Abstract
Objective
To determine whether elderly people can learn to use the inhaler used to deliver zanamivir (Relenza Diskhaler) as effectively as the Turbohaler and to identify which aspects of inhaler technique are most problematic.
Design
Randomised, controlled, intervention study.
Setting
Wards for acute elderly care in a large district general hospital.
Participants
73 patients who were unfamiliar with the use of an inhaler, aged 71 to 99 (mean 83) years.
Main outcome measures
Initial scores and changes in scores 24 hours later using a 10 point scoring system of five aspects of inhaler technique.
Results
38 patients were allocated the Relenza Diskhaler and 35 the Turbohaler. The mean total score was significantly greater in the Turbohaler than Diskhaler groups both initially (8.74 v 7.05) and after 24 hours (8.28 v 5.43). The major difference between inhalers was in loading and priming. After tuition 50% (19 of 38) of patients allocated the Diskhaler were unable to load and prime the device and 65% (24 of 37) were unable to do so 24 hours later. Of those allocated the Turbohaler, two patients were unable to load and prime the device after initial review and one after 24 hours.
Conclusion
Most elderly people cannot use the inhaler device used to deliver the anti-influenza drug zanamivir. Treatment with this drug is unlikely to be effective in elderly people unless the delivery system is improved.
Introduction
Influenza causes an acute respiratory illness, mainly during a two month period in the winter. It affects people of all ages, but 80% of deaths occur in elderly people—that is, those aged over 65—who are more likely to develop complications than younger people. Bronchitis and pneumonia may supervene, resulting in hospital admission and sometimes death.1 Vaccination is effective in preventing or ameliorating influenza in elderly people and is recommended.2 Each year less than half the elderly population are vaccinated, leaving many at risk.3
Zanamivir (Relenza, GlaxoWellcome) is an inhibitor of influenza A and B virus neuraminidase, marketed for the treatment of influenza. It is delivered to the lungs by a dry powder inhaler, the Diskhaler, which is also available as a delivery system for salbutamol and beclomethasone. A five day course of inhaled zanamivir twice daily has been shown to reduce the duration and severity of influenza symptoms.4,5 The ability of inhaled zanamivir to reduce disease severity and hospital admissions among elderly people remains unproved. In September 1999 the National Institute for Clinical Excellence advised against funding for zanamivir treatment as there was insufficient evidence of benefit to elderly patients and those at high risk. In November 2000 the institute recommended zanamivir treatment for patients who were at risk (including people aged over 65 years) who presented with influenza within 48 hours of symptoms. No trial designed specifically to test zanamivir's effectiveness in elderly people with influenza has been published, and the evidence of effectiveness in elderly people comes from subgroup analysis of trials recruiting both young and old patients.
If a significant amount of an inhaled drug is to reach a patient's lungs then the patient must be able to use an inhaler. Inhaler technique can be considered in five stages: loading and priming of the device, exhaling to residual volume, hand and breath coordination of inhalation, breath holding, and awareness of an empty inhaler. Elderly people often have difficulty in using inhaler devices.6,7 Reasons include arthritis, weakness, poor dexterity, and poor vision. Learning to use an inhaler also requires good cognitive function. Those with Hodgkinson mental test scores8 of less than seven out of 10 are unlikely to have adequate inhaler technique.9 Inhalers not requiring hand and breath coordination are more suitable for elderly people, and metered dose inhalers are commonly given with a spacer such as the Volumatic (Allen and Hanbury) to improve inhaler technique.10 A study of elderly people unfamiliar with the use of an inhaler has shown that the dry powder device Turbohaler (Astra) is easily learnt11,12 and proved superior to the metered dose inhalers plus Volumatic spacer combination, which, because it is bulky and has multiple assembly stages, is difficult to load and prime.
Turbohaler is small and does not require inspiration to be coordinated with triggering. Priming consists of two stages: removal of the top and turning the base clockwise and back. An audible click indicates the device is ready to use. The click still occurs even if the device is empty, but a flag in a window shows when no drug remains.
The Diskhaler is pocket sized and does not require inspiration to be coordinated with triggering. The drug is contained in one of four blisters in a disc, inserted on a tray. One blister should be used for each inhalation. The recommended dose of zanamivir is two inhalations (2 × 5 mg) twice daily for five days, providing a total daily inhaled dose of 20 mg. Priming consists of several stages: taking the top off; sliding the tray backwards and forwards to rotate the disc to an intact blister; raising a perforator to 90 degrees, which is then lowered to its original position. This perforates the blister and delivers the drug to the inhaler chamber. If no blisters are intact a new disc must be loaded by unlatching and removing the tray, changing the disc, and replacing the tray.
We aimed to see if elderly people unfamiliar with the use of an inhaler could learn to use the Diskhaler as effectively as the Turbohaler and to identify which aspects of the devices were most problematic.
Participants and methods
After approval from our local research ethics committee, we recruited patients aged over 65 years from seven wards providing acute elderly care at Mayday Hospital. Suitable patients were identified at the daily departmental meeting for the changeover of staff. This meeting is attended by senior house officers, specialist registrars, and consultants. One of the investigators (PD, AH, VJ, MM) reviewed potentially suitable patients on the ward. They were enrolled provided their medical condition was stable and they were either ready or shortly to be discharged from hospital. Patients had to be able to read a sentence; the font of which corresponded in size to the window in the Turbohaler that signals when the inhaler is empty, and approximated to the size of the hole made by the perforator in the blisters of the Diskhaler. Exclusion criteria were previous use of an inhaler, cognitive impairment (defined as a score of less than seven out of 10 on the Hodgkinson mental test), illness affecting ability to use inhalers, such as stroke or arthritis, and due to leave hospital in less than 24 hours.
A series of 100 random numbers (0/1) were computer generated by the hospital's information technology department. These were changed to T (Turbohaler) and D (Diskhaler) and printed as a list. Once informed consent had been obtained the list was consulted and the inhaler allocated.
Before recruitment was started a respiratory nurse advised the investigators on how to teach inhaler usage. The investigators then spent four weeks jointly interviewing ward patients to develop standardised teaching. Investigators took turns explaining inhaler usage, with the other three observing, until all had acquired similar teaching abilities. The investigators also scored patients together, but independently, and then jointly reviewed differences in scores until they were scoring consistently. To confirm consistent scoring, all investigators, without consulting each other, scored a pilot group of 10 patients using both types of inhaler. This confirmed consistent scoring, and recruitment to the study began.
Patients were randomly allocated Diskhaler or Turbohaler and, after 15 minutes' tuition, assessed for their inhaler technique. We considered five aspects: loading and priming, exhaling to residual volume, hand and breath coordination, breath holding for 10 seconds, and awareness that the inhaler was empty. Each aspect was scored for technique: 0 for poor; 1 for moderate; and 2 for perfect. This gave a minimum total score of zero and a maximum of 10.11,13 Assessments of seven aspects of ability to load and prime the Diskhaler were also recorded. These were ability to take the top off, rotate the disc to an intact blister, perforate a blister, replace the perforator, remove the tray, change the disc, and replace the tray. After initial assessment, up to five further minutes' tuition was given if necessary. Assessment was repeated 24 hours later to see if the inhaler could still be used.
This was an observational study with one observer only for the initial and review stages. The inhalers are different in size, shape, and operational characteristics, and masking was therefore not possible. Initial tuition and assessment and the assessment at 24 hours were by different investigators unaware of the previous score.
The primary outcome measure was the differences in mean total scores after initial tuition and at 24 hours. Secondary outcome measures were differences in mean scores for each of five aspects of inhaler technique. Tertiary measures were the proportion of patients allocated Diskhaler who were able to manage each of seven aspects of loading their inhaler.
Statistical analysis
Power was calculated with data from a study that compared Turbohaler with the metered dose inhaler and Volumatic delivery system, which, similar to the Diskhaler, has multiple stages for loading and priming.11 This showed that at least 35 patients needed to be recruited into each group to have a 90% chance of detecting a difference of 1.5 in mean total score at the 5% significance level.
We carried out an independent t test (group statistics) for the variables of age and mental test score to ensure groups did not differ. The sex distribution of the two groups did not differ. The independent t test compared the mean differences in scores of the five aspects of inhaler technique and the mean differences of the summated total scores of these five aspects between the Turbohaler and Diskhaler groups.
Results
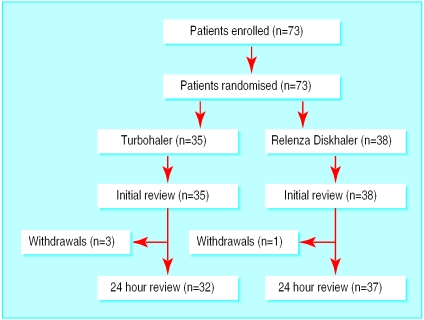
We enrolled 73 patients into the study: 35 were allocated the Turbohaler and 38 the Diskhaler. Three patients from the Turbohaler group and one from the Diskhaler group withdrew between the initial and 24 hour reviews (figure); one patient in the Turbohaler group refused to continue with the study when reviewed at 24 hours and the other three were discharged from hospital before the 24 hour review because hospital transport came early to collect them. The age, sex ratio, and mean mental test scores were similar in each group, but the number and percentage of patients with mental test scores of 10 was higher in the Diskhaler group (table 1).
Table 1.
Characteristics of patients allocated inhalers
| Inhaler device
|
||
|---|---|---|
| Turbohaler (n=35) | Diskhaler (n=38) | |
| No (%) female | 25 (71) | 28 (74) |
| Mean age (range) | 84.0 (76-95) | 82.7 (71-99) |
| Mean mental test score | 9.28 | 9.58 |
| No (%) with maximum mental test scores | 20 (57) | 26 (68) |
After enrolment 20 (57%) of the patients in the Turbohaler group and 10 (26%) in the Diskhaler group achieved perfect scores. These were sustained at 24 hours by 15 of 32 (47%) patients allocated Turbohaler and 5 of 37 (13%) allocated Diskhaler. Mean total scores were significantly higher in the Turbohaler than the Diskhaler group; the difference between groups was greater at the 24 hour review (table 2) The biggest difference in aspects of inhaler technique was in the patients' ability to load and prime the devices. Mean loading and priming scores for the Diskhaler were significantly lower both after the initial review and at 24 hours. More patients in the Diskhaler than Turbohaler group had poor (zero) scores, consistent with the inability to load and prime the device. Nineteen of 38 patients in the Diskhaler group had a poor score for loading and priming on initial review and 24 of 37 after 24 hours, whereas only 2 of 35 patients in the Turbohaler group had a poor score on initial review and 1 of 32 after 24 hours.
Table 2.
Mean (SD) initial and review scores for patients learning to use the Diskhaler or Turbohaler devices
| Diskhaler | Turbohaler | Mean difference (95% CI) | P value | |
|---|---|---|---|---|
| Initial scores | n=38 | n=35 | ||
| Load and prime | 0.87 (0.93) | 1.83 (0.53) | 0.96 (0.68 to 1.31) | <0.001 |
| Exhale to residual volume | 1.60 (0.72) | 1.61 (0.77) | 0.005 (−0.35 to 0.34) | 0.976 |
| Hand and breath coordination | 1.77 (0.54) | 1.94 (0.24) | 0.18 (−0.18 to 0.37) | 0.068 |
| Breath holding | 1.74 (0.50) | 1.80 (0.47) | 0.06 (−0.16 to 0.29) | 0.583 |
| Aware of empty device | 1.08 (0.97) | 1.57 (0.78) | 0.49 (0.2 to 0.9) | 0.019 |
| Total | 7.05 (2.46) | 8.74 (1.98) | 1.69 (0.65 to 2.73) | 0.002 |
| Review scores | n=37 | n=32 | ||
| Load and prime | 0.54 (0.80) | 1.81 (0.47) | 1.27 (0.95 to 1.58) | <0.001 |
| Exhale to residual volume | 1.24 (0.86) | 1.63 (0.71) | 0.38 (−0.45 to 0.76) | 0.48 |
| Hand and breath coordination | 1.43 (0.73) | 1.78 (0.49) | 0.35 (4.5 to 0.64) | 0.021 |
| Breath holding | 1.30 (0.74) | 1.53 (0.76) | 0.23 (−0.13 to 0.60) | 0.202 |
| Aware of empty device | 0.92 (1.01) | 1.53 (0.76) | 0.61 (0.19 to 1.04) | 0.006 |
| Total | 5.43 (2.82) | 8.28 (1.90) | 2.85 (1.70 to 3.99) | <0.001 |
Two patients in the Turbohaler group and one in the Diskhaler group failed to remove the inhaler top. Apart from removing the top, 24 of 38 patients in the Diskhaler group were unable to perform at least one of the other stages of loading and priming after initial review and 30 of 37 patients at 24 hours. Most patients found removal of the tray difficult. The tray could not be removed by 22 of 38 patients after initial tuition and by 28 of 37 patients at 24 hours (table 3).
Table 3.
Numbers (percentages) of patients managing to perform different aspects of priming and loading the Relenza Diskhaler at initial review and at 24 hours
| Aspect of technique | Time of review
|
|
|---|---|---|
| Initial (n=38) | At 24 hours (n=37) | |
| Removing top | 37 (97) | 37 (100) |
| Rotating disc for intact blister | 26 (68) | 14 (38) |
| Perforating blister | 26 (68) | 19 (51) |
| Replacing perforator | 23 (61) | 19 (51) |
| Removing tray | 16 (42) | 9 (24) |
| Changing disc | 17 (45) | 14 (38) |
| Replacing tray | 16 (42) | 14 (38) |
Discussion
Most of the elderly people in our study were unable to use the Diskhaler device used to deliver zanamivir satisfactorily, but those allocated the Turbohaler were more successful. Patients scored significantly better in the Turbohaler than Diskhaler group both initially and at 24 hours. Patients in the Turbohaler group also had a higher proportion of perfect scores than those in the Diskhaler group at 24 hours.
The main difference between inhalers was in loading and priming and awareness of an empty inhaler. Loading and priming is a crucial aspect of inhaler use because if this is incorrectly done no drug can be delivered, no matter how good are other aspects of inhaler technique. Most patients allocated the Diskhaler were unable to load the device.
The two groups had similar age and sex distributions. The mean mental test scores and numbers with perfect scores were lower in the Turbohaler group than in the Diskhaler group. As cognitive function is an important factor in determining inhaler technique,9 the higher mental test scores of the Diskhaler group was an advantage that might have been expected to lead to better inhaler technique and higher scores with this device.
That the Turbohaler was easier to load and prime than the Diskhaler is not surprising as the Diskhaler requires several stages. Apart from removing the top—which all patients were able to do at 24 hours—each task requires good eyesight and dexterity. In particular removing the tray is difficult; three quarters of the patients in the Diskhaler group were unable to do this. Difficulties in loading and priming the Diskhaler contributed to subsequent poor scores for other aspects of inhaler technique.
Although our patients were in hospital, they were in the recovery stage of their illness when recruited. Elderly patients with influenza may be confused and very ill making them more likely to have difficulties using the Diskhaler than those patients in our study. In addition we excluded patients with poor cognitive function and gave up to 15 minutes of personal tuition in inhaler usage before and up to five minutes after initial assessment. Such levels of selection and tuition are impractical for elderly patients presenting with influenza to their doctor in the community. It is likely that elderly patients with influenza will have more difficulties using the Diskhaler than our patients who were about to return to the community.
Elderly people are at particular risk of serious illness if they contract influenza. It is possible that inhaled zanamivir is effective in ameliorating the symptoms, shortening the course of the disease, and reducing complications. More studies of the effectiveness of zanamivir treatment of influenza are needed, but without an improved delivery system they will be difficult to interpret. Our study shows that zanamivir treatment for elderly people with influenza is unlikely to be effective. Better delivery systems for inhalers should be used or developed.
What is already known on this topic
Inhaled zanamivir is effective in reducing the symptoms and duration of influenza
Elderly people have difficulty in using inhalers
What this study adds
Elderly patients are unlikely to be able to use the dry powder inhaler that is used to deliver zanamivir
Improvements should be made to the inhaler
Particular attention should be paid to the loading and priming of the device
Figure.
Trial profile
Acknowledgments
We thank Rosemary Kahn, respiratory nurse at the Mayday Chest clinic, for advice and assistance in developing standardised tuition for and assessment of inhaler usage. Placebo Turbohalers were provided by Astra and placebo Diskhalers by Glaxo Wellcome.
Footnotes
Funding: None.
Competing interests: None declared.
References
- 1.Monto AS. Influenza: quantifying morbidity and mortality. Am J Med. 1998;32:20–25. doi: 10.1016/0002-9343(87)90556-0. [DOI] [PubMed] [Google Scholar]
- 2.Department of Health. Immunisation against infectious diseases. London: DoH; 1996. pp. 120–133. [Google Scholar]
- 3.Anon. Influenza vaccination and older people. Effectiveness Matters. 1996;2:1–4. [Google Scholar]
- 4.Hayden FG, Osterhaus ADME, Treanor JJ, Fleming DM, Aoki FY, Nicholson KG, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza virus infections. New Engl J Med. 1997;337:874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- 5.MIST Study Group. Randomised trial of efficacy and safety of inhaled zanamivir in treatment of influenza A and B virus infections. Lancet. 1998;352:1877–1881. [PubMed] [Google Scholar]
- 6.Armitage JM, Williams SJ. Inhaler technique in the elderly. Age Ageing. 1988;17:275–278. doi: 10.1093/ageing/17.4.275. [DOI] [PubMed] [Google Scholar]
- 7.Elizabeth JE. Problems elderly patients have using metered dose inhalers. Geriatr Med. 1988;18:11–12. [Google Scholar]
- 8.Hodgkinson HM. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing. 1972;1:233–238. doi: 10.1093/ageing/1.4.233. [DOI] [PubMed] [Google Scholar]
- 9.Allen SC. Competence thresholds for the use of inhalers in people with dementia. Age Ageing. 1997;26:83–86. doi: 10.1093/ageing/26.2.83. [DOI] [PubMed] [Google Scholar]
- 10.Connolly M. Inhaler technique of elderly patients: comparison of metered-dose inhalers and large volume spacer devices. Age Ageing. 1995;24:190–192. doi: 10.1093/ageing/24.3.190. [DOI] [PubMed] [Google Scholar]
- 11.Jones VA, Fernandez C, Diggory P. A randomised prospective comparison of inhaler technique among elderly people using one of: Easi-breathe, Turbohaler or Metered dose inhaler and Volumatic inhalers for the first time. Age Ageing. 1999;28:481–484. [Google Scholar]
- 12.Pesson G, Gruvstad E, Stahl E. A new multiple dose powder inhaler (Turbohaler) compared with pressurised inhaler in a study of terbutaline in asthmatics. Eur Respir J. 1986;1:681–684. [PubMed] [Google Scholar]
- 13.Diggory P, Bailey R, Vallon A. Effectiveness of inhaled bronchodilator delivery systems in the elderly. Age Ageing. 1991;19:379–382. doi: 10.1093/ageing/20.5.379. [DOI] [PubMed] [Google Scholar]