Abstract
The development of highly selective agonists for the two major subforms of the estrogen receptor (ERa and ERϐ) has produced new experimental methodologies for delineating the distinct functional role each plays in neurobehavioral biology. It has also been suggested that these compounds might have the potential to treat estrogen influenced behavioral disorders, such as anxiety and depression. Prior work has established that the ERϐ agonist, diarylpropionitrile (DPN) is anxiolytic in gonadectomized animals of both sexes, but whether or not this effect persists in gonadally intact individuals is unknown. Isoflavone phytoestrogens, also potent but less selective ERϐ agonists, have also been shown to influence anxiety in multiple species and are becoming more readily available to humans as health supplements. Here we determined the effects of 0.5, 1 or 2 mg/kg DPN, 1 mg/kg of the ERa agonist propyl-pyrazole-triol (PPT), 3 or 20 mg/kg of the isoflavone equol (EQ) and 3 or 20 mg/kg of the isoflavone polyphenol resveratrol (RES) on anxiety behavior in the gonadally intact male rat using the light/dark box and the elevated plus maze. We first determined that DPN can be successfully administered either orally or by subcutaneous injection, although plasma DPN levels are significantly lower if given orally. Once injected, plasma levels peak rapidly and then decline to baseline levels within 3 hours of administration. For the behavioral studies, all compounds were injected and the animals were tested within 3 hours of treatment. None of the compounds, at any of the doses, significantly altered anxiety-related behavior. Plasma testosterone levels were also not significantly altered suggesting that these compounds do not interfere with endogenous androgen levels. The results suggest that the efficacy of ERϐ agonists may depend on gonadal status. Therefore the therapeutic potential of ERϐ selective agonists to treat mood disorders may be limited.
Keywords: DPN, PPT, estrogen, soy isoflavones, testosterone, anxiety, endocrine active compound, resveratrol, equol, plus maze, ERϐ, estrogen receptor beta
Introduction
To date, two major subforms of the estrogen receptor (ERa and ERϐ) have been identified in mammals (Greene et al., 1986; Kuiper et al., 1996). Although the DNA binding domains of the two are nearly identical in structure, the ligand binding domain of ERϐ is only partially homologous to that of ERa and has previously been shown to bind a spectrum of ligands including the androgen metabolite 17beta-diol (3beta-diol) (Handa et al., 2007; Lund et al., 2006), phytoestrogens, and a number of endocrine disrupting compounds (Kuiper et al., 1996; Kuiper et al., 1998). ERα and ERϐ are differentially distributed in both adult (Shughrue et al., 1998; Zhang et al., 2002) and immature brains (Fried et al., 2004; Lemmen et al., 1999; Perez et al., 2003) of rodents and humans suggesting that the two ER subtypes may regulate different aspects of behavior and neuroendocrine function across the lifespan. The development of ligands specific for either ERa or ERϐ has proven to be a powerful tool to help delineate the relative roles each ER subtype plays in the organization and activation of steroid sensitive physiology and behavior. It has also been proposed that these compounds may have therapeutic potential (Harris, 2007; Imamov et al., 2007; Osterlund et al., 2005). However little has been published regarding the basic pharmacokinetics of these compounds or how they alter hormone dependent physiology and behavior in gonadally intact animals. This information is essential in order to evaluate the therapeutic potential of these compounds.
The present study largely focused on the most commonly used ERϐ specific ligand, diarylpropionitrile (DPN), a compound with a 70-fold higher binding affinity for ERϐ than ERa (Katzenellenbogen et al., 2000). This compound has been used by a number of different investigators from a broad spectrum of fields to evaluate the relative contribution ERϐ plays in the organization and activation of estrogen dependent physiology and behavior (Choleris et al., 2008; Harris, 2007). These prior experiments have employed a wide range of DPN doses, spanning 10 μg to 1.5 mg, but circulating levels of DPN were not determined making it difficult to discern which, if any, of these doses produce plasma levels that approximate circulating steroid hormone levels (Frasor et al., 2003; Lund et al., 2005; Meyers et al., 2001; Rhodes and Frye, 2006; Sanchez-Criado et al., 2004; Walf et al., 2004). In addition, all of these studies have administered DPN by subcutaneous (sc) injection. For some experimental designs oral administration may be advantageous because it is less stressful to the animal and would more closely replicate the route of administration for humans. It is currently unclear from the present literature how long DPN remains in plasma after it is administered or if it would survive oral administration. Therefore, the first goal of the present study was to compare plasma DPN levels following oral or sc injection at two different doses. The second goal was to determine how long DPN remains in plasma following a single sc injection or oral dose. We hypothesized that plasma DPN levels would be present but lower following oral administration than when given by sc injection and that, regardless of the route of administration, would rapidly clear from plasma.
We next examined how agonism of ERϐ by DPN affects ERϐ-dependent behavior in gonadally intact male rats. Agonism of ERϐ by DPN has recently been shown to have significant anxiolytic effects in gonadectomized male and female rats using a variety of behavioral paradigms including the forced swim test, the elevated plus-maze and the open field test (Lund et al., 2005; Walf and Frye, 2005; Walf et al., 2004). In contrast, the ERa specific agonist propyl-pyrazole-triol (PPT) has an opposite or no effect. These studies strongly indicate a role for ERϐ in the modulation of anxiety related behaviors but because all of these studies used gonadectomized animals, it remains unclear whether or not the anxiolytic effect of DPN would persist in a gonadally intact animal. This is a key consideration when evaluating the therapeutic potential of ER selective agonists like DPN because in this case the target subject would be a gonadally intact individual. At the very least, administration of DPN to a gonadally intact animal may modify blood steroid hormone levels, an effect which may result in altered physiology or behavior.
To examine these questions we first compared the effects of two doses of DPN (0.5 mg/kg and 2 mg/kg) on anxiety related behavior using the elevated plus maze and the light dark box. Next, we compared the effect of DPN (1 mg/kg) with the ERa agonist PPT (1 mg/kg) and two phytoestrogens on anxiety related behavior and plasma androgen levels in gonadally intact male rats. Phytoestrogens, steroidal chemicals produced by plants, have been found to mimic or interact with hormone signaling in animals and generally have a higher relative binding affinity (RBA) for ERϐ than ERa (Kuiper et al., 1998; Morito et al., 2001) suggesting that they may be particularly potent modulators of ERϐ-dependent physiology and behavior. They are also becoming more widely available in supplement form and are routinely advertised to “improve mood.” We have previously shown that isoflavone-rich diets can be anxiogenic when fed to gonadally intact male rats (Patisaul et al., 2005). Diets containing soy isoflavones have also been shown to alter anxious and aggressive behaviors in gonadally intact males of other species including non-human primates (Hartley et al., 2003; Moore et al., 2004; Simon et al., 2004). These data suggest that agonism of ERϐ in gonadally intact males may produce distinct and opposite effects on anxiety than in castrated males.
The phytoestrogens chosen for the present study were equol (EQ) and resveratrol (RES). The two most prevalent phytoestrogen isoflavones obtained from soy based foods are genistein and daidzein but, in mammals, equol (7-hydroxy-3[4′hydroxyphenyl]-chroman) is the major metabolite of daidzein and is produced exclusively by microflora in the gut (Setchell et al., 2003b). EQ production varies greatly across species, but rats consuming soy rich diets generate especially high levels of EQ (Axelson et al., 1982; Lephart et al., 2000; Setchell et al., 2002). It has also been suggested that humans that can generate EQ are more likely to experience the hypothesized benefits of soy consumption (Setchell et al., 2002; Speroff, 2005). Therefore we hypothesized that the previously observed anxiogenic effect of an isoflavone rich diet on gonadally intact male rats (Patisaul et al., 2005) might largely be attributed to the activity of EQ. The bioflavonoid isoflavone polyphenol RES (trans -3,5,4′-trihydroxystilbene) is a major constituent of red wine and is now available in health food supplements. Interest in RES intensified following a widely publicized report that obese mice, maintained on a diet containing a relatively high dose of RES (approximately 22 mg/kg body weight per day), showed improved overall health, decreased organ pathology, and longer lifespan compared to obese control mice (Baur et al., 2006). RES has also been shown increase lifespan in other species including fish and C. elegans (Howitz et al., 2003; Valenzano et al., 2006). Because RES binds both ER subtypes with equal affinity (Bowers et al., 2000; Kuiper et al., 1997; Levenson et al., 2003; Schreihofer, 2005) and therefore may not be as potent as EQ and DPN at modulating ERϐ-dependent physiology and behavior, we hypothesized that RES would have a lower potential to alter anxiety-related behavior than EQ or DPN. By comparing the effects of these phytoestrogens with the ERϐ agonist DPN on anxiety-related behavior in gonadally intact male rats, we aimed to determine if any of these compounds might have therapeutic potential for mood disorders in humans.
Because we used gonadally intact animals for these experiments, behavioral change following exposure to any of the ERϐ agonists could result from altered plasma testosterone (T) levels rather than as a direct effect of the administered compound. Several studies have demonstrated an anxiolytic effect of T administration in castrated male rats (Bitran et al., 1993; Fernandez-Guasti and Martinez-Mota, 2005; Frye and Seliga, 2001). Additionally, EQ has the unique ability to specifically bind 5 alpha-dihydro-testosterone (DHT), and thus the potential to inhibit the action of this potent androgen. Therefore we quantified plasma T levels following the conclusion of the behavioral experiments to determine if any of the observed changes in behavior were associated with modified plasma T levels.
Methods
Animal Care
All animals (n = 86 Long Evans males, 18 Wistar males, 3–5 months of age) were maintained on a reverse light cycle (lights off at 10:00hr and on at 22:00hr) at 23°C and 50% humidity and housed individually. Because standard lab chows are soy-based and thus contain significant amounts of phytoestrogens (Boettger-Tong et al., 1998; Thigpen et al., 1999) all animals were fed a semi-purified, phytoestrogen-free diet (5K96, Purina Test Diets, Richmond, IN). Animal care, maintenance, and behavioral testing were conducted in accordance with the applicable portions of the Animal Welfare Act and the U.S. Department of Health and Human Services “Guide for the Care and Use of Laboratory Animals.” The protocol was approved by the North Carolina State University Animal Care and Use Committee and supervised by animal care personnel and veterinary staff.
Experiment 1: Oral versus sc injection administration of DPN
Animals (n = 12 Long Evans males; 3 months of age) were divided into four groups (n = 3 per group) and administered DPN (TocrisBiosciences, Ellisville, MS) either by subcutaneous (sc) injection (1 or 3 mg/kg body weight (bw)) or oral administration (1 or 3 mg/kg bw) daily for three days. Oral doses were prepared by dissolving the appropriate amount of compound in 0.1 ml dimethyl sulfoxide (DMSO, Sigma, Saint Louis, MO) and dispensed on mini vanilla wafer cookies as described previously (Hays et al., 2002). The animals were then fed the wafers. We preferred this method to oral gavage because it is significantly less stressful to the animals. Keeping stress to a minimum was critical because stress can affect performance on the anxiety tests (see below). Animals given DPN by injection (1 or 3 mg/kg bw dissolved in 0.2 mL DMSO) were given cookies containing an equal amount of DMSO. All animals were given cookies for two days prior to the first day of dosing to ensure that they were familiar with the food item and would consume it completely. Animals were sacrificed three to four hours after the final dose by CO2 asphyxiation followed by decapitation. Trunk blood was collected and centrifuged at 5,000 g for 10 minutes at 4°C. The plasma was removed and stored at −20°C until analysis.
Experiment 2: Detection of plasma DPN levels one to two hours after oral administration
In the prior experiment, plasma levels following oral administration were close to the limit of detection indicating that, either oral administration was not effective, or that we waited too long to collect the samples (3–4 hours post administration). To determine if plasma levels were higher in the first two hours after consumption, animals (n = 10 Long Evans males; 3 months of age) were given a single 3 mg/kg bw dose of DPN by oral administration in the morning (09:00) by the same method used in Experiment 1 and then sampled either in the first hour after consumption (n = 5) or in the second hour after consumption (n = 5). Blood was collected from the saphenous vein and centrifuged at 5,000 g for 10 minutes at 4°C. The plasma was collected and stored at −20°C until analysis.
Experiment 3: Duration of detectable plasma DPN levels following a single sc injection
Animals (n = 18 Wistar males; 4 months of age) were given a single 1 mg/kg bw sc injection of DPN in the morning (10:30) and then sacrificed by CO2 asphyxiation followed by decapitation in a sequential series beginning 60 minutes after the injection and ending five hours after the injection. DPN was prepared by first dissolving the compound in 100% EtOH and then adding this solution to sesame oil (Sigma, Saint Louis, MO) to achieve a final ratio of 20% EtOH and 80% oil. Injection volume was 0.2 mL per animal. Five additional animals were injected with EtOH/sesame oil vehicle and served as controls. The change in vehicle was necessary because we found that the DMSO produced skin irritation in the prior experiment. We have used the oil/ethanol vehicle previously with good success (Patisaul et al., 2006). DPN easily dissolves in the EtOH and the oil solution does not produce skin irritation or lesions. Wistar males were used instead of Long Evans males because an appropriate number of Long Evans rats were not available. Although strain differences in plasma DPN levels are possible, we presumed that substantive differences were unlikely.
Experiment 4: Effect of low and high dose DPN on anxiety-related behavior
Anxiety was assessed using the light/dark box and the elevated plus maze as described previously (Morgan and Pfaff, 2001; Patisaul et al., 2005; Pellow and File, 1986). Gonadally intact Long Evans males maintained on a reverse light schedule were randomly assigned to treatment groups (n = 8 per group; 4 – 5 months of age) and administered 0.2 mL vehicle (EtOH/sesame oil solution as described above), 0.5 mg/kg DPN, or 2 mg/kg DPN by sc injection daily for four days. These doses were chosen because they are at the low and high end of what has previously been used by multiple labs to modify estrogen sensitive physiology and behavior including anxiety (Frasor et al., 2003; Harris et al., 2002; Lund et al., 2005). The duration of DPN administration is also consistent with prior studies (Lund et al., 2005).
Animals were subjected to the light/dark test on the third day of DPN administration and the plus maze on the final day of DPN administration. Testing began 40 minutes after the final injection and was completed within three hours. All testing was conducted in the first three hours of the dark cycle. To minimize transport stress, testing was conducted in an alcove of the room where the animals were housed. The lidded light/dark testing chamber (89 × 38 × 38 cm) was constructed of Plexiglas and consisted of two sides, one clear and one dark, separated by a partition. An opening (10 ×10 cm) allowed for passage between the two compartments. The light compartment was illuminated by two 40-W clip lamps placed above the compartment. All behavior was videotaped from a camera facing the apparatus. The animal was gently placed into the dark compartment, and allowed to explore the testing chamber for 5 min. Measures included the number of transitions into the light compartment (with a transition defined as all 4 paws crossing into the light compartment), and total time spent in the light compartment. The plus maze (Stoelting, Wood Dale, IL) was illuminated by red lamps (70 lux) and behavior was videotaped from a camera suspended above the maze. All animals were placed in the center of the maze facing an open arm and allowed to freely explore the maze for five minutes. Measures included the number of open arm entries, total time spent in the open arms, the number of closed arm entries and the time spent in the closed arms, a reporting method that is consistent with what we and others have done previously (Lund et al., 2005; Patisaul et al., 2005; Walf and Frye, 2005). Maze performance was evaluated by well established criteria (Handley and McBlane, 1993; Morgan and Pfaff, 2001; Pellow et al., 1985). An arm entry was defined as the animal having four paws on the arm and an exit was defined as two paws leaving the arm.
Behavior in the light/dark box and the plus maze was scored live by a silent observer, blind to the treatment groups, and consistently positioned out of animal’s sight lines, using the behavioral analysis software program Stopwatch (Center for Behavioral Neuroscience, Emory University). All behavioral measures were then validated from the video by a second observer. To validate the data, a randomly selected subset of the trials was scored from the tape. If the data was reasonably matched, then the live data was determined to be valid. In all cases the data collected live was determined to be valid and therefore used for the analysis.
Experiment 5: Comparison of DPN, PPT, low dose EQ and low dose RES effects on anxiety-related behavior
Gonadally intact Long Evans males maintained on a reverse light schedule were randomly assigned to treatment groups (n = 8 per group; 3 months of age; not the same animals used for the prior experiments) and exposed to 0.2 ml vehicle (EtOH/sesame oil solution as described above), 1 mg/kg PPT (Tocris Biosciences, Ellisville, MS), 1 mg/kg DPN, 3 mg/kg EQ (a racemic mixture generously supplied by Mike Adams of Wake Forest University) or 3 mg/kg RES (Sigma, Saint Louis, MO) by sc injection for 3 days. This dose of EQ is approximately equivalent to the total isoflavone content of soy infant formula and some soy based supplements (Setchell et al., 1986; Setchell et al., 2001; Setchell and Cole, 2003). This dose of DPN and PPT has previously been used by multiple labs to modify estrogen sensitive physiology and behavior including anxiety (Frasor et al., 2003; Harris et al., 2002; Lund et al., 2005) and is midway between the two doses used for Experiment 4. Testing in the light/dark box began 40 minutes after the final (third) injection and was completed over 2 hours as described above.
Experiment 6: Comparison of DPN, PPT, high dose EQ and high dose RES effects on anxiety-related behavior
Thirty eight days after the conclusion of the light/dark testing, the same animals from Experiment 5 were again randomly assigned to treatment groups (n = 8 per group; now 4 months of age) and exposed to 0.2 ml vehicle (EtOH/sesame oil solution described above), 1 mg/kg PPT, 1 mg/kg DPN, 20 mg/kg EQ or 20 mg/kg RES for 3 days. This dose of RES has previously been shown to improve health in obese mice (Baur et al., 2006) and the dose of EQ was chosen to match. Anxiety was assessed using the elevated plus maze as described above. Testing began 30 minutes after the final injection and was completed within 2 hours. All animals were placed in the center of the maze facing an open arm and allowed to freely explore the maze for five minutes. If an animal fell from the maze, it was replaced in the center and testing resumed (n = 1 EQ). If an animal fell twice (n = 1,RES, 1 OIL) it was returned to its home cage and not used for the analysis.
Experiment 7: Effect of DPN, PPT, high dose EQ and high dose RES on plasma testosterone levels
At the end of the plus maze test, each male was removed from the maze and quickly transported to an adjoining room were it was euthanized by CO2 asphyxiation. Trunk blood was collected and centrifuged at 5,000 g for 10 minutes at 4°C. The plasma was then removed and frozen at −20°C. Serum levels of total testosterone were quantified by radioimmunoassay (RIA) using the Coat-A-Count Total Testosterone Kit (Siemens). All samples were run in duplicate. Plasma DPN levels were quantified in the DPN treated and control animals only.
Analysis of Plasma DPN Levels
To precipitate proteins, 100 μL of plasma was transferred to 1.5 mL plastic vials and saturated with sodium chloride. Recovery samples were spiked at this time with DPN in 90:10 water:methanol (v/v) for a final concentration of 50 ng/mL. The plasma was extracted 3 times with 250 μL ethyl acetate by vortex mixing for 30 seconds. The extracts were centrifuged for 5 minutes at 10 rcf. The ethyl acetate fractions were combined and evaporated to dryness under nitrogen. Extracts were brought to 100 μL in 80:20 water:methanol (v/v). The compound 4-(3-hydroxyphenyl)benzonitrile was added to the final extract as the internal standard. All solvents were purchased from Burdick and Jackson.
Samples were analyzed by LC-ESI-MS/MS using a Thermo (San Jose, CA) LTQ linear ion trap mass spectrometer coupled to Surveyor MS Pump and Autosampler (Thermo). Analytes were separated on a 5 μm, 150×2mm Hypersil GOLD® column with a Javelin® guard column (Thermo). Samples (5 μL) were injected onto the column equilibrated in 90:10:0 A:B:C, where A = 50mM acetic acid in water, B= acetonitrile, C = 2-propanol, flow rate 250 μL/min. A 20 minute linear gradient 0:10:90 was employed to elute the analytes of interest. All sample extracts were maintained in the autosampler at 5°C while awaiting injection.
DPN was detected by MS/MS, precursor ion m/z 238 with normalized collision energy of 35% and quantifying product ions m/z 211 and m/z 232. The internal standard was detected by MS/MS, precursor ion m/z 194, normalized collision energy of 40% and quantification was based on the resulting total ion chromatogram although the ion experienced little fragmentation. For the time course study (Experiment 2), however, quantification of the internal standard was based on ion m/z 194 (MS/MS mode).
Data Analysis
For each of the two doses, mean plasma DPN levels following injection or oral administration were compared using a t-test. Pharmacokinetic analysis of the DPN plasma concentration-time curve was performed using a Levenberg-Marquardt fitting method to determine the decay constant (?) and half life (t1/2). As expected, no DPN was detected in the oil treated control animals. Differences in the number of entries into the open and closed arms and the total time spent in each of the arms were detected by a one-way ANOVA with treatment as the factor. Plasma testosterone levels were also analyzed by one-way ANOVA. Main effects were followed up with either Fisher’s Least Significant Difference test or Dunnett’s two sided post-hoc test to specifically compare each treatment group to the control group. In all cases, results were considered statistically significant when P ≤ 0.05.
Results
Experiment 1: Plasma DPN levels were significantly higher following sc injection versus oral administration
For both doses, plasma DPN levels were higher following sc injection rather than oral administration (Table 1). An oral dose of 3 mg/kg bw produced plasma DPN levels approximately equivalent to an injected dose of 1 mg/kg bw indicating that although oral administration is possible, higher doses are needed to achieve the same plasma levels of DPN. Plasma DPN levels from the animals fed 1 mg/kg bw were close to the method detection limit.
Table 1.
Plasma DPN levels following sc injection or oral administration
| Expt | Dose | n | Route | Time post-injection | Mean Plasma DPN (ng/ml) |
|---|---|---|---|---|---|
| Expt 1 | 1 mg/kg bw | 3 | sc injection | 180 – 240 min | 2.71 ± 0.61 |
| Expt 1 | 1 mg/kg bw | 3 | oral | 180 – 240 min | 0.786 ± 0.43 |
| Expt 1 | 3 mg/kg bw | 3 | sc injection | 180 – 240 min | 13.57 ± 2.14 |
| Expt 1 | 3 mg/kg bw | 3 | oral | 180 – 240 min | 1.86 ± 0.27 |
| Expt 2 | 3 mg/kg bw | 5 | oral | 30 – 60 min | 9.19 ± 2.30 |
| Expt 2 | 3 mg/kg bw | 5 | oral | 80 – 120 min | 6.07 ± 1.20 |
Data presented collectively represent the results from Experiment 1 (Expt 1) and Experiment 2 (Expt 2). Regardless of dose, plasma DPN levels were highest in the injected animals compared to the orally treated animals. When dosed orally at 3 mg/kg, plasma DPN levels were highest in the first 60 minutes after administration but near the limit of detection by 3 – 4 hours after administration. (Means ± S.E.)
Experiment 2: Plasma DPN levels are detectable within the first 2 hours after administration
Plasma DPN levels following a single, oral 3 mg/kg bw dose averaged 9.19 ng/ml within the first hour post-exposure and 6.07 ng/ml within the second hour (Table 1). Collectively, the results from this and the prior experiment suggest that, although DPN can successfully be administered orally, plasma levels are relatively low compared to those following sc injection and decline to near the limit of detection within 3–4 hours post exposure. Based on these results, sc injection was used as the exposure route for all subsequent behavioral experiments in this study.
Experiment 3: Plasma DPN levels dropped within three hours of administration by sc injection
Following a single 1 mg/kg bw injection, plasma DPN levels were highest one hour after injection (in the range of 300 – 400 ng/ml) and rapidly declined to near the limit of detection by 3 hours post-injection (Fig. 1). Analysis with a Levenberg-Marquardt fitting method revealed that DPN levels decay exponentially (goodness of fit correlation equaled 0.936) with a decay constant (?) of 2.43 resulting in a half life (t1/2) of 0.29 hours.
Fig. 1.
Time course of plasma DPN levels following a single injection of 1 mg/kg bw. Samples (one per animal; Wistar males) were collected over a five hour window beginning one hour after the injection. Plasma DPN levels declined exponentially with a half life of 0.29 hours. As expected, no DPN was detected in the plasma samples from the oil treated control animals (n = 5), and that data is not depicted on the graph. (Means ± S.E.)
Experiment 4: Neither the high (2 mg/kg) nor the low (0.5 mg/kg) dose of DPN altered anxiety behavior in gonadally intact adult male rats
Performance in the light/dark box was unaffected by either dose of DPN (Fig. 2A and B). The number of light side entries (F(2,21) = 0.01, P = 0.99) and the time spent in the light side (F(2,21) = 0.02, P = 0.98) was nearly identical across all groups, and there was no main effect of treatment for either measure. In the elevated plus maze (Fig 2C and D), neither the low nor the high dose of DPN significantly affected either the number of open arm entries (F(2,21) = 0.188, P= 0.83) or the time spent on the open arms (F(2,21) = 1.78, P = 0.193). The overall total number of entries, regardless of arm type, also did not differ between groups (F(2, 21) = 0.33, P = 0.72) suggesting that locomotor and general exploratory behavior was not significantly affected by either of the DPN treatments. Similarly, the proportion of open arm entries made (F(2,21) = 0.16, P = 0.85) and the proportion of time spent in the open arms (F(2,21) = 1.65, P = 0.22) did not significantly differ among the groups. Although time in the open arms appeared elevated in the high dose DPN group, a Dunnett post-hoc test confirmed that there was no significant difference between this group and the oil treated controls (P = 0.16).
Fig. 2.
Performance in the light/dark box was not significantly affected by treatment with either the high (2 mg/kg) or low (0.5 mg/kg) dose of DPN. The number of light side transitions (A) and the time spent in the light side (B) were nearly identical for all groups. Similarly, behavior in the plus maze did not significantly differ between groups. The number of open arm entries (C) was not affected by either the high or low dose of DPN. Time spent in the open arms generally increased with dose (D) but the effect was not statistically significant and was largely attributable to some of the animals “freezing” on the open arms and therefore spending a disproportionate amount of time on the open arms. The proportion of time spent on the open arms and the percentage of open arm entries was also not significantly different between groups (not depicted). (Means ± S.E.)
Experiment 5: Anxiety behavior in the light/dark box was not affected by the 1 mg/kg dose of DPN or PPT, or the low (3 mg/kg) dose of EQ or RES
In the light/dark box test, there was no main effect of treatment on either the number of entries made into the light chamber (F(4,35) = 1.78, P = 0.16; Fig. 3A) or the amount of time spent in the light chamber (F(4,35) = 1.23, P = 0.32; Fig. 3B). Time spent in the light chamber appeared to be notably lower in both the DPN and PPT groups compared to the control group. However, the Dunnett post-hoc test revealed that time spent in the light chamber was not significantly lower in either the DPN (P = 0.30) or the PPT (P = 0.27) group compared to the oil treated control group indicating that none of the compounds significantly affected anxiety-related behavior at the dose administered.
Fig. 3.
Performance in the light/dark box was not significantly affected by the 1 mg/kg dose of DPN or PPT, or the low (3 mg/kg) dose of EQ or RES. Neither the number of entries into the light side (A) nor the amount of time spent in the light side (B) differed among the groups. (Means ± S.E.)
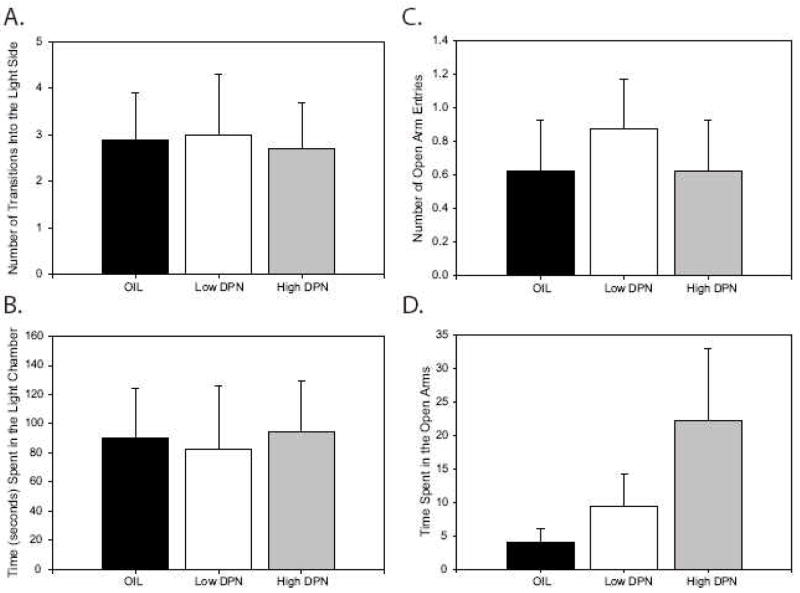
Experiment 6: Anxiety behavior in the plus maze was not affected by the 1 mg/kg dose of DPN or PPT or the high (20 mg/kg) dose of EQ or RES
In the plus maze test, there was no main effect of treatment on the number of open arm entries (F(4,30) = 1.19, P = 0.34; Fig. 4A) but a Dunnett post-hoc test revealed that the DPN animals made significantly fewer open arm entries than the control animals (P = 0.05). However the total number of entries made into the arms, regardless of arm type, was also significantly affected by treatment (F(4,30) = 3.30, P = 0.02; Fig. 4C) with the DPN animals making significantly fewer entries than all of the other groups, including the control animals (P ≤ 0.01). Therefore, the proportion of open arm entries, relative to the total number of arm entries, was analyzed to account for this decrease in overall activity. There was no significant effect of treatment on the proportion of open arm entries (F(4,30) = 0.32, P = 0.86; Fig 4D). Total time spent in the open arms (F(4,30) = 0.31, P = 0.87; Fig. 4B) was not significantly affected by treatment and, similarly, the proportion of time spent on the open arms was also not influenced by treatment (F(4,30) = 0.46, P = 0.77). Collectively these data suggest that none of the compounds significantly influenced anxiety-related behavior at the dose administered but that locomotor and exploratory behavior was lower in the DPN treated animals relative to the other groups.
Fig. 4.
In the experiment comparing high dose (20 mg/kg) phytoestrogen exposure to DPN and PPT (1 mg/kg) exposure, DPN treated animals made fewer open arm entries relative to control animals (A) but there was no significant effect of treatment on time spent in the open arms (B). However, the DPN animals made significantly less arm entries, regardless of type, suggesting that they had lower overall levels of exploratory behavior compared to the other groups (C). Therefore, the proportion of open arm entries was analyzed (D), and no significant effect of treatment was found. These results suggest that exploratory behavior, rather than anxiety related behavior, was affected by DPN. (*P ≤ 0.05; Means ± S.E.)
Experiment 7: Plasma testosterone levels were unaffected by DPN, PPT, EQ or RES
Plasma testosterone levels (Table 2) were measured in all groups and plasma DPN levels were measured in the DPN and OIL groups only (Table 2). There was no main effect of treatment on plasma testosterone levels. Testosterone was highest in the RES males and lowest in the DPN males, nut neither of these two groups was statistically different from the OIL treated control group. Plasma DPN levels averaged 40 ng/mL in the DPN treated group (Table 2).
Table 2.
Plasma testosterone and DPN levels following behavioral testing
| Treatment | Testosterone (ng/ml) | DPN (ng/ml) |
|---|---|---|
| Control | 5.23 ± 1.6 | 0.44 ± 0.12 |
| 1 mg/kg PPT | 6.34 ± 1.52 | N/A |
| 1 mg/kg DPN | 4.94 ± 1.5 | 41.55 ± 8.72 |
| 20 mg/kg EQ | 3.37 ± 1.0 | N/A |
| 20 mg/kg RES | 7.54 ± 1.70† | N/A |
None of the treatments produced serum testosterone levels that were significantly different from the control group. Plasma testosterone was lowest in the EQ group and highest in the RES group. DPN levels, obtained from plasma collected within the first two hours of exposure, were appreciable in the DPN treated group but below the limit of detection, and therefore effectively zero, in the control group. (Means ± S.E.)
Discussion
The results from the present study demonstrate that DPN can be successfully administered either orally or by sc injection. Once administered, plasma DPN levels peak rapidly and decline to near the limit of detection within 3–4 hours. Plasma testosterone levels are not significantly affected by DPN administration or phytoestrogen exposure relative to control animals. The anxiolytic effect of DPN, previously reported in gonadectomized animals (Koehler et al., 2005; Lund et al., 2005; Walf and Frye, 2006), was not observed in the gonadally intact males of the present study. The phytoestrogens RES and EQ also failed to affect anxiety behavior. These data do not support the hypothesis that ERϐ selective agonists or phytoestrogen rich supplements have the potential to improve mood in humans.
Since human exposure to most endocrine active compounds, including birth control pills, health food supplements, and endocrine disruptors derived from plastics and other products, occurs orally, it is often advantageous to use an oral route of administration in animal studies designed to model the effects of these compounds in humans. Here we have shown that oral administration of 3 mg/kg DPN produced appreciable plasma DPN levels within the first two hours after administration, demonstrating that this is a viable method of delivery. However our data suggest that to produce plasma DPN levels that are roughly equivalent, when given orally, the dose must be considerably higher than when administered by injection. Plasma levels within the first 60 minutes after ingestion of the 3 mg/kg dose were approximately 40 times lower than those observed in animals given 1 mg/kg by injection. It is possible that oral administration of DPN produces different metabolites than if DPN is given by sc injection. A thorough analysis of how DPN is metabolized following each route of exposure, and the potential for those metabolites to interact with ERs is crucial but beyond the scope of the present study. It is also important to note that plasma DPN levels may not be directly indicative of target tissue levels. It remains to be determined how much DPN, following either sc injection or oral administration, reaches the brain, the adrenal glands, the prostate, or other ERϐ rich tissues. Prior studies have found that many phytoestrogens are found at higher concentrations in the prostate (Morton et al., 1997) and semen (Dehennin et al., 1982) than in plasma suggesting that it is possible for ERϐ agonists to become sequestered in reproductive organs. DPN, or one of its metabolites, may also be found at higher levels in ER rich tissues such as the brain, a possibility that warrants further investigation. Collectively our data suggest that plasma DPN levels do not peak as dramatically when given orally (compared to sc injection), and produce plasma DPN levels, within the first 1–3 hours of administration, that are reasonably close to plasma androgen levels. This lower and more stable level of exposure may prove to be more useful than sc injection for some experimental designs.
The rapid depletion of plasma DPN does not necessarily indicate a rapid decline in DPN activity. For example, sc injection of 17ϐ-estradiol has previously been shown to produce a similar spike in blood levels followed by precipitous decline but, because it can be retained by estrogen receptors, 17ϐ-estradiol remains active for hours after it is no longer detectable in plasma (Blaustein et al., 1979). Thus, the physiological effects of 17ϐ-estradiol administration may persist for hours to days. Lund and colleagues, using an in vitro binding approach incorporating differential centrifugation to separate bound and unbound receptors, estimated that DPN occupies neural ERs with a half life of about 8 hours (Lund et al., 2005). In addition, Walf and colleagues have reported significant anxiolytic effects of DPN in ovariectomized female rats 48 hours after sc injection (Walf and Frye, 2005; Walf et al., 2004). Collectively these studies suggest that DPN can be sequestered by ERϐ and influence cellular physiology and behavior long after plasma levels have declined. These data also support the possibility that DPN concentrations in ER rich tissues including, perhaps, the brain, may be significantly higher than plasma levels.
While ERa is known to be crucial for reproductive behavior (Hewitt and Korach, 2003; Kudwa et al., 2006; Rissman et al., 1999; Walf and Frye, 2006) ERϐ is thought to play a more significant role in the regulation of non-reproductive behaviors including anxiety and depression. This hypothesis stems from prior studies demonstrating an anxiolytic effect of DPN in gonadectomized rodents of both sexes but a null or opposite effect of the ERa agonist PPT (Lund et al., 2005; Walf and Frye, 2005; Walf et al., 2004). In the present experiment, anxiety related behaviors were not significantly affected by DPN, at any of the doses used, in either testing apparatus. Time in the open arms of the plus maze appeared to increase with dose in Experiment 4 (Fig. 2) but the effect was not statistically significant and was not accompanied by an increased number of open arm entries. A few of the animals, particularly in the 2 mg/kg DPN group, “froze” when they entered an open arm and remained still rather than exploring the arm. This behavior contributed to the higher mean time spent on the open arm in the 2 mg/kg DPN group compared to the OIL group. This type of behavior suggests that exploratory behavior may be suppressed by DPN and is consistent with an anxious reluctance to explore the open arms. Plus maze behavior was similar in animals given 1 mg/kg DPN. They made significantly fewer open arm entries than the oil treated controls, PPT, or phytoestrogen exposed animals (Fig. 4A). However, this effect was largely attributable to reduced overall activity in the maze (Fig. 4C), an observation which supports the supposition that DPN can impact exploratory, locomotor behavior. The proportion of open arm entries relative to the total number of entries, a measure which accounts for reduced overall activity, was not significantly lower in the 1 mg/kg DPN animals compared to the other groups (Fig. 4D). Animals given 1 mg/kg DPN did not make fewer entries into the light side of the light/dark box, but generally retreated quickly and thus spent less time there than the control animals. Collectively, the data from all of the tests suggest that DPN may suppress exploratory behavior in gonadally intact males and may mildly, but certainly not significantly, enhance anxiogenic behavior. This outcome is not consistent with the anxiolytic effect observed in gonadectomized males.
The gonadal status of the males, and therefore the presence of endogenous gonadal hormones, is likely primarily responsible for the failure of DPN to have an anxiolytic effect in either testing apparatus in the present study. However, plasma testosterone levels were not significantly affected by DPN demonstrating that disruption of circulating androgens is not the mechanism by which the anxiolytic potential of DPN is counteracted. Modulation of hormone receptors within the brain, particularly estrogen receptors, may have effectively muted the response, a possibility we are currently exploring. Factors other than gonadal status may also have contributed to the absence of an anxiolytic effect of DPN. The doses used for the present experiments may not be appropriate to generate an effect in gonadally intact animals, even though all three doses used are consistent with what has previously proven to be effective in gonadectomized males (Choleris et al., 2008; Lund et al., 2005). Interestingly, if these doses were insufficient and a higher (or lower) dose of DPN would enhance the observed response, our data suggest that the effect is likely to be anxiogenic and therefore opposite to what has previously been observed in gonadectomized males. This effect would be consistent with the anxiogenic effect of a soy rich diet we and others have observed in gonadally intact males of multiple species (Hartley et al., 2003; Moore et al., 2004; Patisaul et al., 2005; Simon et al., 2004) and suggest that ERϐ plays a mechanistic role.
We hypothesized that the anxiogenic effect of soy consumption we have observed previously (Patisaul et al., 2005) might largely be attributable to EQ because EQ is a metabolite of the soy isoflavone daidzein (Setchell et al., 2003a). Compared to other species, including humans, rats are highly efficient at generating EQ. In the present study, EQ did not affect anxiety related behavior at either the low (3 mg/kg bw) or high (20 mg/kg bw) dose and therefore the data do not support this hypothesis. It is possible that longer exposure periods are needed to produce an appreciable effect. Animals were exposed for 3 days in the present study but 7 days in our prior study. Lephart and colleagues have also reported a dose dependent anxiolytic effect of a phytoestrogen rich diet following either a life long exposure beginning prenatally or consumption for 250 days beginning on post natal day 50 (Lephart et al., 2004). Collectively these studies suggest that soy phytoestrogens may be anxiogenic following short term exposures in adulthood, but anxiolytic when consumed over a longer period of time. It remains to be seen whether or not EQ specifically plays a mechanistic role in this effect. It is also possible that, by using a racemic mixture of EQ for the present studies, a potentially significant effect of EQ was diluted. It is now well accepted that s-equol is the bioactive enantiomeric form of EQ, and the only form produced by human intestinal bacterial flora (Setchell et al., 2005). For the present study, a racemic mixture was used because, at the time, purified s-equol was prohibitively expensive and difficult to obtain.
In contrast to EQ and other soy-based phytoestrogens, very little is known about how RES affects steroid mediated, non-reproductive behaviors. A recent study has shown that a single, 100 mg/kg bw, dose of RES given by interaperitoneal injection, reduced anxiety in immature rats following a traumatic brain injury (Sonmez et al., 2007). The mechanism by which this occurs is unknown but may result from the antioxidant properties of RES rather than its effects through ERs (Signorelli and Ghidoni, 2005). We found no effect of RES on anxiety at either the low (3 mg/kg bw) or high (20 mg/kg bw) dose demonstrating that RES is unlikely to modify anxiety-related behavior in the gonadally intact, uninjured animal.
Interestingly, testosterone levels were lowest in the EQ males and highest in the RES males, although neither was significantly different from the control males. EQ has previously been shown to bind 5 alpha-dihydrotestosterone (DHT) in circulation and possibly within cells but not the androgen receptor or ERa (Lund et al., 2004). The decreased plasma testosterone levels observed in the present study may result from this combined effect of DHT sequestration and ERϐ activation. Elevated plasma testosterone following exposure to RES has been observed previously (Juan et al., 2005; Shin et al., 2008) and hypothesized to result from the increased secretion of gonadotrophins in response to interactions with ERs, therefore leading to elevated testosterone levels.
Compounds selective for ERϐ such as DPN have proven to be valuable tools for exploring the role of ERϐ in the organization and activation of estrogen mediated physiology and behavior. However, the feasibility of using ERϐ agonists like DPN and the phytoestrogens to treat disease requires that they function as needed in a gonadally intact individual without generating significant side effects. It would also be particularly useful for the compound to survive oral administration. Here we have shown that while oral administration of DPN is a viable option, its potential to significantly influence anxiety in an otherwise normal animal is likely minimal at the doses tested. Similarly, the phytoestrogens EQ and RES are not likely to produce an appreciably effect on mood in the male. A Wyeth research group has recently hypothesized that, in the intact animal, the endogenous function of ERϐ can only become apparent when the animal is stressed, ill, or otherwise compromised (Harris, 2007; Jelinsky et al., 2003). If true, then it may take a mitigating factor to reveal a significant anxiolytic effect of DPN and other ERϐ agonists in the gonadally intact animal. Aging, a process that is accompanied by declining hormone levels, may be one of those factors and it is possible that a significant effect of DPN might be seen in older males. The potential for ERϐ agonists to affect anxiety in a post-menopausal female, a time when steroid hormones are low, also remains to be evaluated and would also be of significant clinical interest.
Acknowledgments
The authors are grateful to Mike Adams of Wake Forest University for supplying the equol and Gail Mahnken for conducting the HPLC analysis. We also thank BJ Welker and Linda Hester for their assistance with animal husbandry as well as the students and staff members of the College of Agricultural Engineering at NCSU for constructing the light/dark boxes. This work was supported by NIEHS grant R01 ES016001 to H.B. Patisaul and the College of Agriculture and Life Sciences at NCSU. Work on this project by K.T. Burke and R.E. Hinkle was conducted as part of their undergraduate honors thesis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Axelson M, Kirk DN, Farrant RD, Cooley G, Lawson AM, Setchell KD. The identification of the weak oestrogen equol [7-hydroxy-3-(4′-hydroxyphenyl)chroman] in human urine. Biochem J. 1982;201:353–7. doi: 10.1042/bj2010353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitran D, Kellogg CK, Hilvers RJ. Treatment with an anabolic-androgenic steroid affects anxiety-related behavior and alters the sensitivity of cortical GABAA receptors in the rat. Horm Behav. 1993;27:568–83. doi: 10.1006/hbeh.1993.1041. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Dudley SD, Gray JM, Roy EJ, Wade GN. Long-term retention of estradiol by brain cell nuclei and female rat sexual behavior. Brain Res. 1979;173:355–9. doi: 10.1016/0006-8993(79)90637-1. [DOI] [PubMed] [Google Scholar]
- Boettger-Tong H, Murthy L, Chiappetta C, Kirkland JL, Goodwin B, Adlercreutz H, Stancel GM, Mäkelä S. A case of a laboratory animal feed with high estrogenic activity and its impact on in vivo responses to exogenously administered estrogens. Environmental Health Perspectives. 1998;106:369–373. doi: 10.1289/ehp.98106369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers JL, Tyulmenkov VV, Jernigan SC, Klinge CM. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors α and ϐ. Endocrinology. 2000;141:3657–3667. doi: 10.1210/endo.141.10.7721. [DOI] [PubMed] [Google Scholar]
- Choleris E, Clipperton AE, Phan A, Kavaliers M. Estrogen receptor beta agonists in neurobehavioral investigations. Curr Opin Investig Drugs. 2008;9:760–73. [PubMed] [Google Scholar]
- Dehennin L, Reiffsteck A, Jondet M, Thibier M. Identification and quantitative estimation of a lignan in human and bovine semen. J Reprod Fertil. 1982;66:305–9. doi: 10.1530/jrf.0.0660305. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Martinez-Mota L. Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrinology. 2005;30:762–70. doi: 10.1016/j.psyneuen.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Frasor J, Barnett DH, Danes JM, Hess R, Parlow AF, Katzenellenbogen BS. Response-specific and ligand dose-dependent modulation of estrogen receptor (ER) alpha activity by ERbeta in the uterus. Endocrinology. 2003;144:3159–66. doi: 10.1210/en.2002-0143. [DOI] [PubMed] [Google Scholar]
- Fried G, Andersson E, Csoregh L, Enmark E, Gustafsson JA, Aanesen A, Osterlund C. Estrogen receptor beta is expressed in human embryonic brain cells and is regulated by 17beta-estradiol. Eur J Neurosci. 2004;20:2345–54. doi: 10.1111/j.1460-9568.2004.03693.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Seliga AM. Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cogn Affect Behav Neurosci. 2001;1:371–81. doi: 10.3758/cabn.1.4.371. [DOI] [PubMed] [Google Scholar]
- Greene G, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986;231:1150–1153. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: Activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5alpha-androstane-3beta,17beta-diol. Horm Behav. 2007 doi: 10.1016/j.yhbeh.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley SL, McBlane JW. An assessment of the elevated X-maze for studying anxiety and anxiety-modulating drugs. J Pharmacol Toxicol Methods. 1993;29:129–38. doi: 10.1016/1056-8719(93)90063-k. [DOI] [PubMed] [Google Scholar]
- Harris HA. Estrogen receptor-beta: recent lessons from in vivo studies. Mol Endocrinol. 2007;21:1–13. doi: 10.1210/me.2005-0459. [DOI] [PubMed] [Google Scholar]
- Harris HA, Katzenellenbogen JA, Katzenellenbogen BS. Characterization of the biological roles of the estrogen receptors, ERalpha and ERbeta, in estrogen target tissues in vivo through the use of an ERalpha-selective ligand. Endocrinology. 2002;143:4172–7. doi: 10.1210/en.2002-220403. [DOI] [PubMed] [Google Scholar]
- Hartley DE, Edwards JE, Spiller CE, Alom N, Tucci S, Seth P, Forsling ML, File SE. The soya isoflavone content of rat diet can increase anxiety and stress hormone release in the male rat. Psychopharmacology (Berl) 2003;167:46–53. doi: 10.1007/s00213-002-1369-7. [DOI] [PubMed] [Google Scholar]
- Hays LE, Carpenter CD, Petersen SL. Evidence that GABAergic neurons in the preoptic area of the rat brain are targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin during development. Environ Health Perspect. 2002;110 Suppl 3:369–76. doi: 10.1289/ehp.02110s3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SC, Korach KS. Oestrogen receptor knockout mice: roles for oestrogen receptors alpha and beta in reproductive tissues. Reproduction. 2003;125:143–9. doi: 10.1530/rep.0.1250143. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–6. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Imamov O, Yakimchuk K, Morani A, Schwend T, Wada-Hiraike O, Razumov S, Warner M, Gustafsson JA. Estrogen receptor beta-deficient female mice develop a bladder phenotype resembling human interstitial cystitis. Proc Natl Acad Sci U S A. 2007;104:9806–9. doi: 10.1073/pnas.0703410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinsky SA, Harris HA, Brown EL, Flanagan K, Zhang X, Tunkey C, Lai K, Lane MV, Simcoe DK, Evans MJ. Global transcription profiling of estrogen activity: estrogen receptor alpha regulates gene expression in the kidney. Endocrinology. 2003;144:701–10. doi: 10.1210/en.2002-220728. [DOI] [PubMed] [Google Scholar]
- Juan ME, Gonzalez-Pons E, Munuera T, Ballester J, Rodriguez-Gil JE, Planas JM. trans-Resveratrol, a natural antioxidant from grapes, increases sperm output in healthy rats. J Nutr. 2005;135:757–60. doi: 10.1093/jn/135.4.757. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen BS, Choi I, Delage-Mourroux R, Ediger TR, Martini PG, Montano M, Sun J, Weis K, Katzenellenbogen JA. Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology. J Steroid Biochem Mol Biol. 2000;74:279–85. doi: 10.1016/s0960-0760(00)00104-7. [DOI] [PubMed] [Google Scholar]
- Koehler KF, Helguero LA, Haldosen LA, Warner M, Gustafsson JA. Reflections on the discovery and significance of estrogen receptor beta. Endocr Rev. 2005;26:465–78. doi: 10.1210/er.2004-0027. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Michopoulos V, Gatewood JD, Rissman EF. Roles of estrogen receptors alpha and beta in differentiation of mouse sexual behavior. Neuroscience. 2006;138:921–8. doi: 10.1016/j.neuroscience.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Häggblad J, Hilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and ϐ. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Kuiper GGJM, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GGJM, Lemmen JG, Carlsson B, Corton JC, Safe SH, Van Der Saag PT, Van Der Berg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor ϐ. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Lemmen JG, Broekhof JL, Kuiper GG, Gustafsson JA, van der Saag PT, van der Burg B. Expression of estrogen receptor alpha and beta during mouse embryogenesis. Mech Dev. 1999;81:163–7. doi: 10.1016/s0925-4773(98)00223-8. [DOI] [PubMed] [Google Scholar]
- Lephart ED, Setchell KD, Handa RJ, Lund TD. Behavioral effects of endocrine-disrupting substances: phytoestrogens. Ilar J. 2004;45:443–54. doi: 10.1093/ilar.45.4.443. [DOI] [PubMed] [Google Scholar]
- Lephart ED, Thompson JM, Setchell KD, Adlercreutz H, Weber KS. Phytoestrogens decrease brain calcium-binding proteins but do not alter hypothalamic androgen metabolizing enzymes in adult male rats. Brain Res. 2000;859:123–31. doi: 10.1016/s0006-8993(00)01968-5. [DOI] [PubMed] [Google Scholar]
- Levenson AS, Gehm BD, Pearce ST, Horiguchi J, Simons LA, Ward JE, 3rd, Jameson JL, Jordan VC. Resveratrol acts as an estrogen receptor (ER) agonist in breast cancer cells stably transfected with ER alpha. Int J Cancer. 2003;104:587–96. doi: 10.1002/ijc.10992. [DOI] [PubMed] [Google Scholar]
- Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci. 2006;26:1448–56. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TD, Munson DJ, Haldy ME, Setchell KD, Lephart ED, Handa RJ. Equol is a novel anti-androgen that inhibits prostate growth and hormone feedback. Biol Reprod. 2004;70:1188–95. doi: 10.1095/biolreprod.103.023713. [DOI] [PubMed] [Google Scholar]
- Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–51. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Moore TO, Karom M, O’Farrell L. The neurobehavioral effects of phytoestrogens in male Syrian hamsters. Brain Res. 2004;1016:102–10. doi: 10.1016/j.brainres.2004.04.073. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Pfaff DW. Effects of estrogen on activity and fear-related behaviors in mice. Horm Behav. 2001;40:472–82. doi: 10.1006/hbeh.2001.1716. [DOI] [PubMed] [Google Scholar]
- Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, Ogawa S, Inoue S, Muramatsu M, Masamune Y. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull. 2001;24:351–6. doi: 10.1248/bpb.24.351. [DOI] [PubMed] [Google Scholar]
- Morton MS, Chan PSF, Cheng C, Blacklock N, Matos-Ferreira A, Abranches-Monteiro L, Correia R, Lloyd S, Griffiths K. Lignans and isoflavonoids in plasma and prostatic fluid in men: samples from Portugal, Hong Kong, and the United States. The Prostate. 1997;32:122–128. doi: 10.1002/(sici)1097-0045(19970701)32:2<122::aid-pros7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Witt MR, Gustafsson JA. Estrogen action in mood and neurodegenerative disorders: estrogenic compounds with selective properties-the next generation of therapeutics. Endocrine. 2005;28:235–42. doi: 10.1385/ENDO:28:3:235. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Blum A, Luskin JR, Wilson ME. Dietary soy supplements produce opposite effects on anxiety in intact male and female rats in the elevated plus-maze. Behav Neurosci. 2005;119:587–94. doi: 10.1037/0735-7044.119.2.587. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, Polston EK. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol Teratol. 2006;28:111–8. doi: 10.1016/j.ntt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24:525–9. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- Perez SE, Chen EY, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain Res Dev Brain Res. 2003;145:117–39. doi: 10.1016/s0165-3806(03)00223-2. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. ERbeta-selective SERMs produce mnemonic-enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiol Learn Mem. 2006;85:183–91. doi: 10.1016/j.nlm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Wersinger SR, Fugger HN, Foster TC. Sex with knockout models: behavioral studies of estrogen receptor alpha. Brain Res. 1999;835:80–90. doi: 10.1016/s0006-8993(99)01452-3. [DOI] [PubMed] [Google Scholar]
- Sanchez-Criado JE, Martin De Las Mulas J, Bellido C, Tena-Sempere M, Aguilar R, Blanco A. Biological role of pituitary estrogen receptors ERalpha and ERbeta on progesterone receptor expression and action and on gonadotropin and prolactin secretion in the rat. Neuroendocrinology. 2004;79:247–58. doi: 10.1159/000079100. [DOI] [PubMed] [Google Scholar]
- Schreihofer DA. Transcriptional regulation by phytoestrogens in neuronal cell lines. Mol Cell Endocrinol. 2005;231:13–22. doi: 10.1016/j.mce.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Setchell K, Welsh M, Lim C. HPLC analysis of phytoestrogens in soy preparations using ultraviolet, electrochemical and thermospray mass spectrometric detection. Journal of Chromatography A. 1986;368:315–323. doi: 10.1016/s0021-9673(01)94608-4. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirschner AS, Cassidy A, Heubi JE. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131:1362S–75S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Brown NM, Desai PB, Zimmer-Nechimias L, Wolfe B, Jakate AS, Creutzinger V, Heubi JE. Bioavailability, disposition, and dose-response effects of soy isoflavones when consumed by healthy women at physiologically typical dietary intakes. J Nutr. 2003a;133:1027–35. doi: 10.1093/jn/133.4.1027. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–84. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, Wolfe BE, Nechemias-Zimmer L, Brown NM, Lund TD, Handa RJ, Heubi JE. S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr. 2005;81:1072–9. doi: 10.1093/ajcn/81.5.1072. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Cole SJ. Variations in isoflavone levels in soy foods and soy protein isolates and issues related to isoflavone databases and food labeling. J Agric Food Chem. 2003;51:4146–55. doi: 10.1021/jf026199b. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Faughnan MS, Avades T, Zimmer-Nechemias L, Brown NM, Wolfe BE, Brashear WT, Desai P, Oldfield MF, Botting NP, Cassidy A. Comparing the pharmacokinetics of daidzein and genistein with the use of 13C-labeled tracers in premenopausal women. Am J Clin Nutr. 2003b;77:411–9. doi: 10.1093/ajcn/77.2.411. [DOI] [PubMed] [Google Scholar]
- Shin S, Jeon JH, Park D, Jang MJ, Choi JH, Choi BH, Joo SS, Nahm SS, Kim JC, Kim YB. trans-Resveratrol relaxes the corpus cavernosum ex vivo and enhances testosterone levels and sperm quality in vivo. Arch Pharm Res. 2008;31:83–7. doi: 10.1007/s12272-008-1124-7. [DOI] [PubMed] [Google Scholar]
- Shughrue P, Lane M, Scrimo P, Merchenthaler I. Comparative distribution of estrogen receptor-α (ERα) and ϐ (ERϐ) mRNA in the rat pituitary, gonad, and reproductive tract. Steroids. 1998;63:498–504. doi: 10.1016/s0039-128x(98)00054-3. [DOI] [PubMed] [Google Scholar]
- Signorelli P, Ghidoni R. Resveratrol as an anticancer nutrient: molecular basis, open questions and promises. J Nutr Biochem. 2005;16:449–66. doi: 10.1016/j.jnutbio.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Simon NG, Kaplan JR, Hu S, Register TC, Adams MR. Increased aggressive behavior and decreased affiliative behavior in adult male monkeys after long-term consumption of diets rich in soy protein and isoflavones. Horm Behav. 2004;45:278–84. doi: 10.1016/j.yhbeh.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Sonmez U, Sonmez A, Erbil G, Tekmen I, Baykara B. Neuroprotective effects of resveratrol against traumatic brain injury in immature rats. Neurosci Lett. 2007;420:133–7. doi: 10.1016/j.neulet.2007.04.070. [DOI] [PubMed] [Google Scholar]
- Speroff L. Alternative therapies for postmenopausal women. Int J Fertil Womens Med. 2005;50:101–14. [PubMed] [Google Scholar]
- Thigpen JE, Setchell KDR, Ahlmark KB, Locklear J, Spahr T, Caviness GF, Goelz MF, Haseman JK, Newbold RR, Forsythe DB. Phytoestrogen content of purified open- and closed-formula laboratory animal diets. Laboratory Animal Sciences. 1999;49:530–536. [PubMed] [Google Scholar]
- Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. ERbeta-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005;30:1598–609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Antidepressant effects of ERbeta-selective estrogen receptor modulators in the forced swim test. Pharmacol Biochem Behav. 2004;78:523–9. doi: 10.1016/j.pbb.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Zhang JQ, Cai WQ, Zhou de S, Su BY. Distribution and differences of estrogen receptor beta immunoreactivity in the brain of adult male and female rats. Brain Res. 2002;935:73–80. doi: 10.1016/s0006-8993(02)02460-5. [DOI] [PubMed] [Google Scholar]