Abstract
Color vision is mediated by cone photoreceptors that express opsin photopigments with sensitivities to different light wavelengths. Most mammals, including mice, differentially express M and S opsins for response to medium-long and short wavelengths, respectively. Previous studies demonstrated that a thyroid hormone receptor (TRβ2) is critical for opsin patterning: in TRβ2-deficient mice, M opsin is lost and all cones instead express S opsin. Here, to investigate the requirement for thyroid hormone in cone development, we studied Tshr−/−mice as a model of congenital hypothyroidism. The onset of M opsin expression in Tshr−/−mice was severely delayed until after postnatal d 17 (P17), and M opsin expression failed to attain normal levels at older adult ages. S opsin showed a subtler change with an extended distribution pattern over the superior-inferior axis of the retina. Similar opsin abnormalities were detected in wild-type C57BL/6J mice made hypothyroid by methimazole treatment. In Tshr−/− mice, T3 treatment from P8 recovered significant M opsin expression at P17. Tshr−/− mice produced normal numbers of cones, indicating that the major requirement for thyroid hormone is in opsin patterning rather than in cone generation. The phenotype is similar to, although milder than, that caused by loss of TRβ2 and indicates the necessity for thyroid hormone for cone maturation.
Hypothyroidism in mice retards expression of cone opsins, the photopigments required for color vision, highlighting the sensitivity of sensory systems to thyroid hormone.
Most mammalian species possess dichromatic color vision that is mediated by two opsin photopigments with peak sensitivities to medium-long (M, green) and short (S, blue) wavelengths of light (1,2). M and S opsins are differentially expressed in cone photoreceptors, a cell type that is derived from multipotent progenitor cells at early stages of retinal neurogenesis in utero (3,4,5). In mice, immature cones first express S opsin near birth and M opsin about a week after birth (6). M and S opsins are expressed in opposing gradients such that varying amounts of both opsins are coexpressed in cones in midretinal regions, whereas M opsin predominates in superior (dorsal) regions and S opsin predominates in inferior (ventral) regions (2,6). The controls over cone differentiation and opsin patterning remain poorly understood.
We previously identified a thyroid hormone receptor (TRβ2), encoded by the Thrb (Nr1a2) gene, that is expressed in newly generated cones and that is critical for opsin patterning (7,8,9). In TRβ2-deficient mice, M opsin expression is severely impaired and all cones instead express S opsin (8,10,11). Thus, TRβ2 mediates induction of M opsin and regional suppression of S opsin to give a graded distribution of M and S opsins across the retina. This unexpectedly critical function for a thyroid hormone receptor raises the question of the role of thyroid hormone itself in cone development. Treatment of newborn wild-type mice with excessive T3, the primary active form of thyroid hormone, has been shown to suppress S opsin expression (11), but the impact of a developmental lack of the hormone is undefined. The question is significant because developmental hypothyroidism is a known cause of neurological retardation (12,13). In human populations, early-onset hypothyroidism can be caused by iodine deficiency (14,15) or by sporadic congenital defects that arise in about one in 3000 newborns (16,17). However, the color visual system has been neglected in studies of hypothyroidism.
Hypothyroidism might have different outcomes depending on the role of ligand in regulating TRβ2 activity in cones. The TRβ and TRα1 receptor isoforms, encoded by Thrb and Thra genes, respectively, are transcription factors that may mediate both T3-dependent and T3-independent functions. Thus, ligand need not necessarily be required for TR functions in certain situations (18,19). Alternatively, lack of ligand may produce a worse outcome than lack of receptor. Such an explanation has been proposed to account for the cerebellar defects that result from hypothyroidism but not from loss of TRα1. In the cerebellum, hypothyroidism is thought to lock TRα1 chronically in a ligand-independent state that causes greater transcriptional dysregulation than does absence of TRα1 (20,21). Here, we report opsin abnormalities in TSH receptor-deficient (Tshr−/−) mice that have severe hypothyroidism with very low T4 and T3 levels (22). The study was corroborated in mice made hypothyroid by methimazole treatment. The results reveal M and S opsin abnormalities in hypothyroid mice resembling those of TRβ2-deficient mice, although milder in degree, thereby indicating the need for thyroid hormone in cone development. The results further suggest that there are distinctions in the T3-independent functions of different TR isoforms, namely, TRβ2 in cone cells and TRα1 in the cerebellum, which is discussed.
Materials and Methods
Mouse strains
The Tshr knockout mutation was maintained on a mixed background of C57BL/6J × 129/Sv substrains (22) and was backcrossed for a further generation onto the C57BL/6J strain. Tshr+/− parents were intercrossed to generate +/+, +/−, and −/− littermates for study. To ensure survival, Tshr−/− progeny were not separated at normal weaning age but were kept with the dam until the required age for analysis (up to P80). Saline vehicle or T3 in saline (1.5 μg in 100 μl volume) was administered daily by ip injection. Pregnant C57BL/6J female mice were made hypothyroid by treatment with methimazole (MMI, 0.05% wt/vol) and potassium perchlorate (1.0% wt/vol) in the drinking water beginning at gestational d 14 and continuing after giving birth until progeny were analyzed. Pooled serum was collected from at least n = 3 mice at any given age for hormone measurements. Animal experiments were performed in accordance with the ethical guidelines of the Endocrine Society and approved institutional protocols at the National Institutes of Health.
Hormone measurements
Serum total T4 and total T3 levels were determined by RIA with Coat-A-Count reagents (Diagnostic Systems Laboratories Inc., Webster, TX), as described (23).
In situ hybridization
Digoxigenin-labeled antisense and sense riboprobes were synthesized using as templates cDNA clones of mouse M (592 bases) and S opsins (629 bases) (8) and a rhodopsin cDNA cloned into pGEM3 generated by RT-PCR (300 bases, coordinates 27–327, accession no. NM145383). Retinas were fixed for 3 h in PBS containing 4% paraformaldehyde, cryoprotected in 30% sucrose, and then embedded in OCT for preparation of 10-μm cyrosections. Sections were postfixed in 4% paraformaldehyde in PBS, acetylated and permeabilized with 1% Triton X-100, and then hybridized with probe overnight at 70 C. Sections were washed in 0.2% standard saline citrate, blocked with PBS containing 10% goat serum, and then incubated with antidigoxigenin alkaline phosphatase conjugate (Roche Diagnostics, Indianapolis, IN) for 4 h. Color product was detected using substrate solution containing nitroblue-tetrazolium chloride and 5-bromo-4-chloro-3-indolyl-phosphate (NBT/BCIP).
Antibodies and Western blot analysis
The following antibodies (source and dilution) were used: mouse monoclonal antirhodopsin (04886; Sigma-Aldrich, St. Louis, MO; 1:2000), rabbit antiopsin blue (AB5407; Chemicon, Temecula, CA; 1:1000), rabbit antiopsin red/green (AB5405; Chemicon; 1:1000), and mouse monoclonal antiactin (MAB 1501, pan-actin; Chemicon; 1:3000). For Western blots, the retina was dissected from the eye after removal of the lens and pigmented epithelium. Retinas from three to six eyes from at least three animals were pooled and homogenized in buffer containing 20 mm Tris-Cl (pH 7.5), 0.2 m NaCl, 0.5% Nonidet P-40, 0.5 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, and 10 mm dithiothreitol. Protein samples (20 or 25 μg) were mixed with Novex Tris-glycine-SDS sample buffer (Invitrogen, Carlsbad, CA) without boiling and loaded onto a Tris-glycine 10% polyacrylamide gel. After electrophoresis, proteins were electrotransferred onto nitrocellulose membranes. Membranes were probed with primary antibodies diluted in PBS containing 5% nonfat milk and 0.2% Tween 20 overnight at 4 C and then incubated with second antibody horseradish peroxidase-conjugated goat antirabbit or mouse IgG (Zymed, San Francisco, CA) diluted at 1:10,000 for 1 h at room temperature and then developed with Amersham ECL Plus Western blotting kit (GE Healthcare, Little Chalfont, UK). Bands on autoradiographs were quantified by densitometry.
Immunohistochemistry
Cryosections of 10 μm thickness were blocked with PBS containing 1.5% goat serum, 0.1% BSA, and 0.4% Triton X-100, then incubated with antibodies overnight (S or L/M opsins, source as above, 1:2000 dilution) at room temperature, and then incubated with biotinylated goat antirabbit antibodies and AB reagents Vector ABC Elite kit (Vector Laboratories, Burlingame, CA). Antiserum against the specific N-terminal domain of mouse TRβ2 was raised in rabbit and used at 1:2000 dilution (Ng, L., and D. Forrest, manuscript in preparation). Color development solutions contained 3,3′-diaminobenzidine (Vector). For immunofluorescence, second antibodies were conjugated with fluorescein isothiocyanate; peanut agglutinin (PNA) was conjugated with rhodamine (Vector).
Histology and transmission electron microscopy
Eyes were fixed in 4% glutaraldehyde in PBS for 30 min and then fixed in 10% phosphate-buffered formalin (VWR, West Chester, PA; VW3239-4) for 24 h. For histology, one eye per mouse was dehydrated in a series of ethanol solutions and then embedded in methacrylate-JB4 (Polysciences, Warrington, PA). Two-micrometer-thick sections were prepared and stained with hematoxylin and eosin. To count photoreceptor cells, cone and rod cell bodies were counted in two representative fields per section viewed under a light microscope (field area, 0.018 mm2; fields located in zones 2 and 3 in the vertical plane, indicated in Fig. 1E). Counts were determined for three sections per eye from three eyes from three mice for each genotype, represented as mean number ± sd per area. For electron microscopy, the second eye was postfixed in osmium tetroxide in PBS for 1 h at 4 C and then embedded in epoxy resin (LX-112; Ladd Research Industries, Burlington, VT). One eye from each of n = 3 Thrb2−/− mice and n = 3 +/+ control mice were similarly analyzed by electron microscopy. Ultrathin sections were stained with uranyl acetate and lead citrate for examination under a JEM-1010 microscope (24).
Figure 1.
Developmental abnormalities in opsin expression in Tshr−/− mice. A, Left panels, Delayed M opsin expression in Tshr−/− mice shown by immunostaining of sections of superior and inferior retina at different ages (see panel E for orientation). M opsin protein is detected in cone outer segments (OS) adjacent to the retinal pigmented epithelium (RPE) but is undetectable in Tshr−/− mice at P17 (arrowheads). After onset of expression, slightly greater numbers of M opsin-positive cones are detected in the superior than inferior retina at P20, similar to the distribution in +/+ mice. For simplicity, the RPE is annotated only at P17. Right panels, Extended distribution of S opsin. In +/+ mice, there are greater numbers of S opsin-positive cones in the inferior than superior retina. In Tshr−/− mice, there are increased numbers of S opsin-positive cones in superior zones relative to +/+ mice (shown at P17 by black dots below cones in superior panels). Scale bar, 10 μm. B, In situ hybridization analysis of opsin mRNA expression. Opsin mRNA is detected in cone cell bodies in the ONL. Left panels, M opsin mRNA shows delayed expression. Right panels, S opsin mRNA shows extended distribution with more S opsin-positive cells in superior zones than in +/+ mice. Scale bar, 10 μm. C, In situ hybridization with a rhodopsin probe shows no obvious difference in expression between +/+ and −/− mice (superior region shown). Scale bar, 10 μm. D, Representative control hybridization with a sense probe complementary to the M opsin probe used in B (superior region shown). Scale bar, 10 μm. E, Diagram of a vertical section through the eye indicating superior and inferior zones depicted in A–D; arrowheads indicate orientation from RPE to ONL. Numbers (1–4) refer to zones in which cell counts were performed (see Fig. 5).
Results
Abnormal opsin expression in Tshr−/− mice
Immunostaining showed a severe retardation of M opsin expression in Tshr−/− mice (Fig. 1A). In wild-type (+/+) mice at postnatal d 17 (P17), M opsin was detectable in the cone outer segments (OS) that have formed by this stage. Beyond P17, the segments elongated and opsin signals grew stronger. In contrast, in Tshr−/− mice at P17, little or no M opsin was detected. M opsin became detectable by P20 and increased thereafter, but signals remained below normal strength by P60. The onset of M opsin expression at approximately P20 in Tshr−/− mice reflected a delay of about 10 d compared with +/+ mice in which M opsin expression begins at approximately P9 (6). In both Tshr−/− and +/+ mice, once M opsin expression did initiate, signals were greater in cones in the superior than inferior retina. Thus, in Tshr−/− mice, M opsin expression was delayed, but it followed a normal distribution over the retina. The orientation of superior and inferior retinal domains is indicated in Fig. 1E.
S opsin expression was not delayed in Tshr−/− mice, but it showed an extended distribution across the superior-inferior axis of the retina compared with +/+ mice. In +/+ mice, S opsin showed an opposite distribution to that of M opsin with more S opsin-positive cones in the inferior than superior retina. However, in Tshr−/− mice, S opsin was detected in increased numbers of cones in superior regions than in +/+ mice.
In situ hybridization analysis indicated that the changes in opsin expression occurred at the mRNA level in Tshr−/− mice (Fig. 1B). Opsin mRNA signals were detected in cone cell bodies in the outer nuclear layer (ONL). In Tshr−/− mice, expression of M opsin mRNA was delayed with little or no specific signal detectable above background at P17. M opsin mRNA became detectable by P23, but signals were weaker than normal. Once M opsin mRNA was expressed, it showed a similar distribution in Tshr−/− and +/+ mice with strongest signals in the superior retina. S opsin mRNA showed an extended distribution with increased numbers of positive cells in the superior retina in Tshr−/− mice than in +/+ mice. The second major class of photoreceptors, rods, which mediate vision in dim light, are more abundant than cones and express the distinct photopigment rhodopsin. Rhodopsin mRNA expression was not obviously altered in Tshr−/− mice (Fig. 1C).
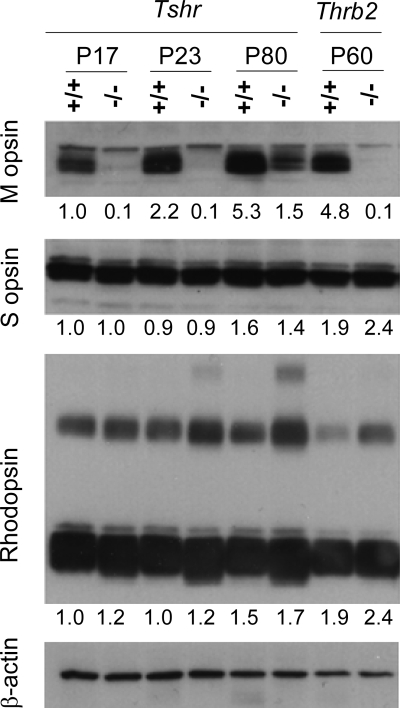
Western blot analysis confirmed the retardation of M opsin expression in Tshr−/− mice (Fig. 2). Very little M opsin was detected at P23 or younger ages. M opsin levels increased at older ages but remained below normal. At P80, M opsin signals were about one third of wild-type strength. Little change in S opsin levels was detected in Tshr−/− mice, consistent with the subtle changes only in the distribution of S opsin noted above. Rhodopsin expression levels were marginally greater than normal in Tshr−/− mice at each age studied, suggesting that there may be subtle consequences of hypothyroidism on rods. In comparison with Tshr−/− mice, M opsin expression in TRβ2-deficient (Thrb2−/−) mice was permanently retarded with little or no detectable M opsin at P60.
Figure 2.
Western blot analysis of opsins in retinal development. Protein extracts were analyzed from +/+ and Tshr−/− retina at several ages and, for comparison, from TRβ2-deficient (Thrb2−/−) mice at P60. Major bands for M opsin (∼40 kDa), S opsin (∼38 kDa), and rhodopsin (∼40 kDa) were quantified by densitometry with normalization to signals for actin: numbers below each lane give an expression level relative to the value for +/+ mice at P17, set arbitrarily at 1.0. Rhodopsin is detected as a major band and as minor, slower-migrating multimeric complexes. Each lane contains 25 μg protein except for Thrb2−/− and +/+ lanes, which contain 20 μg each.
Cone development in Tshr−/− mice
To demonstrate that cones were present in Tshr−/− mice at P17, despite the lack of M opsin, sections were stained with PNA, a lectin that detects cone inner and outer segments (25) (Fig. 3A). PNA is a general cone marker and detects different entities in the cone than do the opsin antibodies, which are limited to detection of M and S opsin-positive subpopulations of cells. PNA-positive cones were readily detected in Tshr−/− mice at P17, indicating that hypothyroidism did not cause a general block in cone development but more specifically altered opsin expression. At early stages in utero, a newly generated population of TRβ2-positive cones was detected in both Tshr−/− and +/+ embryos at embryonic d 18.5 (E18.5) (Fig. 3B). Unlike in TRβ2-deficient embryos (8), S opsin was not overtly overexpressed in cones at E18.5 in Tshr−/− mice (data not shown). Although Tshr−/− and Thrb2−/− mice have similar opsin phenotypes postnatally, the evidence suggests that there are subtle distinctions at embryonic stages. These distinctions may be explained if maternal thyroid hormone is sufficient to maintain suppression of S opsin in Tshr−/− embryos, whereas in Thrb2−/− embryos, the lack of receptor precludes suppression of S opsin regardless of thyroid hormone availability.
Figure 3.
Cone development in Tshr−/− mice. A, In +/+ mice at P17, fluorescent analysis with the lectin PNA (red) and antiserum against M opsin (green) identified many doubly labeled cones (yellow). In Tshr−/− mice, PNA-positive cones are present despite the retarded M opsin expression. A minimal M opsin signal is detected by fluorescence in Tshr−/− mice in the superior retina, indicating that M opsin is beginning to be expressed in a normal gradient. Scale bar, 10 μm. OS, Outer segments. B, A population of newly generated cones is present in both +/+ and Tshr−/− embryos at E18.5, detected by immunostaining for TRβ2. ONBL, Outer neuroblastic layer. Scale bars, 10 μm. RPE, Retinal pigmented epithelium. C, Cone and rod counts were normal in Tshr−/− mice at P24. Mean numbers of cones and rods per unit area of retinal section (0.018 mm2, on 2.0-μm-thick plastic sections) were not significantly different between genotypes (cones: +/+, 25 ± 1 vs.−/−, 23 ± 1, P = 0.06; rods: +/+, 699 ± 59 vs.−/−, 717 ± 28, P = 0.36; Student’s t test). Counts were performed in representative fields in superior and inferior zones 2 and 3 depicted in Fig. 1E, on three sections per eye, on three eyes from three mice. D, Transmission electron microscopy analysis of thin sections showing nuclei of rods (r) and cones (arrowheads) in +/+ and Tshr−/− mice at P24. Some cone cell nuclei appeared somewhat less defined and less rounded in Tshr−/− mice than in +/+ mice. IS, Inner segment layer. E, Transmission electron microscopy analysis in Thrb2−/− mice showed relatively little change in the appearance of most cone cell nuclei (arrowheads) compared with +/+ mice at P24.
Retinal morphology in Tshr−/− mice
Morphology of the retina including the ONL, containing cone and rod cell bodies, and the inner and outer segment layers, had a largely normal appearance in Tshr−/− mice at P24 in histological sections (not shown). Consistent with the generation of a normal population of immature cones in the embryo detected by TRβ2 immunostaining (Fig. 3B), Tshr−/− mice at P24 had normal numbers of both cones and rods, identified morphologically on histological sections (Fig. 3C). Cone cell nuclei were distinguished by their larger size and looser chromatin than the denser, smaller nuclei of the more numerous rods (26). Analysis of ultrastructure by transmission electron microscopy revealed that some cone cell nuclei displayed a less rounded and less well-defined appearance than normal in Tshr−/− mice at P24 (Fig. 3D), suggesting that hypothyroidism may produce subtle morphological changes in addition to alterations in opsin expression. Most cone cell nuclei in Thrb2−/− embryos showed a more normal appearance (Fig. 3E). The possibility that hypothyroidism produces an incidence of subtle change in cone nuclear morphology and whether this is caused directly or indirectly may merit further investigation.
Opsin changes in chemically induced hypothyroidism
As an independent model of hypothyroidism, pregnant female mice of the C57BL/6J strain were treated with MMI in the drinking water beginning at gestational d 14.5 and continuing after birth until the time of analysis of progeny (Fig. 4). MMI treatment of the dam was begun at gestational d 14.5 to ensure that the cumulative action of the chemical treatment produced a state of hypothyroidism in the progeny by the time of birth, when opsin expression and patterning is initiated.
Figure 4.
MMI-induced hypothyroidism in wild-type C57BL6/J mice produces similar opsin abnormalities as those found in Tshr−/− mice. A, MMI treatment was maintained from gestational d 14.5 until pups were analyzed. At P16, M opsin expression was delayed with very little signal detectable by immunostaining (arrowheads). S opsin showed an extended distribution with increased numbers of S opsin-positive cones in the superior retina compared with untreated mice (indicated by dots). Scale bar, 12 μm. OS, Outer segments. B, In situ hybridization analysis of opsin mRNA confirmed the M and S opsin expression abnormalities shown in A. Scale bar, 10 μm. C, Serum T4 levels were chronically low in MMI-treated mice in postnatal development compared with untreated groups (u). For each age, T4 determinations were performed on pools of serum, representing n = 3–7 mice.
In MMI-treated mice, M opsin showed delayed expression, with little or no M opsin detectable at P16. M opsin became detectable later, as shown at P26 (Fig. 4A). S opsin showed an extended distribution with increased numbers of S opsin-positive cones in the superior retina compared with untreated mice at both ages. These abnormalities closely resembled those in Tshr−/− mice, indicating that the opsin phenotype was a result of hypothyroidism and not of differences in mouse strains or in the chemical or genetic means of producing hypothyroidism. The similar outcome in both models of hypothyroidism was confirmed by in situ hybridization analysis in MMI-treated pups. As in Tshr−/− pups, M opsin mRNA was undetectable at P17 (Fig. 4B). S opsin mRNA showed a somewhat extended distribution with more positive cells in the superior retina than in untreated controls.
MMI treatment resulted in very low serum T4 levels in progeny between P2 and P35 compared with untreated control mice over this age range (Fig. 4C). Tshr−/− pups experience hypothyroidism at birth when their thyroid glands fail to become active. In utero, the development of the Tshr−/− embryo is supported to an extent by maternal thyroid hormone provided by the Tshr+/− dam. In contrast, MMI treatment produces a combined maternal-fetal hypothyroidism, such that by the time of birth, the neonate may have been subject to some degree of prior hypothyroidism. Postnatally, the hypothyroidism is similar in both cases and is associated with growth retardation. At P17, Tshr−/− pups weighed about 20% less than +/+ littermates (5.9 ± 0.7 and 7.5 ± 0.8 g, respectively; n ≥ 9; P < 0.01). At P17, MMI-treated pups weighed about 15% less than untreated controls (5.7 ± 0.4 and 6.6 ± 0.3 g, respectively; n ≥ 5; P < 0.01).
Recovery of opsin expression after T3 administration
The hypothyroid state of Tshr−/− mice suggested that thyroid hormone insufficiency caused the opsin phenotype. To support this proposal, T3 was administered to Tshr−/− and +/+ littermates (1.5 μg, ip, daily), from P8–P17, at which stage opsin expression was analyzed. This age range encompassed the period during which M opsin is normally induced in mice (6). T3 but not saline treatment recovered near-normal numbers of M opsin-positive cones in Tshr−/− mice (Fig. 5A). The recovered M opsin expression showed a near-normal distribution in cones across the retina with somewhat greater numbers of M opsin-positive cones in the superior and fewer in inferior zones (Fig. 5B). Unexpectedly, T3 treatment gave a slight reduction in the numbers of M opsin-positive cones in all retinal zones in +/+ mice. Thus, although T3 is required for M opsin induction, this observation suggests that excessive T3 also disturbs expression of M opsin such that the final level of M opsin is modestly suppressed.
Figure 5.
Opsin expression after administration of saline vehicle or T3 (1.5 μg/d, ip) to Tshr−/− and +/+ mice from P8–P17. A and B, In Tshr−/− mice, T3 stimulated recovery of M opsin expression shown by immunostaining (A) and counts of M opsin-positive cones (B). In +/+ mice, T3 treatment gave marginal changes in numbers of M opsin-positive cones compared with saline treatment. C and D, T3 slightly suppressed numbers of S opsin-positive cones in all retinal zones in −/− mice and in inferior zones in +/+ mice. In the most superior zone in +/+ mice, T3 gave increased numbers of S opsin-positive cones. Saline did not alter expression of M or S opsin compared with untreated mice. Scale bar, 10 μm. Counts were performed in representative fields (0.035 mm2) in superior and inferior retina (zones 1–4, depicted in Fig. 1E) on three sections per eye, on three eyes from three mice. Significance of differences between saline and T3 treatment: *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student’s t test). E, Serum T4 levels were low or undetectable in development in Tshr−/− mice. For each age, three or four serum samples were measured, each representing a pool of two to three mice or individual mice in the case of some P60 samples. F, T3 treatment gave increases in serum T3 levels in both +/+ and Tshr−/− mice, measured about 2 h after the last injection. As expected, T3 treatment suppressed T4 levels in +/+ mice. For each genotype, two to four pooled serum samples were measured, representing in total three to eight mice. In E and F, significance of differences between +/+ and −/− genotypes: **, P < 0.01; ***, P < 0.001. OS, Outer segments.
T3 treatment slightly reduced the number of S opsin-positive cones in inferior retinal zones in both +/+ and Tshr−/− mice at P17 (Fig. 5, C and D). Interestingly, in the superior zone of +/+ mice, T3 gave increased rather than decreased numbers of S opsin-positive cones. In superior zones in Tshr−/− mice, T3 treatment only partly suppressed the numbers of S opsin-positive cones, such that final numbers were similarly slightly elevated above normal in both +/+ and Tshr−/− mice upon T3 treatment. Thus, T3 treatment over this period had different outcomes depending on the zone of the retina: S opsin was mildly suppressed in inferior zones but, unexpectedly, was induced in superior zones. Most obviously, over this age range (P8–P17), suppression of S opsin by T3 was much less marked than that previously observed in neonatal +/+ pups (11), indicating that sensitivity declines with developmental stage.
Serum T4 levels were very low or undetectable in Tshr−/− mice at P5, P17, and P60, indicating that the hypothyroidism in this mouse strain (22) had an early onset, encompassing the period when opsin patterning would normally be established (Fig. 5E). T3 injections gave substantially increased serum T3 levels in both Tshr−/− mice and +/+ littermates when measured soon (∼2 h) after the last injection (∼90-fold over levels in saline-treated +/+ mice at P17) (Fig. 5F). Serum T3 levels were low in untreated or saline-treated Tshr−/− mice at P17. As expected, T3 but not saline treatment suppressed serum T4 levels in +/+ mice.
Discussion
Retarded opsin expression in hypothyroidism
This study shows that developmental hypothyroidism causes marked retardation of expression of M opsin and subtler changes in the distribution of S opsin in cone photoreceptors in mouse retina. The results reveal a requirement for thyroid hormone for the normal expression of cone opsins in mammalian development.
The phenotype of Tshr−/− mice closely resembles that of TRβ2-deficient mice, indicating that the function of TRβ2 in cone photoreceptors requires some level of ligand stimulation. However, the eventual, partial induction of M opsin in Tshr−/− mice represents a milder final outcome than in TRβ2-deficient mice in which there is a permanent loss of M opsin. The milder outcome in Tshr−/− mice probably reflects a gradual induction of M opsin by residual low levels of T3. It has been reported that propylthiouracil-induced hypothyroidism in adult wild-type mice caused no change in M or S opsin expression, based on immunohistochemical staining shown in the supplemental data of Ref. 27. A recent report suggested that MMI-induced hypothyroidism produced modest changes in S opsin distribution in adult mice similar to those we observe (28). However, because these studies referred only to adults and did not quantify opsin levels, they did not reveal the pronounced developmental retardation and persistent reduction of M opsin expression reported here.
The evidence points to the existence of changing developmental windows of sensitivity during which thyroid hormone controls the expression of cone opsins. In neonatal mice, the serum level of ligand is too low to induce M opsin, although it has a partial suppressive action over S opsin expression particularly in the superior retina. The neonatal levels of ligand are not so high as to block S opsin expression completely; it is known that excessive T3 suppresses S opsin expression in neonatal pups (11). As development progresses, S opsin becomes less sensitive to T3, whereas M opsin begins to be induced by rising T3 levels about a week after birth.
The distinct temporal responses of M and S opsins are probably the net result of changes in serum T3 concentrations together with more local changes in the sensitivity of the cone cell and the state of transcriptional competence of the opsin genes. Cellular sensitivity may be governed, for example, by transporters for T3 in the retina (29,30), whereas at the gene level, sensitivity may be determined by transcription factors that modify the chromatin structure of the M and S opsin genes or by cofactors that modify TRβ2 activity. The means by which the spatial distribution of M and S opsins is imposed are unclear but might involve subtle gradients of T3 in the retina (11) or a role for the retinoid X receptor RXRγ, a potential heterodimerization partner of TRβ2 (31). Our cotransfection studies in 293T kidney cells or Weri retinoblastoma cells have failed to demonstrate a direct response of S and M opsin gene reporters to TRβ2 or T3 (data not shown), suggesting that additional cell-type-specific factors are required or that TRβ2 acts indirectly through intermediary factors.
Implications for TRβ2 activity and cone development in hypothyroidism
The related outcomes caused by lack of hormone or lack of TRβ2 indicate that the unoccupied receptor does not acquire a strongly deleterious activity in cones. This finding contrasts with the situation in the cerebellum in which it is thought that the major defects caused by hypothyroidism are a result of unoccupied TRα1 producing greater transcriptional dysregulation than the lack of TRα1 (13,21). This intriguing distinction between tissues may be explained if target genes in the cerebellum possess a particular chromatin configuration that makes them prone to dysregulation by the unoccupied TR, whereas target genes in the cone cell possess a less receptive configuration for the unoccupied TR. Alternatively, the distinction may be explained by structural differences between the TR isoforms. In the absence of ligand, TRα1 may possess intrinsic features that confer deleterious activity, whereas TRβ2 may adopt a more benign structure. These contrasting examples concerning the role of TRα1 in the cerebellum and TRβ2 in cone cells raise the possibility that in terms of ligand-independent activity, major functional differences exist between TR isoforms in specific tissues.
Hypothyroidism in humans is known to cause mental retardation and hearing deficits, but the possibility of color visual defects has received little attention. If thyroid hormone insufficiency occurs at or after birth, only mild opsin abnormalities may arise because of the relatively mature state of cone opsin patterning before birth in humans compared with mice (5). In the human fetus, TRβ2 is detected in cones at gestational wk 12 (32); moreover, human fetal retinal cultures can respond to T3 with increases in numbers of PNA- and recoverin-positive cone-like cells (33). Thus, maternal thyroid hormone may play a role in opsin patterning in utero even if the fetus has a defective thyroid gland. However, the relative maturity of human cones before birth suggests that another potential risk may arise from maternal hypothyroidism in pregnancy before fetal thyroid function begins. Investigation of the central, visual-evoked potential in infants born to hypothyroid mothers has indicated reduced contrast sensitivity, although studies of cone function have not been reported so far (34). More pronounced opsin abnormalities might arise if a combined maternal-fetal hypothyroidism occurs such as may be caused by chronic iodine deficiency.
Although systematic studies are awaited that address the consequences of hypothyroidism on human color vision, evidence suggests that human opsins are sensitive to thyroid hormone. Color visual defects have been noted in rare cases of resistance to thyroid hormone (35,36), a syndrome that is associated with TRβ mutations (37). Humans, unlike most mammals, are trichromatic because they have a duplicated array of L and M opsin genes for long (L, red) and medium (M, green) wave responses, instead of a single M opsin-like gene (2). The L and M opsin genes are under the control of a common locus control region such that hypothyroidism may retard expression of both L and M opsins. S opsin may display only subtle changes by analogy with the findings in mice. In human Weri retinoblastoma cells, which express TRβ2 and other cone genes (9,38), T3 can induce L/M opsin mRNA (39).
The expression of TRβ2 in cone photoreceptors represents one of the most cell-specific and conserved expression patterns known for a TR isoform. However, there are likely to be species differences in the response to thyroid hormone of different retinal cell types given that thyroid hormone provides an adaptable signal for varying needs in mammalian and nonmammalian development (19,40,41). There has been little investigation of opsin expression in hypothyroidism in other species, although in the immature trout, which has a more complex set of opsins than mammals, the addition of thyroid hormone alters expression of some opsins (42). In rats, MMI-induced hypothyroidism produces a general reduction in the thickness of the neural retinal layers and disorganization of outer segments (43,44,45). M and S opsin expression was not investigated in these rat studies, but similar defects to those found in the mouse may be predicted.
Acknowledgments
We thank D. St. Germain for providing resources for T4 and T3 assays, supported by National Institutes of Health (NIH) Grant DK054716 and Mary Alice Crawford at NIH/National Eye Institute (NEI) for expert assistance with electron microscopy.
Footnotes
This work was supported by the Intramural Research Program at the NIH/National Institute of Diabetes and Digestive and Kidney Diseases (D.F.), and NIH/NEI (C.-C.C.), a Hirschl Trust Award (D.F.), NIH Grants DK52464 and DK069713 (T.F.D.) and the Veterans Affairs Merit Award Program (T.F.D.) and NIH Grant DK054716 (A.H.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online October 30, 2008
Abbreviations: E18.5, Embryonic d 18.5; MMI, methimazole; ONL, outer nuclear layer; P17, postnatal d 17; PNA, peanut agglutinin; TRβ2, thyroid hormone receptor β2.
References
- Mollon JD 1999 Color vision: opsins and options. Proc Natl Acad Sci USA 96:4743–4745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J 1999 The evolution and physiology of human color vision: insights from molecular genetic studies of visual pigments. Neuron 24:299–312 [DOI] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM 1979 Rods and cones in the mouse retina. II. Autoradiographic analysis of cell generation using tritiated thymidine. J Comp Neurol 188:263–272 [DOI] [PubMed] [Google Scholar]
- Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D 1996 Cell fate determination in the vertebrate retina. Proc Natl Acad Sci USA 93:589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish EE, Xiao M, Yang Z, Provis JM, Hendrickson AE 2004 The role of opsin expression and apoptosis in determination of cone types in human retina. Exp Eye Res 78:1143–1154 [DOI] [PubMed] [Google Scholar]
- Szel A, Rohlich P, Mieziewska K, Aguirre G, van Veen T 1993 Spatial and temporal differences between the expression of short- and middle-wave sensitive cone pigments in the mouse retina: a developmental study. J Comp Neurol 331:564–577 [DOI] [PubMed] [Google Scholar]
- Sjöberg M, Vennström B, Forrest D 1992 Thyroid hormone receptors in chick retinal development: differential expression of mRNAs for α and N-terminal variant β receptors. Development 114:39–47 [DOI] [PubMed] [Google Scholar]
- Ng L, Hurley JB, Dierks B, Srinivas M, Saltó C, Vennström B, Reh TA, Forrest D 2001 A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat Genet 27:94–98 [DOI] [PubMed] [Google Scholar]
- Jones I, Ng L, Liu H, Forrest D 2007 An intron control region differentially regulates expression of thyroid hormone receptor β2 in the cochlea, pituitary, and cone photoreceptors. Mol Endocrinol 21:1108–1119 [DOI] [PubMed] [Google Scholar]
- Shibusawa N, Hashimoto K, Nikrodhanond AA, Liberman MC, Applebury ML, Liao XH, Robbins JT, Refetoff S, Cohen RN, Wondisford FE 2003 Thyroid hormone action in the absence of thyroid hormone receptor DNA-binding in vivo. J Clin Invest 112:588–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MR, Srinivas M, Forrest D, Morreale de Escobar G, Reh TA 2006 Making the gradient: thyroid hormone regulates cone opsin expression in the developing mouse retina. Proc Natl Acad Sci USA 103:6218–6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovet J, Daneman D 2003 Congenital hypothyroidism: a review of current diagnostic and treatment practices in relation to neuropsychologic outcome. Paediatr Drugs 5:141–149 [DOI] [PubMed] [Google Scholar]
- Bernal J 2007 Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab 3:249–259 [DOI] [PubMed] [Google Scholar]
- DeLong GR, Stanbury JB, Fierro-Benitez R 1985 Neurological signs in congenital iodine-deficiency disorder (endemic cretinism). Dev Med Child Neurol 27:317–324 [DOI] [PubMed] [Google Scholar]
- Morreale de Escobar G, Obregon MJ, Escobar del Rey F 2004 Role of thyroid hormone during early brain development. Eur J Endocrinol 151(Suppl 3):U25–U37 [DOI] [PubMed] [Google Scholar]
- Kopp P 2002 Perspective: genetic defects in the etiology of congenital hypothyroidism. Endocrinology 143:2019–2024 [DOI] [PubMed] [Google Scholar]
- Harris KB, Pass KA 2007 Increase in congenital hypothyroidism in New York State and in the United States. Mol Genet Metab 91:268–277 [DOI] [PubMed] [Google Scholar]
- Damm K, Thompson CC, Evans RM 1989 Protein encoded by v-erbA functions as a thyroid-hormone receptor antagonist. Nature 339:593–597 [DOI] [PubMed] [Google Scholar]
- Havis E, Le Mevel S, Morvan Dubois G, Shi DL, Scanlan TS, Demeneix BA, Sachs LM 2006 Unliganded thyroid hormone receptor is essential for Xenopus laevis eye development. EMBO J 25:4943–4951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Curty FH, Borges PP, Lee CE, Abel ED, Elmquist JK, Cohen RN, Wondisford FE 2001 An unliganded thyroid hormone receptor causes severe neurological dysfunction. Proc Natl Acad Sci USA 98:3998–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morte B, Manzano J, Scanlan T, Vennström B, Bernal J 2002 Deletion of the thyroid hormone receptor α1 prevents the structural alterations of the cerebellum induced by hypothyroidism. Proc Natl Acad Sci USA 99:3985–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marians RC, Ng L, Blair HC, Unger P, Graves PN, Davies TF 2002 Defining thyrotropin-dependent and -independent steps of thyroid hormone synthesis by using thyrotropin receptor-null mice. Proc Natl Acad Sci USA 99:15776–15781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A, Martinez ME, Fiering S, Galton VA, St Germain D 2006 Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Invest 116:476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuo J, Bojanowski CM, Zhou M, Shen D, Ross RJ, Rosenberg KI, Cameron DJ, Yin C, Kowalak JA, Zhuang Z, Zhang K, Chan CC 2007 Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration. Invest Ophthalmol Vis Sci 48:3827–3836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanks JC, Johnson LV 1983 Selective lectin binding of the developing mouse retina. J Comp Neurol 221:31–41 [DOI] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM 1979 Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J Comp Neurol 188:245–262 [DOI] [PubMed] [Google Scholar]
- Applebury ML, Farhangfar F, Glosmann M, Hashimoto K, Kage K, Robbins JT, Shibusawa N, Wondisford FE, Zhang H 2007 Transient expression of thyroid hormone nuclear receptor TRβ2 sets S opsin patterning during cone photoreceptor genesis. Dev Dyn 236:1203–1212 [DOI] [PubMed] [Google Scholar]
- Pessoa CN, Santiago LA, Santiago DA, Machado DS, Rocha FA, Ventura DF, Hokoc JN, Pazos-Moura CC, Wondisford FE, Gardino PF, Ortiga-Carvalho TM 2008 Thyroid hormone action is required for normal cone opsin expression during mouse retinal development. Invest Ophthalmol Vis Sci 49:2039–2045 [DOI] [PubMed] [Google Scholar]
- Abe T, Suzuki T, Unno M, Tokui T, Ito S 2002 Thyroid hormone transporters: recent advances. Trends Endocrinol Metab 13:215–220 [DOI] [PubMed] [Google Scholar]
- Visser WE, Friesema EC, Jansen J, Visser TJ 2008 Thyroid hormone transport in and out of cells. Trends Endocrinol Metab 19:50–56 [DOI] [PubMed] [Google Scholar]
- Roberts MR, Hendrickson A, McGuire CR, Reh TA 2005 Retinoid X receptor-γ is necessary to establish the S-opsin gradient in cone photoreceptors of the developing mouse retina. Invest Ophthalmol Vis Sci 46:2897–2904 [DOI] [PubMed] [Google Scholar]
- Lee TC, Almeida D, Claros N, Abramson DH, Cobrinik D 2006 Cell cycle-specific and cell type-specific expression of rb in the developing human retina. Invest Ophthalmol Vis Sci 47:5590–5598 [DOI] [PubMed] [Google Scholar]
- Kelley MW, Turner JK, Reh TA 1995 Regulation of proliferation and photoreceptor differentiation in fetal human retinal cell cultures. Invest Ophthalmol Vis Sci 36:1280–1289 [PubMed] [Google Scholar]
- Mirabella G, Westall CA, Asztalos E, Perlman K, Koren G, Rovet J 2005 Development of contrast sensitivity in infants with prenatal and neonatal thyroid hormone insufficiencies. Pediatr Res 57:902–907 [DOI] [PubMed] [Google Scholar]
- Newell FW, Diddie KR 1977 Typical monochromacy, congenital deafness, and resistance to intracellular action of thyroid hormone. Klin Monatsbl Augenheilkd 171:731–734 [PubMed] [Google Scholar]
- Lindstedt G, Lundberg PA, Sjogren B, Ernest I, Sundquist O 1982 Thyroid hormone resistance in a 35-year old man with recurrent goitre. Scand J Clin Lab Invest 42:585–593 [PubMed] [Google Scholar]
- Refetoff S, Weiss RE, Usala SJ 1993 The syndromes of resistance to thyroid hormone. Endocr Rev 14:348–399 [DOI] [PubMed] [Google Scholar]
- Li A, Zhu X, Brown B, Craft CM 2003 Gene expression networks underlying retinoic acid-induced differentiation of human retinoblastoma cells. Invest Ophthalmol Vis Sci 44:996–1007 [DOI] [PubMed] [Google Scholar]
- Liu Y, Fu L, Chen DG, Deeb SS 2007 Identification of novel retinal target genes of thyroid hormone in the human WERI cells by expression microarray analysis. Vision Res 47:2314–2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh-Armstrong N, Huang H, Remo BF, Liu TT, Brown DD 1999 Asymmetric growth and development of the Xenopus laevis retina during metamorphosis is controlled by type III deiodinase. Neuron 24:871–878 [DOI] [PubMed] [Google Scholar]
- Mader MM, Cameron DA 2006 Effects of induced systemic hypothyroidism upon the retina: regulation of thyroid hormone receptor α and photoreceptor production. Mol Vis 12:915–930 [PubMed] [Google Scholar]
- Veldhoen K, Allison WT, Veldhoen N, Anholt BR, Helbing CC, Hawryshyn CW 2006 Spatio-temporal characterization of retinal opsin gene expression during thyroid hormone-induced and natural development of rainbow trout. Vis Neurosci 23:169–179 [DOI] [PubMed] [Google Scholar]
- Takeda M, Onoda N, Suzuki M 1994 Characterization of thyroid hormone effect on the visual system of the adult rat. Thyroid 4:467–474 [DOI] [PubMed] [Google Scholar]
- Sevilla-Romero E, Munoz A, Pinazo-Duran MD 2002 Low thyroid hormone levels impair the perinatal development of the rat retina. Ophthalmic Res 34:181–191 [DOI] [PubMed] [Google Scholar]
- Pinazo-Duran MD, Iborra FJ, Pons S, Sevilla-Romero E, Gallego-Pinazo R, Munoz A 2005 Postnatal thyroid hormone supplementation rescues developmental abnormalities induced by congenital-neonatal hypothyroidism in the rat retina. Ophthalmic Res 37:225–234 [DOI] [PubMed] [Google Scholar]