Abstract
Parkinson’s disease is characterized by the progression of the disease from the early stages where it still has little functional consequence for afflicted patients, to an advanced stage disease with large consequences in terms of function, quality of life and individual and societal costs. Motor fluctuations and symptoms of levodopa overdosage may occur in parallel with increasing Parkinsonian symptoms. This leads to a narrower therapeutic window which causes problems with traditional oral medication. Various ways of optimizing oral treatment should be tried but often have limited effects. In addition to the previous alternatives of neurosurgery (especially deep brain stimulation of the subthalamic nuclei) and continuous apomorphine treatment there is now also the alternative of continuous enteral levodopa administration via a trans-abdominal tube. The effect of the treatment may be tested individually via naso-duodenal administration before a decision is made whether to continue with permanent treatment. In the present article, the challenges to treatment of Parkinson’s disease in these phases are described as well as the various treatment alternatives available. Focus is mainly on the clinical studies of continuous levodopa infusion therapies, especially enteral administration of levodopa/carbidopa gel. The place of enteral levodopa/carbidopa gel treatment among the other treatment methods is also discussed.
Keywords: Parkinson’s disease, levodopa, carbidopa, levodopa/carbidopa gel
Introduction – Parkinson’s disease and its traditional treatment
Parkinson’s disease, as defined by the classical triad of brady-/a-kinesia, rigidity, and tremor (Hughes et al 1992), has a prevalence in Europe of approximately 108–257 per 100,000 according to a recent review (von Campenhausen et al 2005). The majority of Parkinson patients have an early phase disease (Hoehn and Yahr stages I–II) with symptoms that are, in general, well controlled on standard oral medications. Their disease, consequently, causes little handicap and does not affect their quality of life to a great extent. The mainstay of treatment during these early phases has, ever since its discovery, been oral treatment with levodopa (Olanow et al 2004). Initially, very high doses of levodopa were administered with good symptomatic relief but with problematic side-effects due to the need to administer high doses to overcome peripheral degradation. Later on, even more disturbing long-term side-effects developed with extreme dyskinesisas being induced in most patients. The situation was improved with the discovery and addition of peripheral decarboxylase inhibitors. Since then, a number of modifications have been made to increase the pharmacological bioavailability of administered levodopa, thus precluding the need to administer the very high doses. Even so, involuntary movements develop over time after levodopa treatment has been started. A strong trend for developing alternative treatment strategies not associated with these problems has been the result, and consequently the dopaminergic agonists have recently received much attention as an alternative to levodopa. Agonists are now often successfully used in monotherapy or with selegiline during the early phases of Parkinson’s disease. However, for most Parkinson patients the disease progresses over time through these more easily managed phases to a complicated disease. This is especially notable for patients in whom the disease first appears early on in life and who, as a result, have many years with the disease. These patients have severe handicaps, reduced quality of life and the disease severely affects their ability to work and function independently in society (Dodel et al 2001). In addition to the initial symptoms of Parkinsonism, this phase is characterized by severe problems involving the medication used for symptom relief. Such medication-related problems which are largely absent during the early phases of illness, strongly affect the ability of the treating neurologist to control optimally the symptoms of the patient. The problems both affect the motor functioning of the patient with a more narrow optimal dosage window defined by overdosage and underdosage symptoms, as well as cognitive and psychiatric functioning. In addition, other non-motor symptoms such as pain and psychiatric problems are often accentuated during these later phases.
During these phases the therapeutic alternatives are few and a careful evaluation and monitoring of the patients is required by neurologists with experience in treating such patients. Patients need to be considered individually and treatment regimes need to be individually tailored. Therefore, it is important to have a range of different treatment alternatives to choose between. The present review focuses on one such alternative, that of continuous levodopa administration and compares this strategy with other treatments for advanced fluctuating Parkinson’s disease patients.
Advanced Parkinson’s disease (PD)
The end of the first, usually reasonably uncomplicated phases of PD, is often heralded by a change in the duration of effect of each individual levodopa dose. The patient experiences reduced functioning before he has taken the next dose of medication. This phenomenon is commonly described as “end of dose wearing off ”. Initially, wearing off symptoms come gradually and in a predictable pattern which can usually be alleviated by taking the next dose somewhat earlier, ie, with higher number of doses per day as a result. Later on (though in some patients this might even appear earlier) the phases of reduced function may appear more suddenly and sometimes in a more unpredictable pattern, not clearly related to time of medication. These sudden “off-phases” naturally confer upon the patient a feeling of insecurity since he or she can never be sure to be able to fulfil tasks, social obligations, and so on, with a good or at least predictable, functional level. The patient also feels an increased sensitivity to external factors sometimes beyond the influence of the patient, such as stress, the need for unforeseen physical exertion, and varying times and compositions of meals. All these factors may drastically affect the degree or duration of symptomatic relief that the patient experiences from the oral medication. A typical example is the patient who goes out shopping without any problems initially, only to find himself suddenly frozen in place in the queue of a busy shop, unable to pay, move or even to explain his problem to the shop attendants. It can easily be understood that such a situation must strongly influence the subjectively experienced quality of life (Dodel et al 2001).
Another “new” problem which may appear in patients at this stage is the appearance of motor symptoms of over dosage. Initially this may be only an internal “restlessness” subjectively experienced by the patient. However, for some patients this internal restlessness may well be particularly noticeable as the more open involuntary dyskinetic movements that may also appear. The dyskinesias most commonly appear first on the expected maximum of the serum levodopa curve, so called “peak-dose dyskinesias” but may also appear at more or less fixed levels of the serum curve as it increases or decreases (“bi-phasic dyskinesia”) or even in a more random erratic fashion. Dyskinesias also tend to be strongly affected by stress and are often worsened in situations when the patient feels under close observation. Initially and with limited amounts of involuntary movements, they do not appear to be such an additional burden to the patient as one might expect. The patients, in our experience, certainly appear keener on avoiding under-functioning than on worrying about involuntary movements.
Too little attention has probably, until very recently, been paid to the non-motor symptoms seen in advanced Parkinson’s disease, possibly with the exception of hallucinations. Hallucinations have long been recognized as a symptom of dopaminergic over activity in advanced patients, and often develop in parallel with the above described motor symptoms of over dosage. A recent review by Chaudhuri and co-authors summarizes the main non-motor symptoms (Chaudhuri et al 2006a). These include the following: depression and anxiety, hallucinations/psychsis, cognitive impairment, constipation, sexual dysfunction, dysautonomic symptoms such as orthostatism, pain, and reduced olfaction. Using a new 30-item non-motor symptom screening questionnaire (the “NMSQuest”), Chaudhuri and co-workers reported significantly higher scores among PD patients for dribbling, impaired taste/smell, impaired swallowing, constipation, urinary urgency, weight loss, forgetfulness, sadness, reduced concentration, hallucinations, anxiety, sexual dysfunction, falling, daytime sleepiness, vivid dreams, and sweating (Chaudhuri et al 2006b). There was a significant association with Hoehn and Yahr disease stage and duration showing the increased burden of these symptoms in advanced PD. Such non-motor symptoms are also important in determining the quality of life burden of the disease (Schrag et al 2000; Global Parkinson’s disease survey steering committee 2002) and nursing home placement (Aarsland et al 2000). There is so far insufficient evidence regarding the effect of dopaminergic treatment both on the development and on the alleviation of non-motor symptoms. Certainly any treatment for advanced phase Parkinson’s disease should be considered also in this light. We find that especially clear over-dose associated symptoms such as hallucinations and orthostatism and off-related symptoms such as pain and off-period anxiety and depression are important factors to consider when treating advanced phase patients. In addition, long-term development of non-motor symptoms such as cognitive and psychiatric functioning must be evaluated for all long-term treatments.
Strategies for the prevention of development of motor complications
During the last decade, new evidence has been accumulating about mechanisms involved especially in the development of fluctuations. It has been known for a long time that fluctuations develop quickly once levodopa treatment has been initiated (Olanow et al 2001). This observation has previously been taken to suggest that levodopa in itself may be toxic, something that has been demonstrated clearly in experimental systems in several studies (reviewed by Olanow et al 2004). Because of this, many authorities and experts advocate a delayed start of levodopa treatment especially for younger Parkinson patients, thus hoping to delay the start of the count-down to fluctuations and dyskinesia development (Olanow et al 2001). Thus dopaminergic agonists have received much attention and are recommended for treatment of early Parkinson’s disease (Olanow et al 2001). The agonists are not dependent on metabolic change in the remaining dopaminergc cells in the brain but are assumed to act directly on dopaminergic receptors. Thus, any possible toxicity elicited through metabolism of levodopa is avoided. It must be kept in mind, however, that once the patient has developed symptoms strong enough to cause functional problems, agonists are often not enough. There is no other treatment more effective than levodopa for controlling these symptoms and treatment should then not be withheld due to an exaggerated fear of levodopa toxicity (Olanow et al 2004).
There is now considerable evidence that, rather than through general substance toxicity, levodopa may cause the development of fluctuations through the intermittent or pulsatile stimulation of dopaminergic systems which is a consequence of its short half-life combined with intermittent oral dosing schedules (Olanow et al 2004; Stocchi 2005). It has been demonstrated that different strategies for smoothing out the levodopa stimulation pattern by the early use of agonists, as mentioned above as well as with inhibitors of levodopa degradation (catechol-O-methyl transferase or COMT inhibitors), all delay the development of these complications (Olanow et al 2001; Stocchi and Olanow 2004). It thus seems that it is the administration pattern rather than the drug itself that is associated with toxicity. There are so far no studies of patients treated with continuous levodopa already from the early phases of disease.
Alternative treatments for advanced disease
Adapted use of oral medication
Oral medication has been, and continues to be, the main treatment for the majority of patients also in late phase Parkinson’s disease. Standard medication regimes used during earlier phases, eg, levodopa three times daily, are only rarely sufficient. The use of levodopa itself can be modified in various ways to fit better the individual patient. Often the dosage in terms of milligrams levodopa per day will be a compromise between a dose that is sufficient to relieve the patient from the most disabling symptoms of Parkinsonism, and the major overdosage symptoms (especially dyskinesias/hyperkinesias and hallucinations). Patients usually tend to prefer overdosage to underdosage. The distribution of the levodopa intake over the day will often need to be modified with more doses with shorter intervals between doses and the patterns of rest/activity and meals over the day may need to be coordinated with medication times. Sustained release tablets or capsules are often more difficult to control and evaluate than traditional tablets and are rarely used by most neurologists (Widner 2003). However, in some cases, for example where compliance for multi-intake dosing is low, it may be considered as an attempt to smooth out the serum concentration curve somewhat. A perhaps more rational approach is to attempt to reduce the peripheral break-down of administered levodopa further by use of COMT inhibitors such as tolcapone and entacapone either in addition to the previously used levodopa preparations or in combination preparations. This strategy has been shown to increase trough levodopa levels without increasing peak levels, to prolong the effect duration of each levodopa dose, and to improve fluctuations and reduce dyskinesias (Olanow et al 2001).
A slightly different strategy is the addition of other classes of anti-parkinson medications which may even out dopaminergic function. In many cases, the addition of dopaminergic agonists at this stage of disease may be beneficial and serve to reduce fluctuations. However, often the patient has been on agonists already, since the early use of agonists has, in many countries, been recommended as first or second line treatments for early Parkinon’s disease. Thus, for many patients the early use of the agonists may lead to no further benefit when the patient subsequently progresses to advanced, fluctuating disease. Furthermore, as stated above, one of the main non-motor complications of advanced disease is hallucinations and this may be aggravated by agonist addition, precluding their use (Olanow et al 2001). However, if the patient tolerates the possible side-effects and does not develop hallucinations, improvement of fluctuations and functions may be observed. Agonists include cabergoline, bromocriptine, pramipexole, ropinirole, and pergolide. Of these, some are ergotamine derivatives (cabergoline, pergolide) which have a long half-life enabling attainement of a more smooth serum curve. Cabergoline with the longest half-life may be dosed once daily. These are theoretically attractive characteristics in the context of treating fluctuations. However, the ergotamine derivatives have recently been suggested to have severe side-effects (pleuropulmonary and valvular fibrosis) which have not been clearly reported with non-ergot agonists (Horvath et al 2004; van Camp et al 2004; Dhawan et al 2005). The discussion is ongoing as to whether these side-effects are dose dependent and may be prevented by keeping a lower dose, or whether a change to non-ergot agonists should be done for these patients.
Continuous agonist treatment
As opposed to levodopa, some agonists have pharmacological properties that make them suitable for parenteral administration subcutaneously or trans dermally. Apomorphine, one of the oldest dopamine agonists in clinical use (Schwab et al 1951; Frankel et al 1990), has some characteristics (rapid uptake, close to 100% bioavailability after subcutaneous injection and short time delay to effect) that have led to it being utilized as an injectable “rescue drug” for sudden severe off states (Steiger et al 1992). A randomized, double-blind trial has confirmed apomorphine’s effectiveness in reducing “off” manifestations (Dewey et al 2001). The subcutaneous use of apomorphine is, in an evidence-based review by a movement disorder task force, considered to be efficacious in treating motor complications and reducing off times (Goetz et al 2006). The use of easily handled pen injectors similar to insulin pens may in some cases clearly improve the situation for fluctuating patients with such sudden short offs. For patients in whom the off-state in itself is not the problem, but rather the on-off fluctuations in general and dyskinesias are the central problems, the short duration of effect of single injections reduces the utility of apomorphine in this form. Some of these patients may, however, benefit from continuous subcutaneous infusion of the drug, a strategy which in spite of the short half-life of the drug enables a smooth serum curve to be attained (Nicole et al 1993). Modern systems with small pumps similar to the insulin pumps used by diabetics are practical and easy to carry and maintain. The side-effects include dopaminergic and largely dose-dependent ones such as hallucinations and nausea especially at start-up. In addition, the medication may give local reactions at the injection site such as inflammation, pigmentation, and fibrosis which is something that almost all patients develop over time (Stocchi et al 2001). Despite these side-effects, the treatment has been demonstrated to give a lasting improvement of dyskinesias and to reduce off-time significantly (Colzi et al 1998; Stocchi et al 2001; Kanovsky et al 2002). In studies evaluating long-term treatment, no worsening of neuropsychiatric parameters has been described (Di Rosa et al 2003). Dosage of levodopa can be considerably reduced without loss of symptom control but some levodopa usually must be maintained (Colzi et al 1998; Stocchi et al 2001). The fact that operative treatment is not required for continuous apomorphine treatment is an advantage.
Another substance that has been tested for subcutaneous use is the ergot derivative lisuride. This substance also has a short half-life and since its solubility is similar to that of apomorphine and its oral bioavailability is low, the drug is suitable for continuous subcutaneous infusion. Continuous subcutaneous infusion of lisuride gave stable serum concentrations (Krause et al 1988). Infusion over the long term had a good effect on levodopa response with positive effects on the size of the therapeutic window between Parkinsonian motor underfunction and dyskinesia, but psychiatric side-effects were common (Vaamonde et al 1991).
Recently, a new attractive strategy for continuous agonist treatment has been introduced (Pfeiffer 2005). This involves transcutaneous delivery of agonists via a dermal patch or through the application on the skin of a microemulsion of the agonist (apomorphine in this case) which is then covered by an occlusive membrane (Priano et al 2004). Levodopa cannot be used in this form due to solubility problems. So far only transdermal patch treatment with the new agonist rotigotine has reached the stage where it is available for clinical usage. It has been tested both for early monotherapy (The Parkinson study group, 2003; Poewe and Luessi 2005) as well as for late fluctuating Parkinson’s disease (Verhagen Metman et al 2001). Other agonist substances are also under study for possible administration via this route, which include lisuride mentioned above, as one of the most promising (Pfeiffer 2005) as well as the rotigotine-like substance 5-OH-DPAT (Nugroho et al 2005).
If drugs with sufficient symptomatic effect can be easily and practically administered transdermally then this may represent a new possibility for achieving continuous dopaminergic stimulation which may be of benefit also to advanced Parkinson’s patients. At present it is difficult to envisage such treatment for this patient category without parallel levodopa treatment, and further studies are required to evaluate effects on fluctuations and dyskinesias as well as other advanced stage parkinsonian symptoms.
Neurosurgical therapy
In two large recent reviews, therapeutic alternatives for Parkinson’s disease have been considered including therapy alternatives for advanced disease with motor complications and dyskinesias. In the first of these reviews, an evidence-based update on pharmacological and surgical treatments in Parkinson’s disease, unilateral pallidotomy was considered efficacious for treatment of motor complications such as dyskinesias and fluctuations and for use as a symptomatic adjunct to levodopa (Goetz et al 2006). It was regarded as “clinically useful” in terms of practiced implications. The only other neurosurgical technique for treating Parkinson’s disease that was also classified as efficacious and clinically useful was deep brain stimulation of the subthalamic nucleus (DBS-STN) used as a symptomatic adjunct to levodopa (Goetz et al 2006). In an evidence-based review focusing explicitly on treatment of motor fluctuations and dyskinesia, the Quality Standards Subcommittee of the American Academy of Neurology did not include pallidotomy but only DBS of STN, globus pallidus, and thalamus (Pahwa et al 2006). The authors concluded that only DBS-STN was possibly effective in improving motor function, reducing fluctuations, and dyskinesias and reducing drug usage, while all other methods of DBS had insufficient evidence. DBS-STN was recommended as a treatment option for these endpoints based on level C evidence (Pahwa et al 2006). In many countries lesional surgery has largely been replaced by DBS strategies. DBS has been extensively described in a review by Benabid (2003). The effect of the treatment is in most patients very good with lasting improvement of off-medication motor symptoms (Herzog et al 2003; Rodriguez-Oroz et al 2005) and quality of life lasting at least 2–5 years (Lagrange et al 2002) (reviewed by Diamond and Jankovic 2005). In a recent multicenter study reporting results from 69 patients (49 receiving bilateral STN and 20 receiving bilateral globus pallidus stimulation) lasting dramatic improvement especially of off-state severity and length per day were seen with both localizations of the electrodes (Rodriguez-Oroz et al 2005). In this study, levodopa requirement was reduced with STN stimulation but not with pallidal stimulation. In another, larger study of 95 consecutive patients treated with bilateral STN stimulation, there was also a clear improvement of off-medication scores as well as blinded motor scores and quality of life over 12 months (Fraix et al 2006). Levodopa dosage was less than half of pre-operative values after surgery in this study, other studies have reduced levodopa postoperatively slightly less (Esselink et al 2004; Rodriguez-Oroz et al 2005).
Side-effects of deep brain stimulation include cerebral hemorrhage. This has been reported in 1%–3% of cases ((Deuschl et al 2003), with some authors reporting higher levels (Lagrange et al 2002; Fraix et al 2006) but with only a few percent with lasting sequelae. However, some cases have resulted in death, and together with other severe complications such as fatal postoperative pneumonia, a recent randomized trial by Deuschl et al (2006) reported a procedure-related mortality of 2%. Other directly procedure-related adverse effects include infections, improper placement of electrodes, transient seizures, and confusion. The frequencies of such side-effects vary greatly in different reports. Speech problems (mainly dysarthria), gait and balance problems, psychiatric problems such as depressive symptoms including suicides, as well as cognitive decline are other reported side-effects. In long-term studies such side-effects are frequent (Rodriguez-Oroz et al 2005), occurring in the majority of patients. Psychiatric side-effects are reported in 8%–20% of patients (Lagrange et al 2002; Herzog et al 2003). Most of these are temporary but there are also several cases of severe depressions even to the extent of including suicide attempts (Lagrange et al 2002; Burkhard et al 2004). It has been suggested that some of these side-effects may be related to the reduced dose of levodopa after surgery (Benabid 2003; Deuschl et al 2003). In this context it is interesting that pallidal stimulation, which does not enable clear reduction of levodopa after surgery, has fewer of these side-effects and, indeed, seems to have fewer side-effects in general, while possibly being slightly less effective in relieving motor symptoms (Esselink et al 2004; Rodriguez-Oroz et al 2005).
Due to perioperative risk and risk of symptomatic intracranial bleeding, a reasonably strict age limit of 70 years has usually been practiced in the past. However, this has recently been challenged because subgroups of over 70-year-old Parkinson patients have been found to have benefited from the treatment (Russmann et al 2004; Tagliati et al 2005). This issue is also discussed in the AAN review by Pahwa and co-workers where older age and longer duration of disease are concluded to be predictive of a worse outcome after DBS-STN (level C evidence) (Pahwa et al 2006). However, the main conclusion of this review (level B evidence) was that preoperative levodopa response is the best predictive factor for outcome of DBS-STN. Fraix and co-workers also found on-medication functioning to be the best predicitive factor of postoperative motor outcome (Fraix et al 2006)
The total cost of deep brain stimulation has not been addressed in many studies so far. However, Fraix et al (2006) calculated the direct costs before STN operation and up to 1 year afterwards as well as the costs related to the surgery itself (Fraix et al 2006). The 6 month cost decreased from €10087 to €1673 after surgery. Surgery-related costs were €36904 per patient. However, the follow-up hospitalizations and the outpatient controls (total approx €5500 during the first year) were not included in the postoperative costs but were regarded as surgery-related costs. In our experience, these costs continue since patients need regular polyclinical controls often both by a neurosurgical and a neurological department for control and adjustment of stimulator function, battery level and adjustment of concomitant pharmacological therapy. In addition, indirect costs such as employment situation and sick leave were not reported and are therefore difficult to evaluate.
Patients may also have fear of a neurosurgical operation and the importance of this should not be underestimated. Patients should be approached with an understanding of these fears and not with an outright “now or never” ultimatum. This may put undue pressure on patients, later on possibly leading to many more problems than need be in case they feel pressurized into taking an operation and experience side-effects. Proper information from a physician with knowledge of the procedure and with a good relationship with and knowledge of the patient and his or her situation, as well as time, may prevent later problems. How many patients offered the treatment decline this offer has also not, to our knowledge, been reported so far.
Continuous levodopa treatment
Intravenous
In 1975, Shoulson described 7 Parkinson’s patients with severe on/off fluctuations on oral medication who were administered intravenous levodopa continuously. The administration, encouragingly, led to the virtual disappearance of the fluctuations (Shoulson et al 1975). Several small additional trials in the UK and the US supported these findings (Nutt et al 1984; Quinn et al 1984). The efficiency of continuous intravenous treatment in reducing fluctuations led these researchers to propose the development of strategies for continuous treatment with levodopa-analogues for such patients (Quinn et al 1984). Hardie and co-workers performed a meticulous study focussing mainly on fluctuating symptoms such as dyskinesias and off-dose dystonias. They used careful monitoring of oral medication with both serum levodopa concentration measurements and clinical evaluation of patients as well as similar monitoring after i.v. administration and response to the dopamine agonists lisuride and apomorphine (Hardie et al 1984). Again it was demonstrated that continuous intravenous administration gave more stable serum curves and that this clearly reduced fluctuations. The pharmacochemical characteristics of levodopa itself meant that it could only be administered intravenously or orally. Especially the low aqeous solubility of levodopa made it impossible to consider subcutaneous administration since considerable volumes would have been required. In the intravenous studies volumes over 2 L of solute (saline or dextrose and water) per day with less than 1 mg/mL of levodopa were often administered, making this administration very cumbersome. The acidity of the infusion substance further made thrombophlebitis a distinct possibility, and to reduce this risk central venous access was often utilized.
Intra-duodenal
Kurlan and co-workers as well as Sage and co-workers demonstrated that direct intra-duodenal infusion was possible and also reduced fluctuations to a similar extent to i.v. infusions (Kurlan et al 1986, 1988, Sage et al 1988a). An Italian group reported a single case with very advanced disease which they treated with levodopa methyl ester (250 mg/mL levodopa) intraduodenally, thus considerably reducing volume requirements (Ruggieri et al 1989). This group has later continued the use of this strategy for enteral levodopa administration for more patients with good result (Stocchi et al 1992). Later studies have further corroborated this and especially shown good improvement of the hyperkinesias and dyskinesias but also increased on-times and improved motor scores (Syed et al 1998; Stocchi et al 2005).
Pharmacokinetics of levodopa infusion therapies
The first solutions that were used for enteral infusion of levodopa contained 1 g/L levodopa and 250 mg/L carbidopa which again meant that large volumes had to be used (Kurlan et al 1988). Kurlan et al compared standard oral medication of levodopa with levodopa administered either intermittently to simulate oral intake patterns but with intragastric or intra-duodenal administration, or with continuous administration to these same sites (Kurlan et al 1988). In 10 patients continuous intra-duodenal administration gave the best result and the lowest coefficient of variation of plasma levels of levodopa.
In several studies, intra-duodenal levodopa administration has been compared to oral medication including sustained release tablets. Bredberg found a coefficient of variation of 45% in 5 patients in a crossover study of 5 patients on oral medication which decreased to 12% on intra-duodenal medication (Bredberg et al 1993). In a randomized cross-over study of the same intra-duodenal levodopa/carbidopa gel (Duodopa®) versus levodopa/carbidopa SR tablets a lower coefficient of variation of the serum concentration was found for the intra-duodenal gel administration than for oral tablets (14% vs 34%) (Nyholm et al 2003).
Using a different intra-duodenal levodopa solution, Kurth et al did a double blind placebo controlled cross over trial in 10 patients and found a lower plasma levodopa variability as compared with oral medication (17% vs 38%) (Kurth et al 1993). Only Stocchi et al found plasma variability not to be improved by continuous enteral infusion but they used the difference between the lowest and highest plasma levels and not coefficient of variation to address this issue in three patients (Stocchi et al 2005). In addition, measurements were collected only hourly suggesting the possibility that some of the variability in serum levels may have been missed. However, Stocchi et al as well as Sage et al note that the troughs in the plasma levodopa curve are avoided by continuous infusion ie, that the area under the curve is increased and suggest that this mechanism may underlie some of the improvement in functional on time per day (Sage et al 1988a; Stocchi et al 2005).
Using intravenous continuous levodopa administration Nutt et al (1984) found that intake of meals high in phenylalanine, leucine, and isoleucine reversed the effect of i.v. levodopa despite serum levodopa levels remaining unchanged. This suggested that the uptake of levodopa over the blood–brain barrier is inhibited by large amounts of these amino acids. In a later study, the same authors further demonstrated that part of the variability in motor function was explained by plasma variations in amino acid levels (Nutt et al 1997). Similar results have been found with intra-duodenal administration where oral protein intake reduced the effect of administered levodopa on motor functioning while serum levodopa levels were not affected (Frankel et al 1989).
Intravenous infusion rates required based on several of the published iv studies have been approximately 1 mg/kg/hour of levodopa or about 50–100 mg/hour (Nutt et al 1984, 1985, 1997; Hardie et al 1984, 1986; Ruggieri et al 1988). In intra-duodenal infusions, rates have been very similar with about 30–90 mg/hour, although some studies used slightly higher levels, above 100 mg/hour.
In studies of infusion administration over a long time, plasma levodopa levels were gradually significantly reduced, thus providing evidence against continuous infusion inducing tolerance (Nilsson et al 1998). In a study of all 65 patients who had been treated with intra-duodenal levodopa/carbidopa gel, over 60% of the patients decreased their levodopa dosage over the course of the long time treatment, with levodopa dosage decreasing by 5% on average between the initial titration and the last available control visit (Nyholm et al 2006). In a study with the aim of observing the effect of continuous intravenous levodopa, Schuh and co-workers showed that in 6 patients with severe dyskinesias, uptitration of levodopa to 2.4 mg/kg/hour led to a 40% reduction of dyskinesia scores with a rightward shift of the dyskinesia dose-response curve. The dose-response curve for anti-Parkinsonian effects was not shifted, thus leading to a larger therapeutic window and suggesting separate mechanisms of the two levodopa effects (Schuh and Bennett 1993).
There have been suggestions that administration of levodopa over 24 hours without a night time “drug holiday” may lead to the development of tolerance (Cedarbaum et al 1990). However, a recent report of 5 patients on 24-hour treatment for 13–37 months showed only a small increase in average infusion rates (14%) from start to final follow up with 2 patients reducing their infusion rate slightly (Nyholm et al 2005a).
Method of enteral levodopa/carbidopa gel (Duodopa) administration
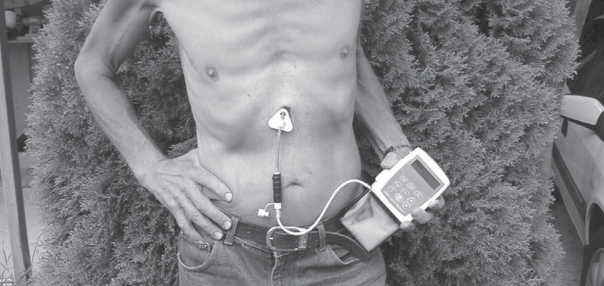
The levodopa/carbidopa gel developed by Neopharma AB, Uppsala, Sweden (presently Solvay Pharma GmbH, Hannover, Germany) is a carboxymethylcellulose aqueous gel containing 20 mg/mL of levodopa and 5 mg/mL of carbidopa. It is supplied in cassettes containing 100 mL of gel solution (2 g levodopa). The content of the cassettes is administered by a portable infusion pump (CADD-Legacy-Duodopa, Smiths Medical, MN, USA) and via a naso-duodenal or transabdominal duodenal tube to the duodenum proximal jejunum (see Figure 1). For short-term treatment and clinical test periods, the naso-duodenal route is used to avoid surgery. Fluoroscopy is usually used for correct placement of the tube. For longer-term treatment, usually after such a test phase, the tube is introduced directly into the gastric tract by means of PEG (percutaneous endoscopic gastrostomy) surgery and guided to the same position in the duodenum/jejunum with the gastroscope. The position is later controlled by fluorography. All surgical procedures are performed using local anesthesia. The pump is carried in a special bag or harness with infusion ongoing throughout the hours of the day (usually approx. 16 hours). The pump is usually stopped at night and a new medication cassette is connected every morning.
Figure 1.
The administration system used for continuous enteral administration of levodopa/carbidopa gel (Duodopa®).
Clinical studies of levodopa infusion
The promising results of the first continuous intravenous levodopa studies, which showed clear improvement of fluctuations in patients who had previously been severely handicapped (Shoulson et al 1975; Hardie et al 1984; Nutt et al 1984; Quinn et al 1984), led to suggestions that medication strategies involving continuous dopaminergic stimulation should be developed (Quinn et al 1984). Since agonists, with the possible exception of apomorphine, are less effective than levodopa in controlling Parkinsonian symptoms (Olanow et al 2004), not all patients can be controlled fully by addition of agonists since dyskinesias often lead to a necessity for reducing oral levodopa, with the result that the patient becomes under-dosed. In addition, if the patient has developed cognitive side-effects and hallucinations, it may be necessary also to keep agonist dose at a minimum. For these reasons there has continued to be some interest in continuous levodopa treatment strategies, with a consequent upswing in trials using continuous enteral infusion over the long term since the late 1980s (Sage et al 1988a, b, 1989b; Nilsson et al 1998; Syed et al 1998; Nilsson et al 2001).
The first open clinical attempts having produced clear improvements of fluctuations, Hardie and co-workers did one of the few double-blind crossover studies of i.v. infusion in 14 patients over 3 days and were able to show significant improvement of motor function (25% median improvement in % on-time) and a significantly reduced number of on-off cycles per day (Hardie et al 1984). Similarly other authors demonstrated almost continuous mobility in a partly blinded study over 8 hours with minimal side-effects (Quinn et al 1984).
In studies of intra-duodenal administration, blinded placebo-controlled studies have been performed only over short periods and using naso-duodenal administration. Significantly increased time in good function was found in parallel with the decreased plasma variability described (Kurth et al 1993).
However, as is well known from studies of neurosurgical treatment methods such as DBS, clinical studies of surgical treatments for Parkinson’s disease pose a number of methodological problems. As for neurosurgery, it is very difficult and ethically almost impossible to envisage double-blind, placebo-controlled studies using some type of sham operation with placebo administration. In addition, due to the strong symptomatic effect and short interval between changed administered dose and clinical effect, patients notice very quickly whether they have effective infusion or not. In addition, the act of simply observing an unstable and fluctuating Parkinson patient often affects the symptoms. Many patients are strongly affected by stress and a hospital stay with very different surroundings, food, and other circumstances. This presents a very different setting compared with the home situation, which is a problem for long-term studies. Therefore, combinations of home on-off diary self-registration of symptoms and clinical observation in the hospital have been used. In order to reduce observer bias based on open observational studies, various techniques have been used, such as video-based scoring by several independent investigators as well as less subjective functional assessments using electronic movement registration (eg, Nyholm et al 2003; Nilsson et al 2001)). Video-scoring may also be used in a blinded fashion (see below).
In Uppsala, Sweden, a series of studies using levodopa/carbidopa gel were done using such strategies to objectify the observations regarding motor function (Bredberg et al 1993; Nilsson et al 1998; Nilsson et al 2001). In these open studies, intra-duodenal adminstration was again compared with standard oral levodopa and clear improvement in motor function was found. Using opto-electronic movement registration and video-based on-off scoring in 9 patients, a significantly improved motor function and reduced fluctuations were found. The patients were followed openly over long-term treatment via a PEG stoma for, on average 4.7 years, with very little disease progress seen (Nilsson et al 2001). An increased part of the day in functional “near normal” phase was seen as compared with the situation at start-up.
Video-based, blinded, on-off scoring was also used in a later cross-over study of 12 patients treated by oral levodopa sustained-release tablets or duodenal levodopa/cabidopa gel infusion over 3 weeks using naso-duodenal catheters (Nyholm et al 2003). These studies verified the previous results of significantly lower levodopa plasma concentration coefficient of variation with enteral administration and a significantly improved motor function both assessed by Unified Parkinson’s Disease Rating Scale (UPDRS) total scores and UPDRS motor subscores. In addition, significantly increased time in functionally good phase (described as “near normal” functioning) was seen. In a follow-up study (the DIREQT study—Duodopa Infusion: Randomized Efficacy and Quality of Life Trial), 25 patients participated in a 3 + 3 week randomized cross-over study using naso-duodenal administration of the levodopa/carbidopa gel (Nyholm et al 2005b). In this study, the treatment alternatives were either levodopa/carbidopa infusion via the naso-duodenal route or the optimized conventional oral medication that the patients were using at baseline. Asssessment was by blinded video-based on-off recording with conventional therapy patients using a dummy tube during videofilming. In addition, a home diary was used and quality of life parameters were examined using PDQ-39 as well as 15D quality of life instruments. Analysis was done both based on intention to treat and per protocol. Significantly improved time in good function was found as well as significantly improved quality of life and improved total UPDRS as well as subscores of the UPDRS I, II, and IV. According to self-assessment diaries there were improvements mainly in questions regarding physical functioning such as walking, turning in bed, and ability to do daily chores, while reports of sleep, feeling depressed and dyskinesia did not significantly differ between the treatments (Nyholm et al 2005b). Tolerability was good with only one serious adverse effect suspected to be related to infusion treatment (confusion and insomnia in a 75-year-old patient. The frequency of other side-effects was lower than with the conventional treatment.
Information has also been collected on all patients treated between 1991 and 2002, for diagnosed Parkinson’s disease with fluctuations, with levodopa/carbidopa gel (only one of all consecutive patients did not agree to participate) (Nyholm et al 2007). Adverse effects related to the duodenal tube or PEG were the most common and consisted mainly of displacement of the duodenal tube. Adverse effects directly related to the stoma surgery in the gastric wall were more rare, as were problems with malfunctioning infusion pumps. Altogether, frequency of adverse events was lower on the enteral treatment than the frequency of events with oral treatment at baseline prior to start of infusion treatment. Some patients died while on treatment but the deaths were not judged to be procedure or treatment related. Altogether, the relative safety of this treatment method seems good also in the long run (Nyholm et al 2007). It seems from these studies as well as from others that motor effects remain stable also over long-term treatment while the natural progress of the non-motor Parkinson-related symptoms may be less affected.
A recent statistical analysis of predictive factors for predicting outcome of levodopa/carbidopa infusion based on two studies by Nyholm et al (2003, 2005b) found that the most important predictive factors were severity of Parkinsonism at baseline (Westin et al 2006). This was assessed as UPDRS scores, where motor UPDRS as well as total UPDRS scores were significant outcome predictors. Also, blinded rating of on-off percentage at baseline was a significant predictor. As stated by the authors and also from our experience, such prediction of outcome based on statistical material is not as important as individual test of the treatment based on non-invasive naso-duodenal administration prior to start-up of the more committed invasive treatment. We test each patient individually this way and use the individual improvement or lack of improvement to predict the final outcome and decide whether the patient should have permanent treatment. We have found that results seen on naso-duodenal testing are virtually the same as those seen on the final treatment both regarding individual optimized dosage, gain in on-time, and UDRS on and off scores (unpublished results).
There have been some other open clinical studies of continuous long-term intra-duodenal levodopa using other preparations. The group of Syed and Sage and co-workers in New Jersey described 22 patients treated (with an aqueous solution of 1g levodopa and 250 mg/L of carbidopa) on average for 47 months (summarized in Syed et al 1998). They compared the group of patients who decided to stop infusion therapy with those who continued at the latest control or continued until time of death. Both groups had significantly improved % on times compared with tablet treatment. In addition, the group who continued treatment also had reduced dyskinesia scores and more severe dyskinesias at baseline. Levodopa methyl ester has been used in studies by Stocchi and co-workers in Italy (Stocchi et al 2005). In 6 patients treated over 6 months, they found improved UPDRS ADL scores and dyskinesia scores, while motor scores in on and off were unchanged. There was also a significant improvement in on time without dyskinesia and reduction in off times.
In addition to the use for severely fluctuating patients, strategies using long-term enteral infusion of levodopa have also been used for treating special forms of dyskinesias (Sage et al 1989a), and night-time infusion has been shown to have good effects on severe sleeping problems (Sage and Mark 1991; Nyholm et al 2005a). In our clinical experience, one of the major positive effects we have had in using this treatment strategy in severe fluctuators is that the degree of pain that some of our patients have had has been clearly reduced (personal observation). These aspects of Parkinsonian symptoms deserve to be examined further where the possibility to fine titrate the levodopa serum concentration can be of considerable advantage. The same may also apply to psychiatric symptoms such as depression and anxiety, which are often very difficult to treat in patients with Parkinsonism and anti-Parkinsonian medication. In addition, since psychiatric symptoms are often seen as contraindicating neurosurgical treatment, medical optimization using continuous treatment strategies may be the last resort for these patients.
Lack of comparative studies
As stated above, DBS treatment (especially of the nucleus subthalamicus), continuous levodopa, and continuous apomorphine treatment seem to focus on the same patient categories. So far, in most countries neurosurgical treatment has been most commonly used. However, there are as yet no comparative studies comparing the three treatments which could help to guide our choice in a setting where two or more alternative treatments may be available.
A few studies compare the hitherto most common treatments, apomorphine versus DBS. Similar efficiency in reducing off time was found in an open non-randomized study (12 + 13 patients), with both treatment types (51% for apomorphine and 76% for DBS) (de Gaspari et al 2006). Abnormal movements were reduced only by DBS. Only DBS-treated patients, however, had worsening of neuropsychatric index scores after 12 months.
Few treatment centers have more than one of these alternative treatment methods in their arsenal. In most Western countries there is a possibility of referring at least younger patients and patients without cognitive or psychiatric problems to evaluation of neurosurgical treatment. If available, the other methods have so far mainly been reserved for patients where neurosurgery is felt to be contraindicated. In the past, continuous apomorphine has been more readily available in most countries but there has, during the past decade, been an upswing in availability of enteral levodopa. This has been especially in the Scandinavian countries, the UK, and USA, and recently the levodopa/carbidopa gel has been registered on the European and Scandinavian markets (Duodopa). Different treatment centers prefer different methods for their patients, usually based on the treatments that they are most familiar with and which are most readily available to them. Table 1 illustrates pros and cons to consider in the comparison between the three treatment methods. Comparative studies where patients are randomized to one treatment or the other to enable direct head-to-head comparison of indications, effect, side-effects, and costs are urgently needed.
Table 1.
Factors to be considered in choice between various treatments for advanced fluctuating Parkinson’s disease
| Neurosurgery (DBS of the STN, pallidotomy) |
Pros: Well established, good effect on motor complications when used as an adjunct to levodopa (reduces fluctuations and dyskinesia), lasting effect, may be cheaper (though total cost uncertain)
Cons: Major surgery with larger peroperative risk, small risk of IC bleeding, risk of worsening of depression and cognitive function, risk of speech problems/dysarthria and gait problems, not individually testable, irreversible, (pallidotomy (unilateral) possibly associated with less risk), also requires levodopa, continuous technical support required at high level |
| Continuous subcutaneous apomorphine |
Pros: Reasonably well established, good effect especially in treating motor complications and reducing off-time, non-invasive method, individually testable without committed surgical step, does not require surgery, easily reversible, no worsening of depression or cognition, small pump and subcutaneous administration, technical support may be easier due to subcutaneous technique
Cons: Local side-effects in most patients (fibrosis and local inflammation), tolerance? Problematic side effects for some especially at start up (nausea), cumbersome individual titration, many patients require levodopa as well, cost? |
| Intra-duodenal levodopa gel |
Pros: Good effect in treating fluctuations, improving on time and reducing off, most effective for worst patients, approved treatment, uses most symptomatically effective drug, monother apy without need for additional oral medication possible in most, individually testable without operation, reversible, minor surgical procedure/risk, rare and predictable adverse effects (based on oral treatment), adverse reactions
Cons: Cumbersome individual titration, need to carry large pump, continuous technical support required esp. regarding pump and tubing problems during first year, cost, requires minor surgery for long-term treatment |
Costs of Parkinson’s disease and its treatment
The total costs of Parkinson’s disease have recently been addressed based on studies in 5 European countries (Lindgren et al 2005). There are only a few studies and many uncertainties but some approximations may be made. Thus the mean total cost per year is €10,000–20,000 with slightly more than half of this as direct costs (including drug costs) and the rest as indirect costs. However, it was clear that costs increased dramatically as Parkinson’s disease progressed, with Hoehn and Yahr stage 5 patients totaling €20,000 to €30,000 while the cost associated with stage 1 patients was about 20%–25% of this.
Neurosurgery is associated with high surgery-associated costs but lower yearly costs after successful surgery (surgery-related €36900, yearly costs after surgery €1673) (Fraix et al 2006). However, control costs after surgery (€5500) were not included in the yearly costs, and it is our experience that these costs continue since further anti-Parkinson medication is also almost always required after surgery. In addition some controls are required for the stimulator system including battery change and further adjustment. It would seem reasonable to expect yearly costs at the level of an average Parkinson patient also after surgery, ie, in the range of €10,000–20,000. One decisive factor when calculating yearly costs of neurosurgical DBS therapy is the number of years over which the surgery-related and device-related costs should be discounted.
Apomorphine costs vary greatly in the published literature depending on whether total cost for subcutaneously administered apomorphine (including subcutaneous injections) or just continuous subcutaneous infusion treatment is included. A German study by Dodel et al (1998) based on retrospective analysis of 409 patients found that the apomorphine patients (with continuous subcutaneous infusion) among these had medication costs of approximately €13,500 per year. This was just over 3 times as much as conventionally treated advanced Parkinson patients (Hoehn and Yahr stages IV–V). In a small analysis based on pure medication costs of a few patients in Germany using apomorphine pump treatment, the yearly medication cost was estimated at €73,000–91,000 (Meissner et al 2001).
Duodopa, in comparison, is also an expensive treatment with simple medication costs of almost €50,000 per year in Europe. One study has been peformed of Duodopa treatment costs but results have so far only been presented in oral and abstract form (Kristiansen et al 2005). According to this, two years of Duodopa treatment costs US$93,600 which should be compared with US$28,700 for the previous conventional treatment. The cost per additional quality adjusted life year based on these calculations was US$1.02 million which, according to these authors, is above conventional cost-effectiveness thresholds. However, there are several uncertainties associated with these calculations warranting careful interpretation of the results. The present day status of Duodopa as an orphan drug also naturally affects treatment costs and the price may thus also change in the future.
What is clear, however, is that the cost of administration of duodenal levodopa must be weighed against improvement in quality of life for treated patients. Further studies regarding this are necessary. At present, cost may be the greatest single factor preventing the more widespread use of this treatment.
Conclusion – place of intra-duodenal levodopa in treatment of fluctuating, advanced phase Parkinson’s disease
Continuous administration of levodopa given intra-duodenally via an intestinal tube with the tip in the duodenum/proximal jejunum is a safe treatment method with a clear clinical gain for advanced Parkinson patients fluctuating between hyper-/dyskinesias and off-phases. The only commercially available such treatment involves the use of levodopa/carbidopa caboxymethylcellulose gel, which is also the most well-documented method. It is an invasive method but involves only minor surgery performed under local anesthesia. However, the surgical risk, although small and in our view acceptable for most advanced Parkinson patients without serious comorbidity, must not be ignored. The treatment, therefore, has its place only after oral non-invasive treatment possibilities have been optimally tried and when these no longer are deemed sufficient.
Indications for the treatment are similar to indications for the alternative treatments using stereotactic brain surgery (especially DBS of the subthalamic nuclei, possibly pallidotomy) and continuous subcutaneous apomorphine infusion. Patients must have levodopa-responsive Parkinson’s disease fluctuating between off-phases and dyskinetic/hyperkinetic phases in such a way that the patients’ function and quality of life are reduced. Patients should be cognitively intact enough to manage the administration system (possibly with help from home nurse or relatives) and not severely affected by psychiatric symptoms such as uncontrolled depression or psychosis. Psychiatric complications, especially depression, may be less of a counter-indication against enteral levodopa and apomorphine than for deep brain stimulation though more data are required regarding this. Age alone need not be a counter-indication for continuous medical treatment.
The lack of direct comparator studies makes evaluation of comparative treatment effects difficult to evaluate. Due to a combination of the neurosurgical treatments being more readily available and there being more documentation on their effects, a major group of patients for enteral levodopa and apomorphine, so far, have been patients considered for, but not offered, neurosurgical treatment. This may be because of age, comorbidity with increased surgical risk, or psychiatric problems. In addition, some patients who have previously been unsuccessfully or insufficiently treated by neurosurgery may also be candidates, as well as patients admitted for neurosurgery but who have long time delays before surgery can take place. Also patients not wishing surgery of the brain are possible candidates for the less invasive techniques. Whether or not continuous infusion of levodopa should also be considered in younger patients, and its placement on the treatment ladder relative to neurosurgery, are questions that are yet to be answered. Effect, especially long-term effect, and side-effects, as well as risk, costs, and quality of life are all factors that must be considered.
Regarding treatment costs, not enough data have been published so far to enable choice between the three treatment methods based on cost, though it is clear that all three treatments are expensive. It may be that neurosurgery is slightly less costly but costs for ineffective neurosurgery and follow-up treatment as well as for potentially more serious complications should also be considered.
It is possible to test the method for enteral levodopa treatment individually via naso-duodenal administration, prior to any invasive surgical steps. This enables a better basis for a decision about treatment both on the part of the physician and the involved patient. We would suggest that such a test should always be made before surgery. Such a test enables the patient and the physician to make better informed and individually based decisions about treatment. Similarly, apomorphine treatment does not require a committed step involving surgery before treatment can be tested.
The inclusion of enteral levodopa in the treatment arsenal thus enables more individually tailored treatment of a small but complicated group of patients. Further results from ongoing studies and clinical series will further clarify criteria for optimal selection of the target population.
Acknowledgments
Thanks to Ian F Lawrence and Christine Hage-Lundqvist for proofreading the manuscript.
Footnotes
Disclosures
The author has received research project sponsorship from Solvay-Pharma AS, Norway, the producers of Duodopa®, and has participated in scientific meetings sponsored by the same company. He has also received sponsorship for participation in various meetings by Orion Pharma AS, Pfizer Pharma AS, and GlaxoSmithKline Pharma AS, Norway who also produce medication used for Parkinson’s disease and has participated in clinical research projects sponsored by Schwarz Pharma AS, Norway.
References
- Aarsland D, Larsen JP, Tandberg E, et al. Predictors of nursing home placement in Parkinsons disease: a population-based prospective study. J Am Geriatr Soc. 2000;48:938–42. doi: 10.1111/j.1532-5415.2000.tb06891.x. [DOI] [PubMed] [Google Scholar]
- Benabid AL. Deep brain stimulation for Parkinson’s Disease. Curr Opin Neurobiol. 2003;13:696–706. doi: 10.1016/j.conb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Bredberg E, Nilsson D, Johansson K, et al. Intraduodenal infusion of a water-based levodopa dispersion for optimisation of the therapeutic effect in severe Parkinson’s disease. Eur J Clin Pharmacol. 1993;45:117–22. doi: 10.1007/BF00315491. [DOI] [PubMed] [Google Scholar]
- Burkhard PR, Vingerhoets FJG, Berney A, et al. Suicide after successful deep brain stimulation for movement disorders. Neurology. 2004;63:2170–2. doi: 10.1212/01.wnl.0000145603.48221.b5. [DOI] [PubMed] [Google Scholar]
- Cedarbaum JM, Silvestr M, Kutt H. Sustained enteral aministration of levodopa increases and interrupted infusion decreases levodopa dose requirements. Neurology. 1990;40:995–7. doi: 10.1212/wnl.40.6.995. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Healy DG, Schapira AHV. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006a;5:235–45. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Martinez-Martin P, Schapira AHV, et al. International multicenter pilot study of the first comprehensive selfcompleted nonmotor symptoms questionnaire for Parkinsons disease: the NMSQuest Study. Move disord. 2006b doi: 10.1002/mds.20844. (E-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- Colzi A, Turner K, Lees AJ. Continuous subcutaneous waking day apomorphine in the long term treatment of levodopa induced interdose dyskinesias in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1998;64:573–6. doi: 10.1136/jnnp.64.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gaspari D, Siri C, Landi A, et al. Clinical and neuropsychological follow up at 12 months in patients with complicated Parkinsosn’s disease treated with subcutaneous apomorphine infusion or deep brain stimulation of the subthalamic nucleus. J Neurol Neurosurg Psychiatry. 2006;77:450–3. doi: 10.1136/jnnp.2005.078659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinsons disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Wenzelburger R, Kopper F, et al. Deep brain stimulation of the subthalamic nucleus for Parkinson’s disease: a therapy approaching evidence-based standards. J Neurol. 2003;250(Suppl 1):I43–6. doi: 10.1007/s00415-003-1109-8. [DOI] [PubMed] [Google Scholar]
- Dewey RB, Hutton T, LeWitt PA, et al. A randomized, double-blind, placebo-controlled trial of subcutaneously injected apomorphine for Parkinsonian off-state events. Arch Neurol. 2001;58:1385–92. doi: 10.1001/archneur.58.9.1385. [DOI] [PubMed] [Google Scholar]
- Dhawan V, Medcalf P, Stegie F, et al. Retrospective evaluation of cardio-pulmonary fibrotic side effects in symptomatic patients from a group of 234 Parkinsons disease patents treated with cabergoline. J Neural Transm. 2005;112:661–8. doi: 10.1007/s00702-005-0289-1. [DOI] [PubMed] [Google Scholar]
- Di Rosa AE, Epifanio A, Antonini A, et al. Continuous apomorphine infusion and neuropsychiatric disorders: a controlled study in patients with advanced Parkinson’s disease. Neurol Sci. 2003;24:174–5. doi: 10.1007/s10072-003-0116-0. [DOI] [PubMed] [Google Scholar]
- Diamond A, Jankovic J. The effect of deep brain stimulation on quality of life in movement disorders. J Neurol Neurosurg Psychiatry. 2005;76:1188–93. doi: 10.1136/jnnp.2005.065334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodel R, Eggert KM, Singer MS, et al. Costs of drug treatment in Parkinsons disease. Mov disord. 1998;13:249–54. doi: 10.1002/mds.870130209. [DOI] [PubMed] [Google Scholar]
- Dodel RC, Berger K, Oertel WH. Health-related quality of life and healthcare utilisation in patients with Parkinson’s disease: impact of motor fluctuations and dyskinesias. Pharmacoeconomics. 2001;19:1013–38. doi: 10.2165/00019053-200119100-00004. [DOI] [PubMed] [Google Scholar]
- Esselink RAJ, deBie EMA, de Haan RJ, et al. Unilateral pallidotomy versus bilateral subthalamic nucleus stimulation in PD. Neurology. 2004;62:2017. doi: 10.1212/01.wnl.0000103235.12621.c3. [DOI] [PubMed] [Google Scholar]
- Fraix V, Houeto J-L, Lagrange E, et al. Clinical and economic results of bilateral subthalamic nucleus stimulation in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2006;77:443–9. doi: 10.1136/jnnp.2005.077677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel JP, Kempster PA, Bovingdon M, et al. The effects of oral protein on the absorption of intraduodenal levodopa and motor performance. J Neurol Neurosurg Psychiatry. 1989;52:1063–7. doi: 10.1136/jnnp.52.9.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel JP, Lees AJ, Kempster PA, Stern GM. Subcutaneous apomorphine in the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1990;53:96–101. doi: 10.1136/jnnp.53.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Parkinson’s disease survey steering committee. Factors impacting on quality of life in Parkinson’s disease: results from an international survey. Mov disord. 2002;17:60–7. doi: 10.1002/mds.10010. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Poewe W, Rascol O, et al. Eidence based medical review update: pharmacological and surgical treatments of Parkinsons disease: 2001 to 2004. Mov disord. 2006;20:523–39. doi: 10.1002/mds.20464. [DOI] [PubMed] [Google Scholar]
- Hardie RJ, Lees AJ, Stern GM. On-off fluctuations in Parkinson’s disease. a clinical and neuropharmacological study. Brain. 1984;107:487–506. doi: 10.1093/brain/107.2.487. [DOI] [PubMed] [Google Scholar]
- Hardie RJ, Malcolm SL, Lees AJ, et al. The pharmacokinetics of intravenous and oral evodopa in patients with Parkinson’s disease who exhibit on-off fluctuations. Br J Clin Pharmacol. 1986;22:429–36. doi: 10.1111/j.1365-2125.1986.tb02913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog J, Volkmann J, Krack P, et al. Two-year follow-up of subthalamic deep brain stimulation in Parkinson’s disease. Mov Disord. 2003;18:1332–7. doi: 10.1002/mds.10518. [DOI] [PubMed] [Google Scholar]
- Horvath J, Fross RD, Kleiner-Fisman G, et al. Severe multivalvular heart disease: a new complication of the ergot derivative dopamine agonists. Mov disord. 2004;19:656–62. doi: 10.1002/mds.20201. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological Study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanovsky P, Kubova D, Bares M, et al. Levodopa-induced dyskinesias and continuous subcutaneous infusions of apomorphine: results of a two-year, prospective follow-up. Mov Disord. 2002;17:188–91. doi: 10.1002/mds.1276. [DOI] [PubMed] [Google Scholar]
- Krause W, Niewueboer B, Ruggieri S, et al. Pharmacokinetics of lisuride after subcutaneous infusion. J Neural Transm. 1988;(Suppl 27):71–4. doi: 10.1007/978-3-7091-8954-2_8. [DOI] [PubMed] [Google Scholar]
- Kristiansen IS, Bingefors C, Nyholm D, et al. The cost-effectiveness of continuous duodenal delivery of levodopa (Duodopa) in patients with severe Parkinsons disease [abstract] Mov disord. 2005;20(Suppl 10):S80. [Google Scholar]
- Kurlan R, Nutt JG, Woodward WR, et al. Duodenal and gastric delivery of levodopa in Parkinsonism. Ann Neurol. 1988;23:589–95. doi: 10.1002/ana.410230611. [DOI] [PubMed] [Google Scholar]
- Kurlan R, Rubin AJ, Miller C, et al. Duodenal delivery of levodopa for on-off fluctuations in Arkinsonism: preliminary observations. Ann Neurol. 1986;20:262–5. doi: 10.1002/ana.410200213. [DOI] [PubMed] [Google Scholar]
- Kurth MC, Tetrud JW, Tanner CM, et al. Double-blind, placebo-controlled, cross-over study of duodenal infusion of levodopa/carbidopa in Parkinson’s disease patients with “on-off ” fluctuations. Neurology. 1993;43:1698–703. doi: 10.1212/wnl.43.9.1698. [DOI] [PubMed] [Google Scholar]
- Lagrange E, Krack P, Moro E, et al. Bilateral subthalamic nucleus stimulation improves health-related quality of life in PD. Neurology. 2002;59:1976–8. doi: 10.1212/01.wnl.0000037486.82390.1c. [DOI] [PubMed] [Google Scholar]
- Lindgren P, von Campenhausen S, Spottke E, et al. Cost of Parkinsons disease in Europe. Eur J Neurol. 2005;12:68–73. doi: 10.1111/j.1468-1331.2005.01197.x. [DOI] [PubMed] [Google Scholar]
- Meissner W, Trottenberg T, Klaffke S, et al. Apomorphinterapi versus tiefe hirnstimulation. Nervenarzt. 2001;72:924–7. doi: 10.1007/s001150170004. [DOI] [PubMed] [Google Scholar]
- Nicole E, Pollak P, Serre-Debeauvais F, et al. Pharmacokinetics of apomorphine in Parkinsonian patients. Fundam Clin Pharmacol. 1993;7:245–52. doi: 10.1111/j.1472-8206.1993.tb00238.x. [DOI] [PubMed] [Google Scholar]
- Nilsson D, Hansson LE, Johansson K, et al. Long-term intraduodenal infusion of a water based levodopa-carbidopa dispersion in very advanced Parkinson’s disease. Acta Neurol Scand. 1998;97:175–83. doi: 10.1111/j.1600-0404.1998.tb00633.x. [DOI] [PubMed] [Google Scholar]
- Nilsson D, Nyholm D, Aquilonius SM. Duodenal levodopa infusion in Parkinson’s disease—long-term experience. Acta Neurol Scand. 2001;104:343–8. doi: 10.1034/j.1600-0404.2001.00153.x. [DOI] [PubMed] [Google Scholar]
- Nugroho AK, Li L, Dijkstra D, et al. Transdermal iontophoresis of the dopamine agonist 5-OH-DPAT in human skin in vitro. J Contr Rel. 2005;103:393–403. doi: 10.1016/j.jconrel.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Carter JH, Lea ES, et al. Motor fluctuations during continuous levodopa infusions in patients with Parkinson’s disease. Mov Disord. 1997;12:285–92. doi: 10.1002/mds.870120304. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Woodward WR, Anderson JL. The “on-off” phenomenon in Parkinsons disease. Relation to levodopa absorption and transport. N Engl J Med. 1984;310:483–8. doi: 10.1056/NEJM198402233100802. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Woodward WR, Anderson JL. The effect of carbidopa on the pharmacokinetics of intravenously administered levodopa: the mechanism of action in the treatment of Parkinsonism. Ann Neurol. 1985;18:537–43. doi: 10.1002/ana.410180505. [DOI] [PubMed] [Google Scholar]
- Nyholm D, Askmark H, Gomes-Trolin C, et al. Optimizing levodopa pharmacokinetics: intestinal infusion versus oral sustained-release tablets. Clin Neuropharmacol. 2003;26:156–63. doi: 10.1097/00002826-200305000-00010. [DOI] [PubMed] [Google Scholar]
- Nyholm D, Jansson R, Willows T, et al. Long-term 24 hour duodenal infusion of levodopa: outcome and dose requirements. Neurology. 2005a;65:1506–7. doi: 10.1212/01.wnl.0000183146.78892.3f. [DOI] [PubMed] [Google Scholar]
- Nyholm D, Lewander T, Johansson A, et al. Enteral levodopoda/carbidopa infusion in advanced Parkinson’s disease: long term exposure. Clin Neuropharmacol. 2007 doi: 10.1097/WNF.0b013e3180ed449f. [DOI] [PubMed] [Google Scholar]
- Nyholm D, Nilsson Remahl I, et al. Duodenal levodopa infusion monotherapy vs oral polypharmay in advanced Parkinson disease. Neurology. 2005b;64:216–23. doi: 10.1212/01.WNL.0000149637.70961.4C. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Agid Y, Mizuno Y, et al. Levodopa in the treatment of Parkinson’s disease: current controversies. Mov disord. 2004;19:997–1005. doi: 10.1002/mds.20243. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Watts RL, Koller WC. An algorithm (decision tree) for the management of Parkinsons disease (201): Treatment Guidelines. Neurology. 2001;56:88. doi: 10.1212/wnl.56.suppl_5.s1. [DOI] [PubMed] [Google Scholar]
- Pahwa R, Factor SA, Lyons KE, et al. Practice parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based Review): Report of the Quality Standards Subcommittee of the American Academy of Neruology. Neurology. 2006;66:983–95. doi: 10.1212/01.wnl.0000215250.82576.87. [DOI] [PubMed] [Google Scholar]
- Pfeiffer RE. A. Promising new technology for Parkinsons disease. Neurology. 2005;65:S6–10. doi: 10.1212/wnl.65.2_suppl_1.s6. [DOI] [PubMed] [Google Scholar]
- Poewe W, Luessi F. Clinical studies with transdermal rotigotine in early Parkinsons disease. Neurology. 2005;65:S11–14. doi: 10.1212/wnl.65.2_suppl_1.s11. [DOI] [PubMed] [Google Scholar]
- Priano L, Albani G, Brioschi A, et al. Transdermal apomorphine permeation from microemulsions: A new treatment in Parkinson’s disease. Mov disord. 2004;19:937–42. doi: 10.1002/mds.20054. [DOI] [PubMed] [Google Scholar]
- Quinn N, Parkes JD, Marsden CD. Control of on/off phenomenon by continuous intravenous infusion of levodopa. Neurology. 1984;34:1131–6. doi: 10.1212/wnl.34.9.1131. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Oroz MC, Obeso JA, Lang AE, et al. Bilateral deep brain stimulation in Parkinson’s disease: a multicentre study with 4 years follow-up. Brain. 2005;128:2240–9. doi: 10.1093/brain/awh571. [DOI] [PubMed] [Google Scholar]
- Ruggieri S, Stocchi F, Carta A, et al. Jejunal delivery of levodopa methyl ester. Lancet. 1989;2:45–6. doi: 10.1016/s0140-6736(89)90285-7. [DOI] [PubMed] [Google Scholar]
- Ruggieri S, Stocchi F, Carta A, et al. Comparison between levodopa and lisuride intravenous infusions: A clinical study. Mov disord. 1988;3:313–19. doi: 10.1002/mds.870030405. [DOI] [PubMed] [Google Scholar]
- Russmann H, Ghika J, Villemure J-G, et al. Subthalamic nucleus deep brain stimulation in Parkinson disease patients over age 70 years. Neurology. 2004;63:1952–4. doi: 10.1212/01.wnl.0000144198.26309.d8. [DOI] [PubMed] [Google Scholar]
- Sage JI, Mark MH. Nighttime levodopa infusions to treat motor Fluctuations in advanced Parkinsosn disease: preliminary observations. Ann Neurol. 1991;30:616–17. doi: 10.1002/ana.410300417. [DOI] [PubMed] [Google Scholar]
- Sage JI, McHale DM, Sonsalla P, et al. Continuous levodopa infusions to treat complex dystonia in Parkinson’s disease. Neurology. 1989a;39:888–91. doi: 10.1212/wnl.39.7.888. [DOI] [PubMed] [Google Scholar]
- Sage JI, Schuh L, Heikkila RE, et al. Continuous duodenal infusions of levodopa: plasma concentrations and motor fluctuations in Parkinson’s disease. Clin Neuropharmacol. 1988a;11:36–44. [PubMed] [Google Scholar]
- Sage JI, Trooskin S, Sonsalla P, et al. Experience with continuous enteral levodopa infusions in the treatment of 9 patients with advanced Parkinsons disease. Neurology. 1989b;39:60–3. [PubMed] [Google Scholar]
- Sage JI, Trooskin S, Sonsalla PK, et al. Long-term duodenal infusion of levodopa for motor fluctuations in Parkinsonism. Ann Neurol. 1988b;24:87–9. doi: 10.1002/ana.410240116. [DOI] [PubMed] [Google Scholar]
- Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinsons disease. J Neurol Neurosurg Psychiatry. 2000;69:308–12. doi: 10.1136/jnnp.69.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh LA, Bennett JP., Jr Suppression of dyskinesias in advanced Parkinson’s disease. I. Continuous intravenous levodopa shifts dose response for production of dyskinesias but not for relief of Parkinsonism in patients with advanced Parkinson’s disease. Neurology. 1993;43:1545–50. doi: 10.1212/wnl.43.8.1545. [DOI] [PubMed] [Google Scholar]
- Schwab RS, Amador LV, Lettvin JY. Apomorphine in Parkinson’s disease. Trans Am Neurol Assoc. 1951;56:251–3. [PubMed] [Google Scholar]
- Shoulson I, Glaubiger GA, Chase TN. On-off response. Clinical and biochemical correlations during oral and intravenous levodopa administration in Parkinsonian patients. Neurology. 1975;25:1144–8. doi: 10.1212/wnl.25.12.1144. [DOI] [PubMed] [Google Scholar]
- Steiger MJ, Quinn NP, Marsden CD. The clinical use of apomorphine in Parkinson’s disease. J Neurol. 1992;239:389–93. doi: 10.1007/BF00812157. [DOI] [PubMed] [Google Scholar]
- Stocchi F. Optimising levodopa therapy for the management of Parkinsons disease. J Neurol. 2005;252:IV/43–IV/48. doi: 10.1007/s00415-005-4009-4. [DOI] [PubMed] [Google Scholar]
- Stocchi F, Olanow CW. Continuous dopaminergic stimulation in early and advanced Parkinson’s disease. Neurology. 2004;62:S56–S63. doi: 10.1212/wnl.62.1_suppl_1.s56. [DOI] [PubMed] [Google Scholar]
- Stocchi F, Ruggieri S, Carta A, et al. Intravenous boluses and continuous infusion of L-dopa methyl ester in fluctuating patients with Parkinson’s disease. Mov disord. 1992;7:249–56. doi: 10.1002/mds.870070311. [DOI] [PubMed] [Google Scholar]
- Stocchi F, Vacca L, De Pandis MF, et al. Subcutaneous continuous apomorphine infusion in fluctuating patients with Parkinson’s disease: long-term results. Neurol Sci. 2001;22:93–4. doi: 10.1007/s100720170062. [DOI] [PubMed] [Google Scholar]
- Stocchi F, Vacca L, Ruggieri S, et al. Intermittent vs. continuous levodopa administration in patients with advanced Parkinson’s disease. Arch Neurol. 2005;62:905–10. doi: 10.1001/archneur.62.6.905. [DOI] [PubMed] [Google Scholar]
- Syed N, Murphy J, Zimmerman T, et al. Ten years experience with enteral levodopa infusions for motor fluctuations in Parkinson’s disease. Mov disord. 1998;13:336–8. doi: 10.1002/mds.870130222. [DOI] [PubMed] [Google Scholar]
- Tagliati M, Pourfar MH, Alterman RL. Comment on: subthalamic nucleus deep brain stimulation in Parkinson disease patients over age 70 years. Neurology. 2005;65:179–80. doi: 10.1212/wnl.65.1.179-a. [DOI] [PubMed] [Google Scholar]
- The Parkinson study group. A controlled trial of rotigotine monotherapy in early Parkinsons disease. Arch Neurol. 2003;60:1721–8. doi: 10.1001/archneur.60.12.1721. [DOI] [PubMed] [Google Scholar]
- Vaamonde J, Luquin MR, Obeso JR. Subcutaneous lisuride infusion in Parkinson’s disease: response to chronic administration in 34 patients. Brain. 1991;114:601–17. doi: 10.1093/brain/114.1.601. [DOI] [PubMed] [Google Scholar]
- van Camp G, Flamez A, Cosyns B, et al. Treatment of Parkinsons disease with pergolide and relation to restrictive valvular heart disease. Lancet. 2004;363:1179–83. doi: 10.1016/S0140-6736(04)15945-X. [DOI] [PubMed] [Google Scholar]
- Verhagen Metman L, Gillespie M, Farmer C, et al. Continuous transdermal dopaminergic stimulation in advanced Parkinsons disease. Clin Neuropharmacol. 2001;24:163–9. doi: 10.1097/00002826-200105000-00008. [DOI] [PubMed] [Google Scholar]
- von Campenhausen S, Bornschein B, Wick R, et al. Prevalence and incidence of Parkinsons disease in Europe. Eur Neuropsychopharmacol. 2005;15:473–90. doi: 10.1016/j.euroneuro.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Westin J, Nyholm D, Groth T, et al. Outcome prediction of enteral levodopa/carbidopa infusion in advanced Parkinsons disease. Parkinsonism Relat Disord. 2006 doi: 10.1016/j.parkreldis.2006.02.006. (E-pub ahead of print ) [DOI] [PubMed] [Google Scholar]
- Widner H. Strategies to modify levodopa treatment. Adv Neurol. 2003;91:229–36. [PubMed] [Google Scholar]