Abstract
Medulloblastoma (MD) is the most common malignant brain tumor in children. These invasive neuroectodermal tumors arise from cerebellar granule cell-like precursors. In the developing cerebellum, estrogen influences growth and viability of granule cell precursors that transiently express elevated levels estrogen receptor-β (ERβ) during differentiation. Immunoanalysis revealed that ERβ was expressed in the maturing human cerebellum, in all 22 primary MD tumors analyzed, and in two MD-derived cell lines (D283Med and Daoy). Very low levels of ERα-like proteins were detected in each cell line and 41% of tumor samples. Physiological concentrations of the 17β-estradiol- or the ERβ-selective agonist 2,3-bis(4-hydroxyphenyl)-propionitrile diarylpropionitrile dose-dependently increased MD growth and cellular migration. In contrast, the ERα-selective agonist (4-propyl-[1H]pyrazole-1,3,5-triyl) trisphenol did not influence MD growth. Similar to previous studies in normal cerebellar granule cell precursors, these studies demonstrate that the physiological actions of estrogens in MD are mediated by ERβ. Preclinical studies assessing the therapeutic efficacy of antiestrogen chemotherapeutics for treating human MD were performed. It was found that pharmacological inhibition of ER-mediated signaling with the ER antagonist drug Faslodex (ICI182,780) blocked all estrogen-mediated effects in both cell culture and xenograft models of human MD. These studies have revealed that functional ERβ expression is a fundamental aspect of MD biology and has defined antiestrogen therapy as a potentially efficacious clinical approach to improve the long-term outcomes for MD patients.
Estrogens, via ERβ, stimulate growth of medulloblastoma. ICI182,780 and the antiestrogen drug Faslodex block estrogen-stimulated tumor growth in vitro and in xenograft models of medulloblastoma.
For children and adolescents less than 20 yr old, neoplasms of the central nervous system (CNS) are the second most frequent childhood cancer; they account for more than 16% of all childhood malignancy (1). Medulloblastoma (MD) are invasive primitive neuroectodermal tumors (PNET) that arise in the cerebellum from granule cell-like precursors that have escaped terminal differentiation. As a histological class, MD are the most common pediatric brain tumor (2,3).
Despite recent advances in understanding the molecular mechanisms involved in the etiology of MD, the 5-yr survival rate for MD patients remains between 50 and 70%. For young children and infants, the survival rate is even lower (1,4). The continued refinement of aggressive multimodal therapy that includes craniotomy and tumor resection, radiation, and chemotherapy has increased survival rates for children with MD. However, those benefits have come with the cost of life-long therapy-induced side effects (5,6). The aggressive therapeutic approaches used to treat MD result in an array of debilitating complications and increased risks for adverse endocrine and cardiovascular effects in adulthood (5,6,7,8). Thus, there is a need for improved management of MD that focuses on increased cure rate while decreasing negative side effects induced primarily by radiation therapy.
In the brain, 17β-estradiol (E2) acts as a mitogen and trophic factor that influences neuronal development (9). Both estrogen receptors (ERα and ERβ) are expressed in the developing and mature brain with distinctive cellular and developmental profiles (10,11,12,13,14,15). In addition to gonadal estrogens of females, estrogens are also synthesized locally in the brain from androgens by cytochrome P450 aromatase (CYP19). These locally synthesized estrogens, especially in males, are responsible for much of the estrogenic signaling activity in the developing brain. As in other estrogen-responsive tissues, in the estrogen-sensitive cells of the nervous system, the binding of estradiol at membrane-associated and intracellular ERs regulates the activity of various growth factor-like signaling pathways and the transcription of estrogen-responsive genes (16,17,18). Initial indications for a possible role of ERs in MD came from studies showing that ERβ was transiently expressed at high levels in differentiating cerebellar granule cell precursors and that low concentrations of E2 regulated granule cell precursor proliferation and viability (12,13,19,20,21).
The role of ERα in the etiology and progression of estrogen-dependent breast cancer is fairly well characterized. In estrogen-responsive tissues such as breast, uterus, and prostate, estrogens play major roles in the pathophysiology of hormone-responsive tumors (22). Chemotherapeutic agents such as the selective estrogen receptor modulator tamoxifen and the ER transcriptional antagonist ICI182,780 are used clinically as first-line and adjuvant pharmacotherapy to block ER activity and decrease growth of estrogen-responsive tumors (23). However, neither the role of ERβ in estrogen-responsive cancers nor the potential involvement of ERα or ERβ in CNS neoplasms is known.
In light of the spatiotemporal pattern of ERβ expression in maturing granule cell precursors and the shared developmental linage of granule cells and MD, it was hypothesized that growth and migration of MD are regulated by ER-dependent mechanisms (13). Results of immunostaining studies that determined the patterns of ER expression in developing human cerebellum, archival samples of MD, and human MD-derived cell lines are reported. Additionally, results of in vitro studies investigating the mechanism of estrogen/ER-dependent regulation of MD cell growth and migration in culture and in vivo xenograft studies demonstrating the therapeutic potential of antiestrogens to inhibit MD tumor growth are presented.
Materials and Methods
Steroids and pharmacological agents
Dimethylsulfoxide (DMSO) (batch no. 00451HE) and estradiol benzoate (E8515) were from Sigma-Aldrich (St. Louis, MO), and E2 (E2E0950; batch B0356) was from Steraloids (Newport, RI). ICI182,780, 4,4′,4″-(4-propyl-[1H]pyrazole-1,3,5-triyl) trisphenol (PPT), 2,3-bis(4-hydroxyphenyl)-propionitrile diarylpropionitrile (DPN), and LY294002 were from Tocris Cookson (Ellisville, MO). Clostridium difficile toxin B (List Biologicals, Campbell, CA), U0126 (Promega, Madison, WI), and Faslodex (AstraZeneca, Wilmington, DE) were used as inhibitors. Pharmaceutical-grade castor oil (CAS RN, 8001-79-4; lot A0204107001) was from Acros Organics (Geel, Belgium).
Human tissues
MD samples were obtained as 5-μm serial sections from the Cooperative Human Tissue Network (National Cancer Institute, Rockville, MD). Other investigators may have received specimens from the same subjects. Diagnostic surgical histopathology reports were supplied by the Cooperative Human Tissue Network with tumor grades confirmed and reported according to the criteria of Eberhart et al. (24). The studied patient population was comprised of 27% females and 73% males, median age 7.5 yr (range, 4 months to 18 yr), which is representative of the incident rates for MD (1). Approval for use of human tissues was obtained from the University of Cincinnati Institutional Review Board.
Immunohistochemistry
Normal cerebellar tissue was from the vermis of an 8.5-month-old male (cause of death was cardiorespiratory insufficiency with septic state, fever, and pneumonia; no neurological abnormalities presented, a gift from Dr. Laszlo Seress). The blocks were incubated at 4 C in a mixture of 4% paraformaldehyde and 2% acrolein for 2 d. Sagittal and coronal cerebellar sections were cut at 50 μm. Sections were incubated for 2 d at 4 C with 1 μg/ml anti-ERα 6F11 (Novocastra, Newcastle upon Tyne, UK) or ERβ antisera PA1-310b (Affinity BioReagents, Golden, CO), or 06-629 (Upstate Biotechnology, Lake Placid, NY). Immunoreactivity was visualized with nickel-diaminobenzidine by the avidin-biotin peroxidase complex (ABC) method (Vector Laboratories, Burlingame, CA).
Paraffin-embedded sections were dewaxed and rehydrated through graded alcohol. Heat-mediated antigen retrieval was performed at 100 C in 1 liter 0.01 m citrate buffer (pH 6.0). Sections were incubated at 4 C for 24–48 h with 5 μg/ml anti-ERα 6F11, anti-ERβ 14C8 (GeneTex, San Antonio, TX), or PA1-310b. Immunoreactivity was visualized with diaminobenzidine and hematoxylin counterstaining. Specificity controls included blocking of antigen binding sites with immunogenic peptide and replacement of primary or secondary antibodies with nonspecific serum (13,14,15). Microscopic examination of immunostained material was carried out using a Nikon Eclipse 55i microscope using a DS-Fi1 CCD camera controlled with Digital Sight software. Final graphics were generated and labeled using Photoshop version 7.01.
Cell culture
Cell lines were acquired from the American Type Culture Collection and propagated in Eagle’s MEM supplemented with 10% fetal bovine serum (FBS) or 10% charcoal/methyl-β-cyclodextrin- stripped fetal bovine serum, and 100 U/ml penicillin, 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA). Primary cerebellar cultures were prepared from neonatal female Sprague Dawley rats (16–17 g) without enzymatic treatment. Procedures were in accordance with approved Institutional Animal Care and Use Committee protocols. Primary cerebellar cultures of maturing granule cell neurons were maintained free of serum and exogenous steroid hormones as previously described (19,25).
Western blot analysis
Generation of protein lysates, SDS-PAGE, and Western blotting were performed using standard protocols. Recombinant human ERα and ERβ1 proteins (25 ng/lane; Panvera Corp., Madison, WI), rat uterus, and ovary protein lysates (12.5 μg/lane) were used as controls for specificity of immunoreactivity. Cerebellum, ovary, and uterus tissue was isolated from young female Sprague Dawley rats (20 or 250–300 g; Charles-River, Wilmington, MA) and homogenized on ice in lysis buffer. Total protein in each lysate was quantified using a Lowry assay (DC protein assay; Bio-Rad, Hercules, CA) and fractionated in 10% Tris-HCl SDS-polyacrylamide gels and electrotransferred to polyvinylidene difluoride membranes. Membranes were blocked with 3% nonfat dry milk and then incubated overnight at 4 C with 0.5 μg/ml PA1-310b or 0.2 μg/ml anti-ERα serum (MC-20; Santa Cruz Biotechnology, Santa Cruz, CA) in Tris-buffered saline with Tween 20 and 1% dry milk. Blots were washed and incubated at room temperature for 4 h with 25 pg/ml peroxidase-conjugated goat antirabbit IgG (Kirkegaard and Perry Laboratories, Gaithersburg, MD) and visualized by enhanced chemiluminescence (SuperSignal West Pico; Pierce, Rockford, IL). Controls in which deletion of primary antibodies or immunoabsorption with immunogenic peptide were performed for all antisera (13,14,19). Digital images of the blots were captured and analyzed with the EDAS290 imaging system (Kodak, Rochester, NY).
In vitro cell migration analysis
The responsiveness of MD cell migration to estrogens was assessed using a modified Boyden chamber assay as described previously (26). MD cells were incubated for 22 h in phenol-free RPMI/10% charcoal/cyclodextrin-stripped serum and an additional 2 h in the presence of 0.1 m mitomycin-C. Cells were dissociated, washed, and suspended in phenol-free RPMI supplemented with methyl-β-cyclodextrin-treated 0.2% BSA (BSA; fraction V). Cells (3.0 × 104) were seeded in 0.1 ml RPMI/BSA into the upper well of a 24-well transwell chambers (8.0-μm pore size; Corning, Corning, NY). Lower wells contained 0.6 ml RPMI/BSA plus 10% FBS (positive control), 0.001% DMSO (vehicle) or E2. After 48 h of incubation at 37 C cells on the lower surface of the filter were fixed with 100% methanol (−20 C) and stained with hematoxylin. For each well, digital images of five random ×10 objective fields of cells were captured. The mean cell number per field was calculated. For inhibitor studies, 30 min before E2 exposure, cells in the upper chamber were pretreated with 10 nm ICI182,780, 10 μm U0126, 10 μm LY294002, 2–50 pg/ml C. difficile toxin B, or an equal volume of inhibitor vehicle.
In vitro analysis of E2-responsive growth
Growth assays were performed as described previously (26). Growing cultures in phenol red-free Eagle’s MEM/10% FBS were dissociated, washed, and resuspended into phenol red-free RPMI 1640 plus 10% charcoal-stripped FBS. Viable cell numbers were determined and seeded into appropriate culture vessels. Cultures were untreated, or treated with DMSO (0.001%), various concentrations of E2, PPT, or DPN, with or without 10−8 m ICI182,780. Each compound was serially diluted into fresh DMSO/PBS vehicle to obtain an equal 0.001% final DMSO concentration in all cultures, and 10% FBS served as a positive control. At 24, 48, 72, and 96 h after treatment, viable cell numbers were determined by direct counting of trypan blue-excluding cells. The amount of 5-bromo-2′-deoxyuridine (BrdU) incorporated into newly synthesized DNA at the 24-h time point was monitored using an ELISA-based approach (19).
Xenograft studies of E2-responsive growth
All animal procedures were done in accordance with approved Institutional Animal Care and Use Committee protocols. Intact or gonadectomized nude mice (Hsd:Athymic Nude-Foxn1ν; Harlan Sprague Dawley, Indianapolis, IN) were acquired at 3–4 wk of age. Upon arrival, mice were housed under controlled laboratory conditions in a pathogen-free animal care facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. All mice were maintained ad libitum on defined phytoestrogen-free synthetic diet (Basal Diet 5755; TestDiet, Richmond, IN). Mice received 0.1 ml bilateral sc injections of 1 × 107 D283Med cells per site in phenol red-free/growth factor-reduced Matrigel (BD Biosciences, San Diego, CA). Inoculated animals were observed daily with tumors measured at least weekly using vernier calipers (Mitutoyo, Kawasaki, Japan). Tumor volumes were estimated from surface measurements using the equation π/6(d1)(d2)2 where d is tumor diameter, with d1 measured at the point of cross-sectional maximum, with d2 measured perpendicular to d1 such that d1 ≥ d2 (27).
Preliminary studies indicated that if tumor volumes were less than 700–800 mm3 by about 4 wk after inoculation, either tumor implantation was not successful or spontaneous regression of the tumor was likely. Tumor regression was observed in all groups of animals and was independent of sex, gonadal status, or hormonal milieu. Tumors not reaching about 700–800 mm3 or greater at time of treatments were excluded from further study. Tumor-bearing mice were randomly assigned to treatment or control groups at the time of treatment initiation. Animals were treated three times per week with a 50-μl sc injection of 10 μg estradiol benzoate. Faslodex (1 mg) was administered as a weekly 20-μl injection. Hormone and inhibitor treatments were based on established protocols for dosage and administration that have previously demonstrated efficacy (23,28,29,30,31). Animals in control groups received equal volume injections of the castor oil vehicle. For most experiments, there were five animals in each group. Animals were killed at the end of the indicated treatment period, at the first sign of distress, or when tumor size was estimated to exceed 20% of body weight.
Statistical analysis
Differences between treatment groups, or with in a group at different times, was assessed using an ANOVA with posttest comparison using the Bonferroni’s multiple comparison test. A minimal level of statistical significance for differences between groups or different data points was considered P < 0.05. Data were analyzed with Excel (Microsoft) and GraphPad Prism version 5.0.
Results
ER expression in human cerebellum
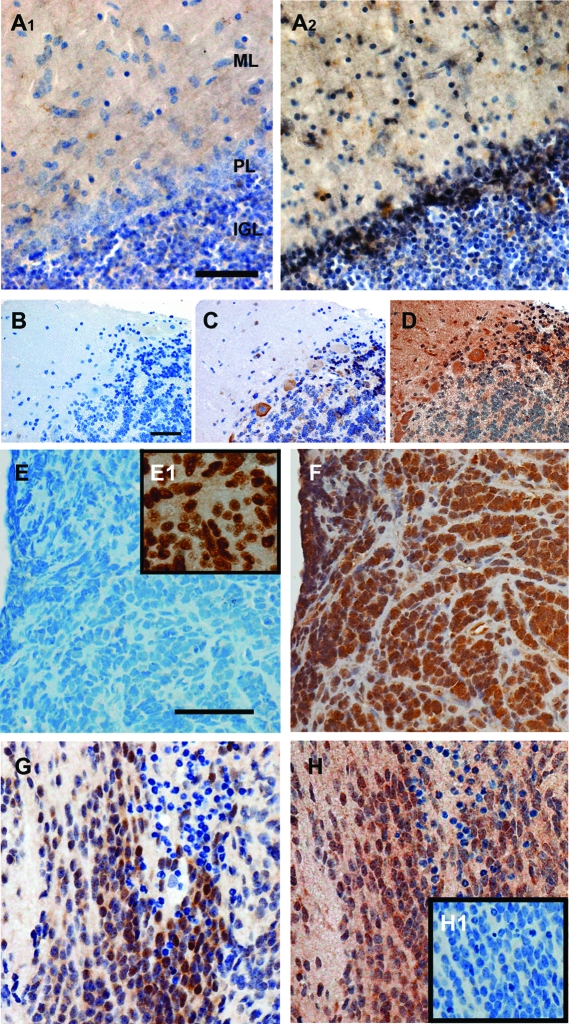
Establishing a connection between human cerebellar development and MD, with respect to possible roles of ER-mediated signaling, required demonstrating ER expression in maturing granule cells from nonmalignant human cerebellar tissue. Immunostaining of postmortem cerebellar tissue from an 8.5-month-old male with ERα- or ERβ-specific antiserum revealed ER patterns that correlated well with patterns observed in the developing rodent cerebellum (13). Detectable levels of ERα immunoreactivity were not observed (Fig. 1A1). In more mature anterior and posterior basal regions of primary cerebellar lobules, ERβ was observed at low levels in Purkinje cells and the internal granule cell layer (IGL). Strong ERβ staining was evident in the later maturing apical tips of tertiary lobules primarily present in scattered small radially migrating granule cell-like precursors (Fig. 1A2). Medium-size stellate- and basket-like neurons in the molecular layer (ML) were also immunopositive. Highest levels of staining were localized to profiles associated with the Purkinje cell layer (PL) with lower levels of ERβ immunoreactivity in granule cells settled into the IGL. This pattern of staining was similar to that observed in regions of the rat cerebellum at an equivalent developmental stage (13).
Figure 1.
Immunohistochemical analysis of ER expression in human cerebellar tissue and MD. A, Representative sections from the cerebellar vermis of an 8.5-month-old male: A1, expression of ERα-like immunoreactivity was below the level of detection; A2, expression of ERβ-like immunoreactivity was restricted to the apical tips of tertiary cerebellar lobules. Immunopositive granule cells and interneurons are observed in the immature ML. Numerous labeled interneurons and scattered immunopositive migrating granule cells are evident. The uppermost part of the IGL contained many labeled granule cells as well as heavy staining associated with the PL. B–D, Photomicrographs of serial sections from a MD from an 8-yr-old male that contained regions of the cerebellum: B, negative control section of the sample stained after treatment with nonspecific control serum; C, section after staining with anti-ERα monoclonal antibody 6F11 showing specific ERα immunoreactivity localized to Purkinje cells and stellate-like cells in the ML, low levels of staining are also present in MD cells infiltrating the cerebellar IGL; D, serial section from the same sample stained with anti-ERβ serum. Diffuse staining of the ML and IGL are evident. Strong staining of the cell bodies of Purkinje cells and interneurons in the ML is observed. As for ERα, increased expression of ERβ is observed in MD cells infiltrating the cerebellar IGL. E–H, Immunohistochemical analysis of ER expression in primary MD tumors: E, photomicrograph of a MD tumor section from a 5-yr-old male after staining with anti-ERα monoclonal antibody 6F11 showing no specific ERα immunoreactivity or background staining; E1, positive control immunohistochemical detection of ERα in a known ER-positive breast carcinoma stained with anti-ERα 6F11; F, serial section from the same MD tumor stained with anti-ERβ monoclonal 14C8 demonstrating moderately strong ERβ-specific immunostaining; G, region of a MD section from a 1.25-yr-old male with mixed ERα-positive and negative cells; H, serial section from the same region of tumor displaying ERβ immunoreactivity; H1, negative control, a serial section of the same tumor stained after treatment with nonspecific control serum. Scale bar for all images, 50 μm.
Immunostaining of sections from a surgically resected MD from an 8-yr-old male that contained nonmalignant regions of the cerebellum revealed staining consisted with previous observations in the adult cerebellum (Fig. 1, B–D). Low levels of ERα were observed in Purkinje cells and scattered satellite-like neurons in the ML and Golgi-like cells in the IGL. Immunopositive malignant cells were observed associated with the IGL (Fig. 1C; upper right quadrant). For ERβ, the strongest staining was in Purkinje cells with diffuse staining of the ML as a result of ERβ immunoreactivity distributed into Purkinje cell dendrites, and staining of some interneuron-like cell bodies (Fig. 1D). Lower levels of immunostaining were observed in mature granule cells of the IGL and intensified staining demarcating regions of malignant cells infiltrating the IGL (Fig. 1D; upper right quadrant).
ER expression in MD
Detectable levels of ERβ were observed in each primary MD sample analyzed (Table 1). The distribution and intensity of ERβ varied from tumor to tumor, and regionally within the same section. Strong nuclear localized staining with diffuse staining of cytoplasmic elements was most common (e.g. Fig. 1, F and H). However, populations of mixed positive and negative cells and regions of tumor cells devoid of ERβ were often observed. In 41% of tumors, nuclear localized ERα was observed in small focal clusters representing less than 1% of the total number of tumor cells. There were no observable differences in ER expression related to patient age or sex. Figure 1, E and F, shows results from serial regions of the same tumor stained for ERα or ERβ. This desmoplastic tumor was ERα negative (Fig. 1E) and ERβ positive (Fig. 1F). Figure E1, inset, shows staining of a known ER-positive breast carcinoma stained for ERα (positive control). Serial sections of a different MD tumor demonstrate a focus of overlapping ERα and ERβ expression (Fig. 1, G and H). A negative control staining of a serial section from the same tumor region is shown as an inset in Fig. H1.
Table 1.
ER expression in medulloblastoma
| Age (yr) | Sex | Gradea | ERα, 6F11 | ERβ
|
|
|---|---|---|---|---|---|
| 310B | 14C8 | ||||
| 0.3 | M | Slight anaplasia | 0 | ++ | ++ |
| 1.25 | M | Desmoplastic/MBEN | ++ | ++ | + |
| 2 | M | Classical | + | ++ | + |
| 2 | M | Desmoplastic | +/− | + | + |
| 5 | M | Desmoplastic | 0 | ++ | ++ |
| 5 | M | Desmoplastic | +/− | +++ | ++ |
| 6 | M | Anaplastic | 0 | +++ | +++ |
| 6 | M | Classical | 0 | +++ | +++ |
| 7 | M | Classical | 0 | + | + |
| 7 | M | Classical | 0 | ++ | +++ |
| 7 | F | Desmoplastic | 0 | ++ | + |
| 8 | M | Classical | + | +++ | ++ |
| 9 | F | Anaplastic | 0 | +++ | +++ |
| 9 | F | Classical | +/− | +++ | +++ |
| 9 | M | Desmoplastic | 0 | ++ | ++ |
| 11 | F | Classical | +/− | + | ND |
| 12 | F | Classical | 0 | + | ++ |
| 14 | M | Classical | 0 | +++ | ++ |
| 15 | F | Anaplastic | 0 | ++ | +++ |
| 16 | M | Classical | 0 | ++ | ND |
| 17 | M | Classical | ++ | ++ | +/− |
| 18 | M | Classical | +/− | ++ | + |
The relative positive cell density and intensity of immunostaining is indicated as follows: 0, no detected staining; +/−, positive staining in less than 10% of cells; +, scattered or low intensity; ++, moderate intensity in many cells; +++, high intensity in most or all cells. F, Female; M, male; MBEN, medulloblastoma extensive nodularity; ND, not done.
According to Eberhart et al. (24).
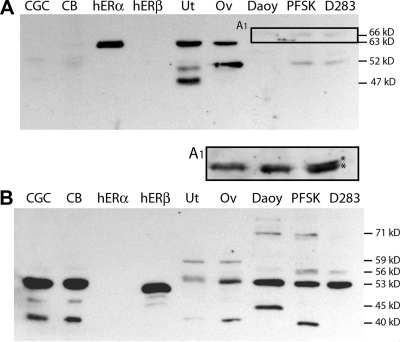
For each MD cells culture model, immunoblot analysis was used to compare expression of ER-like proteins in the MD-derived neuron-like (D283Med) and glia-like (Daoy) cell lines. Low levels of 66- and 52-kDa ERα-like proteins were detected (Fig. 2A). Overexposure of the 64- to 70-kDa region of this blot demonstrates that the 66-kDa immunoreactive species are doublets, suggesting that posttranslational modification such as phosphorylation may contribute to the observed decreased mobility (Fig. 2A1). However, trivial explanations (differences of ionic or protein concentrations) may contribute to the apparent differences in molecular weights. As in the primary tumors, the significance of these very low levels of ERα-like immunoreactivity is unclear.
Figure 2.
Comparative Western blot analysis of ER expression in MD cells. Shown are representative Western blots of SDS-PAGE gels containing protein from lysates of primary cerebellar granule cell cultures (CGC; 30 μg), P12 neonatal rat cerebellum (CB; 30 μg), purified recombinant human ERα protein (rhERα; 25 ng), purified recombinant human ERβ protein (rhERβ; 25 ng), adult rat uterus (Ut; 12.5 μg), adult rat ovary (Ov; 12.5 μg), and whole-cell protein lysates (30 μg) from Daoy, PFSK1, and D283Med (D283) cells. A, Representative blot that was analyzed with ERα-specific antiserum. Low levels of a 66-kDa immunoreactive protein in each cell line and a 52-kDa species in PFSK and D283 cells were detectable. A, An overexposure of the 64- to 70-kDa region of the lanes from each cell line reveal that the ERα-like 66-kDa band is a doublet. B, Representative blot that was analyzed with ERβ-specific antiserum. A major ERβ-like immunoreactive species of 53 kDa was present in all MD cells; immunoreactive proteins of 40, 46, 56, and 71 kDa were also evident.
Western blot analysis of ERβ protein expression revealed a major ERβ-like band of 53 kDa in each cell line. Lower levels of a 56-kDa ERβ2-like protein were also present in lysates from D283Med and PFSK1 cells (Fig. 2B). Additional ERβ-immunoreactive proteins with mobilities similar to previously described functional isoforms of human ERβ were differentially present (32,33,34,35). The notable differences in ERβ-like immunoreactive species may account for the differences in E2 sensitivity of each cell line. The results of the Western analysis demonstrate that MD cell lines recapitulate the elevated levels of ERβ expressed in maturing granule cell precursors and in primary MD tumor cells.
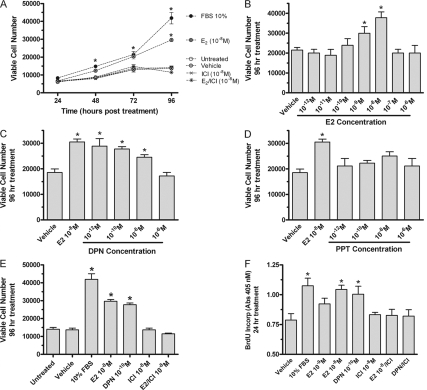
Estradiol regulates MD growth in culture
Direct counting of D283Med cells grown in a basal medium supplemented with 10 nm estradiol revealed a significant ER-dependent increase in viable cell numbers compared with control cultures lacking estradiol (Fig. 3A). In estradiol-treated cultures at the 48- and 72-h time points, the number of viable cells was increased about 1.5-fold. At 96 h, estradiol further increased D283Med cell growth to more than 2.1-fold above control. The steroidal inhibitor of ER-mediated transactivation ICI182,780 blocked estradiol-induced increases in cell numbers (Fig. 3, A and E). Concentration-response analysis showed that 10 nm estradiol exerted maximal effects on D283Med growth (Fig. 3B). The inverted U-shaped dose-response relationship observed for growth stimulation is typical of estradiol’s stimulatory effects on responsive tumors. Growth of D283Med cells was dose-dependently stimulated by the low concentrations (1 pm to 10 nm) of the ERβ-selective agonist DPN (Fig. 3C). The ERα-selective agonist PPT did not influence viable cell numbers at any concentrations tested (Fig. 3D). Results of BrdU incorporation experiments revealed an ERβ-mediated dose-dependent mitogenic response to 1 or 10 nm estradiol or 0.1 nm DPN (Fig. 3F). The ERα-selective agonist PPT was without effect. Estradiol and DPN each stimulated BrdU incorporation in cultures of D283Med cells by more than 1.4-fold 24 h after treatment (Fig. 3F). In all cases, ICI182,780 blocked estrogenic growth stimulation (Fig. 3, A, E, and F). These results demonstrate that ERβ expressed in D283Med MD cells is functional and that ERβ-induced alterations in estrogen-responsive gene expression are required for the growth-stimulatory effects of E2.
Figure 3.
Effects of estradiol on the growth of D283Med cells. In each experiment, 3000 cells/ml were cultured in basal medium containing 10% charcoal-cyclodextrin-stripped serum or 10% FBS (positive control). A, At the time of plating, medium was either untreated or supplemented with 10 nm E2, 0.001% DMSO (vehicle), 10 nm ICI182,780 (ICI), or 10 nm E2 plus 10 nm ICI (E2/ICI). At 24, 48, 72, and 96 h after seeding/treatments, the number of viable cells present in each culture was determined by direct cell counting. Viable cell numbers at each time point are plotted. B–D, For concentration-response analysis, Med283D cells were plated as above and treated with either DMSO vehicle or the indicated final concentration of each ER agonist. Viable cell numbers were determined after 96 h incubation. B, Concentration-response analysis for the effects of E2 on cell numbers revealed significant increases in cell growth in the presence of 10−9 and 10−10 m E2. C, Concentration-response analysis for effects of the ERβ-selective agonist DPN. Increases in cell numbers were observed in cultures containing from 10−12 to 10−8 m DPN. D, Concentration-response analysis for effects of the ERα-selective agonist PPT on D283Med cell growth. Increases in cell numbers were not observed in response to PPT. E and F, Mitogenic actions of estrogens in D283Med cells. E, At 96 h after seeding/treatments, the number of viable cells present in each culture was determined as above by direct counting. In the presence of the ER antagonist ICI182,780 (10−8 m), the growth-stimulating actions of E2 (10−8 m) were completely abrogated. Untreated cultures and cultures treated with vehicle, 10% serum, DPN (10−10 m), or DPN/ICI182,780 (10−8 m) were included for comparison or as controls. F, At the 24-h time point, the effect of E2 (10−8 and 10−9 m) and DPN (10−10 m) with or without ICI182,780 (10−8 m) on the amount of BrdU incorporated into newly synthesized DNA of dividing Med283D cells was assessed using an ELISA-based method. The results of the analysis of BrdU incorporation in the presence of E2 or DPN show that mitosis was increased. Those mitogenic actions were completely blocked in the presence of ICI182,780. All results are expressed as the mean ± sem derived from at least three different experiments. A level of significant differences between experimental treatment and its appropriate corresponding control groups were determined by ANOVA and indicated above the error bars; *, P < 0.05. Each treatment group contained at least three independent samples.
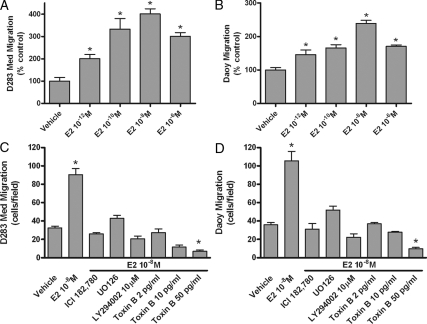
Estradiol regulates MD cell migration
Dose-response analysis using estradiol as a chemoattractant revealed that concentrations of estradiol as low as 1 pm increased cell migration of both D283Med and Daoy cells (Fig. 4, A and B). Maximal effects were induced by 10 nm estradiol and estradiol’s ability to stimulate migration was completely inhibited by the presence of ICI182,780 (Fig. 4, C and D). Additional inhibitor studies were done to investigate the importance of ERK1/2 MAPK and phosphatidylinositol 3-kinase signaling pathways in E2-medated MD migration. Blockade of MAPK kinase-1 kinase activity with U0126 or phosphatidylinositol 3-kinase activity with LY294002 completely inhibited E2-induced increases in cell migration (Fig. 4, C and D). Members of the Rho family of small GTP-binding proteins are known to be important regulators of actin assembly and the dynamic organization of the actin cytoskeleton during cell migration. Toxin B, a specific inhibitor of Rho GTPases (36), fully blocked the ability of estradiol to increase migration of D283Med and Daoy cells (Fig. 4, C and D). Those results suggest that estradiol sensitivity of MD cell migration involves integrated changes in estrogen-responsive gene expression and growth-factor-like signaling pathways to regulate actin cytoskeleton dynamics and tumor cell migration (37).
Figure 4.
Estrogen-mediated MD cell migration. D283Med (A) or Daoy (B) cells were seeded into the top wells of transwell Boyden chambers and allowed to migrate in the absence (vehicle) or presence of a gradient of estradiol chemoattractant in the bottom chamber. For estradiol dose response, concentrations of E2 between 10−12 and 10−6 m acted as a chemoattractant to stimulate increased D283Med cell migration. C and D, Inhibitors of classical ER-mediated signaling or growth factor-like signaling completely blocked estradiol-induced (10−8 m) increases in Med283D and Daoy cell migration. Results are expressed as the mean ± sem derived from multiple experiments (n = 6). Significant differences between values for vehicle control and treatment groups are indicated. *, P < 0.05.
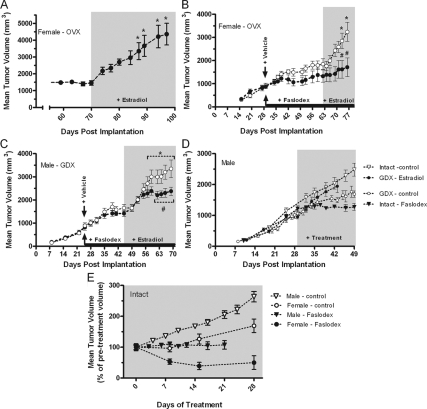
Antiestrogen blockade of estradiol-regulated MD growth in vivo
Xenograft models of human MD in athymic nude mice were used to determine whether MD growth was responsive to gonadal hormones in vivo and whether inhibition of ER-dependent signaling impacted tumor growth. Initial studies in which D283Med tumor cells were implanted in ovariectomized (ovx) nude mice revealed that tumor implantation and growth were not dependent upon gonadal hormones. However, in the absence of gonadal hormones, tumor growth reached a plateau by approximately 40 d after inoculation. Tumor size became arrested at a volume of about 1500 mm3 (e.g. Fig. 5, A–C). In the ovx females over the next 30 d, a further increase in tumor size was not observed (Fig. 5A). In animals without hormone supplementation or in vehicle-treated animals, renewed tumor growth was not observed. However, exogenous estradiol induced a robust stimulation of tumor growth (Fig. 5A). The antiestrogen drug Faslodex completely inhibited estradiol-stimulated tumor growth (Fig. 5, B and C). Similar tumor growth characteristics and responsiveness to estradiol were observed in castrated males (Fig. 5D). Castration significantly reduced tumor growth in males, and in the presence of estradiol, tumor growth rate in castrated males was restored to the rate observed in intact males (Fig. 5D). In intact males and females, Faslodex treatment inhibited tumor growth (Fig. 5, D and E). In both gonadectomized males and females, treatment with Faslodex did not significantly influence the growth of tumors in the absence of estradiol, although in females, Faslodex appeared to decrease tumor size. Although this effect was not statistically significant in the ovx female model (Fig. 5B), in normal females, the mean tumor size was observed to decrease by approximately 50% (Fig. 5E). A similar reduction in tumor size upon Faslodex treatment was not observed in males, and tumor growth in intact males was consistently more rapid than in females, suggesting that other sex-specific factors might influence the ability of ER signaling to stimulate MD growth.
Figure 5.
Effects of estradiol and Faslodex on ER-mediated growth of D283Med xenografts in gonadectomized nude mice. Animals received bilateral inoculations of approximately 107 D283Med cells on d 0, and tumors were allowed to develop in the absence of hormonal supplementation. Tumor growth was measured at least weekly, and animals were randomly assigned to treatment groups on the day treatments were initiated. Supplementation with E2 by sc injection every other day is indicated by gray shading. A, In ovx females, tumors were allowed to develop in the absence of gonadal hormones for 70 d. Over this period, increases in tumor size became arrested. Estradiol supplementation initiated on d 70 induced a rapid increase in tumor growth. Similar increases in animals without hormone supplementation or in vehicle-treated animals were never observed. *, Significant (P < 0.05; n = 6) increase in volume compared with the volume on d 70. B, Analysis of the effect of Faslodex (1 mg/wk) on MD growth in ovx females. After inoculation, tumors were allowed to grow to a mean volume of 872 mm3, at which time animals were treated with either Faslodex (n = 4; 1 mg/wk sc) or an equal volume of castor oil vehicle (n = 6). Initiation of treatments is indicated with arrows, and the duration of Faslodex treatment is indicated with a solid bar. Estradiol treatment was initiated on d 62. Faslodex blocked estrogen-induced increases in tumor growth. *, Significant (P < 0.05) increase in volume compared with the volume on d 62; #, mean volume for Faslodex and vehicle-treated groups were significantly different. C, Analysis of the effect of Faslodex (1 mg/wk) on MD growth in castrated males. Experimental procedures were similar to those used above for females. Tumors had reached a mean volume of 891 mm3 on d 25, at which time treatments were initiated. Estradiol treatment was initiated on d 45. Faslodex inhibited estrogen-induced increases in tumor growth. *, Significant (P < 0.05) increase in volume compared with the volume on d 65; #, mean volume for Faslodex-treated group (n = 8) was significantly different from the vehicle-treated control (n = 8). Within the Faslodex-treated group, differences in volumes between d 45 and any subsequent treatment day were not significant. D, Comparison of Faslodex actions on MD growth in intact or gonadectomized male and female nude mice. Intact or castrated (GDX) male nude mice were inoculated, and tumor growth was monitored. Estradiol, Faslodex, or vehicle treatments were started on d 29 when tumors had reached a volume of about 1000 mm3. Intact control n = 13, and Faslodex n = 5; GDX control n = 10, and estradiol n = 7. Differences between values for treated and control groups were significantly different after 1 wk treatment. E, The response of MD tumor growth to Faslodex treatment in intact males and females. For comparisons between different experiments, tumor volumes for each treatment group were transformed to a percentage of the tumor volume at initiation of treatment with growth compared beginning on the day treatment was started. Differences between values for treated and control groups were significantly different after 1 wk treatment. The volumes for the male and female control groups at d 28 were significantly different from each other. Female control: n = 7, and Faslodex n = 6; male control: n = 13, and Faslodex n = 5.
Discussion
Estrogens are major regulators of normal brain development, maturation, and function in both males and females. Estradiol is also a well-known carcinogen that plays a central role in the etiology and progression of hormone-responsive tumors. MD arise from a failure of cerebellar granule cell precursors to appropriately terminate normal development and differentiate correctly into mature neurons. Based on previous analyses showing that ERβ was up-regulated in differentiating granule cell precursors, we reasoned that ERβ-mediated mechanisms might contribute to the etiology or progression of MD. The results presented above support the developmental parity of ERβ function during granule cell ontogeny and MD oncogenesis.
Immunohistochemical studies demonstrated that ERs were expressed in primary MD tumors from both male and female patients and immature granule cells of the normally maturing human cerebellum. Significantly, ERβ-like immunoreactivity was detected in malignant cells of every MD tumor analyzed and also in the well-characterized human MD cell lines D283Med and Daoy. In contrast, ERα-like immunoreactivity was detected at only low levels in 41% of the patient tumors. The results of pharmacological studies demonstrating that activation of ERβ, but not ERα, was required for the growth-stimulatory effects of E2 revealed that estrogen responsiveness in MD is mediated by ERβ. Although this study was not designed to elucidate whether ER levels are a prognostic marker of clinical outcome, it is notable that tumors anticipated to have better clinical outcomes, classical or desmoplastic MD (24), exhibited lowest levels of ERβ expression. In contrast, the more aggressive anaplastic tumors were characterized as having the highest levels of ERβ staining and lacked detectable ERα expression. Because the primary concern of the study was to assess whether ERs were expressed in MD, this study was limited in power to detect specific associations between receptor expression and clinically related endpoints. Additional research into the association between expression of specific ERs, receptor variants, and clinical outcomes of MD patients is needed. However, our analysis has revealed that functional ERβ expression is a fundamental aspect of MD biology.
The cerebellum is one of the latest developing regions of the brain; in humans, significant amounts of neuronal proliferation, differentiation, and maturation continue for many years after birth. During infancy and early childhood, the mitotic granule cell precursors and maturing postmitotic cerebellar neurons are sensitive to numerous autocrine, paracrine, endocrine, and environmental factors that regulate or impact neuronal proliferation and maturation. Because of the protracted perinatal duration of cerebellar development, it is likely that increased postnatal exposures of undifferentiated cerebellar granule cell precursors and malignant MD precursors to the influences of the hormonal milieu contributes to the predominance of cerebellar tumors in children. It is noteworthy that coincident with the time the cerebellum reaches an essentially mature state the incidence of MD sharply declines (1).
In the ERβ-positive D283Med cells and xenografts, physiological concentrations of E2 were found to dose-dependently stimulate mitogenesis and tumor growth. In vivo and in vitro experiments using ER-selective agonists and the ER antagonist ICI182,780 demonstrated that the growth-stimulating actions of estradiol were mediated by ERβ-dependent mechanisms. Additionally, E2 was found to have extremely potent, growth factor-like effects on MD cell migration. Those data strongly support our hypothesis that MD are E2-responsive tumors and that growth and tumor cell invasion can be blocked by inhibition of ERβ activity. Related in vitro studies had demonstrated previously that cerebrocortical-derived PNET cells also express active ER (26). As in MD cells, E2 activation of ERβ increased ER- and ERK-dependent cell migration, suggesting that rapid signaling actions of E2 and ER-mediated regulation of estrogen-responsive gene expression contributed to the invasive phenotype of PNETs (26).
Previous research has made it clear that loss of normal developmental regulation of signaling mechanisms is a contributing factor in the etiology of MD. Along with being associated with dysregulation of Hedgehog or Wnt signaling, other growth factor signaling mechanisms can play functional roles in the etiology, progression, and dissemination of MD (2,16,17). In MD and PNET cells, growth factor receptor-induced ERK signaling clearly plays an important role in regulating tumor cell migration and invasion (26,37). It is therefore not surprising that estrogen-signaling mechanisms could impact the regulation of MD pathophysiology via specific intracellular signaling cascades. In neuronal cells, growth factor signaling pathways are often coordinately integrated with ER-dependent signaling (9,38). Because mechanisms that facilitate regulatory cross talk between growth factor signaling and classical ER-mediated actions of estradiol are well known, we investigated whether those mechanisms might be involved in the pathophysiology of MD. Blockade of either growth factor signaling cascades or ER-mediated transactivation were effective in blocking E2-mediated chemotaxis. It is probable that growth factor-like E2-signaling via ERβ and changes in classical E2-responsive gene expression are important cooperative regulatory mechanisms responsible for the physiological action of E2 in MD.
Neither the role of ERβ in estrogen-responsive cancers nor the potential involvement of either ERα or ERβ in CNS neoplasms has been clearly defined. Retrospective studies analyzing expression of ERβ in breast cancer tumors suggest that ERβ expression might be a predictive indicator of prognosis or response to tamoxifen treatment in both ERα-positive and -negative breast carcinomas (39,40,41). Furthermore, expression of a specific alternatively spliced isoform of ERβ, which may act in a dominant-negative fashion to selectively decrease ERα transcriptional activity, may potentially decrease ERα-positive tumor responsiveness to tamoxifen (42). Thus, there is strong, although mainly circumstantial, evidence for a clinically important role of ERβ signaling in hormone-responsive breast cancers.
Before the studies reported here, there was little direct evidence that inhibitors of ER-mediated signaling would be efficacious therapeutic agents for treating CNS-derived tumors. Evidence for a functional role of ERs in CNS tumors comes from numerous studies that have detected expression of ERs in various cell lines initially derived from a variety of central or sympathetic nervous system tumors (26,43,44). Those observations were taken to suggest that estrogens and ERs might have various roles in many different types of nervous system tumors (neuroblastoma, glioma, etc.). Additionally, retrospective clinical studies comparing differential ER expression in astrocytic carcinomas found that ERβ was expressed in some astrocytic tumors. Because ERβ expression was limited to the low-grade tumors, those findings were taken to indicate that ERβ might be an indicator of less aggressive tumors and thus a potential marker of favorable treatment response and clinical outcome (45).
Results from studies employing glioma-derived cell lines showed that in vitro high concentrations of tamoxifen increase the cytotoxic sensitivity of tumor cells to chemotherapeutic drugs and radiation. Rather than disruption of ER-mediated signaling mechanisms, the rationale for high-dose tamoxifen therapy for treating those CNS malignancies is based on its ability to sensitize these glial-derived tumor cells in vitro to other chemotherapeutic agents and radiation via inhibition of PKC. Although tamoxifen therapy has revolutionized the treatment of breast cancer, the use of high-dose tamoxifen for treatment of astrocytic brain tumors in adults and children has proven disappointing (46,47,48). Not only have recent clinical studies employing tamoxifen as adjuvant therapy failed to prove beneficial, some studies have found high-dose tamoxifen to increase recurrence of multifocal tumors in glioblastoma patients (49).
In contrast to the use of tamoxifen as a chemosensitizing agent, our results suggest a rational application of ER-signaling inhibitors for treatment of MD. Results of human MD xenograft experiments demonstrated that lacking estrogen, or when ER activity is inhibited, MD tumor growth becomes arrested. Clinically, the loss of estrogen’s growth-stimulating actions could likely aid in MD treatment and potentially limit the dose of radiotherapy required to cure MD. Because peak incidence of MD is around age 5, blockade of estrogen signaling as a temporary adjuvant treatment for MD would be expected to have minimal long-term side effects in the majority of MD patients. Thus, in most patients, antiestrogen treatments are anticipated to be efficacious while avoiding critical periods of most estrogen-mediated effects, including those involved with development of secondary sexual characteristics during puberty and the much earlier critical periods of estrogen-sensitive CNS development that controls sexual specification of the brain. Agents that selectively inhibit ERβ signaling may prove the most effective antiestrogen treatments for MD by increasing further the specificity of the antiestrogen effects to those mediated via ERβ. However, well-characterized ERβ-selective drugs are not as yet clinically available. Any antiestrogen signaling treatment modalities developed must keep in mind the potential impact of specific drug metabolites and the impact that blockade will have on other estrogen-responsive processes, such as bone growth. It is also noteworthy that the youngest of patients are likely exposed to the highest levels of estrogenic endocrine-disrupting chemicals and are typically most sensitive to the disruptive actions of some of these environmental estrogens (50). Simply by limiting the exposure of MD patients to dietary phytoestrogens and estrogenic compounds migrating from medical devices, there may be immediate benefits that would result from decreasing patient exposure to environmental estrogens. Thus, it is felt that careful attention should now be paid to MD patient exposure to compounds with estrogen-like activities.
Acknowledgments
We acknowledge excellent technical assistance of Dr. Robert Jakab, Michelle Kirby, and Lynda Spurling.
Footnotes
This work was supported by National Institutes of Health National Institute of Environmental Health Sciences Grant R01-ES015145, the University of Cincinnati Center for Environmental Genetics (P30-ES06096), and a Translational Research Initiative grant from Cincinnati Children’s Hospital Research Foundation.
Disclosure Statement: The authors have nothing to disclose.
First Published Online November 13, 2008
Abbreviations: BrdU, 5-Bromo-2′-deoxyuridine; CNS, central nervous system; DMSO, dimethylsulfoxide; DPN, 2,3-bis(4-hydroxyphenyl)-propionitrile diarylpropionitrile; E2, 17β-estradiol; ER, estrogen receptor; FBS, fetal bovine serum; IGL, internal granule cell layer; MD, medulloblastoma; ML, molecular layer; PL, Purkinje cell layer; PNET, primitive neuroectodermal tumor; PPT, 4,4′,4″-(4-propyl-[1H]pyrazole-1,3,5-triyl) trisphenol; ovx, ovariectomized.
References
- Ries LA, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, Bunin GR 1999 Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995, National Cancer Institute, SEER Program. National Institutes of Health (NIH) Publication No. 99-4649. Bethesda, MD: NIH, Department of Health and Human Services [Google Scholar]
- Rubin JB, Rowitch DH 2002 Medulloblastoma: a problem of developmental biology. Cancer Cell 2:7–8 [DOI] [PubMed] [Google Scholar]
- Wechsler-Reya R, Scott MP 2001 The developmental biology of brain tumors. Annu Rev Neurosci 24:385–428 [DOI] [PubMed] [Google Scholar]
- Gatta G, Capocaccia R, Coleman MP, Ries LA, Berrino F 2002 Childhood cancer survival in Europe and the United States. Cancer 95:1767–1772 [DOI] [PubMed] [Google Scholar]
- Gurney JG, Kadan-Lottick NS, Packer RJ, Neglia JP, Sklar CA, Punyko JA, Stovall, M, Yasui Y, Nicholson HS, Wolden S, McNeil DE, Mertens AC, Robison LL 2003 Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood Cancer Survivor Study. Cancer 97:663–673 [DOI] [PubMed] [Google Scholar]
- Gurney JG, Ness KK, Stovall M, Wolden S, Punyko JA, Neglia JP, Mertens AC, Packer RJ, Robison LL, Sklar CA 2003 Final Height and Body Mass Index among Adult Survivors of Childhood Brain Cancer: Childhood Cancer Survivor Study. J Clin Endocrinol Metab 88:4731–4739 [DOI] [PubMed] [Google Scholar]
- Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE 2004 Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol 5:399–408 [DOI] [PubMed] [Google Scholar]
- Packer RJ 1999 Childhood medulloblastoma: progress and future challenges. Brain Dev 21:75–81 [DOI] [PubMed] [Google Scholar]
- Lee SJ, McEwen BS 2001 Neurotrophic and neuroprotective actions of estrogens and their therapeutic implications. Annu Rev Pharmacol Toxicol 41:569–591 [DOI] [PubMed] [Google Scholar]
- Takeyama J, Suzuki T, Inoue S, Kaneko C, Nagura H, Harada N, Sasano H 2001 Expression and cellular localization of estrogen receptors α and β in the human fetus. J Clin Endocrinol Metab 86:2258–2262 [DOI] [PubMed] [Google Scholar]
- Fried G, Andersson E, Csöregh L, Enmark E, Gustafsson JA, Aanesen A, Osterlund C 2004 Estrogen receptor β is expressed in human embryonic brain cells and is regulated by 17β-estradiol. Eur J Neurosci 20:2345–2354 [DOI] [PubMed] [Google Scholar]
- Belcher SM 1999 Regulated expression of estrogen receptor α and β mRNA in granule cells during development of the rat cerebellum. Brain Res Dev Brain Res 115:57–69 [DOI] [PubMed] [Google Scholar]
- Jakab RL, Wong JK, Belcher SM 2001 Estrogen receptor-β immunoreactivity in differentiating cells of the developing rat cerebellum. J Comp Neurol 430:396–409 [DOI] [PubMed] [Google Scholar]
- Zsarnovszky A, Belcher SM 2001 Identification of a developmental gradient of estrogen receptor expression and cellular localization in the developing and adult female rat primary somatosensory cortex. Brain Res Dev Brain Res 129:39–46 [DOI] [PubMed] [Google Scholar]
- Zsarnovszky A, Smith T, Hajos F, Belcher SM 2002 Estrogen regulates GFAP-expression in specific subnuclei of the female rat interpeduncular nucleus: a potential role for estrogen receptor β. Brain Res 958:488–496 [DOI] [PubMed] [Google Scholar]
- McEwen B 2002 Estrogen actions throughout the brain. Recent Prog Horm Res 57:357–384 [DOI] [PubMed] [Google Scholar]
- Belcher SM, Zsarnovszky A 2001 Estrogenic actions in the brain: estrogen, phytoestrogens, and rapid intracellular signaling mechanisms. J Pharmacol Exp Ther 299:408–414 [PubMed] [Google Scholar]
- Falkenstein E, Tillmann HC, Lost D, Feuring M, Lost D 2000 Multiple actions of steroid hormones: a focus on rapid, nongenomic effects. Pharmacol Rev 52:513–556 [PubMed] [Google Scholar]
- Wong JK, Le HH, Zsarnovszky A, Belcher SM 2003 Estrogens and ICI182,780 (Faslodex) modulate mitosis and cell death in immature cerebellar neurons via rapid activation of p44/p42 mitogen-activated protein kinase. J Neurosci 23:4984–4995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsarnovszky A, Le HH, Wang HS, Belcher SM 2005 Ontogeny of rapid estrogen-mediated ERK1/2 signaling in the rat cerebellar cortex in vivo: potent non-genomic agonist and endocrine disrupting activity of the xenoestrogen bisphenol A. Endocrinology 146:5388–5396 [DOI] [PubMed] [Google Scholar]
- Sasahara K, Shikimi H, Haraguchi S, Sakamoto H, Honda S, Harada N, Tsutsui K 2007 Mode of action and functional significance of estrogen-inducing dendritic growth, spinogenesis, and synaptogenesis in the developing Purkinje cell. J Neurosci 27:7408–7417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flototto T, Djahansouzi S, Glaser M, Hanstein B, Niederacher D, Brumm C, Beckmann MW 2001 Hormones and hormone antagonists: mechanisms of action in carcinogenesis of endometrial and breast cancer. Horm Metab Res 33:451–457 [DOI] [PubMed] [Google Scholar]
- Jordan VC, Brodie AM 2007 Development and evolution of therapies targeted to the estrogen receptor for the treatment and prevention of breast cancer. Steroids 72:7–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart CG, Kepner JL, Goldthwaite PT, Kun LE, Duffner PK, Friedman HS, Strother DR, Burger PC 2002 Histopathologic grading of medulloblastomas: a Pediatric Oncology Group study. Cancer 94:552–560 [DOI] [PubMed] [Google Scholar]
- Wong JK, Kennedy PR, Belcher SM 2001 Simplified serum- and steroid-free culture conditions for the high-throughput viability analysis of primary cultures of cerebellar neurons. J Neurosci Methods 110:45–55 [DOI] [PubMed] [Google Scholar]
- Kirby M, Zsarnovszky A, Belcher SM 2004 Estrogen receptor expression in a human primitive neuroectodermal tumor cell line from the cerebral cortex: estrogen stimulates rapid ERK1/2 activation and receptor-dependent cell migration. Biochem Biophys Res Commun 319:753–758 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Hongo K, Tada T, Kobayashi S 2003 What is the best method for reporting tumor diameter in vestibular schwannoma? Neurosurgery 53:634–638 [DOI] [PubMed] [Google Scholar]
- Yue W, Zhou D, Chen S, Brodie A 1994 A new nude mouse model for postmenopausal breast cancer using MCF-7 cells transfected with the human aromatase gene. Cancer Res 54:5092–5095 [PubMed] [Google Scholar]
- Yue W, Wang J, Savinov A, Brodie A 1995 Effect of aromatase inhibitors on growth of mammary tumors in a nude mouse model. Cancer Res 55:3073–3077 [PubMed] [Google Scholar]
- McLeskey SW, Zhang L, El-Ashry D, Trock BJ, Lopez CA, Kharbanda S, Tobias CA, Lorant LA, Hannum RS, Dickson RB, Kern FG 1998 Tamoxifen-resistant fibroblast growth factor-transfected MCF-7 cells are cross-resistant in vivo to the antiestrogen ICI 182,780 and two aromatase inhibitors. Clin Cancer Res 4:697–711 [PubMed] [Google Scholar]
- Jelovac D, Macedo L, Goloubeva OG, Handratta V, Brodie AMH 2005 Additive antitumor effect of aromatase inhibitor letrozole and antiestrogen fulvestrant in a postmenopausal breast cancer model. Cancer Res 65:5439–5444 [DOI] [PubMed] [Google Scholar]
- Choi I, Ko C, Park-Sarge OK, Nie R, Hess RA, Graves C, Katzenellenbogen BS 2001 Human estrogen receptor β-specific monoclonal antibodies: characterization and use in studies of estrogen receptor β protein expression in reproductive tissues. Mol Cell Endocrinol 181:139–150 [DOI] [PubMed] [Google Scholar]
- Ogawa S, Inoue S, Watanabe T, Orimo A, Hosoi T, Ouchi Y, Muramatsu M 1998 Molecular cloning and characterization of human estrogen receptor βcx: a potential inhibitor of estrogen action in human. Nucleic Acids Res 26:3505–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat RA, Harnish DC, Stevis PE, Lyttle CR, Komm BS 1998 A novel human estrogen receptor β: identification and functional analysis of additional N-terminal amino acids. J Steroid Biochem Mol Biol 67:233–240 [DOI] [PubMed] [Google Scholar]
- Moore JT, McKee DD, Slentz-Kesler K, Moore LB, Jones SA, Horne EL, Su JL, Kliewer SA, Lehmann JM, Willson TM 1998 Cloning and characterization of human estrogen receptor β isoforms. Biochem Biophys Res Commun 47:75–78 [DOI] [PubMed] [Google Scholar]
- Just I, Fritz G, Aktories K, Giry M, Popoff MR, Boquet P, Hegenbarth S, Eichel-Streiber C 1994 Clostridium difficile toxin B acts on the GTP-binding protein Rho. J Biol Chem 269:10706–10712 [PubMed] [Google Scholar]
- MacDonald TJ, Brown KM, LaFleur B, Peterson K, Lawlor C, Chen Y, Packer RJ, Cogen P, Stephan DA 2001 Expression profiling of medulloblastoma: PDGFRA and the RAS/MAPK pathway as therapeutic targets for metastatic disease. Nat Genet 29:143–152 [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Kow LM, Pfaff D 2005 Integration of steroid hormone initiated membrane action to genomic function in the brain. Steroids 70:388–396 [DOI] [PubMed] [Google Scholar]
- Skliris GP, Carder PJ, Lansdown MR, Speirs V 2001 Immunohistochemical detection of ERβ in breast cancer: towards more detailed receptor profiling? Br J Cancer 84:1095–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp TA, Weiss HL, Parra IS, Cui Y, Osborne CK, Fuqua SAW 2004 Low levels of estrogen receptor β protein predict resistance to tamoxifen therapy in breast cancer. Clin Cancer Res 10:7490–7499 [DOI] [PubMed] [Google Scholar]
- Gruvberger-Saal SK, Bendahl PO, Saal LH, Laakso M, Hegardt C, Eden P, Peterson C, Malmstrom P, Isola J, Borg A, Ferno M 2007 Estrogen receptor β expression is associated with tamoxifen response in ERα-negative breast carcinoma. Clin Cancer Res 13:1987–1994 [DOI] [PubMed] [Google Scholar]
- Saji S, Omoto Y, Shimizu C 2002 Expression of estrogen receptor (ER) βcx protein in ERα-positive breast cancer: specific correlation with progesterone receptor. Cancer Res 62:4849–4853 [PubMed] [Google Scholar]
- Ma ZQ, Spreafico E, Pollio G, Santagati S, Conti E, Cattaneo E, Maggi, A 1993Activated estrogen receptor mediates growth arrest and differentiation of a neuroblastoma cell line. Proc Natl Acad Sci USA 90:3740–3744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie KK, Keefe D, Powell S, Naftolin F 1994 Estrogen receptors are identified in the glioblastoma cell line U138MG. J Soc Gynecol Investig 1:238–244 [DOI] [PubMed] [Google Scholar]
- Batistatou A, Stefanou D, Goussia A, Arkoumani E, Papavassiliou AG, Agnantis NJ 2004 Estrogen receptor β (ERβ) is expressed in brain astrocytic tumors and declines with dedifferentiation of the neoplasm. J Cancer Res Clin Oncol 130:405–410 [DOI] [PubMed] [Google Scholar]
- Broniscer A, Leite CdC, Lanchote VL, Machado TMS, Cristofani LM 2000 Radiation therapy and high-dose tamoxifen in the treatment of patients with diffuse brainstem gliomas: results of a Brazilian cooperative study. J Clin Oncol 18:1246–1253 [DOI] [PubMed] [Google Scholar]
- Robins HI, Won M, Seiferheld WF, Schultz CJ, Choucair AK, Brachman DG, Demas WF, Mehta MP 2006 Phase 2 trial of radiation plus high-dose tamoxifen for glioblastoma multiforme: RTOG protocol BR-0021. Neuro Oncol 8:47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence AM, Peterson RA, Scharnhorst JD, Silbergeld DL, Rostomily RC 2004 Phase II study of concurrent continuous temozolomide (TMZ) and tamoxifen (TMX) for recurrent malignant astrocytic gliomas. J Neurooncol 70:91–95 [DOI] [PubMed] [Google Scholar]
- Puchner MJ, Giese A, Lohmann F, Cristante L 2004 High-dose tamoxifen treatment increases the incidence of multifocal tumor recurrences in glioblastoma patients. Anticancer Res 24:4195–4203 [PubMed] [Google Scholar]
- vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, Farabollini F, Guillette LJ, Hauser R, Heindel JJ, Ho SM, Hunt PA, Iguchi T, Jobling S, Kanno J, Keri RA, Knudsen KE, Laufer H, LeBlanc GA, Marcus M, McLachlan JA, Myers JP, Nadal A, Newbold RR, Olea N, Prins GS, Richter CA, Rubin BS, Sonnenschein C, Soto AM, Talsness CE, Vandenbergh JG, Vandenberg LN, Walser-Kuntz DR, Watson CS, Welshons WV, Wetherill Y, Zoeller RT 2007 Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol 24:131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]