Abstract
Thyroid hormones are essential for normal development and metabolism. Thyroid hormone biosynthesis requires iodide uptake into the thyrocytes and efflux into the follicular lumen, where it is organified on selected tyrosyls of thyroglobulin. Uptake of iodide into the thyrocytes is mediated by an intrinsic membrane glycoprotein, the sodium-iodide symporter (NIS), which actively cotransports two sodium cations per each iodide anion. NIS-mediated transport of iodide is driven by the electrochemical sodium gradient generated by the Na+/K+-ATPase. NIS is expressed in the thyroid, the salivary glands, gastric mucosa, and the lactating mammary gland. TSH and iodide regulate iodide accumulation by modulating NIS activity via transcriptional and posttranscriptional mechanisms. Biallelic mutations in the NIS gene lead to a congenital iodide transport defect, an autosomal recessive condition characterized by hypothyroidism, goiter, low thyroid iodide uptake, and a low saliva/plasma iodide ratio. Pendrin is an anion transporter that is predominantly expressed in the inner ear, the thyroid, and the kidney. Biallelic mutations in the SLC26A4 gene lead to Pendred syndrome, an autosomal recessive disorder characterized by sensorineural deafness, goiter, and impaired iodide organification. In thyroid follicular cells, pendrin is expressed at the apical membrane. Functional in vitro data and the impaired iodide organification observed in patients with Pendred syndrome support a role of pendrin as an apical iodide transporter.
This review shows how the sodium-iodide symporter mediates the active transport of iodide at the basolateral membrane of thyrocytes and discusses biallelic mutations in NIS and the effects of pendrin.
The iodide-containing thyroid hormones T3 and its precursor T4 are crucial for normal development, growth, and regulation of numerous metabolic pathways. The main function of the thyroid gland is to concentrate iodide and to make it available for biosynthesis of thyroid hormones. The significance of this mechanism is evident in light of the scarcity of iodide in most of the environment and the fact that insufficient dietary supply of iodide remains a major public health issue in many parts of the world (1).
The synthesis of thyroid hormones requires a normally developed thyroid gland, an adequate nutritional intake of iodide, and a series of sequential biochemical steps. Thyroid hormone synthesis takes place in the follicles, the functional units of the gland (2). Each follicle consists of a single layer of thyroid epithelial cells surrounding the follicular lumen. The follicular lumen is filled with colloid, which is predominantly composed of thyroglobulin, a large glycoprotein that serves as the scaffold for thyroid hormone synthesis (3).
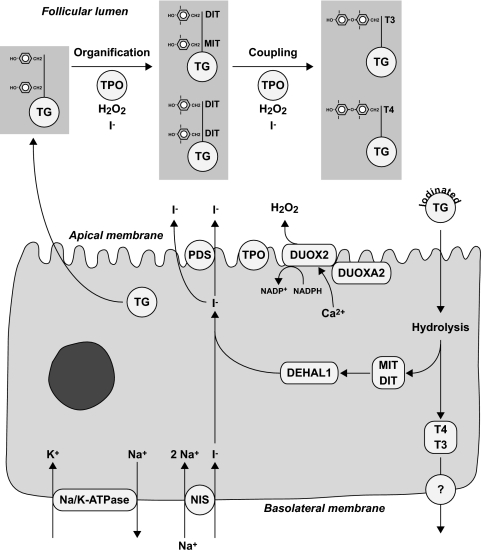
The synthesis of thyroid hormones requires uptake of iodide across the basolateral membrane into the thyrocytes, transport across the cell, and efflux through the apical membrane into the follicular lumen. Uptake of iodide is mediated by the sodium-iodide symporter (NIS), which cotransports two sodium ions along with one iodide ion, with the sodium gradient serving as the driving force (Fig. 1) (1). The energy required to produce the sodium gradient is provided by the ouabain-sensitive Na+/K+-ATPase (4). The efflux of iodide across the apical membrane is mediated, at least in part, by pendrin (5). Once iodide reaches the cell-colloid interface, it is oxidized and rapidly organified by incorporation into selected tyrosyl residues of thyroglobulin. This reaction, referred to as organification, is catalyzed by thyroid peroxidase (TPO) in the presence of hydrogen peroxide and results in the formation of mono- and diiodotyrosines (MIT and DIT). The generation of hydrogen peroxide is mediated by the calcium-dependent reduced nicotinamide adenine dinucleotide phosphate (NADPH) dual oxidase type 2 (DUOX2). TPO also catalyzes the coupling of two iodotyrosines to form either T3 or T4. To release thyroid hormones, thyroglobulin is engulfed by pinocytosis, digested in lysosomes, and then secreted into the bloodstream at the basolateral membrane (2). The mechanisms of hormone secretion at the basolateral membrane and the involved channel(s) have not been characterized.
Figure 1.
Main steps in thyroid hormone synthesis. At the basolateral membrane of thyroid follicular cells, which form the follicles, iodide is transported into thyrocytes by the NIS. NIS is dependent on the sodium gradient created by the Na/K-ATPase. At the apical membrane, iodide efflux is, in part, mediated by pendrin (PDS/SLC26A4). At the cell-colloid interface, iodide is oxidized by TPO in the presence of H2O2. H2O2 is produced by the calcium- and reduced nicotinamide adenine dinucleotide phosphate-dependent (NADPH) enzyme DUOX2. DUOX2 requires a specific maturation factor, DUOXA2. Thyroglobulin (TG), which is secreted into the follicular lumen, serves as matrix for synthesis of T4 and T3. First, TPO catalyzes iodination of selected tyrosyl residues (organification), which results in the formation of MIT and DIT. Subsequently, two iodotyrosines are coupled to form either T4 or T3 in a reaction that is also catalyzed by TPO. Iodinated thyroglobulin is stored as colloid in the follicular lumen. Upon a demand for thyroid hormone secretion, thyroglobulin is internalized into the follicular cell by pinocytosis and digested in lysosomes, which generates T4 and T3 that are released into the bloodstream through unknown mechanisms. The unused MIT and DIT are retained in the cell and deiodinated by the iodotyrosine dehalogenase 1 (DEHAL1). The released iodide is recycled for thyroid hormone synthesis.
NIS
NIS is an integral plasma membrane glycoprotein localized at the basolateral plasma membrane of thyrocytes (6). This protein plays an essential role in thyroid physiology by mediating uptake of iodide into the thyrocytes, a key step in thyroid hormone synthesis. The ability of the thyroid gland to accumulate radioiodine is also a cornerstone in the diagnosis and treatment of thyroid disorders (7).
The molecular characterization of NIS began in 1996 when the cDNA encoding rat NIS was isolated by expression-cloning in Xenopus laevis oocytes (6). Subsequently, the human cDNA has been cloned by a RT-PCR approach taking advantage of the homology to rat NIS (8). Rat NIS is predicted to have 618 amino acids with a relative molecular mass of 65,196 Daltons (6), and human NIS contains 643 amino acids and exhibits an 84% amino acid identity and 93% similarity to rat NIS (9). The human NIS gene is located on chromosome 19p12-13.2 and contains 14 introns and 15 exons (9).
NIS (SLC5A5) belongs to the solute carrier family 5A (SLC5A). All members of this protein family depend on an electrochemical sodium gradient as the driving force for transport of anions across the plasma membrane (10). The current secondary structure model predicts that NIS contains 13 transmembrane domains with the amino terminus located extracellularly and the carboxy terminus facing the cytosol (1). The mature NIS protein is approximately 87 kDa in size and has three asparagine-linked glycosylation sites (1). Glycosylation does not seem to be required for stability, activity, or targeting of the NIS molecule to the plasma membrane (11). The expression of NIS is differentially regulated and is subjected to various posttranslational modifications in each tissue in which it is expressed (1,12).
In addition to thyroid follicular cells, NIS is expressed in several other tissues, including the salivary glands, gastric mucosa, and the lactating mammary gland, where it mediates active transport of iodide (1). In the lactating mammary gland, NIS plays an important role by concentrating iodide in the milk, thereby supplying newborns with iodide for thyroid hormone synthesis.
Although NIS has a high affinity for iodide, it is able to mediate transport of other ions (13). Large anions such as thiocyanate and perchlorate can inhibit accumulation of iodide in the thyroid by competing with iodide (14,15). Perchlorate is 10–100 times more potent than thiocyanate in inhibiting iodide accumulation in the thyroid (1). The ability of perchlorate to block iodide transport has been used in the therapy of hyperthyroidism, and it is used in the perchlorate discharge test, which serves to detect defects in iodide organification (16). In normal individuals, administration of perchlorate blocks subsequent accumulation of iodide in the thyrocytes but does not cause any discharge of previously accumulated radioiodine because of its rapid organification. In contrast, in individuals with a total or partial iodide organification defect, administration of perchlorate results in the rapid release of the unorganified fraction of the tracer from the thyrocytes (16). Until recently, it has been controversial whether perchlorate acts as a blocker or as a substrate that is transported by NIS (13,17,18). Two recent studies provide evidence that perchlorate is actively transported by NIS (19,20). Perchlorate transport could be demonstrated in a polarized cell system (19) as well as by direct measurement with mass spectrometry (20). Moreover, it has been shown that perchlorate, a widely found pollutant, is transported into the milk (19). Remarkably, the stoichiometry of the NIS-mediated Na+/ClO4− transport is electroneutral, which contrasts with the electrogenic transport of iodide (two Na+ and one I−) (19). These findings indicate that NIS is able to transport different substrates with distinct stoichiometries (19).
Regulation of NIS protein expression
TSH is the major regulator of thyroid cell proliferation, differentiation, and function, including iodide uptake (21). The effects of TSH are primarily mediated through the activation of the cAMP cascade via the GTP-binding protein Gsα (22). TSH stimulates iodide accumulation by positively regulating NIS expression at the protein and mRNA level via the cAMP pathway (23). Hypophysectomized rats with low circulating levels of TSH have a decreased protein expression of NIS, whereas a single injection of TSH leads to a prompt increase in NIS expression (24). Rats maintained on an iodide-deficient diet or treated with propylthiouracil, an agent blocking iodide organification, have high concentrations of TSH, which correlates with an increase in NIS protein expression. These findings are in agreement with the results obtained in human thyroid primary cultures (25,26) and the rat thyroid FRTL-5 cell line (23). In FRTL-5 cells, withdrawal of TSH results in a decrease in intracellular cAMP concentration and iodide uptake activity (23). Re-addition of TSH increases NIS mRNA and protein expression and subsequently restores iodide uptake activity.
Recent studies have shown that TSH not only regulates NIS transcription and biosynthesis but also mediates NIS activity by posttranscriptional mechanisms (27). In the presence of TSH, NIS is active and inserted in the basolateral membrane of thyrocytes (27). Upon TSH withdrawal, NIS protein half-life decreases from 5 to 3 d, and it translocates from the plasma membrane to intracellular compartments (27). As of yet, the mechanisms regulating the subcellular distribution of NIS are only partially elucidated. It is known that NIS has several consensus sites for kinases, including protein kinase A (PKA) and protein kinase C (1). Although it has been shown that NIS is phosphorylated in vivo (27), the functional significance of this modification needs further characterization. A more recent study identified five amino acid residues in NIS that are phosphorylated in vivo, but the phosphorylation status of these amino acid residues does not affect targeting of NIS to the plasma membrane (28). NIS contains several sorting sequences that are known to play a role in targeting, retention, and endocytosis of other membrane proteins (29). For example, the PDZ motif (T/S-X-V/L) located at the carboxy terminus of NIS is one of the sequences involved in protein-protein interactions (1). The PDZ motif is recognized by PDZ-binding proteins that have been implicated in internalization of other transporters (29). NIS also has a dileucine motif, L557L558, which is known to interact with the clathrin-coated system (30). This interaction leads to incorporation of integral membrane proteins into coated vesicles that are then carried to different destinations within the cell (31).
Iodide is another factor that can regulate iodide accumulation in the thyroid. In 1948, Wolff and Chaikoff (32) reported that high doses of iodide block iodide organification in the rat thyroid in vivo. This phenomenon, known as the acute Wolff-Chaikoff effect, is a reversible process, because iodide organification resumes when the iodide concentration in the serum decreases. The mechanisms underlying the Wolff-Chaikoff effect are complex and involve acute regulation of several key genes and proteins within the thyrocytes. Several studies have examined the effect of iodide on NIS mRNA and protein expression in vivo and in vitro (33,34,35). In vivo data suggest that high concentrations of iodide lead to reduction in both NIS mRNA and protein levels, partially by a transcriptional mechanism. In vitro results suggest that exposure to high doses of iodide results in a decrease in NIS protein levels that is, at least in part, due to an increase in NIS protein turnover (33,34,35).
Congenital iodide transport defect (ITD)
Biallelic mutations in the NIS gene cause a congenital ITD. ITD is an autosomal recessive condition characterized by hypothyroidism, goiter, reduced or absent thyroid uptake of radioiodide, and a low saliva/plasma iodide ratio (1,36). Currently, at least 12 ITD-causing mutations of NIS have been identified (37). Six of these mutations, namely 226delH, T354P, G395R, Q267E, G543E, and V59E have been characterized more thoroughly (38,39,40,41). The G543E substitution leads to retention of NIS in intracellular compartments as a result of improper maturation and trafficking of the protein, the other mutants are still targeted to the membrane but result in a loss of function (38,39,40,41). Structural and functional analysis of the T354P mutant protein demonstrated that a hydroxyl group at the β-carbon of the residue at position 354 is crucial for proper NIS function (38). Substitutions of the glycine residue at position 395 residue with several amino acids indicated that the presence of a small and an uncharged amino acid residue at this position is required for NIS function (39). Lastly, a recent study revealed that the histidine residue at position 226 is important for the iodide transport activity of NIS (37).
Pendrin
Pendrin is a highly hydrophobic membrane protein located at the apical membrane of thyrocytes (2,4). In addition to the thyroid, pendrin is also expressed in the kidney and in the inner ear (42,43). In the kidney, pendrin plays an important role in acid-base metabolism as an exchanger of chloride and bicarbonate in β-intercalated cells (44). In the inner ear, pendrin is important for generation of the endocochlear potential (45).
Pendrin belongs to the SLC26A family, which includes several anion transporters, as well as the motor protein prestin that is expressed in outer hair cells (46,47). Pendrin is encoded by the SLC26A4 gene, which was cloned in 1997 (48). The SLC26A4 gene is located on chromosome 7q21-31 and contains 21 exons with an open reading frame of 2343 bp (48).
Pendrin is a glycoprotein composed of 780 amino acids (2). It contains three putative extracellular asparagine-glycosylation sites (4,49). Pendrin usually appears as a single protein band with a molecular mass of 110–115 kDa when isolated from human thyroid membranes (49). Pendrin is proposed to have 12 transmembrane domains with both amino and carboxy termini located inside the cytosol (4,50). Like other members of the SLC26A family, pendrin contains a so-called STAS (sulfate transporter and antisigma factor antagonist) domain (51). The exact function of this domain has not been elucidated. Recent studies, however, suggest that the STAS domain can interact with the regulatory domain of CFTR (cystic fibrosis transmembrane conductance regulator) in certain epithelial cells (52,53,54).
Pendred syndrome
Mutations in the SLC26A4 gene lead to Pendred syndrome (2). Pendred syndrome is an autosomal recessive disorder characterized by sensorineural deafness, goiter, and a partial defect in iodide organification (55,56). Deafness or hearing impairment is the leading clinical sign of Pendred syndrome (56). In many patients, hearing loss is prelingual; in some individuals, however, the hearing loss develops later in childhood (57). Patients with Pendred syndrome display an enlarged endolymphatic sac and duct (58,59,60). A subset of patients presents with a so-called Mondini defect, which is characterized by replacement of the cochlear turns by a single cavity or a rudimentary cochlea (57,58). Variability in the hearing loss observed in patients with SLC26A4 mutations suggests that the phenotype is influenced by environmental factors and/or genetic modifiers (61).
Goiter usually develops during childhood. There is, however, a substantial variation within and between families and different geographic regions (62,63,64). Nutritional iodide intake appears to play an important role as a modifier of the thyroidal phenotype (65,66). Under conditions of high iodide intake, most individuals have no or only a mild enlargement of the thyroid (60,67). If the nutritional iodide is scarce, patients with Pendred syndrome not only develop goiter but may also present with mild or overt hypothyroidism (68,69).
Mutations in the SLC26A4 gene, found in patients with Pendred syndrome, are highly heterogeneous (56). Currently, more than 150 mutations of the SLC26A4 gene have been reported (56). Most of the mutations are missense mutations, and a smaller number of mutations consists mainly of nonsense and intronic mutations (56). Loss of function of some of the mutants results from the retention of the mutated and misfolded protein in intracellular compartments, most likely the endoplasmic reticulum (70,71).
Role of pendrin in the thyroid
Initial functional studies of pendrin in Xenopus oocytes have demonstrated that pendrin is able to mediate transport of chloride and iodide (5) and that it can act as a chloride/formate exchanger (72). The ability of pendrin to mediate iodide efflux (5), the localization of pendrin at the apical membrane of thyrocytes (4,73), as well as the defect in iodide organification observed in patients with Pendred syndrome (62,74), suggested that pendrin could function as an apical iodide transporter in thyroid cells (46). The results obtained from a number of independent studies performed in heterologous systems support the role of pendrin in mediating, at least in part, apical iodide efflux. Yoshida et al. (75) have demonstrated that iodide efflux is much higher in nonpolarized Chinese hamster ovary cells expressing NIS and pendrin than in cells expressing NIS alone. Electrophysiological studies with transfected COS-7 cells also indicate that pendrin mediates iodide transport and that it is more efficient at high extracellular concentrations of chloride (76). In addition, iodide efflux/chloride influx appears to be more efficient than chloride efflux/iodide influx (76). These findings are consistent with results obtained in polarized Madin-Darby canine kidney (MDCK) cells (50). MDCK cells expressing NIS and pendrin independently or simultaneously were cultured in a bicameral system, which allowed measuring iodide uptake at the basolateral membrane and iodide efflux at the apical membrane. Cells transfected with NIS alone have a significant increase in intracellular iodide uptake compared with untransfected control cells. In contrast, cells expressing NIS and pendrin show a significant increase in iodide transport into the apical chamber and consequently a significant decrease in the intracellular iodide content. These findings support the notion that pendrin may have a role in facilitating vectorial iodide transport at the apical membrane (50). The partial organification defect found in patients with Pendred syndrome suggests, however, that iodide can reach the follicular lumen independently of the presence of pendrin.
Questions concerning the role of pendrin as an apical iodide transporter
The traditional concept held that iodide simply crosses the apical membrane due to the electrochemical gradient that is present between the cytosol and the follicular lumen (77). However, autoradiography studies revealed that iodide first accumulates in the cytosol and subsequently moves to the follicular lumen (78). This transport of iodide across the apical membrane is rapidly stimulated by TSH (64,79). Hence, it has been proposed that apical iodide efflux is mediated through a specific transporter or channel. This concept has risen based on several findings. Electrophysiological studies performed with thyroid membrane vesicles suggested the presence of two apical iodide transporters (80). Functional studies performed in heterologous cells, including polarized cells (50,75,76,81), along with the iodide organification defects found in patients with Pendred syndrome (62,74), suggested that pendrin mediates apical iodide efflux in thyrocytes. The physiological role of pendrin has, however, been questioned for several reasons (77). Patients with biallelic mutations in the SLC26A4 gene display a mild or no thyroidal phenotype under conditions of sufficient iodide intake (65). The pendrin knockout mice, studied under normal iodide intake conditions, do not develop a goiter or abnormal thyroid hormone levels (45). In addition, it is intriguing that pendrin may have distinct roles in the thyroid, the inner ear, and the kidney (77). This led to the proposal that pendrin may be a part of multiprotein complex, the composition of which may vary among different cell types, thereby potentially explaining a variability in anion selectivity of pendrin (77). Other proteins (SLC5A8 and chloride channel 5 ClCn5) have been proposed to mediate apical iodide efflux (82,83). Functional studies performed in Xenopus oocytes and polarized MDCK cells clearly demonstrate that SLC5A8, originally designated as human apical iodide transporter (hAIT) (82), does not mediate iodide uptake or efflux (84). Localization of the ClCn5 protein at the apical membrane of thyrocytes and a thyroidal phenotype of the ClCn5-deficient mice that is reminiscent of Pendred syndrome suggest that ClCn5 could be, possibly in conjunction with other chloride channels, involved in mediating apical iodide efflux or iodide/chloride exchange (83). This possibility has, as of yet, not been corroborated by further experimental data.
Regulation of pendrin expression
TSH stimulates iodide efflux across the apical membrane of thyrocytes (79,85,86). After exposure to TSH, iodide efflux is rapidly stimulated in FRTL-5 cells (85) and in polarized porcine thyrocytes (79,86). In polarized porcine thyrocytes grown in a bicameral system, measurement of iodide transport in both directions demonstrates that TSH up-regulates iodide efflux at the apical membrane, whereas efflux across the basolateral membrane does not change (79). Treatment of rat thyroid PCCl3 cells with TSH for a short time results in the rapid translocation of pendrin from intracellular compartments, specifically endosomes, to the plasma membrane, thus suggesting a role of pendrin in rapid regulation of apical iodide efflux (87). Insertion of pendrin in the apical membrane correlates with the phosphorylation of pendrin, but it remains unknown whether phosphorylation is necessary or sufficient for this translocation. These events occur through the PKA pathway and can be inhibited by H89, a specific PKA inhibitor (87). Interestingly, translocation of pendrin from the cytosol to the plasma membrane through a PKC-dependent pathway has been demonstrated after exposure of cultured rat thyroid cells to insulin for 10, 20, and 40 min (88).
At least in rat FRTL-5 cells, TSH does not significantly modify SLC26A4 gene expression (4). Interestingly, thyroglobulin has been shown to up-regulate SLC26A4 mRNA levels in FRTL-5 cells while suppressing expression of several thyroid-specific genes, including the TSH receptor, NIS, TPO, and TG genes (4). Treatment with iodide does not affect expression of the SLC26A4 gene (89). Exposure to thyroglobulin leads to a decreased NIS gene and protein expression and subsequently results in a reduced iodide uptake in vitro (90). In contrast, accumulation of thyroglobulin in the follicular lumen suppresses iodide uptake in vivo (90). It has been suggested that the inverse relationship between the concentration of thyroglobulin in the follicular lumen and iodide uptake in vivo may be important in the regulation of thyroid function under constant TSH levels (91) and promote iodide efflux into the follicular lumen (89).
Conclusions
NIS mediates the active transport of iodide at the basolateral membrane of thyrocytes.
Biallelic mutations in NIS cause a congenital iodide transport defect, an autosomal recessive condition, characterized by hypothyroidism, goiter, low thyroid iodide uptake, and low saliva/plasma iodide ratio.
Pendrin is involved in the apical iodide efflux in thyroid cells. It can also exchange chloride and bicarbonate.
Pendrin is encoded by the SLC26A4 gene. Biallelic mutations in the SLC26A4 gene cause Pendred syndrome, an autosomal recessive disorder, characterized by deafness, goiter, and impaired iodide organification.
In the inner ear, pendrin is important for anion and fluid transport and for maintenance of the endocochlear potential.
In the kidney, pendrin plays a role in acid-base metabolism as a chloride/bicarbonate exchanger.
In addition to pendrin, other apical iodide channels or transporters may be involved in regulation of apical iodide efflux in thyrocytes.
Footnotes
Part of this work has been supported by Grant 1R01DK63024-01 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (to P.K.).
First Published Online February 5, 2009
Abbreviations: CICn5, Chloride channel 5; DIT, diiodotyrosine; DUOX2, dual oxidase type 2; ITD, iodide transport defect; MDCK, Madin-Darby canine kidney; MIT, monoiodotyrosine; NIS, sodium-iodide symporter; PKA, protein kinase A; SLC5A, solute carrier 5A; TPO, thyroperoxidase.
References
- Dohan O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, Ginter CS, Carrasco N 2003 The sodium/iodide symporter (NIS): characterization, regulation, and medical significance. Endocr Rev 24:48–77 [DOI] [PubMed] [Google Scholar]
- Kopp P 2005 Thyroid hormone synthesis: thyroid iodine metabolism. In: Braverman L, Utiger R, eds. Werner and Ingbar’s the thyroid: a fundamental and clinical text. 9th ed. New York: Lippincott Williams Wilkins; 52–76 [Google Scholar]
- Arvan P, Di Jeso B 2005 Thyroglobulin structure, function, and biosynthesis. In: Braverman L, Utiger R, eds. Werner and Ingbar’s the thyroid: a fundamental and clinical text. New York: Lippincott Williams Wilkins; 77–95 [Google Scholar]
- Royaux IE, Suzuki K, Mori A, Katoh R, Everett LA, Kohn LD, Green ED 2000 Pendrin, the protein encoded by the Pendred syndrome gene (PDS), is an apical porter of iodide in the thyroid and is regulated by thyroglobulin in FRTL-5 cells. Endocrinology 141:839–845 [DOI] [PubMed] [Google Scholar]
- Scott DA, Wang R, Kreman TM, Sheffield VC, Karniski LP 1999 The Pendred syndrome gene encodes a chloride-iodide transport protein. Nat Genet 21:440–443 [DOI] [PubMed] [Google Scholar]
- Dai G, Levy O, Carrasco N 1996 Cloning and characterization of the thyroid iodide transporter. Nature 379:458–460 [DOI] [PubMed] [Google Scholar]
- Mazaferri EL 2000 Carcinoma of the follicular epithelium. In: Braverman L, Utiger R, eds. Thyroid: a fundamental and clinical text. 8th ed. New York: Lippincott Williams & Wilkins; 904–930 [Google Scholar]
- Smanik PA, Liu Q, Furminger TL, Ryu K, Xing S, Mazzaferri EL, Jhiang SM 1996 Cloning of the human sodium iodide symporter. Biochem Biophys Res Commun 226:339–345 [DOI] [PubMed] [Google Scholar]
- Smanik PA, Ryu KY, Theil KS, Mazzaferri EL, Jhiang SM 1997 Expression, exon-intron organization, and chromosome mapping of the human sodium iodide symporter. Endocrinology 138:3555–3558 [DOI] [PubMed] [Google Scholar]
- Reizer J, Reizer A, Saier Jr MH 1994 A functional superfamily of sodium/solute symporters. Biochim Biophys Acta 1197:133–166 [DOI] [PubMed] [Google Scholar]
- Levy O, De la Vieja A, Ginter CS, Riedel C, Dai G, Carrasco N 1998 N-linked glycosylation of the thyroid Na+/I− symporter (NIS). Implications for its secondary structure model. J Biol Chem 273:22657–22663 [DOI] [PubMed] [Google Scholar]
- De La Vieja A, Dohan O, Levy O, Carrasco N 2000 Molecular analysis of the sodium/iodide symporter: impact on thyroid and extrathyroid pathophysiology. Physiol Rev 80:1083–1105 [DOI] [PubMed] [Google Scholar]
- Eskandari S, Loo DD, Dai G, Levy O, Wright EM, Carrasco N 1997 Thyroid Na+/I− symporter. Mechanism, stoichiometry, and specificity. J Biol Chem 272:27230–27238 [DOI] [PubMed] [Google Scholar]
- Carrasco N 1993 Iodide transport in the thyroid gland. Biochim Biophys Acta 1154:65–82 [DOI] [PubMed] [Google Scholar]
- Wolff J 1964 Transport of iodide and other anions in the thyroid gland. Physiol Rev 44:45–90 [DOI] [PubMed] [Google Scholar]
- Baschieri L, Benedetti G, Deluca F, Negri M 1963 Evaluation and limitations of the perchlorate test in the study of thyroid function. J Clin Endocrinol Metab 23:786–791 [DOI] [PubMed] [Google Scholar]
- Yoshida A, Sasaki N, Mori A, Taniguchi S, Mitani Y, Ueta Y, Hattori K, Sato R, Hisatome I, Mori T, Shigemasa C, Kosugi S 1997 Different electrophysiological character of I−, ClO4−, and SCN− in the transport by Na+/I− symporter. Biochem Biophys Res Commun 231:731–734 [DOI] [PubMed] [Google Scholar]
- Yoshida A, Sasaki N, Mori A, Taniguchi S, Ueta Y, Hattori K, Tanaka Y, Igawa O, Tsuboi M, Sugawa H, Sato R, Hisatome I, Shigemasa C, Grollman EF, Kosugi S 1998 Differences in the electrophysiological response to I− and the inhibitory anions SCN− and ClO4−, studied in FRTL-5 cells. Biochim Biophys Acta 1414:231–237 [DOI] [PubMed] [Google Scholar]
- Dohan O, Portulano C, Basquin C, Reyna-Neyra A, Amzel LM, Carrasco N 2007 The Na+/I− symporter (NIS) mediates electroneutral active transport of the environmental pollutant perchlorate. Proc Natl Acad Sci USA 104:20250–20255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran N, Valentin-Blasini L, Blount BC, McCuistion CG, Fenton MS, Gin E, Salem A, Hershman JM 2008 Thyroid-stimulating hormone increases active transport of perchlorate into thyroid cells. Am J Physiol Endocrinol Metab 294:E802–E806 [DOI] [PubMed] [Google Scholar]
- Vassart G, Dumont JE 1992 The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev 13:596–611 [DOI] [PubMed] [Google Scholar]
- Laglia G, Zeiger MA, Leipricht A, Caturegli P, Levine MA, Kohn LD, Saji M 1996 Increased cyclic adenosine 3′,5′-monophosphate inhibits G protein-coupled activation of phospholipase C in rat FRTL-5 thyroid cells. Endocrinology 137:3170–3176 [DOI] [PubMed] [Google Scholar]
- Weiss SJ, Philp NJ, Ambesi-Impiombato FS, Grollman EF 1984 Thyrotropin-stimulated iodide transport mediated by adenosine 3′,5′-monophosphate and dependent on protein synthesis. Endocrinology 114:1099–1107 [DOI] [PubMed] [Google Scholar]
- Levy O, Dai G, Riedel C, Ginter CS, Paul EM, Lebowitz AN, Carrasco N 1997 Characterization of the thyroid Na+/I− symporter with an anti-COOH terminus antibody. Proc Natl Acad Sci USA 94:5568–5573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Endo T, Kawaguchi A, Ikeda M, Nakazato M, Kogai T, Onaya T 1997 Increased expression of the Na+/I− symporter in cultured human thyroid cells exposed to thyrotropin and in Graves’ thyroid tissue. J Clin Endocrinol Metab 82:3331–3336 [DOI] [PubMed] [Google Scholar]
- Kogai T, Curcio F, Hyman S, Cornford EM, Brent GA, Hershman JM 2000 Induction of follicle formation in long-term cultured normal human thyroid cells treated with thyrotropin stimulates iodide uptake but not sodium/iodide symporter messenger RNA and protein expression. J Endocrinol 167:125–135 [DOI] [PubMed] [Google Scholar]
- Riedel C, Levy O, Carrasco N 2001 Post-transcriptional regulation of the sodium/iodide symporter by thyrotropin. J Biol Chem 276:21458–21463 [DOI] [PubMed] [Google Scholar]
- Vadysirisack DD, Chen ES, Zhang Z, Tsai MD, Chang GD, Jhiang SM 2007 Identification of in vivo phosphorylation sites and their functional significance in the sodium iodide symporter. J Biol Chem 282:36820–36828 [DOI] [PubMed] [Google Scholar]
- Fanning AS, Anderson JM 1999 PDZ domains: fundamental building blocks in the organization of protein complexes at the plasma membrane. J Clin Invest 103:767–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan PK, Waites C, Liu Y, Krantz DE, Edwards RH 1998 A leucine-based motif mediates the endocytosis of vesicular monoamine and acetylcholine transporters. J Biol Chem 273:17351–17360 [DOI] [PubMed] [Google Scholar]
- Marks MS, Ohno H, Kirchnausen T, Bonracino JS 1997 Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell Biol 7:124–128 [DOI] [PubMed] [Google Scholar]
- Wolff J, Chaikoff IL 1948 Plasma inorganic iodide as a homeostatic regulator of thyroid function. J Biol Chem 174:555–564 [PubMed] [Google Scholar]
- Eng PH, Cardona GR, Fang SL, Previti M, Alex S, Carrasco N, Chin WW, Braverman LE 1999 Escape from the acute Wolff-Chaikoff effect is associated with a decrease in thyroid sodium/iodide symporter messenger ribonucleic acid and protein. Endocrinology 140:3404–3410 [DOI] [PubMed] [Google Scholar]
- Eng PH, Cardona GR, Previti MC, Chin WW, Braverman LE 2001 Regulation of the sodium iodide symporter by iodide in FRTL-5 cells. Eur J Endocrinol 144:139–144 [DOI] [PubMed] [Google Scholar]
- Spitzweg C, Joba W, Morris JC, Heufelder AE 1999 Regulation of sodium iodide symporter gene expression in FRTL-5 rat thyroid cells. Thyroid 9:821–830 [DOI] [PubMed] [Google Scholar]
- Wolff J 1983 Congenital goiter with defective iodide transport. Endocr Rev 4:240–254 [DOI] [PubMed] [Google Scholar]
- Wu SL, Ho TY, Liang JA, Hsiang CY 2008 Histidine residue at position 226 is critical for iodide uptake activity of human sodium/iodide symporter. J Endocrinol 199:213–219 [DOI] [PubMed] [Google Scholar]
- Levy O, Ginter CS, De la Vieja A, Levy D, Carrasco N 1998 Identification of a structural requirement for thyroid Na+/I− symporter (NIS) function from analysis of a mutation that causes human congenital hypothyroidism. FEBS Lett 429:36–40 [DOI] [PubMed] [Google Scholar]
- Dohan O, Gavrielides MV, Ginter C, Amzel LM, Carrasco N 2002 Na+/I− symporter activity requires a small and uncharged amino acid residue at position 395. Mol Endocrinol 16:1893–1902 [DOI] [PubMed] [Google Scholar]
- De La Vieja A, Ginter CS, Carrasco N 2004 The Q267E mutation in the sodium/iodide symporter (NIS) causes congenital iodide transport defect (ITD) by decreasing the NIS turnover number. J Cell Sci 117:677–687 [DOI] [PubMed] [Google Scholar]
- De la Vieja A, Ginter CS, Carrasco N 2005 Molecular analysis of a congenital iodide transport defect: G543E impairs maturation and trafficking of the Na+/I− symporter. Mol Endocrinol 19:2847–2858 [DOI] [PubMed] [Google Scholar]
- Soleimani M, Greeley T, Petrovic S, Wang Z, Amlal H, Kopp P, Burnham CE 2001 Pendrin: an apical Cl−/OH−/HCO3− exchanger in the kidney cortex. Am J Physiol Renal Physiol 280:F356–F364 [DOI] [PubMed] [Google Scholar]
- Everett LA, Morsli H, Wu DK, Green ED 1999 Expression pattern of the mouse ortholog of the Pendred’s syndrome gene (Pds) suggests a key role for pendrin in the inner ear. Proc Natl Acad Sci USA 96:9727–9732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED 2001 Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA 98:4221–4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett LA, Belyantseva IA, Noben-Trauth K, Cantos R, Chen A, Thakkar SI, Hoogstraten-Miller SL, Kachar B, Wu DK, Green ED 2001 Targeted disruption of mouse Pds provides insight about the inner-ear defects encountered in Pendred syndrome. Hum Mol Genet 10:153–161 [DOI] [PubMed] [Google Scholar]
- Everett LA, Green ED 1999 A family of mammalian anion transporters and their involvement in human genetic diseases. Hum Mol Genet 8:1883–1891 [DOI] [PubMed] [Google Scholar]
- Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P 2000 Prestin is the motor protein of cochlear outer hair cells. Nature 405:149–155 [DOI] [PubMed] [Google Scholar]
- Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis AD, Sheffield VC, Green ED 1997 Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat Genet 17:411–422 [DOI] [PubMed] [Google Scholar]
- Porra V, Bernier-Valentin F, Trouttet-Masson S, Berger-Dutrieux N, Peix JL, Perrin A, Selmi-Ruby S, Rousset B 2002 Characterization and semiquantitative analyses of pendrin expressed in normal and tumoral human thyroid tissues. J Clin Endocrinol Metab 87:1700–1707 [DOI] [PubMed] [Google Scholar]
- Gillam MP, Sidhaye AR, Lee EJ, Rutishauser J, Stephan CW, Kopp P 2004 Functional characterization of pendrin in a polarized cell system. Evidence for pendrin-mediated apical iodide efflux. J Biol Chem 279:13004–13010 [DOI] [PubMed] [Google Scholar]
- Aravind L, Koonin EV 2000 The STAS domain: a link between anion transporters and antisigma-factor antagonists. Curr Biol 10:R53–R55 [DOI] [PubMed] [Google Scholar]
- Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S 2002 A molecular mechanism for aberrant CFTR-dependent HCO3− transport in cystic fibrosis. EMBO J 21:5662–5672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S 2004 Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol 6:343–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcheynikov N, Ko SB, Zeng W, Choi JY, Dorwart MR, Thomas PJ, Muallem S 2006 Regulatory interaction between CFTR and the SLC26 transporters. Novartis Found Symp 273:177–186; discussion 186–192, 261–264 [PubMed] [Google Scholar]
- Morgans ME, Trotter WR 1958 Association of congenital deafness with goitre; the nature of the thyroid defect. Lancet 1:607–609 [DOI] [PubMed] [Google Scholar]
- Kopp P, Pesce L, Solis SJ 2008 Pendred syndrome and iodide transport in the thyroid. Trends Endocrinol Metab 19:260–268 [DOI] [PubMed] [Google Scholar]
- Reardon W, Coffey R, Phelps PD, Luxon LM, Stephens D, Kendall-Taylor P, Britton KE, Grossman A, Trembath R 1997 Pendred syndrome: 100 years of underascertainment? QJM 90:443–447 [DOI] [PubMed] [Google Scholar]
- Phelps PD, Coffey RA, Trembath RC, Luxon LM, Grossman AB, Britton KE, Kendall-Taylor P, Graham JM, Cadge BC, Stephens SG, Pembrey ME, Reardon W 1998 Radiological malformations of the ear in Pendred syndrome. Clin Radiol 53:268–273 [DOI] [PubMed] [Google Scholar]
- Fugazzola L, Mannavola D, Cerutti N, Maghnie M, Pagella F, Bianchi P, Weber G, Persani L, Beck-Peccoz P 2000 Molecular analysis of the Pendred’s syndrome gene and magnetic resonance imaging studies of the inner ear are essential for the diagnosis of true Pendred’s syndrome. J Clin Endocrinol Metab 85:2469–2475 [DOI] [PubMed] [Google Scholar]
- Pryor SP, Madeo AC, Reynolds JC, Sarlis NJ, Arnos KS, Nance WE, Yang Y, Zalewski CK, Brewer CC, Butman JA, Griffith AJ 2005 SLC26A4/PDS genotype-phenotype correlation in hearing loss with enlargement of the vestibular aqueduct (EVA): evidence that Pendred syndrome and non-syndromic EVA are distinct clinical and genetic entities. J Med Genet 42:159–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azaiez H, Yang T, Prasad S, Sorensen JL, Nishimura CJ, Kimberling WJ, Smith RJ 2007 Genotype-phenotype correlations for SLC26A4-related deafness. Hum Genet 122:451–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser GR, Morgans ME, Trotter WR 1960 The syndrome of sporadic goitre and congenital deafness. Q J Med 29:279–295 [PubMed] [Google Scholar]
- Fraser GR 1965 Association of congenital deafness with goitre (Pendred’s syndrome): a study of 207 families. Ann Hum Genet 28:201–249 [DOI] [PubMed] [Google Scholar]
- Nilsson LR, Borgfors N, Gamstorp I, Holst HE, Liden G 1964 Nonendemic goitre and deafness. Acta Paediatr 53:117–131 [DOI] [PubMed] [Google Scholar]
- Sato E, Nakashima T, Miura Y, Furuhashi A, Nakayama A, Mori N, Murakami H, Naganawa S, Tadokoro M 2001 Phenotypes associated with replacement of His by Arg in the Pendred syndrome gene. Eur J Endocrinol 145:697–703 [DOI] [PubMed] [Google Scholar]
- Gausden E, Coyle B, Armour JA, Coffey R, Grossman A, Fraser GR, Winter RM, Pembrey ME, Kendall-Taylor P, Stephens D, Luxon LM, Phelps PD, Reardon W, Trembath R 1997 Pendred syndrome: evidence for genetic homogeneity and further refinement of linkage. J Med Genet 34:126–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami S, Abe S, Weston MD, Shinkawa H, Van Camp G, Kimberling WJ 1999 Non-syndromic hearing loss associated with enlarged vestibular aqueduct is caused by PDS mutations. Hum Genet 104:188–192 [DOI] [PubMed] [Google Scholar]
- Kopp P, Arseven OK, Sabacan L, Kotlar T, Dupuis J, Cavaliere H, Santos CL, Jameson JL, Medeiros-Neto G 1999 Phenocopies for deafness and goiter development in a large inbred Brazilian kindred with Pendred’s syndrome associated with a novel mutation in the PDS gene. J Clin Endocrinol Metab 84:336–341 [DOI] [PubMed] [Google Scholar]
- Gonzalez Trevino O, Karamanoglu Arseven O, Ceballos CJ, Vives VI, Ramirez RC, Gomez VV, Medeiros-Neto G, Kopp P 2001 Clinical and molecular analysis of three Mexican families with Pendred’s syndrome. Eur J Endocrinol 144:585–593 [DOI] [PubMed] [Google Scholar]
- Rotman-Pikielny P, Hirschberg K, Maruvada P, Suzuki K, Royaux IE, Green ED, Kohn LD, Lippincott-Schwartz J, Yen PM 2002 Retention of pendrin in the endoplasmic reticulum is a major mechanism for Pendred syndrome. Hum Mol Genet 11:2625–2633 [DOI] [PubMed] [Google Scholar]
- Schnyder S, Chen L, Chan L, Turk A, Gillam MP, Kopp P 2005 Pendrin mutations that are retained in intracellular compartments induce the IRE1/XBP1 and the ATF6 unfolded protein response pathways. Thyroid 15(Suppl 1):S-4–S-5 (Abstract O11) [Google Scholar]
- Scott DA, Karniski LP 2000 Human pendrin expressed in Xenopus laevis oocytes mediates chloride/formate exchange. Am J Physiol Cell Physiol 278:C207–C211 [DOI] [PubMed] [Google Scholar]
- Bidart JM, Mian C, Lazar V, Russo D, Filetti S, Caillou B, Schlumberger M 2000 Expression of pendrin and the Pendred syndrome (PDS) gene in human thyroid tissues. J Clin Endocrinol Metab 85:2028–2033 [DOI] [PubMed] [Google Scholar]
- Kopp P 1999 Pendred’s syndrome: clinical characteristics and molecular basis. Current Opin Endocrinol Diabetes 6:261–269 [Google Scholar]
- Yoshida A, Taniguchi S, Hisatome I, Royaux IE, Green ED, Kohn LD, Suzuki K 2002 Pendrin is an iodide-specific apical porter responsible for iodide efflux from thyroid cells. J Clin Endocrinol Metab 87:3356–3361 [DOI] [PubMed] [Google Scholar]
- Yoshida A, Hisatome I, Taniguchi S, Sasaki N, Yamamoto Y, Miake J, Fukui H, Shimizu H, Okamura T, Okura T, Igawa O, Shigemasa C, Green ED, Kohn LD, Suzuki K 2004 Mechanism of iodide/chloride exchange by pendrin. Endocrinology 145:4301–4308 [DOI] [PubMed] [Google Scholar]
- Wolff J 2005 What is the role of pendrin? Thyroid 15:346–348 [DOI] [PubMed] [Google Scholar]
- Andros G, Wollman SH 1967 Autoradiographic localization of radioiodide in the thyroid gland of the mouse. Am J Physiol 213:198–208 [DOI] [PubMed] [Google Scholar]
- Nilsson M, Bjorkman U, Ekholm R, Ericson LE 1990 Iodide transport in primary cultured thyroid follicle cells: evidence of a TSH-regulated channel mediating iodide efflux selectively across the apical domain of the plasma membrane. Eur J Cell Biol 52:270–281 [PubMed] [Google Scholar]
- Golstein P, Abramow M, Dumont JE, Beauwens R 1992 The iodide channel of the thyroid: a plasma membrane vesicle study. Am J Physiol 263:C590–C597 [DOI] [PubMed] [Google Scholar]
- Taylor JP, Metcalfe RA, Watson PF, Weetman AP, Trembath RC 2002 Mutations of the PDS gene, encoding pendrin, are associated with protein mislocalization and loss of iodide efflux: implications for thyroid dysfunction in Pendred syndrome. J Clin Endocrinol Metab 87:1778–1784 [DOI] [PubMed] [Google Scholar]
- Rodriguez AM, Perron B, Lacroix L, Caillou B, Leblanc G, Schlumberger M, Bidart JM, Pourcher T 2002 Identification and characterization of a putative human iodide transporter located at the apical membrane of thyrocytes. J Clin Endocrinol Metab 87:3500–3503 [DOI] [PubMed] [Google Scholar]
- van den Hove MF, Croizet-Berger K, Jouret F, Guggino SE, Guggino WB, Devuyst O, Courtoy PJ 2006 The loss of the chloride channel, ClC-5, delays apical iodide efflux and induces a euthyroid goiter in the mouse thyroid gland. Endocrinology 147:1287–1296 [DOI] [PubMed] [Google Scholar]
- Paroder V, Spencer SR, Paroder M, Arango D, Schwartz Jr S, Mariadason JM, Augenlicht LH, Eskandari S, Carrasco N 2006 Na+/monocarboxylate transport (SMCT) protein expression correlates with survival in colon cancer: molecular characterization of SMCT. Proc Natl Acad Sci USA 103:7270–7275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SJ, Philp NJ, Grollman EF 1984 Effect of thyrotropin on iodide efflux in FRTL-5 cells mediated by Ca2+. Endocrinology 114:1108–1113 [DOI] [PubMed] [Google Scholar]
- Nilsson M, Bjorkman U, Ekholm R, Ericson LE 1992 Polarized efflux of iodide in porcine thyrocytes occurs via a cAMP-regulated iodide channel in the apical plasma membrane. Acta Endocrinol 126:67–74 [DOI] [PubMed] [Google Scholar]
- Pesce L, Kopp P 2007 Thyrotropin rapidly regulates pendrin membrane abundance via PKA dependent and PKC dependent pathways in rat thyroid cells. Thyroid 17(Suppl 1):S-136 (Abstract 282) [Google Scholar]
- Muscella A, Marsigliante S, Verri T, Urso L, Dimitri C, Botta G, Paulmichl M, Beck-Peccoz P, Fugazzola L, Storelli C 2008 PKC-ε-dependent cytosol-to-membrane translocation of pendrin in rat thyroid PC Cl3 cells. J Cell Physiol 217:103–112 [DOI] [PubMed] [Google Scholar]
- Suzuki K, Kohn LD 2006 Differential regulation of apical and basal iodide transporters in the thyroid by thyroglobulin. J Endocrinol 189:247–255 [DOI] [PubMed] [Google Scholar]
- Suzuki K, Mori A, Saito J, Moriyama E, Ullianich L, Kohn LD 1999 Follicular thyroglobulin suppresses iodide uptake by suppressing expression of the sodium/iodide symporter gene. Endocrinology 140:5422–5430 [DOI] [PubMed] [Google Scholar]
- Kohn LD, Suzuki K, Nakazato M, Royaux I, Green ED 2001 Effects of thyroglobulin and pendrin on iodide flux through the thyrocyte. Trends Endocrinol Metab 12:10–16 [DOI] [PubMed] [Google Scholar]