Abstract
3-Iodothyronamine (T1AM) is an endogenous compound with chemical features that are similar to thyroid hormone. T1AM has a carbon skeleton identical to that of T4 and contains a single carbon-iodine bond. Theoretically, T1AM could be produced from T4 by enzymatic decarboxylation and deiodination. Recent studies show that T1AM and higher iodinated thyronamines are subject to similar metabolic processing as iodothyronines such as T4, suggesting a biological linkage between iodothyronines and iodothyronamines. In addition, single doses of T1AM administered to rodents induce a hypometabolic state that in certain ways resembles hibernation and is opposite to the effects of excess T4. This review will discuss the latest developments on this recently discovered thyroid hormone derivative.
3-Iodothyronamine is a biologically active, endogenous derivative of thyroid hormone that may represent a new arm of the thyroid endocrine system.
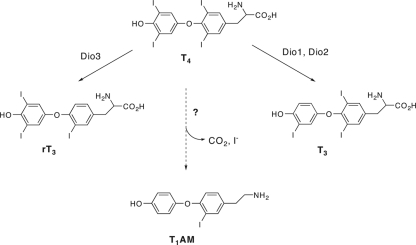
Thyronamines are chemical derivatives of thyronines, which are generally regarded as the principle chemical form of thyroid hormone. T4, the major thyronine secreted from the thyroid gland and most abundant thyroid hormone in circulation is widely viewed as a prohormone (Fig. 1) (1). Outer-ring enzymatic deiodination of T4 gives rise to T3, a high-affinity ligand for the thyroid hormone receptors, whereas inner ring T4 deiodination produces rT3, a T4 metabolite whose biological role is not entirely clear, despite the fact that equal circulating fractions of T3 and rT3 are produced from T4 in healthy individuals, and substantially more rT3 than T3 is produced in disorders that are characterized as nonthyroidal illness (2,3,4). In addition to T3 and rT3, most of the other theoretically possible further deiodinated thyronines have been detected in tissue and circulation (5). Thyronines are amino acids, the decarboxylation of which gives rise to a phenethylamine derivative called a thyronamine (Fig. 1). There are several literature reports describing the synthesis and pharmacological evaluation of different thyronamines, varying from one another in iodine content, dating back to the 1930s, but none of these older reports demonstrated that thyronamines were endogenous substances (6,7,8,9,10,11,12,13,14).
Figure 1.
The chemical structures of thyronines and thyronamines. T4 is converted to both T3 and rT3 by the indicated deiodinases. T1AM, the most studied thyronamine to date, has the same chemical skeleton as T4 and theoretically can be made from T4 by enzymatic decarboxylation and deiodination.
In 2004, Scanlan and co-workers (15) described the synthesis and biological properties of 3-iodothyronamine (T1AM), a novel thyronamine that had not been described previously. Using synthetic T1AM as a standard in liquid chromatography/tandem mass spectrometry assays, T1AM was shown to be an endogenous component of biogenic amine extracts from rodent brain, liver, heart, and blood. With subsequent liquid chromatography/tandem mass spectrometry method development, this assay is now suitable for quantitative analysis of endogenous T1AM and pharmacokinetic analysis of pharmacologically administered T1AM (16). In terms of its actions, T1AM was found to be a potent agonist of trace amine-associated receptor 1 (TAAR1), an orphan G protein-coupled receptor (GPCR) that is highly homologous to adrenergic, dopaminergic, and serotonergic GPCRs (17). T1AM was found to activate rat and mouse TAAR1 with EC50 values of 14 and 112 nm, respectively, and subsequent structure-activity studies have further defined the T1AM ligand pharmacophore that binds to and activates TAAR1 (18,19,20,21). In vivo, a single ip injection of T1AM was found to rapidly decrease core body temperature and heart rate (15). In mice, T1AM induced an approximate 10 C drop in body temperature that peaked about 1 h after injection and returned to normal after 4–6 h, depending on the T1AM dose. The T1AM-induced reduction in cardiac performance was found to be a direct effect and independent of the T1AM-induced hypothermia. In a rat working heart preparation held at 37 C, introduction of T1AM into the perfusion buffer resulted in large and immediate decreases in both heart rate and systolic aortic pressure, and additional studies on the heart further support the direct actions of T1AM on this organ (22,23).
These initial pharmacological findings indicated that single-dose T1AM treatment rapidly induced a hypometabolic state in rodents. The induction of hypothermia was thought to have potential neuroprotective benefit in the case of ischemic injury such as stroke. Indeed, T1AM treatment was found to reduce infarct volume by 40% in a middle cerebral artery occlusion stroke model in mice, and the degree of neuroprotection afforded by T1AM was found to correlate well with the magnitude of hypothermia that was induced by T1AM (24). Along with hypothermia and reduced cardiac function, single-dose T1AM rapidly induces hyperglycemia in mice (25). Mice that receive an ip injection of T1AM show 2- to 5-fold increased blood glucose peaking 1 h after the injection and returning to baseline after 3–4 h. The mechanism of this effect appears to involve T1AM activation of α2A adrenergic receptors (Adra2a) in β-islet cells of the pancreas, because Adra2a knockout mice show no hyperglycemia after T1AM treatment. Ligand activation of α-2A receptors leads to a decrease in cAMP through coupling to Gαi, resulting in inhibition of insulin secretion from β-islet cells. At present, it is not certain whether this effect of T1AM is direct or whether it is purely pharmacological or has physiological significance. In addition to the GPCRs TAAR1 and α-2A, T1AM also acts at plasma membrane and vesicular biogenic amine transporters (26). T1AM is an inhibitor, but not a competitive substrate, of the dopamine and norepinephrine plasma membrane transporters and also an inhibitor of vesicular monoamine transporters that package biogenic amine neurotransmitters into vesicles within neurons. The inhibitory potency of T1AM against these transporters is in the low micromolar range, which is similar in magnitude to the KM values of the endogenous substrates of these transporters. Whether or not endogenous T1AM is present at these levels in synapses or neurons of the central nervous system is presently not known. Interestingly, The T1AM reuptake inhibition is specific for catecholamines because T1AM shows no inhibitory activity toward the serotonin reuptake transporter.
The metabolism of thyroid hormone is directly connected to its actions, and thyronamines appear to share many of the metabolic reactions of thyronines. Like thyronines, thyronamines are substrates for sulfotransferase enzymes that catalyze the sulfation of the phenolic hydroxyl shared between the two classes of compounds (27). T1AM is the most efficiently processed thyronamine substrate using a sulfotransferase preparation from human liver, and the Vmax/KM value for T1AM compares to that of T3, suggesting that similar to T3, sulfation of T1AM may be an important clearance mechanism for regulating free circulating levels. In addition to sulfation, thyronamines are also converted to thyroacetic acids by an amine oxidase activity (28). Upon incubation with HepG2 cells or human thyroid tissue homogenates, T1AM is robustly converted to 3-iodothyroacetic acid, and T3AM is converted into 3,5,3′-triiodothyroacetic acid (Triac), a known active metabolite of T3. This conversion appears to be very efficient and occurs both in vitro and in vivo; the enzymes responsible for this are currently unknown, but monoamine oxidase or semicarbazide-sensitive amine oxidase are reasonable candidates. The biological purpose behind this conversion is also presently unclear. It is possible that this could be a deactivation mechanism, similar to sulfation, although the biological activities (if any) of 3-iodothyroacetic acid are presently unknown. The robust conversion of T3AM to Triac is intriguing and suggests the possibility that this pathway, involving the intermediacy of a thyronamine, is responsible, at least in part, for the endogenous production of Triac from T3.
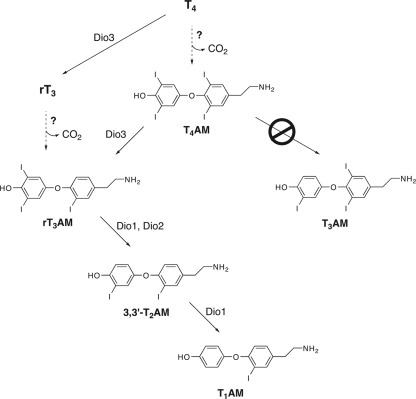
As mentioned above, enzymatic deiodination plays a major role in the activation of thyroid hormone from the latent form, T4, to the active form, T3 (2,3). This activation pathway is mediated by the type I deiodinase (Dio1) and Dio2 isozymes. T4 is also converted to rT3 in what may be a deactivation mechanism, and this process is catalyzed by the Dio3 isozyme (4). In addition to iodothyronines, iodothyronamines are substrates for the three deiodinases, Dio1, Dio2, and Dio3 (29). Iodothyronamines have comparable Vmax/KM values compared with the corresponding iodothyronine, indicating that iodothyronamines would be as readily deiodinated as iodothyronines should they exist at comparable concentrations and encounter the appropriate deiodinase isozyme. Interestingly, the isozyme selectivity of iodothyronamine deiodination differs from that of iodothyronines. T4AM is not a substrate for Dio1 or Dio2 and cannot be deiodinated to T3AM, whereas T4AM is a good substrate for Dio3 and is readily deiodinated to rT3AM. Dio1 and Dio2 can then sequentially deiodinate rT3AM to provide T1AM. This isozyme-selective processing suggests a specific biosynthetic pathway for endogenous T1AM production (Fig. 2) with either T4AM or rT3AM serving as entry points into the pathway, and these entry points would originate from decarboxylation of either T4 or rT3. Although the logic of this proposed biosynthetic pathway is very attractive, the precise relationship between T1AM and thyroid hormone is at present poorly understood, and although T1AM is clearly an endogenous chemical derivative of T4, there is not direct evidence at present, proving that T1AM is a T4 metabolite in vivo. However, viable alternative routes for T1AM production are not obvious, because biosynthesis from tyrosine would require oxidative coupling of two molecules of tyrosine and aromatic ring iodination, which are specialized biochemical reactions catalyzed by thyroperoxidase during thyroid hormone synthesis; alternative robust enzymatic iodination pathways in vertebrates are not presently known.
Figure 2.
Summary of enzyme-catalyzed thyronamine deiodinase reactions. Unlike T4, T4AM is not a substrate for Dio1 and -2 and cannot be deiodinated to T3AM. However, T4AM is readily deiodinated to rT3AM by Dio3, and rT3AM can be further deiodinated to ultimately provide T1AM. This suggests a unique biosynthetic deiodination pathway for T1AM starting from the decarboxylation products of either T4 or rT3.
In addition to the hypometabolic effects of T1AM discussed previously, it was recently discovered that single-dose T1AM dramatically switches fuel utilization away from carbohydrates and toward lipids (30). Siberian hamsters (Phodopus sungorus), a hibernating rodent species, as well as mice, display a complete shift in respiratory quotient (RQ) from a normal, mixed carbohydrate and lipid value (0.90 for hamsters, 0.83 for mice) to an RQ value of approximately 0.7, indicating that a switch to pure lipid burning has occurred upon single-dose T1AM administration. Interestingly, this 0.7 RQ persisted in summer-acclimatized Siberian hamsters for at least 24 h after the T1AM injection, making this fueling shift the effect of longest duration yet to be observed with single-dose T1AM. The kinetics of this effect differs somewhat from the previously studied T1AM pharmacology. The onset of the RQ effect was slower than the onset of T1AM-induced hypothermia, bradycardia, or hyperglycemia; these three effects reach a maximum about 1 h after T1AM injection, whereas the complete RQ shift is reached 4.5 h after injection. Consistent with the shift in RQ toward lipid utilization, T1AM-treated Siberian hamsters had measurable urine ketone content that peaked 16 h after injection, again illustrating the extended duration of the T1AM-induced lipid burning switch compared with hypothermia, bradycardia, or hyperglycemia that typically return to baseline values within 4–6 h after T1AM administration.
Since its discovery, the pharmacology experiments with T1AM have exclusively involved single, high-dose T1AM administration while following biological effects that occur rapidly, generally peaking within 1 h after injection. Interesting biological effects have been observed and reported in these studies, and the full spectrum of T1AM’s pharmacological effects is unlike any other known drug or endogenous biologically active compound. This early work was guided by a working hypothesis that T1AM may be a novel neuromodulator/neurotransmitter, possessing biological actions similar to the chemically related biogenic amines dopamine, norepinephrine, and serotonin (15). This neurotransmitter view, put forward originally by Dratman (31) almost three decades before the discovery of T1AM, has been instructive and useful and may indeed account for the majority of T1AM’s actions. However, given the strong chemical relationship between T1AM and thyroid hormone in terms of both structure and enzymatic processing, coupled to the powerful biological actions of T1AM on metabolism and fueling, prompts one to ask whether T1AM may also possess hormone-like properties akin to those of T4/T3 that involve direct T1AM-induced changes to target gene expression profiles. Such actions would not be readily revealed from single-dose experiments and require the development of multidose pharmacology strategies along with a better understanding of the generation, distribution, and metabolic processing of endogenous T1AM. This future work will be critical for understanding the physiological basis of T1AM as well as identifying pathologies and illness that either involve T1AM or would benefit from T1AM therapy.
Acknowledgments
I sincerely thank all of my co-workers and collaborators whose efforts have been instrumental in illuminating the biological actions of T1AM and defining this new area of research. I also thank Dr. Mary Dratman for her insight and many thought-provoking discussions.
Footnotes
This research was supported by the National Institutes of Health (Grant DK-52798).
Disclosure Statement: The author is an inventor on U.S. Patents 6,979,750 B1; 7,339,079 B2; and 7,355,079 B2.
First Published Online December 30, 2008
Abbreviations: Dio1, Type I deiodinase; GPCR, G protein-coupled receptor; RQ, respiratory quotient; TAAR1, trace amine-associated receptor 1; T1AM, 3-iodothyronamine; Triac, triiodothyroacetic acid.
References
- Yen PM 2001 Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142 [DOI] [PubMed] [Google Scholar]
- Kohrle J 2002 Iodothyronine deiodinases. Methods Enzymol 347:125–167 [DOI] [PubMed] [Google Scholar]
- Bianco A, Kim BW 2006 Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest 116:2571–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SA 2005 Physiology and pathophysiology of type 3 deiodinase in humans. Thyroid 15:875–881 [DOI] [PubMed] [Google Scholar]
- Pittman CS 1979 Hormone metabolism. In: DeGroot LJ, ed. Endocrinology. 1st ed. New York: Grune, Stratton; 365–372 [Google Scholar]
- Stohr R 1931 Synthese des thyronamins. Hoppe-Seyler Z. Physiol Chem 201:142–148 [Google Scholar]
- Thibault O, Lachaze A 1951 Recherches sur la nature de la “thyroxine active.” Renforcement immediat par la thyroxamine des effets de l’adrenaline sur divers muscles lisses. Compt Rend Soc Biol 145:797–800 [PubMed] [Google Scholar]
- Buu-Hoi NP, Chanh PH, Petit L 1966 Some biological effects of thyronamine. Med Pharmacol Exp 15:17–23 [DOI] [PubMed] [Google Scholar]
- Buu-Hoi NP, Chanh PH, Petit L 1969 Thyronamine, a new substance with long-acting positive inotropic effect. Pharmacology 2:281–287 [DOI] [PubMed] [Google Scholar]
- Boissier JR, Giudicelli JF, Larno S, Advenier C 1973 Differential inotropic-chronotropic action of thyronamine. Eur J Pharmacol 22:141–149 [DOI] [PubMed] [Google Scholar]
- Tomita K, Lardy HA 1956 Synthesis and biological activity of some triiodinated analogues of thyroxine. J Biol Chem 219:595–604 [PubMed] [Google Scholar]
- Meyer T, Hesch R-D 1983 Triiodothyronamine-a β-adrenergic metabolite of triiodothyronine? Horm Metab Res 15:602–606 [DOI] [PubMed] [Google Scholar]
- Cote P, Polumbo RA, Harrison DC 1974 Thyronamine, a new inotropic agent: its cardiovascular effects and mechanism of action. Cardiovasc Res 8:721–730 [DOI] [PubMed] [Google Scholar]
- Petit L, Buu-Hoi NP 1961 A synthesis of thyronamine and its lower homolog. J Org Chem 26:3832–3834 [Google Scholar]
- Scanlan TS, Suchland KL, Hart ME, Chiellini G, Huang Y, Kruzich PJ, Frascarelli S, Crossley II DA, Bunzow JR, Ronca-Testoni S, Lin ET, Hatton D, Zucchi R, Grandy DK 2004 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat Med 10:638–642 [DOI] [PubMed] [Google Scholar]
- DeBarber AE, Geraci T, Colasurdo VP, Hackenmueller SA, Scanlan TS 2008 Validation of a liquid chromatography-tandem mass spectrometry method to enable quantification of 3-iodothyronamine from serum. J Chromatogr A 1210:55–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchi R, Chiellini G, Scanlan TS, Grandy DK 2006 Trace amine-associated receptors and their ligands. Br J Pharmacol 149:967–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart ME, Suchland KL, Miyakawa M, Bunzow JR, Grandy DK, Scanlan TS 2006 Trace amine-associated receptor agonists: synthesis and evaluation of thyronamines and related analogues. J Med Chem 49:1101–1112 [DOI] [PubMed] [Google Scholar]
- Tan ES, Miyakawa M, Bunzow JR, Grandy DK, Scanlan TS 2007 Exploring the structure-activity relationship of the ethylamine portion of 3-iodothyronamine for rat and mouse trace amine-associated receptor 1. J Med Chem 50:2787–2798 [DOI] [PubMed] [Google Scholar]
- Tan ES, Groban ES, Jacobson MP, Scanlan TS 2008 Toward deciphering the code to aminergic G protein-coupled receptor drug design. Chem Biol 15:343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snead AN, Miyakawa M, Tan ES, Scanlan TS 2008 Trace amine-associated receptor 1 (TAAR1) is activated by amiodarone metabolites. Bioorg Med Chem Lett 18:5920–5922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiellini G, Frascarelli S, Ghelardoni S, Carnicelli V, Tobias SC, DeBarber A, Brogioni S, Ronca-Testoni S, Cerbai E, Grandy DK, Scanlan TS, Zucchi R 2007 Cardiac effects of 3-iodothyronamine: a new aminergic system modulating cardiac function. FASEB J 21:1597–1608 [DOI] [PubMed] [Google Scholar]
- Frascarelli S, Ghelardoni S, Chiellini G, Vargiu R, Ronca-Testoni S, Scanlan TS, Grandy DK, Zucchi R 2008 Cardiac effects of trace amines: pharmacological characterization of trace amine associated receptors. Eur J Pharmacol 587:231–236 [DOI] [PubMed] [Google Scholar]
- Doyle KP, Suchland KL, Ciesielski TMP, Lessov NS, Grandy DK, Scanlan TS, Stenzel-Poore MP 2007 Novel thyroxine derivatives, thyronamine and 3-iodothyronamine, induce transient hypothermia and marked neuroprotection against stroke injury. Stroke 38:2569–2576 [DOI] [PubMed] [Google Scholar]
- Regard JB, Kataoka H, Cano DA, Camerer E, Yin L, Zheng YW, Scanlan TS, Hebrok M, Coughlin SR 2007 Probing cell type-specific functions of Gi in vivo identifies GPCR regulators of insulin secretion. J Clin Invest 117:4034–4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snead AN, Santos MS, Seal RP, Miyakawa M, Edwards RH, Scanlan TS 2007 Thyronamines inhibit plasma membrane and vesicular monoamine transport. ACS Chem Biol 2:390–398 [DOI] [PubMed] [Google Scholar]
- Pietsch CA, Scanlan TS, Anderson RJ 2007 Thyronamines are substrates for human liver sulfotransferases. Endocrinology 148:1921–1927 [DOI] [PubMed] [Google Scholar]
- Wood WJ, Geraci T, Nilsen A, DeBarber A, Scanlan TS 2009 Iodothyronamines are oxidatively deaminated to iodothyroacetic acids in vivo. Chembiochem 10:361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piehl S, Heberer T, Balizs G, Scanlan TS, Smits R, Koksch B, Kohrle J 2008 Thyronamines are isozyme-specific substrates of deiodinases. Endocrinology 149:3037–3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braulke LJ, Klingenspor M, DeBarber A, Tobias SC, Grandy DK, Scanlan TS, Heldmaier G 2008 3-Iodothyronamine: a novel hormone controlling the balance between glucose and lipid utilisation. J Comp Physiol B 178:167–177 [DOI] [PubMed] [Google Scholar]
- Dratman MB 1974 On the mechanism of action of thyroxin, an amino acid analog of tyrosine. J Theor Biol 46:255–270 [DOI] [PubMed] [Google Scholar]