is uncertain, because to date, no inactivating mutations in the deiodinase genes in humans have been described. However, Dumitrescu et al. (48) have described two families with partial inactivating mutations in the selenocysteine insertion sequence-binding protein 2, which is essential for the synthesis of selenoproteins (83). Notably, the serum hormone profile in affected members of these families bear a striking resemblance to that of the D1/D2KO mouse: an elevated serum T4 level, a serum TSH level at the upper range of normal or slightly elevated, and a serum T3 level that is in the low normal range or minimally reduced. These findings suggest that the phenotype of the D1/D2KO animal has relevance to the human condition and suggests that there can be life without the ability to convert T4 to T3.
The deiodinases are important determinants of the alterations in systemic TH levels observed in illness and nutritional deficiency
Fasting and severe illness induce profound changes in TH economy marked by decreases in serum T4 and T3 levels without a compensatory rise in the serum TSH level in both humans and animal models (84,85,86). This generalized suppression of the thyroid axis is associated with a significant decline in basal metabolic rate and decreased protein and fat catabolism (87,88), and it has been postulated that this has adaptive benefit to the organism, although there is contradictory evidence (89,90). The pathophysiological mechanisms responsible for these systemic changes are complex and remain poorly understood (84,91). However, alterations in deiodinase activities have been postulated as playing important roles (Fig. 2).
Figure 2.
Proposed roles for the deiodinases in the systemic response of the thyroid axis to illness or nutritional deprivation. Recent studies have called these concepts into question (see text).
Observed decreases in hepatic D1 activity (resulting in a possible decrease in T3 production) and increases in hepatic and skeletal muscle D3 activity (resulting in a possible increase in T3 and T4 degradation) in states of fasting and/or illness have been implicated as causative factors in the declines in serum T3 and T4 levels (84,91). In addition, increased D2 activity in the hypothalamus of ill or fasted rodents has been shown to result in an increased T3 level in this tissue, and this is postulated to result in enhanced feedback suppression on TRH and TSH production despite lowered serum levels of T3 and T4 (92,93). [Note that D2 activity in human skeletal muscle, a potential modulator of systemic T3 levels during acute illness, is unchanged in this condition (94).]
Such concepts, however, are based on indirect evidence whereby deiodinase activities have been correlated with serum and/or tissue TH levels, and it is possible and perhaps likely that some of the observed changes in deiodinase activity (e.g. the decrease in hepatic D1 activity) are a consequence of, rather than a cause of, the decrease in the serum T3 level (95,96). Indeed, in two studies in which the in vivo rate of T4 to T3 conversion was measured directly in fasting animals by injecting [125I]T4 and quantifying [125I]T3 appearance in whole-animal homogenates, the fraction of T4 converted to T3 was approximately doubled and total T3 production was unchanged (97,98). These latter reports call into question the interpretation of kinetic studies from human and animal experiments, which are based solely on data derived from analysis of serum and where the decreased plasma appearance of T3 is assumed to be attributed to a decrease in tissue 5′-deiodinase (98,99). Thus, much of the T3 produced during fasting or illness may remain in tissues and not exchanged with the plasma T3 pool but, rather, metabolized via other degradative pathways or excreted via stool or urine. This concept is supported by recent studies in which the administration of supraphysiological amounts of T4 and/or T3 to rabbits with systemic illness was required to correct the serum levels of these hormones, suggesting that enhanced hormonal degradation and/or excretion, rather than diminished thyroidal secretion or decreased T4 to T3 conversion was playing a dominant role in the response to nonthyroidal illness (100).
Studies in deiodinase-deficient mice raise further questions regarding the roles of these enzymes in the systemic response to fasting and illness. Thus, D3KO mice subjected to a systemic bacterial infection develop marked reductions in their serum T4 and T3 levels comparable to those in infected wild-type animals, demonstrating that at least in this model, D3 is not required for development of a nonthyroidal illness-type syndrome (101). Furthermore, in preliminary studies, we have observed that fasting or the induction of systemic illness by the administration of lipopolysaccharide induces alterations in serum TH levels in D1/D2KO mice that are the same as those observed in fasted or ill wild-type animals, calling into question the importance of changes in the D1 and D2 as mediators of the systemic response to nutritional deprivation (Galton, V. A., and D. L. St. Germain, unpublished observations). Thus, the extent to which changes in deiodinase activities are necessary or responsible for systemic alterations in the thyroid axis in response to illness and nutritional deprivation remains uncertain.
Induction of D3 activity in response to tissue injury may influence healing or regenerative processes
Even if the deiodinases do not play a major role in mediating the systemic response of the thyroid axis to illness or altered nutrition, the changes in deiodinase expression observed in these circumstances could influence TH action locally in individual tissues or subsets of cells within an organ. In this regard, considerable interest has been generated by recent observations that D3 is induced in vivo at sites of tissue injury and inflammation and in cell culture systems by conditions of hypoxic or oxidative stress (102,103,104). Thus, D3 induction has been observed in animal models at sites of local inflammation (105,106), in peripheral nerves in response to cryolesion-induced injury (107), and in the heart in the setting of myocardial infarction (108) and cardiac hypertrophy (109). However, caution should be observed in assigning significance to these observations, because in some cases, the observed level of D3 activity have been exceedingly low (e.g. the reported D3 activity level of 0.1 fmol/min·mg protein in the right ventricle of a rat model of cardiac hypertrophy is approximately 1/60th of that observed in the neonatal mouse hypothalamus (35) and 1/5000th of that noted in the rat pregnant uterus shortly after blastocyst implantation (32) and could represent a nonspecific induction of gene expression with little or no functional consequences. Alternatively, there may be a significant level of D3 expressed in a subset of cells under these circumstances that has important effects on local TH action.
If this is the case, then presumably the induction of D3 leads to a localized region of hypothyroidism within the tissue in question, and this could influence healing or regenerative processes. For example, Li et al. (107) have suggested that the induction of D3 in peripheral nerve after injury may serve to reduce temporarily TH action locally and delay the myelination of regenerating fibers, given that the expression of myelin basic protein is a TH-dependent process (110). This elegant concept requires confirmation in an in vivo system in which the level of D3 activity can be manipulated at sites of nerve injury and the effects on regeneration assessed.
As an additional example, a low level of D3 activity (∼0.5 fmol/min·mg protein) is induced in the heart after myocardial infarction (108). Conceivably, D3 expression in this setting could serve to induce or accentuate a hypothyroid state within cardiac tissue after ischemic injury. This might be protective to cardiac cells in the acute stages of injury, without contributing to the adverse effects (e.g. increased peripheral vascular resistance) that accompany the systemic hypothyroid state. Such an outcome might tend to reinforce any long-term beneficial effects of the nonthyroidal illness syndrome. Alternatively, the D3 induction might have detrimental long-term consequences given that T4, T3, and T4 analogs (e.g. diiodothyropropionic acid) have been shown to stimulate angiogenesis in the postinfarct rat heart (111,112,113,114). Such potentially paradoxical effects highlight the complexities in trying to predict the local functional consequences of deiodinase expression and point toward the need for carefully controlled studies to assess the significance of this D3 stress response.
The D2 is important for brain development and function
Neurological development in rodents, including mice, is known to be dependent on the provision of appropriate TH levels in the brain; severe abnormalities in learning, memory, and locomotion are observed in hypothyroid animals (115) or animals in which TH action has been disrupted (116). Much circumstantial evidence has led to the concept that the D2, and as a corollary the prohormone T4, is critical for brain development and function (117). This concept is derived from studies in rodents where 1) it is estimated that more than 50% of the T3 in the euthyroid adult rat brain is derived from local T4 to T3 conversion (118), 2) the D2 has been demonstrated to be the predominant pathway for extrathyroidal and intracerebral T3 production in the euthyroid neonatal rat (58,119), and 3) it has been observed that only the provision of T4, and not T3, to a hypothyroid pregnant dam provides for restoration of the fetal brain T3 content (120). Given that D2 is required both in utero and later in development for T4 to T3 conversion in the brain, the latter finding implies that normal brain development is dependent largely, if not exclusively, on the D2 to maintain brain TH action.
Observations made on the D2KO mouse call this concept into question; the neurological phenotype of this animal, which manifests a D2 deficiency from the time of conception, is surprisingly mild and manifests essentially none of the severe neurological abnormalities noted when rodents are hypothyroid during the developmental period (121). This is particularly surprising given that the T3 content of the 15-d-old D2KO mouse is reduced by 50% (thereby verifying that the D2 is an important contributor to brain T3 content), the same percentage as that noted in concurrently studied hypothyroid wild-type neonatal mice. Consistent with the mild neurobehavioral phenotype of the D2KO animal are molecular studies showing that alterations in expression of TH-dependent genes in several brain regions are significantly milder than in the hypothyroid neonates.
The reasons for these unexpectedly mild phenotypic features of the D2KO mouse are as yet uncertain but may indicate that T3 obtained from serum, the level of which is normal in D2KO pups, plays a more important role than previously thought in brain development, or that T4 in brain can compensate to a significant extent for the deficiency of T3. (The T4 levels in both serum and brain are approximately doubled in the D2KO pup.) Indeed, the lowering of serum TH levels in the D2KO mouse to render it hypothyroid results in a severe and lethal phenotype (Galton, V. A., unpublished observations), suggesting that the role of the D2 in brain development is particularly important in states of thyroid insufficiency. Alternatively, or in addition, we may not yet be aware of the appropriate subset of TH actions that would allow us to detect compromised neurobehavioral or neuromolecular development in the D2KO mouse.
The D1 serves principally as a scavenger enzyme
Whereas the D2 and D3 appear to have well defined functions, the physiological role of the D1 is difficult to define and to a considerable extent remains an enigma. Based on the prior concept that the majority of circulating T3 was derived from T4 via the catalytic activity of this enzyme, emphasis has been placed on the capability of the D1 for 5′-deiodination of T4 to T3. However, the human and rodent D1 is extremely inefficient in carrying out this reaction when compared either with the D2, which has a 700-fold greater catalytic efficiency for 5′-deiodination of T4 (75), or with its own catalytic capabilities for 5′-deiodinating rT3, rT3S, or T2S or 5-deiodinating T4S or T3S (6,122). Thus, the D1 catalyzes the deiodination of these latter substrates with efficiencies that range from approximately 20-fold (T3S) to over 600-fold (rT3) greater compared with the 5′-deodination of T4. Although the large mass of D1 present in the liver does clearly allow for T4 to T3 conversion, this deiodinase seems much better positioned to clear from the circulation rT3 and other inactive, sulfated, and lesser iodothyronines, compounds generally not degraded efficiently by the D2 or the D3 (122). Indeed, studies in the D1KO mouse have demonstrated that iodothyronines other than T4 are the principal substrates for 5′-deiodination by this enzyme (81), which releases iodine back into the circulation for excretion in the urine or reclamation in the thyroid gland should the iodine supply be deficient. In the absence of the D1, inactive and lesser iodothyronines escape deiodination and are excreted in the feces with the potential loss of the associated iodine. Thus, this scavenging function of the D1 may take on a particularly important role in the setting of iodine deficiency.
This concept of the D1 as a scavenger deiodinase may also hold true regarding its expression in the thyroid gland, where in addition to converting T4 to T3, it may also deiodinate rT3 and lesser iodothyronines released from thyroglobulin. In this role, it might assist the iodotyrosine dehalogenase (DEHAL1), which deiodinates monoiodotyrosine and diiodotyrosine, for recycling iodine within the thyroid gland (123). Evidence for this comes from studies comparing the iodothyronine content of thyroglobulin with the experimentally determined rates of secretion of T4, T3, and rT3 from the human thyroid gland. Notably, the T3/T4 ratio in thyroid secretions is considerably higher than that found in thyroglobulin, whereas just the opposite finding is true regarding the rT3/T4 ratio (70,80,124). These observations suggest that there is a net formation of T3 and a net degradation of rT3 within the thyroid gland after thyroglobulin hydrolysis, presumably by the D1 (and/or D2 in humans).
Current Challenges and Future Opportunities
Much has been learned regarding the structure and function of the deiodinases over the past four decades, yet much remains to be done to achieve the goal of using our current and future knowledge for maintaining health and treating disease. An important aspect of future research will be to move beyond correlative studies, whereby speculation about deiodinase function is based on associated changes in serum or tissue TH levels or some readout of TH action. Although such associations are important for hypothesis generation, they rarely allow for the definitive assignment of functional roles. Among the major challenges in accomplishing this goal are the following.
Relative lack of knowledge regarding the effects of TH in tissues and cells
Given that the function of the deiodinases is to regulate the circulating and prereceptor concentrations of TH, our understanding of their roles in health and disease is dependent on a detailed knowledge of the effects of TH action in individual cells and tissues. Thus, a comprehensive understanding of TH action is needed to allow a less biased and more expansive approach to assessing functional outcomes of altered deiodinase expression. Unfortunately, our knowledge of both the physiological and molecular effects of TH action remains largely incomplete regarding most cells and tissues. For example, it is possible that the mild neurological phenotype of the D2KO mouse results, at least in part, from our ignorance with regard to appropriate molecular and neurobehavioral readouts to assess. Thus, a concerted effort is needed to define in a comprehensive manner the effects of TH at the molecular (i.e. the thyroidome or thyrome) and physiological level in major tissues and cell types to provide additional markers of TH action. This is of particular importance with regard to those cells and tissues where low levels of expression of deiodinases occur. In the case of tissues with heterogeneous cell types, techniques to isolate individual cell types will need to be employed.
Relative lack of knowledge regarding the trafficking of T3 and T4 within and between cells
The recent description of specific transporters that mediate TH entry and exit from certain cells has been a major advance in our understanding of the processes that control TH action. Yet, to understand the function of the deiodinases, we need to learn much more about how TH are trafficked within and between cells. For example, one explanation offered as to why the catalytic action of the D2 contributes to the local T3 content of a tissue is that the enzyme is located in the endoplasmic reticulum, and thus T3 derived from this enzyme is presumed to have ready access to the nucleus and the T3 receptors (125). Yet this hypothesis fails to take into account the observations that in at least some tissues, in particular the central nervous system, T3 derived from the D2 appears to act in cells other than those in which it is formed. One example of this is in the developing cochlea where D2 is localized in the connective tissue that gives rise to the bony labyrinth, whereas TRβ and TRα1 are mainly expressed in the sensory epithelium and spiral ganglion (44). Similarly, in the hypothalamus and other regions of the brain, the D2 is expressed primarily in glial cells and not in neurons, which are thought to be the principal T3-responsive cells (126). Thus, a more complete understanding of cellular transport of TH into, within, and between cells is critical to our appreciating how deiodinases function in complex tissues such as the brain.
Current models for studying the effects of deiodination have significant limitations
Current models for defining the roles of deiodinases include amphibian and rodent models and various cell culture systems. Although extremely useful, these have certain shortcomings including the fact that the expression patterns of D2 and D3 appear to differ somewhat between rodents and humans under both basal conditions, where D2 expression is more widespread in the human (127), and during illness, where D3 activation in human liver and skeletal muscle appears to be more rigorous than in the rodent (128). In addition, the availability of pharmacological inhibitors of the deiodinases is limited. Although propylthiouracil is a relatively specific inhibitor of the D1 and iodinated contrast agents such as iopanoic acid inhibit all three deiodinases, both also have direct inhibitory effects on the function of the thyroid gland, which complicates their use in in vivo studies (129,130). To date, no inhibitors specific for the D2 and D3 have been described. The availability of these would provide useful tools for functional studies.
In place of selective pharmacological inhibitors, genetic models of deiodinase deficiency have been developed and proven useful in the hands of several investigators (34). However, complexities arise in the use of these animals in that the developmental abnormalities noted in the D2KO and D3KO mice, which have constitutive deiodinase deficiencies, persist into adulthood and complicate the assessment of the functional roles of these enzymes later in life. Thus, the development of conditional knockout animals that allow for the targeting of deiodinase deficiencies to specific organs or at specific times in the life cycle of the organism would be of significant benefit. An alternative approach in this regard would be the use of small interfering RNA techniques.
Only limited use has been made of transgenic overexpression models in the study of the deiodinases, but when employed, these have been very instructive. The few examples reported include transgenic tadpoles expressing the D3 (131) and mouse models where D2 expression has been targeted to the myocardium (132,133).
Finally, we need to be more creative in the experimental paradigms employed in defining the roles of these enzymes, because it is likely that environmental stresses will exacerbate phenotypic abnormalities in deiodinase-deficient animals relative to when they are housed under controlled conditions in a vivarium.
Use of the deiodinases to manipulate TH action in selected tissues
Ultimately, a better understanding of the importance of the deiodinases in controlling TH action during both development and in adulthood may lead to translational applications. With this knowledge may come treatments for neurological, psychiatric, metabolic, or cardiac disorders influenced by TH. Paradigms of such research are the discovery that selected bile acids can induce D2 activity, and hence thermogenesis, in selected tissues (52) and a recent report of D2 being targeted for overexpression in the heart (133). This latter maneuver altered gene expression patterns in this organ and negated the adverse effects of pressure overload on the left ventricle.
Thus, eventually we may learn to manipulate local deiodinase activity and TH action in clinical situations through the use of pharmacological agents that can target selective tissues and whose design is based on a more complete understanding of the mechanisms responsible for regulating deiodinase expression and catalytic function (134).
Footnotes
This work was supported by National Institutes of Health Grants 5R01DK54716 and R01HD09020.
Disclosure Statement: The authors have nothing to disclose.
First Published Online January 29, 2009
Abbreviations: BAT, Brown adipose tissue; D1, type 1 deiodinase; D3KO, D3 knockout; P2, postnatal d 2; T2, 3,3′-diiodothyronine; TH, thyroid hormone.
References
- Visser WE, Friesema ECH, Jansen J, Visser TJ 2008 Thyroid hormone transport in and out of cells. Trends Endocrinol Metab 19:50–56 [DOI] [PubMed] [Google Scholar]
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR 2002 Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23:38–89 [DOI] [PubMed] [Google Scholar]
- Kohrle J 2007 Thyroid hormone transporters in health and disease: advances in thyroid hormone deiodination. Best Pract Res Clin Endocrinol Metab 21:173–191 [DOI] [PubMed] [Google Scholar]
- Gereben B, Zeold A, Dentice M, Salvatore D, Bianco AC 2008 Activation and inactivation of thyroid hormone by deiodinases: local action with general consequences. Cell Mol Life Sci 65:570–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg IN 1991 An overview of thyroid hormone deiodinases. In: Wu SY, ed. Thyroid hormone metabolism: regulation and clinical aspects. New York: Blackwell Scientific; 29–39 [Google Scholar]
- Toyoda N, Kaptein E, Berry M, Harney JW, Larsen PR, Visser TJ 1997 Structure-activity relationships for thyroid hormone deiodinases by mammalian type I iodothyronine deiodinases. Endocrinology 138:213–219 [DOI] [PubMed] [Google Scholar]
- St. Germain DL 2001 Selenium, deiodinases, and endocrine function. In: Hatfield DL, ed. Selenium: its molecular biology and role in human health. Boston: Kluwer Academic Publishers; 189–202 [Google Scholar]
- Berry MJ, Maia AL, Kieffer JD, Harney JW, Larsen PR 1992 Substitution of cysteine for selenocysteine in type I iodothyronine deiodinase reduces the catalytic efficiency of the protein but enhances its translation. Endocrinology 131:1848–1852 [DOI] [PubMed] [Google Scholar]
- Buettner C, Harney J, Larsen PR 2000 The role of selenocysteine 133 in catalysis by the human type 2 iodothyronine deiodinase. Endocrinology 141:4606–4612 [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Klootwijk W, Visser TJ 2003 Substitution of cysteine for selenocysteine in the catalytic center of type III iodothyronine deiodinase reduces catalytic efficiency and alters substrate preference. Endocrinology 144:2505–2513 [DOI] [PubMed] [Google Scholar]
- Xu XM, Carlson BA, Zhang Y, Mix H, Kryukov GV, Glass RS, Berry MJ, Gladyshev VN, Hatfield DL 2007 New developments in selenium biochemistry: selenocysteine biosynthesis in eukaryotes and archaea. Biol Trace Elem Res 119:234–241 [DOI] [PubMed] [Google Scholar]
- DePalo D, Kinlaw WB, Zhao C, Engelberg-Kulka H, St Germain DL 1994 Effect of selenium deficiency on type I 5′-deiodinase. J Biol Chem 269:16223–16228 [PubMed] [Google Scholar]
- Meinhold H, Campos-Barros A, Walzog B, Köhler R, Müller F, Behne D 1993 Effects of selenium and iodine deficiency on type I, type II, and type III iodothyronine deiodinases and circulating thyroid hormones in the rat. Exp Clin Endocrinol 101:87–93 [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Nicol F, Beckett GJ, Arthur JR 1996 Selenoenzyme expression in thyroid and liver of second generation selenium- and iodine-deficient rats. J Mol Endocrinol 16:259–267 [DOI] [PubMed] [Google Scholar]
- Morreale de Escobar G 2001 The role of thyroid hormone in fetal neurodevelopment. J Pediatr Endocrinol Metab 14:1453–1462 [PubMed] [Google Scholar]
- Forrest D, Reh TA, Rüsch A 2002 Neurodevelopmental control by thyroid hormone receptors. Curr Opin Neurobiol 12:49–56 [DOI] [PubMed] [Google Scholar]
- Galton VA 2005 The roles of the iodothyronine deiodinases in mammalian development. Thyroid 5:823–834 [DOI] [PubMed] [Google Scholar]
- Galton VA 1992 The role of thyroid hormone in amphibian metamorphosis. Trends Endocrinol Metab 3:96–100 [DOI] [PubMed] [Google Scholar]
- Tata JR 1996 Amphibian metamorphosis: an exquisite model for hormonal regulation of post-embryonic development in vertebrates. Dev Growth Differ 38:223–231 [DOI] [PubMed] [Google Scholar]
- Brown DD 2005 The role of deiodinases in amphibian metamorphosis. Thyroid 15:815–821 [DOI] [PubMed] [Google Scholar]
- Allen BM 1916 Extirpation experiments in Rana pipiens larvae. Science 44:755–757 [DOI] [PubMed] [Google Scholar]
- Becker KB, Stephens KC, Davey JC, Schneider MJ, Galton VA 1997 The type 2 and type 3 iodothyronine deiodinases play important roles in coordinating development in Rana catesbeiana tadpoles. Endocrinology 138:2989–2997 [DOI] [PubMed] [Google Scholar]
- Huang H, Cai L, Remo BF, Brown DD 2001 Timing of metamorphosis and the onset of the negative feedback loop between the thyroid gland and the pituitary is controlled by type II iodothyronine deiodinase in Xenopus laevis. Proc Natl Acad Sci USA 98:7348–7353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Brown DD 2004 Expression of type II iodothyronine deiodinase marks the time that a tissue responds to thyroid hormone-induced metamorphosis in Xenopus laevis. Dev Biol 266:87–95 [DOI] [PubMed] [Google Scholar]
- Cooper E, Gibbens M, Thomas CR, Lowy C, Burke CW 1983 Conversion of thyroxine to 3,3′,5′-triiodothyronine in the guinea pig placenta: in vivo studies. Endocrinology 112:1808–1815 [DOI] [PubMed] [Google Scholar]
- Castro MI, Braverman LE, Alex S, Wu C, Emerson CH 1985 Inner-ring deiodination of 3,5,3′-triiodothyronine in the in situ perfused guinea pig placenta. J Clin Invest 76:1921–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer RH, Galligan JP, Cannell GR, Addison RS, Roberts MS 1996 Maternal to fetal thyroxine transmission in the human term placenta is limited by inner ring deiodination. J Clin Endocrinol Metab 81:2247–2249 [DOI] [PubMed] [Google Scholar]
- Porterfield SP, Hendrich CE 1993 The role of thyroid hormones in prenatal and neonatal neurological development: current perspectives. Endocr Rev 14:94–106 [DOI] [PubMed] [Google Scholar]
- Ares S, Quero J, Morreale de Escobar G 2005 Neonatal iodine deficiency: clinical aspects. J Pediatr Endocrinol 18(Suppl 1):1257–1264 [DOI] [PubMed] [Google Scholar]
- Williams GR 2008 Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol 20:784–794 [DOI] [PubMed] [Google Scholar]
- Roti E, Fang SL, Green K, Emerson CH, Braverman LE 1981 Human placenta is an active site of thyroxine and 3,3′,5-triiodothyronine tyrosyl ring deiodination. J Clin Endocrinol Metab 53:498–501 [DOI] [PubMed] [Google Scholar]
- Galton VA, Martinez E, Hernandez A, St. Germain EA, Bates JM, St. Germain DL 1999 Pregnant rat uterus expresses high levels of the type 3 iodothyronine deiodinase. J Clin Invest 103:979–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SA, Dorfman DM, Genest DR, Salvatore D, Larsen PR 2003 Type 3 iodothyronine deiodinase is highly expressed in the human uteroplacental unit and in fetal epithelium. J Clin Endocrinol Metab 88:1384–1388 [DOI] [PubMed] [Google Scholar]
- St. Germain DL, Hernandez A, Schneider MJ, Galton VA 2005 Insights into the role of deiodinases from studies of genetically modified animals. Thyroid 15:905–916 [DOI] [PubMed] [Google Scholar]
- Hernandez A, Martinez E, Fiering S, Galton VA, St. Germain D 2006 Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Invest 116:476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A, Martinez ME, Liao XH, Van Sande J, Refetoff S, Galton VA, St. Germain DL 2007 Type 3 deiodinase deficiency results in functional abnormalities at multiple levels of the thyroid axis. Endocrinology 148:5680–5687 [DOI] [PubMed] [Google Scholar]
- Kempers MJ, van Tijn DA, van Trotsenburg AS, de Vijlder JJ, Wiedijk BM, Vulsma T 2003 Central congenital hypothyroidism due to gestational hyperthyroidism: detection where prevention failed. J Clin Endocrinol Metab 88:5851–5857 [DOI] [PubMed] [Google Scholar]
- Kempers MJ, van Trotsenburg AS, van Tijn DA, Bakker E, Wiedijk BM, Endert E, de Vijlder JJ, Vulsma T 2005 Disturbance of the fetal thyroid hormone state has long-term consequences for treatment of thyroidal and central congenital hypothyroidism. J Clin Endocrinol Metab 90:4094–4100 [DOI] [PubMed] [Google Scholar]
- Walker P, Courtin F 1985 Transient neonatal hyperthyroidism results in hypothyroidism in the adult rat. Endocrinology 116:2246–2250 [DOI] [PubMed] [Google Scholar]
- Azizi F, Vegenakis AG, Bollinger J, Reichlin S, Braverman LE, Ingbar SH 1974 Persistent abnormalities in pituitary function following neonatal thyrotoxicosis in the rat. Endocrinology 94:1681–1688 [DOI] [PubMed] [Google Scholar]
- Bakke JL, Lawrence NL, Bennett J, Robinson S 1975 The late effects of neonatal hyperthyroidism upon the feedback regulation of TSH secretion in rats. Endocrinology 97:659–664 [DOI] [PubMed] [Google Scholar]
- Dussault JH, Coulombe P, Walker P 1982 Effects of neonatal hyperthyroidism on the development of the hypothalamic-pituitary-thyroid axis in the rat. Endocrinology 110:1037–1042 [DOI] [PubMed] [Google Scholar]
- Marsh-Armstrong N, Huang H, Remo BF, Liu TT, Brown DD 1999 Asymmetric growth and development of the Xenopus laevis retina during metamorphosis is controlled by type III deiodinase. Neuron 24:871–878 [DOI] [PubMed] [Google Scholar]
- Campos-Barros A, Amma LL, Faris JS, Shailam R, Kelley MW, Forrest D 2000 Type 2 iodothyronine deiodinase expression in the cochlea before the onset of hearing. Proc Natl Acad Sci USA 97:1287–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L, Goodyear RJ, Woods CA, Schneider MJ, Diamond E, Richardson GP, Kelley MW, St. Germain DL, Galton VA, Forrest D 2004 Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proc Natl Acad Sci USA 101:3473–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L, Hernandez A, Ren T, He W, Srinvas M, Ma M, Galton VA, St Germain D, Forrest D 18 December 2008 Deafness and premature cochlear maturation caused by deletion of type 3 deiodinase, a thyroid-hormone inactivating enzyme. Endocrinology 10.1210/en.2008-1572 [Google Scholar]
- Schneider MJ, Fiering SN, Pallud SE, Parlow AF, St Germain DL, Galton VA 2001 Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol Endocrinol 15:2137–2148 [DOI] [PubMed] [Google Scholar]
- Dumitrescu AM, Liao XH, Abdullah MS, Lado-Abeal J, Majed FA, Moeller LC, Boran G, Schomburg L, Weiss RE, Refetoff S 2005 Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet 37:1247–1252 [DOI] [PubMed] [Google Scholar]
- Bianco AC, Silva JE 1987 Intracellular conversion of thyroxine to triiodothyronine is required for the optimal thermogenic function of brown adipose tissue. J Clin Invest 79:295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho SD, Kimura ET, Bianco AC, Silva JE 1991 Central role of brown adipose tissue thyroxine 5′-deiodinase on thyroid hormone-dependent thermogenic response to cold. Endocrinology 128:2149–2159 [DOI] [PubMed] [Google Scholar]
- de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, Harney JW, Larsen PR, Bianco AC 2001 The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest 108:1379–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J 2006 Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439:484–489 [DOI] [PubMed] [Google Scholar]
- Zavacki AM, Ying H, Christoffolete MA, Aerts G, So E, Harney JW, Cheng SY, Larsen PR, Bianco AC 2005 Type 1 iodothyronine deiodinase is a sensitive marker of peripheral thyroid status in the mouse. Endocrinology 146:1568–1575 [DOI] [PubMed] [Google Scholar]
- Sagar GDV, Gereben B, Callebaut I, Mornon J-P, Zeold A, da Silva WS, Luongo C, Dentice M, Tente SM, Freitas BCG, Harney JW, Zavacki AM, Bianco AC 2007 Ubiquitination-induced conformational change within the deiodinase dimer is a switch regulating enzyme activity. Mol Cell Biol 27:4774–4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters R, Fekete C, Goncalves C, Legradi G, Tu HM, Harney J, Bianco AC, Lechan RM, Larsen PR 2001 Regional physiological adaptation of the central nervous system deiodinases to iodine deficiency. Am J Physiol 281:E54–E61 [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale HF, Obregon MJ, Hernandez A, Escobar del Rey F, Morreale de Escobar G 1997 Regulation of iodothyronine deiodinase activity as studied in thyroidectomized rats infused with thyroxine or triiodothyronine. Endocrinology 138:2559–2568 [DOI] [PubMed] [Google Scholar]
- van Doorn J, Roelfsema F, van der Heide D 1986 Conversion of thyroxine to 3,5,3′-triiodothyronine in several rat tissues in vivo: the effect of hypothyroidism. Acta Endocrinol 113:59–64 [DOI] [PubMed] [Google Scholar]
- Silva JE, Matthews PS 1984 Production rates and turnover of triiodothyronine in rat-developing cerebral cortex and cerebellum: responses to hypothyroidism. J Clin Invest 74:1035–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech SG, Walker SW, Arthur JR, Lee D, Beckett GJ 1995 Differential control of type-I iodothyronine deiodinase expression by the activation of the cyclic AMP and phosphoinositol signalling pathways in cultured human thyrocytes. J Mol Endocrinol 14:171–177 [DOI] [PubMed] [Google Scholar]
- Toyoda N, Nishikawa M, Horimoto M, Yoshikawa N, Mori Y, Yoshimura M, Masaki H, Tanaka K, Inada M 1990 Graves’ immunoglobulin G stimulates iodothyronine 5′-deiodinating activity in FRTL-5 rat thyroid cells. J Clin Endocrinol Metab 70:1506–1511 [DOI] [PubMed] [Google Scholar]
- Laurberg P, Vestergaard H, Nielsen S, Christensen SE, Seefeldt T, Helleberg K, Pedersen KM 2007 Sources of circulating 3,5,3′-triiodothyronine in hyperthyroidism estimated after blocking of type 1 and type 2 iodothyronine deiodinases. J Clin Endocrinol Metab 92:2149–2156 [DOI] [PubMed] [Google Scholar]
- Huang SA, Tu HM, Harney JW, Venihaki M, Butte AJ, Kozakewich HP, Fishman SJ, Larsen PR 2000 Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Eng J Med 343:185–189 [DOI] [PubMed] [Google Scholar]
- Huang SA, Fish SA, Dorfman DM, Salvatore D, Kozakewich HP, Mandel SJ, Larsen PR 2002 A 21-year-old woman with consumptive hypothyroidism due to a vascular tumor expressing type 3 iodothyronine deiodinase. J Clin Endocrinol Metab 87:4457–4461 [DOI] [PubMed] [Google Scholar]
- Kim BW, Daniels GH, Harrison BJ, Price A, Harney JW, Larsen PR, Weetman AP 2003 Overexpression of type 2 iodothyronine deiodinase in follicular carcinoma as a cause of low circulating free thyroxine levels. J Clin Endocrinol Metab 88:594–598 [DOI] [PubMed] [Google Scholar]
- Miyauchi A, Takamura Y, Ito Y, Miya A, Kobayashi K, Matsuzuka F, Amino N, Toyoda N, Nomura E, Nishikawa M 2008 3,5,3′-Triiodothyronine thyrotoxicosis due to increased conversion of administered levothyroxine in patients with massive metastatic follicular thyroid carcinoma. J Clin Endocrinol Metab 93:2239–2242 [DOI] [PubMed] [Google Scholar]
- Brtko J, Bobalova J, Podoba J, Schmutzler C, Kohrle J 2002 Thyroid hormone receptors and type I iodothyronine 5′-deiodinase activity of human thyroid toxic adenomas and benign cold nodules. Exp Clin Endocrinol Diabetes 110:166–170 [DOI] [PubMed] [Google Scholar]
- Celi FS, Coppotelli G, Chidakel A, Kelly M, Brillante BA, Shawker T, Cherman N, Feuillan PP, Collins MT 2008 The role of type 1 and type 2 5′-deiodinase in the pathophysiology of the 3,5,3′-triiodothyronine toxicosis of McCune-Albright syndrome. J Clin Endocrinol Metab 93:2383–2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler D, Burger AG 1984 The deiodination of the iodothyronines and their derivatives in man. Endocr Rev 5:151–184 [DOI] [PubMed] [Google Scholar]
- Chanoine J, Braverman LE, Farwell AP, Safran M, Alex S, Dubord S, Leonard JL 1993 The thyroid gland is a major source of circulating T3 in the rat. J Clin Invest 91:2709–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegler L, Gillquist J, Lindvall R, Almqvist S 1982 Secretion rates of thyroxine, triiodothyronine, and reverse triiodothyronine in man during surgery. Acta Endocrinol 101:193–198 [DOI] [PubMed] [Google Scholar]
- Oppenheimer JH, Schwartz HL, Surks MI 1972 Propylthiouracil inhibits the conversion of l-thyroxine to l-triiodothyronine. An explanation of the antithyroxine effect of propylthiouracil and evidence supporting the concept that triiodothyronine is the active thyroid hormone. J Clin Invest 51:2493–2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frumess RD, Larsen PR 1975 Correlation of serum triiodothyronine (T3) and thyroxine (T4) with the biological effects of thyroid hormone replacement in propylthiouracail-treated rats. Metabolism 24:547–554 [DOI] [PubMed] [Google Scholar]
- Larsen PR, Frumess RD 1977 Comparison of the biological effects of thyroxine and triiodothyronine in the rat. Endocrinology 100:980–988 [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Chapa F, DiStefano III JJ 1998 Direct measurement of the contributions of type I and type II 5′-deiodinases to whole body steady state 3,5,3′-triiodothyronine production from thyroxine in the rat. Endocrinology 139:4626–4633 [DOI] [PubMed] [Google Scholar]
- Maia AL, Kim BW, Huang SA, harney JW, Larsen PR 2005 Type 2 deiodinase is the major source of plasma T3 in euthyroid humans. J Clin Invest 115:2524–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore D, Tu H, Harney JW, Larsen PR 1996 Type 2 iodothyronine deiodinase is highly expressed in human thyroid. J Clin Invest 98:962–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda N, Nishikawa M, Mori Y, Yoshimura M, Masaki H, Gondou A, Yonemoto T, Inada M 1992 Identification of a 27-kilodalton protein with the properties of type I iodothyronine 5′-deiodinase in human thyroid gland. J Clin Endocrinol Metab 74:533–538 [DOI] [PubMed] [Google Scholar]
- Laurberg P, Boye N 1982 Outer and inner ring monodeiodination of thyroxine by dog thyroid and liver: a comparative study using a particulate cell fraction. Endocrinology 110:2124–2130 [DOI] [PubMed] [Google Scholar]
- Laurberg P 1978 Selective inhibition of the secretion of triiodothyronines from the perfused canine thyroid by propylthiouracil. Endocrinology 103:900–905 [DOI] [PubMed] [Google Scholar]
- Tegler L, Anderberg B, Smeds S 1982 Preferential secretion of triiodothyronine in man. Horm Metab Res 14:593–595 [DOI] [PubMed] [Google Scholar]
- Schneider MJ, Fiering SN, Thai B, Wu SY, St. Germain E, Parlow AF, St. Germain DL, Galton VA 2006 Targeted disruption of the type1 selenodeiodinase gene (Dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology 147:580–589 [DOI] [PubMed] [Google Scholar]
- Galton VA, Schneider M, Clark AS, St. Germain DL, Life without T4 to T3 conversion: studies in mice devoid of the 5′-deiodinases. Endocrinology, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann PR, Berry MJ 2005 Selenoprotein synthesis: a unique translational mechanism used by a diverse family of proteins. Thyroid 15:769–775 [DOI] [PubMed] [Google Scholar]
- DeGroot LJ 2006 Nonthyroidal illness syndrome. In: DeGroot LJ, Jameson JL, eds. Endocrinology. 5th ed. Philadelphia: Elsevier; 2101–2112 [Google Scholar]
- Kmiec Z, Kotlarz G, Smiechowska B, Mysiwski A 1996 Thyroid hormones homeostasis in rats refed after short-term and prolonged fasting. J Endocrinol Invest 19:304–311 [DOI] [PubMed] [Google Scholar]
- Boelen A, Wiersinga WM, Fliers E 2008 Fasting-induced changes in the hypothalamus-pituitary-thyroid axis. Thyroid 18:123–129 [DOI] [PubMed] [Google Scholar]
- Wells S, Campbell R 1986 Decrease in resting metabolic rate during rapid weight loss is reversed by low dose thyroid hormone treatment. Metabolism 35:298–291 [DOI] [PubMed] [Google Scholar]
- Forsum E, Hillman PE, Nesheim MC 1981 Effect of energy restriction on total heat production, basal metabolic rate, and specific dynamic action of food in rats. J Nutr 111:1691–1697 [DOI] [PubMed] [Google Scholar]
- Van den Berghe G, Baxter RC, Weekers F, Wouters P, Bowers CY, Iranmanesh A, Veldhuis JD, Bouillon R 2002 The combined administration of GH-releasing peptide-2 (GHRP-2), TRH and GnRH to men with prolonged critical illness evokes superior endocrine and metabolic effects compared to treatment with GHRP-2 alone. Clin Endocrinol (Oxf) 56:655–669 [DOI] [PubMed] [Google Scholar]
- Van den Berghe G, Wouters P, Weekers F, Mohan S, Baxter RC, Veldhuis JD, Bowers CY, Bouillon R 1999 Reactivation of pituitary hormone release and metabolic improvement by infusion of growth hormone-releasing peptide and thyrotropin-releasing hormone in patients with protracted critical illness. J Clin Endocrinol Metab 84:1311–1323 [DOI] [PubMed] [Google Scholar]
- Chopra IJ 1997 Euthyroid sick syndrome: is it a misnomer? J Clin Endocrinol Metab 82:329–334 [DOI] [PubMed] [Google Scholar]
- Coppola A, Hughes J, Esposito E, Schiavo L, Meli R, Diano S 2005 Suppression of hypothalamic deiodinase type II activity blunts TRH mRNA decline during fasting. FEBS Lett 579:4654–4658 [DOI] [PubMed] [Google Scholar]
- Fekete C, Sarkar S, Christoffolete MA, Emerson C, Bianco AC, Lechan RM 2005 Bacterial lipopolysaccharide (LPS)-induced type 2 iodothyronine deiodinase (D2) activation in the mediobasal hypothalamus (MBH) is independent of the LPS-induced fall in serum thyroid hormone levels. Brain Res 1056:97–99 [DOI] [PubMed] [Google Scholar]
- Mebis L, Langouche L, Visser TJ, Van den Berghe G 2007 The type II iodothyronine deiodinase is up-regulated in skeletal muscle during prolonged critical illness. J Clin Endocrinol Metab 92:3330–3333 [DOI] [PubMed] [Google Scholar]
- O'Mara BA, Dittrich W, Lauterio TJ, St. Germain DL 1993 Pretranslational regulation of type I 5′-deiodinase by thyroid hormones and in fasted and diabetic rats. Endocrinology 133:1715–1723 [DOI] [PubMed] [Google Scholar]
- Debaveye Y, Ellger B, Mebis L, Darras VM, Van den Berghe G 2008 Regulation of tissue iodothyronine deiodinase activity in a model of prolonged critical illness. Thyroid 18:551–560 [DOI] [PubMed] [Google Scholar]
- Kinlaw WB, Schwartz HL, Oppenheimer JH 1984 Decreased serum triiodothyronine in starving rats is due primarily to diminished thyroidal secretion of thyroxine. J Clin Invest 75:1238–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen YM, DiStefano III JJ, Yamada H, Nguyen TT 1994 Direct measurement of whole body thyroid hormone pool sizes and interconversion rates in fasted rats: hormone regulation implications. Endocrinology 134:1700–1709 [DOI] [PubMed] [Google Scholar]
- DiStefano III JJ 1991 Kinetic modeling methods in theory and practice. In: Wu SY, ed. Thyroid hormone metabolism: regulation and clinical implications. Boston: Blackwell Scientific; 65–89 [Google Scholar]
- Debaveye Y, Ellger B, Mebis L, Visser TJ, Darras VM, Van den Berghe G 2008 Effects of substitution and high-dose thyroid hormone therapy on deiodination, sulfoconjugation, and tissue thyroid hormone levels in prolonged critically ill rabbits. Endocrinology 149:4218–4228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelen A, Kwakkel J, Wieland CW, St. Germain DL, Fliers E, Hernandez A 26 November 2008 Impaired bacterial clearance in type 3 deiodinase deficient mice infected with Streptococcal pneumoniae. Endocrinology 10.1210/en.2008-1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SA, Bianco AC 2008 Reawakened interest in type III iodothyronine deiodinase in critical illness and injury. Nat Clin Pract Endocrinol Metab 4:148–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonides WS, Mulcahey MA, Redout EM, Muller A, Zuidwijk MJ, Visser TJ, Wassen FWJS, Crescenzi A, da-Silva WS, Harney J, Engel FB, Obregon MJ, Larsen PR, Bianco AC, Huang SA 2008 Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. J Clin Invest 118:975–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamirand A, Pallud-Mothre S, Ramauge M, Pierre M, Courtin F 2008 Oxidative stress regulates type 3 deiodinase and type 2 deiodinase in cultured rat astrocytes. Endocrinology 149:3713–3721 [DOI] [PubMed] [Google Scholar]
- Boelen A, Kwakkel J, Alkemade A, Renckens R, Kaptein E, Kuiper G, Wiersinga WM, Visser TJ 2005 Induction of type 3 deiodinase in inflammatory cells of mice with chronic local inflammation. Endocrinology 146:5128–5134 [DOI] [PubMed] [Google Scholar]
- Boelen A, Boorsma J, Kwakkel J, Wieland CW, Renckens R, Visser TJ, Fliers E, Wiersinga W 2008 Type 3 deiodinase is highly expressed in infiltrating neutrophilic granulocytes in response to acute bacterial infection. Thyroid 18:1095–1103 [DOI] [PubMed] [Google Scholar]
- Li WW, Le Goascogne C, Ramauge M, Schumacher M, Pierre M, Courtin F 2001 Induction of type 3 iodothyronine deiodinase by nerve injury in the rat peripheral nervous system. Endocrinology 142:5190–5197 [DOI] [PubMed] [Google Scholar]
- Olivares EL, Marassi MP, Fortunato RS, da Silva ACM, Costa-e-Sousa RH, Arau'jo IG, Mattos EC, Masuda MO, Mulcahey MA, Huang SA, Bianco AC, Carvalho DP 2007 Thyroid function disturbance and type 3 iodothyronine deiodinase induction after myocardial infarction in rats: a time course study. Endocrinology 148:4786–4792 [DOI] [PubMed] [Google Scholar]
- Wassen FW, Schiel AE, Kuiper GG, Kaptein E, Bakker O, Visser TJ, Simonides WS 2002 Induction of thyroid hormone-degrading deiodinase in cardiac hypertrophy and failure. Endocrinology 143:2812–2815 [DOI] [PubMed] [Google Scholar]
- Farsetti A, Desvergne B, Hallenbeck P, Robbins J, Nikodem VM 1992 Characterization of myelin basic protein thyroid hormone response element and its function in the context of native and heterologous promoter. J Biol Chem 267:15785–15788 [PubMed] [Google Scholar]
- Tomanek RJ, Zimmerman MB, Suvarna PR, Morkin E, Pennock GD, Goldman S 1998 A thyroid hormone analog stimulates angiogenesis in the post-infarcted rat heart. J Mol Cell Cardiol 30:923–932 [DOI] [PubMed] [Google Scholar]
- Tomanek RJ, Busch TL 1998 Coordinated capillary and myocardial growth in response to thyroxine treatment. Anat Rec 251:44–49 [DOI] [PubMed] [Google Scholar]
- Zheng W, Weiss RM, Wang X, Zhou R, Arlen AM, Lei L, Lazartigues E, Tomanek RJ 2004 DITPA stimulates arteriolar growth and modifies myocardial postinfarction remodeling. Am J Physiol 286:H1994–H2000 [DOI] [PubMed] [Google Scholar]
- Davis FB, Mousa SA, O'Connor L, Mohamed S, Lin HY, Cao HJ, Davis PJ 2004 Proangiogenic action of thyroid hormone is fibroblast growth factor-dependent and is initiated at the cell surface. Circ Res 94:1500–1506 [DOI] [PubMed] [Google Scholar]
- Anthony A, Adams PM, Stein SA 1993 The effects of congenital hypothyroidism using the hyt/hyt mouse on locomotor activity and learned behavior. Horm Behav 27:418–433 [DOI] [PubMed] [Google Scholar]
- Venero C, Guadano-Ferraz A, Herrero AI, Nordstrom K, Manzano J, Moreale de Escobar G, Bernal J, Vennstrom B 2005 Anxiety, memory impairment, and locomotor dysfunction caused by a mutant thyroid hormone receptor α1 can be ameliorated by T3 treatment. Genes Dev 19:2152–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morreale de Escobar G, Obregon MJ, Escobar del Rey F 2000 Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J Clin Endocrinol Metab 85:3975–3987 [DOI] [PubMed] [Google Scholar]
- Crantz FR, Silva JE, Larsen PR 1982 An analysis of the sources and quantity of 3,5,3′-triiodothyronine specifically bound to nuclear receptors in rat cerebral cortex and cerebellum. Endocrinology 110:367–375 [DOI] [PubMed] [Google Scholar]
- Silva JE, Matthews P 1984 Thyroid hormone metabolism and the source of plasma triiodothyronine in 2-week-old rats: effects of thyroid status. Endocrinology 114:2394–2405 [DOI] [PubMed] [Google Scholar]
- Calvo R, Obregón MJ, Ruiz de Ona C, Escobar del Rey F, de Escobar GM 1990 Congenital hypothyroidism, as studied in rats: crucial role of maternal thyroxine but not 3,5,3′-triiodothyronine in the protection of the fetal brain. J Clin Invest 86:889–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galton VA, Wood ET, St. Germain EA, Withrow CA, Aldrich G, St. Germain GM, Clark AS, St. Germain DL 2007 Thyroid hormone homeostasis and action in the type 2 deiodinase-deficient brain during development. Endocrinology 148:3080–3088 [DOI] [PubMed] [Google Scholar]
- Leonard JL, Visser TJ 1986 Biochemistry of deiodination. In: Hennemann G, ed. Thyroid hormone metabolism. New York: Marcel Dekker; 189–229 [Google Scholar]
- Gnidehou S, Caillou B, Talbot M, Ohayon R, Kaniewski J, Noël-Hudson MS, Morand S, Agnangji D, Sezan A, Courtin F, Virion A, Dupuy C 2004 Iodotyrosine dehalogenase 1 (DEHAL1) is a transmembrane protein involved in the recycling of iodide close to the thyroglobulin iodination site. FASEB J 18:1574–1576 [DOI] [PubMed] [Google Scholar]
- Tegler L, Gillquist J, Lindvall R, Almqvist S, Roos P 1983 Thyroid hormone secretion rates: response to endogenous and exogenous TSH in man during surgery. Clin Endocrinol (Oxf) 18:1–9 [DOI] [PubMed] [Google Scholar]
- Baqui MMA, Gerebon B, Harney JW, Larsen PR, Bianco AC 2000 Distinct subcellular localization of transiently expressed types 1 and 2 deiodinases as determined by immunofluorescence confocal microscopy. Endocrinology 141:4309–4312 [DOI] [PubMed] [Google Scholar]
- Guadaño-Ferraz A, Obregón MJ, St. Germain DL, Bernal J 1997 The type 2 iodothyronine deiodinase is expressed primarily in glial cells in the neonatal rat brain. Proc Natl Acad Sci USA 94:10391–10396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore D, Tibor B, Harney JW, Larsen PR 1996 Molecular biological and biochemical characterization of the human type 2 selenodeiodinase. Endocrinology 137:3308–3315 [DOI] [PubMed] [Google Scholar]
- Peeters RP, Wouters PJ, Kaptein E, van Toor H, Visser TJ, Van den Berghe G 2003 Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. J Clin Endocrinol Metab 88:3202–3211 [DOI] [PubMed] [Google Scholar]
- Wu S, Shyh T, Chopra IJ, Solomom DH, Huang H, Chu P 1982 Comparison of sodium ipodate (Oragrafin) and propylthiouracil in early treatment of hyperthyroidism. J Clin Endocrinol Metab 54:630–634 [DOI] [PubMed] [Google Scholar]
- Laurberg P 1985 Multisite inhibition by ipodate of iodothyronine secretion from perfused dog thyroid lobes. Endocrinology 117:1639–1644 [DOI] [PubMed] [Google Scholar]
- Huang H, Marsh-Armstrong N, Brown DD 1999 Metamorphosis is inhibited in transgenic Xenopus laevis tadpoles that overexpress type III deiodinase. Proc Natl Acad Sci USA 96:962–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachucki J, Hopkins J, Peeters R, Tu H, Carvalho SD, Kaulbach H, Abel ED, Wondisford FE, Ingwall JS, Larsen PR 2001 Type 2 iodothyronine deiodinase transgene expression in the mouse heart causes cardiac-specific thyrotoxicosis. Endocrinology 142:13–20 [DOI] [PubMed] [Google Scholar]
- Pascual-Leone AM, Besa E, Hervás F, Escrivá F, Alvarez C 1983 Effects of l-thyroxine treatment on pituitary GH content of adult rats with neonatal thyrotoxicosis. Acta Endocrinol 104:340–344 [DOI] [PubMed] [Google Scholar]
- da-Silva WS, Harney JW, Kim BW, Li J, Bianco SD, Crescenzi A, Christoffolete MA, Huang SA, Bianco AC 2007 The small polyphenolic molecule kaempferol increases cellular energy expenditure and thyroid hormone activation. Diabetes 56:767–776 [DOI] [PubMed] [Google Scholar]