Abstract
Purpose
To determine the tolerability and serum concentration of epratuzumab, a humanized monoclonal antibody targeting CD22, administered alone and in combination with reinduction chemotherapy in children with relapsed acute lymphoblastic leukemia (ALL), and to preliminarily assess tumor targeting and efficacy.
Patients and Methods
Therapy consisted of a single-agent phase (epratuzumab 360 mg/m2/dose intravenously twice weekly × four doses), followed by four weekly doses of epratuzumab in combination with standard reinduction chemotherapy. Morphologic and minimal residual disease (MRD) responses were determined at the end of this 6-week period. Serum concentrations of epratuzumab were determined before and 30 minutes after infusions, and CD22 targeting efficiency was determined by quantifying changes in CD22 expression after epratuzumab administration.
Results
Fifteen patients (12 fully assessable for toxicity) with first or later CD22-positive ALL marrow relapse enrolled on the feasibility portion of this study from December 2005 to June 2006. Two dose-limiting toxicities occurred: one grade 4 seizure of unclear etiology and one asymptomatic grade 3 ALT elevation. In all but one patient, surface CD22 was not detected by flow cytometry on peripheral blood leukemic blasts within 24 hours of drug administration, indicating effective targeting of leukemic cells by epratuzumab. Nine patients achieved a complete remission after chemoimmunotherapy, seven of whom were MRD negative.
Conclusion
Treatment with epratuzumab plus standard reinduction chemotherapy is feasible and acceptably tolerated in children with relapsed CD22-positive ALL. CD22 targeting was efficient, and the majority of patients achieved favorable early responses.
INTRODUCTION
Although 80% of children with newly diagnosed acute lymphoblastic leukemia (ALL) are cured of their disease, outcome is poor when the disease recurs. No greater than one third of children with relapsed ALL survive, independent of salvage regimen and prior therapy.1 The ability to successfully induce a second complete remission (CR2) is also limited compared with the more than 98% CR rate observed at initial diagnosis,2-4 and most patients who do achieve CR continue to have evidence of minimal residual disease (MRD) at the end of remission induction, a harbinger of early disease relapse.5,6 These data highlight the need to improve therapy for children with relapsed ALL, including developing more effective reinduction regimens that can minimize early disease burden.
CD22, a B-cell surface antigen, is highly expressed in more than 90% of cases of childhood B-precursor ALL (unpublished data). Epratuzumab, an investigational humanized monoclonal antibody, binds to the third extracellular domain of CD22. After binding, the receptor/antigen complex is rapidly internalized.7,8 In contrast to rituximab, which is directly cytotoxic to B cells, epratuzumab appears to modulate B-cell activation and signaling. In in vitro studies, mechanisms of action include antibody-dependent cellular cytotoxicity, CD22 phosphorylation, and proliferation inhibition with cross linking.9 Epratuzumab has been evaluated in adult patients with indolent and aggressive B-cell non-Hodgkin's lymphoma,10-14 and more recently has been used to treat adults with autoimmune diseases. 15-17 Favorable responses have been observed, with 43% overall response rates to single-agent epratuzumab therapy (360 mg/m2/dose) in patients with recurrent follicular lymphoma.11
One of the Children's Oncology Group's (COG's) strategies to evaluate novel antileukemia drugs efficiently is to assess the impact of the new agent, when administered as a component of a multidrug reinduction regimen, on early end points, such as remission reinduction rates and MRD burden. The primary objectives of this study were to establish the feasibility and to preliminarily assess the antitumor activity of epratuzumab administered as a single agent and in conjunction with chemotherapy in children with relapsed ALL. Epratuzumab was selected for study because of high CD22 expression levels in B-precursor ALL, a mechanism of action distinct from cytotoxic agents, and a toxicity profile that could allow for combining it with dose-intensive chemotherapy. The response to epratuzumab in adult patients with non-Hodgkin's lymphoma, and the prior success of chemoimmunotherapeutic approaches in other adult hematopoietic tumors,18 further supported this approach for childhood ALL.
PATIENTS AND METHODS
Patients and Eligibility
Patients 2 to 21 years of age with first or later ALL marrow relapse (M3 marrow) occurring at any time after initial diagnosis, with or without extramedullary disease, were eligible provided that their blasts expressed CD22 (≥ 25%). Additional eligibility requirements included a Karnofsky score of at least 50, or a Lansky score of at least 50, adequate organ function, and an initial WBC of no more than 50,000/μL. Although patients were required to have recovered from the acute toxic effects of prior therapy, there was no waiting period for study entry for children who experienced relapse while receiving standard ALL maintenance chemotherapy. Institutional review boards at participating institutions approved the study. Informed consent was obtained from patients age 18 years and older or from parents/legal guardians of children younger than 18 years, with child assent when appropriate, according to individual institutional policies.
Dosage and Drug Administration
Epratuzumab was supplied by Immunomedics Inc (Morris Plains, NJ) as a sterile liquid formulation, which was diluted with normal saline to a final concentration of 1 mg/mL. After premedication with acetaminophen and diphenhydramine, epratuzumab was administered as a slow intravenous infusion starting at a rate of 0.5 mg/kg/h, with gradual incremental increases in the rate to a maximum rate of 400 mg/h, as tolerated. Corticosteroids or meperidine could be administered for infusion reactions, but were not otherwise administered as routine premedication.
Trial Design
Patients received four doses of epratuzumab, 360 mg/m2/dose IV, twice weekly during the 14-day reduction phase, followed by four weekly doses, 360 mg/m2/dose, administered with block 1 chemotherapy (Table 1). After block 1, patients received blocks 2 and 3 of a standard reinduction chemotherapy regimen. The trial was initially designed to explore higher epratuzumab dose levels, but to expedite drug development, the trial was amended to only evaluate the adult phase II dose of 360 mg/m2/dose. Any patient who developed a WBC greater than 100,000/μL or symptoms of hyperleukocytosis during the 14-day reduction phase proceeded directly to block 1 chemoimmunotherapy.
Table 1.
Study Drug Dosing
| Study Phase | Dosing |
|---|---|
| Reduction phase | |
| Epratuzumab 360 mg/m2 | Days −14, −10, −6, and −2 (protocol amendment) |
| IT therapy* | Day −14, (Days −10 and −6, if CNS positive) |
| Block 1 | |
| Epratuzumab 360 mg/m2 | Days 8, 15, 22, and 29 |
| Vincristine 1.5 mg/m2 | Days 1, 8, 15, and 22 |
| Prednisone 40 mg/m2/d | Days 1-29 |
| PEG-asparaginase 2,500 U/m2 | Days 2, 9, 16, and 23 |
| Doxorubicin 60 mg/m2 | Day 1 |
| Dexrazoxane 600 mg/m2 | Day 1 |
| IT therapy | Days 15 and 29 (Days 1 and 15, if CNS positive) |
| Block 2 | |
| Cyclophosphamide 440 mg/m2 | Days 1-5 |
| Etoposide 100 mg/m2 | Days 1-5 |
| Methotrexate 5 g/m2 | Day 22 |
| IT therapy | Days 1 and 22 |
| Block 3 | |
| Cytarabine 3 g/m2 q 12 h | Days 1, 2, 8, and 9 |
| L-asparaginase 6,000 U/m2 | Days 2 and 9 (at hour 42 after cytarabine) |
Abbreviations: IT, intrathecally; PEG, pegylated.
IT cytarabine on day −14 of the reduction phase followed by methotrexate alone for all subsequent doses in patients who were CNS negative, and methotrexate, hydrocortisone, and cytarabine (ITT) for those who were CNS positive. All doses of IT medications were based on age.
Toxicities were graded according to the National Cancer Institute's Common Toxicity Criteria (version 3.0). Dose-limiting nonhematologic toxicity was defined as any grade 3 or 4 adverse event attributable to epratuzumab with the specific exclusion of grade 3 nausea or vomiting, grade 3 hepatic transaminase (AST and/or ALT) elevation returning to grade 1 before the next treatment course, grade 3 fever or infection, and alopecia. Dose-limiting hematologic toxicity was defined as absence of peripheral blood count recovery (absolute neutrophil count > 500/μL and platelet count > 20,000/μL) within 6 weeks of starting block 1 chemotherapy, in those patients who achieved remission, as documented by marrow aplasia, not marrow infiltration.
Response to epratuzumab alone was determined by conventional bone marrow aspirate morphology at the end of the 14-day reduction phase. Regardless of the response, patients went on to receive epratuzumab plus chemotherapy during block 1. At the end of block 1 (day 36), response was again assessed by conventional marrow morphology, and in addition, marrow MRD was measured by flow-cytometry at the COG Reference Laboratory at Johns Hopkins University (Baltimore, MD), as previously described.19
Criteria for Assessment of Response
Complete remission (CR) was defined as attainment of M1 bone marrow (< 5% blasts) with no evidence of circulating blasts or extramedullary disease and with recovery of peripheral counts (absolute neutrophil count > 1000/μL and platelet count > 100,000/μL). Partial remission (PR) was defined as complete disappearance of circulating blasts and achievement of M2 marrow status (≥ 5% to < 25% blast cells and adequate cellularity). Partial-remission cytolytic (PRCL) was defined as complete disappearance of circulating blasts and achievement of at least 50% reduction from baseline in bone marrow blast count. Progressive disease (PD) during the reduction phase was defined as an increase in the WBC to greater than 100,000/μL, or the development of symptoms attributable to a rapidly rising absolute blast count. In blocks 1 to 3 of reinduction therapy, it was defined as an increase of at least 25% in the absolute number of circulating leukemic cells, development of extramedullary disease, or other laboratory or clinical evidence of PD. Patients not fulfilling criteria for CR, PR, PRCL, or PD were considered to have stable disease (SD). Detectable MRD at any level was designated positive. Designation as MRD negative implied a sensitivity of 1/10,000 cells.
Serum Concentration Studies
Participation in the pharmacokinetic portion of the study was optional for patients in accordance with previously published guidelines.20 Blood samples (2 mL) were collected before and 30 minutes after epratuzumab infusions on days −14 and −2, before infusions on days −10 and −6, and at the end of the reduction phase. Epratuzumab concentrations were determined by enzyme-linked immunosorbent assay at Immunomedics Inc.
Flow Cytometry Assessment of CD22 Targeting
Peripheral-blood samples were obtained pretherapy, and on days −13, −6 and 0 of the reduction phase for determination of CD22 expression by flow cytometry. Samples were stained with the four-color combination CD10-fluorescein isothiocyanate/CD22-phycoerythrin (PE)/CD45-peridinin chlorophyll protein/CD19-allophycocyanin along with two different anti-CD22 monoclonal antibodies: SHCL-1 (BD Biosciences [BDB], San Jose, CA) and RFB4 (Caltag, Burlingame, CA). These antibodies bind to different epitopes on the extracellular portion of the CD22 molecule. The RFB4 antibody and epratuzumab competitively bind to the third extracellular domain of CD22, so in the presence of epratuzumab, RFB4 binding is blocked,21 whereas SHCL-1 binds to a non–cross-blocking epitope.22 Antibodies other than RFB4 were obtained from BDB. Samples were analyzed on a FACSCalibur flow cytometer using Cell-Quest software (BDB). Leukemic blasts were gated using a combination of CD45, CD19 and CD10, and fluorescence expression of both SHCL-1 and RFB4 were determined and quantified using Quantibrite software (BDB). Briefly, standard beads with known numbers of PE molecules bound were analyzed under the same conditions used for the experiments, and the geometric mean channel of the beads plotted to obtain a standard curve. Channel values of experimental samples, corrected for nonspecific background fluorescence, were then converted to PE molecules bound using this standard curve. Binding of CD22 (either RFB4 or SHCL-1) after epratuzumab administration was expressed as a percentage of pretreatment values.
RESULTS
From February 2005 to June 2006, 18 patients were enrolled onto the study. The first three patients were treated with epratuzumab, 360 mg/m2 weekly for two doses, before a study amendment to change epratuzumab dosing to twice weekly during the reduction phase. These first three patients are not included in the current analysis and did not experience toxicities that differed from the subsequent 15 patients. Among the 15 patients comprising this report, 12 were fully assessable for toxicity, with a median age of 10 years (range, 3 to 18 years) Two patients did not complete block 1 because of infection not attributed to epratuzumab, and one patient was removed by investigator choice during the reduction phase before receiving protocol-defined epratuzumab doses. Eleven patients were in first (n = 7 early, < 36 months; n = 4 late, ≥ 36 months from initial diagnosis)23 relapse, and four patients were in second or later marrow relapse (Table 2).
Table 2.
Patient Characteristics
| Characteristics | Number of Patients (n = 15) |
|---|---|
| Sex | |
| Male | 8 |
| Female | 7 |
| Age, years | |
| Median | 10 |
| Range | 3-18 |
| Presenting WBC, per μL | |
| Median | 3,950 |
| Range | 100-10,300 |
| Presenting absolute blast count, per μL | |
| Median | 384 |
| Range | 0-9,400 |
| Site/Timing of relapse | |
| First relapse | 11 |
| Early isolated marrow | 7 |
| Late isolated marrow | 1 |
| Late marrow + CNS | 2 |
| Late marrow + testicular | 1 |
| Second or greater relapse | 4 |
| Isolated marrow | 2 |
| Marrow + CNS | 2 |
NOTE. Early relapse, relapse occurring < 36 months from initial diagnosis; late relapse, relapse occurring ≥ 36 months from initial diagnosis.
Toxicity
Overall, epratuzumab was tolerated with acceptable toxicity during the reduction phase and block 1. Grade 1 or 2 infusion reactions, characterized by rigors, fever, and nausea, were observed in 10 of 15 patients during the reduction phase. Reactions occurred with the initial infusion only, and resolved after the infusion was temporarily stopped and additional medications (corticosteroids and/or meperidine) were administered. All patients were then able to resume and complete the initial infusions and did not experience subsequent reactions. Two patients experienced dose-limiting toxicities. One patient had a grade 4 seizure at the end of block 1; the etiology of the seizure was unclear and the patient subsequently developed progressive disease. A second patient experienced a grade 3 ALT elevation that failed to return to grade 1 before the time the block 2 therapy was scheduled to begin. Two patients died as a result of infections while receiving protocol therapy; one patient entered onto the study with a second relapse and a prior period of prolonged neutropenia, and the other child with a first early relapse of infant ALL. The status of their underlying leukemia at the time of death is unknown.
Response
Response to epratuzumab alone was assessed at the completion of the reduction phase. Eleven patients had SD, one patient had a PRCL response, and three had PD (Table 3). Median absolute blast counts decreased from 384/μL (range, 0 to 9400/μL) at study entry to 17/μL (range, 0 to 55,088/μL) at the end of the 2-week reduction phase. Only one patient showed a rise in absolute blast count to more than 50,000/μL during the reduction phase.
Table 3.
Response to Protocol Therapy
| Patient No. | Disease Characteristics | Single-Agent Epratuzumab (reduction phase)
|
Epratuzumab + Block 1 Chemotherapy
|
|||||
|---|---|---|---|---|---|---|---|---|
| Absolute Blast Count (per μL)
|
Marrow Blast (%)
|
|||||||
| Pre | Post | Pre | Post | Response | Response | MRD | ||
| 1 | 1st early M | 440 | 34 | 57 | 11 | SD | ND* | ND* |
| 2 | 1st late M + CNS | 328 | 0 | 94 | 84 | SD | CR | Negative |
| 3 | 2nd M + CNS | 9400 | < 300 | 90 | 95 | SD | ND* | ND* |
| 4 | 1st early M | 2016 | 0 | 71 | 34 | PRCL | CR | Negative |
| 5 | 2nd M + CNS | 384 | 66 | 85 | 37 | SD | CR | Negative |
| 6 | 2nd M | < 100 | 0 | 90 | 96 | SD | SD | Positive |
| 7 | 2nd M | 0 | 0 | 84 | 73 | SD | CR | Negative |
| 8 | 1st late M + T | 544 | 300 | 91 | 90 | SD | CR | Positive |
| 9 | 1st early M | 1863 | ND† | 83 | ND† | PD | ND† | ND† |
| 10 | 1st early M | 0 | 0 | 70 | 89 | SD | CR | Negative |
| 11 | 1st early M | 0 | 55,088 | 81 | 88 | PD | PD | Positive |
| 12 | 1st early M | 130 | 34,875 | 39 | ND‡ | PD | PR | Positive |
| 13 | 1st late M | 1442 | 0 | 90 | 86 | SD | CR | Negative |
| 14 | 1st late M + CNS | 2000 | 1000 | 98 | 82 | SD | CR | Negative |
| 15 | 1st early M | 0 | 0 | 65 | 34 | SD | CR | Positive |
Disease characteristics: M, marrow; T, testicular; CR, complete remission; PR, partial response; PRCL, partial remission cytolytic; SD, stable disease; PD, progressive disease; ND, not done.
Death during block 1.
Patient removed from study after one dose of epratuzumab.
Patient did not complete reduction phase because of PD.
Response to chemoimmunotherapy was determined at the end of block 1 (Table 3). Two patients died as a result of infections during block 1, and one patient was removed from protocol during the reduction phase at the discretion of the treating physician because of a rising WBC count. Nine patients achieved a complete remission. Remission was achieved in two of four patients with second or greater marrow relapse, three of seven patients with early marrow relapse and all four patients with late marrow relapse. Seven of the nine patients achieving a morphologic CR had no detectable MRD at the end of block 1. One additional patient who was MRD positive at the end of block 1 became MRD negative at the end of block 2.
Serum Concentrations of Epratuzumab
Serum epratuzumab concentrations increased with the initial twice-weekly dosing, with median values of 72 μg/mL (range, 36 to 97 μg/mL), 146 μg/mL (range, 64 to 171 μg/mL), and 163 μg/mL (range, 79 to 222 μg/mL) μg/mL determined by enzyme-linked immunosorbent assay in a subgroup of seven patients with samples obtained before their second, third, and fourth doses on days −10, −6 and −2, respectively.
CD22 Targeting
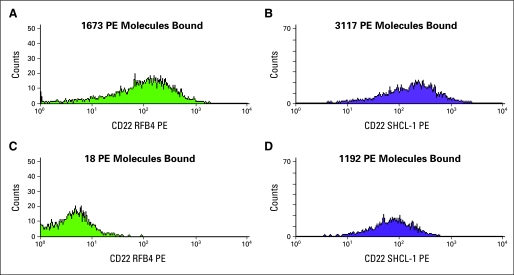
The efficiency of CD22 targeting was determined by quantitatively assessing changes in CD22 expression after epratuzumab administration in 11 patients. Levels of CD22 expression on blood blasts at baseline varied over approximately two logs. Irrespective of baseline antigen levels, 10 of 11 patients who opted to participate in these studies showed at least 98% reduction of RFB4 binding 24 hours after the initial dose of epratuzumab, and the other (#15) showed 95% reduction (Table 4). Persistent targeting of epratuzumab was observed throughout the reduction phase, as evidenced by complete abrogation of RFB4 binding in all but two patients (#4 and #8) that showed incomplete (approximately 85%) blocking of RFB4 at day 0. Data from a representative patient are shown in Figure 1.
Table 4.
Binding of Anti-CD22 Antibodies RFB-4 and SHCL-1 to Leukemic Cells
| No. of PE Molecules Bound at Study Entry
|
% of Pretreatment Levels
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Patient No. | Pretreatment
|
RFB4
|
SHCL1
|
|||||
| RFB4 | SHCL1 | Day −13 | Day −6 | Day 0 | Day −13 | Day −6 | Day 0 | |
| 1 | 19,596 | 27,225 | 2.4 | 1.4 | 0.3 | 15.9 | 9.0 | 16.7 |
| 2 | 3,071 | 4,274 | 1.3 | 0.8 | 0.7 | 29.3 | 27.5 | 39.1 |
| 3 | 10,313 | 13,342 | 1.9 | 0.8 | 0.6 | 19.1 | 14.6 | 16.1 |
| 4 | 227 | 398 | 0 | 0 | 15.9 | 19.3 | 70.4 | 46.7 |
| 5 | 3,779 | 5,158 | 0.1 | 0.9 | 0 | 13.3 | 11.9 | 30.7 |
| 6 | 296 | 438 | 1.7 | 0 | 1.4 | 51.4 | 32.9 | 25.8 |
| 7 | 6,677 | 8,408 | 0.3 | 0.1 | 0 | 22.1 | 19.1 | 26.2 |
| 8 | 5,850 | 7,181 | NT | 0 | 14.1 | NT | 17.5 | 22.3 |
| 9 | 1,673 | 3,117 | 1.1 | 2 | NT | 38.2 | 22.7 | NT |
| 10 | 9,953 | 13,650 | 0.1 | 1.9 | 0.1 | 42.7 | 15.2 | 29.4 |
| 11 | 836 | 1,125 | NT | 1.8 | 2 | NT | 100 | 100 |
| 12 | 2,109 | 3,229 | NT | 0.7 | 1.3 | NT | 21.9 | 26.7 |
| 13 | 5,892 | 7,922 | 0.1 | 0.2 | 0 | 32.5 | 20.7 | 21.8 |
| 14 | NT | NT | NT | NT | NT | NT | NT | NT |
| 15 | 6,011 | 9,207 | 5.3 | NT | NT | 12.5 | NT | NT |
| Median | 4,814 | 6,170 | 1.1 | 0.8 | 0.65 | 22.1 | 21.3 | 26.7 |
Abbreviations: PE, phycoerythrin; NT, not tested, samples not submitted.
Fig 1.
Changes in CD22 antibody expression after administration of epratuzumab. Four-color flow cytometry was performed, and CD22 binding quantified on gated leukemic blasts as described in Patients and Methods. Pretreatment expression of (A) RFB4 and (B) SHCL-1 in patient 9. Expression of (C) RFB4 and (D) SHCL-1 24 hours after administration of epratuzumab to the same patient. PE, phycoerythrin.
After treatment with epratuzumab, there was a significant difference between binding of the non–cross-reacting antibody SHCL-1 and that seen with RFB4. In all but one patient, and at all time points, levels of SHCL1 were lower than what was observed in the pretreatment sample, but the levels of residual binding varied greatly both among patients and at different time points. The lowest level of residual binding seen with any sample was 9% (ie, 91% inhibition) and the highest 70%. These results suggest partial loss of CD22/epratuzumab complex from the cell surface. The remaining patient showed no decrement in levels of baseline binding, suggesting no change in the CD22/epratuzumab complex after drug administration. At day −6 and day 0, but not day −13, the magnitude of the SHCL-1 decrement was correlated with pretreatment levels of CD22, with blasts with the highest levels showing the greatest loss of SHCL-1 binding (data not shown).
DISCUSSION
Despite improvements in outcome for children with newly diagnosed ALL, the treatment of relapsed disease remains a significant challenge.1,2,4,24-26 Epratuzumab is the first new agent to be evaluated in combination with a three-block cytotoxic reinduction regimen for marrow relapse that was originally studied in 127 children in the COG AALL01P2 trial.27 Overall, epratuzumab administration was tolerated with acceptable toxicity in children with relapsed ALL, both as a single agent and when combined with chemotherapy. The most frequent single-agent toxicities observed were grade 1 or 2 infusion reactions that resolved with supportive medications and prolongation of the infusion; reactions did not recur with subsequent doses. The toxicity profile is similar to that observed in adults.11-14 Given the high-risk population, the two deaths secondary to infection observed on the current study were not unexpected. In our prior AALL01P2 study limited to children with first marrow relapse,27 the toxic death rate with block 1 chemotherapy alone was 4%, with a 40% incidence of febrile neutropenia and a 19% incidence of documented infections.
Although efficacy was not a primary objective of this feasibility study, the preliminary outcome of patients enrolled, including the early regression in MRD, an important prognostic indicator at the time of relapse,5,6 was encouraging. Historical remission reinduction rates for early, late, and second or greater marrow relapse are approximately 70%, 95%, and 40%, respectively, despite heterogeneity in the reinduction regimens.2,3,26,28-34 The majority of patients enrolled onto this study had higher risk relapses, which included seven early and four second or greater relapses. At the end of block 1 chemoimmunotherapy, nine patients achieved CR. The responses compared favorably with those observed with the three-block chemotherapy platform alone, in which the CR2 rates at the end of block 1 for early and late first marrow relapse were 68% ± 6% and 96% ± 3%, respectively, and among those in CR2, MRD more than 0.01% was detected at the end of block 1 in 75% ± 7% of those with early relapses versus 51% ± 8% of those with late relapses.27 In this study, seven of nine patients achieving CR had no detectable MRD at the end of block 1.
Similar to its performance in adult studies,35 epratuzumab concentration increased steadily with each administered dose during the reduction phase. During the reduction phase of this study, we also had the opportunity to assess the binding efficiency of CD22. With the use of the RFB4 antibody, which competitively binds to the same extracellular domain of CD22 as epratuzumab,21 we found essentially complete saturation of CD22 by epratuzumab in the vast majority of patients. This pattern of effective targeting was sustained in the majority of patients throughout the reduction phase. The use of the alternate SHCL-1 antibody, which binds to a different epitope of CD22, allowed us to analyze the fate of the CD22/epratuzumab complex. Some residual CD22 expression was seen after epratuzumab administration, although it was greatly reduced from baseline levels. Although we cannot exclude the possibility that this reflects shedding of CD22 bound by epratuzumab from the cell surface, it appears more likely that this reduction is a result of partial internalization of the CD22/epratuzumab complex, which has been demonstrated in vitro in prior studies.8 The degree of internalization correlated to some degree with baseline levels of CD22 antigen expression, with those patients with low levels showing relatively poor internalization. Of interest, the one patient who showed no change in the level of SHCL-1 binding from baseline (and thus no internalization) had progressive disease during the reduction phase.
In conclusion, this initial experience with epratuzumab in children with relapsed CD22-positive ALL has demonstrated that this chemoimmunotherapy combination has an acceptable toxicity profile. CD22 was efficiently targeted over time in the majority of patients, and preliminary evidence of efficacy was observed. The favorable rate of MRD after administration of chemotherapy with epratuzumab suggests that the antibody may enhance response to cytotoxic chemotherapy. Thus, the phase II portion of this study is currently underway to determine whether remission reinduction rates and MRD responses at the end of block 1 with chemoimmunotherapy are superior to those achieved with the chemotherapy platform alone.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: David M. Goldenberg, Immunomedics Inc (C); William A. Wegener, Immunomedics Inc (C) Consultant or Advisory Role: None Stock Ownership: David M. Goldenberg, Immunomedics Inc; William A. Wegener, Immunomedics Inc Honoraria: None Research Funding: Michael J. Borowitz, BD Biosciences; Peter C. Adamson, National Cancer Institute Expert Testimony: None Other Remuneration: David M. Goldenberg, Immunomedics Inc
AUTHOR CONTRIBUTIONS
Conception and design: Elizabeth Raetz, Mitchell S. Cairo, Michael J. Borowitz, William L. Carroll, Peter C. Adamson
Financial support: Michael J. Borowitz, William L. Carroll
Administrative support: Susan M. Blaney, William L. Carroll
Provision of study materials or patients: Mitchell S. Cairo, Susan M. Blaney, David M. Goldenberg, William L. Carroll
Collection and assembly of data: Elizabeth Raetz, Michael J. Borowitz, Susan M. Blaney, Mark D. Krailo, William L. Carroll
Data analysis and interpretation: Elizabeth Raetz, Mitchell S. Cairo, Michael J. Borowitz, Susan M. Blaney, Mark D. Krailo, Tarek Leil, Joel Reid, William Wegener, William L. Carroll, Peter C. Adamson
Manuscript writing: Elizabeth Raetz, Mitchell S. Cairo, Michael J. Borowitz, Susan M. Blaney, David M. Goldenberg, William Wegener, William L. Carroll, Peter C. Adamson
Final approval of manuscript: Elizabeth Raetz, Mitchell S. Cairo, Michael J. Borowitz, Mark D. Krailo, David M. Goldenberg, William Wegener, William L. Carroll, Peter C. Adamson
Supported in part by Children's Oncology Group Grant No. U10 CA98543 from the National Cancer Institute.
Presented in part at 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Saarinen-Pihkala UM, Heilmann C, Winiarski J, et al: Pathways through relapses and deaths of children with acute lymphoblastic leukemia: Role of allogeneic stem-cell transplantation in Nordic data. J Clin Oncol 24:5750-5762, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Einsiedel HG, von Stackelberg A, Hartmann R, et al: Long-term outcome in children with relapsed ALL by risk-stratified salvage therapy: Results of trial acute lymphoblastic leukemia-relapse study of the Berlin-Frankfurt-Munster Group 87. J Clin Oncol 23:7942-7950, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Gaynon PS, Harris RE, Altman AJ, et al: Bone marrow transplantation versus prolonged intensive chemotherapy for children with acute lymphoblastic leukemia and an initial bone marrow relapse within 12 months of the completion of primary therapy: Children's Oncology Group study CCG-1941. J Clin Oncol 24:3150-3156, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Rivera GK, Zhou Y, Hancock ML, et al: Bone marrow recurrence after initial intensive treatment for childhood acute lymphoblastic leukemia. Cancer 103:368-376, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Coustan-Smith E, Gajjar A, Hijiya N, et al: Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia after first relapse. Leukemia 18:499-504, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Eckert C, Biondi A, Seeger K, et al: Prognostic value of minimal residual disease in relapsed childhood acute lymphoblastic leukaemia. Lancet 358:1239-1241, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Shih LB, Lu HH, Xuan H, et al: Internalization and intracellular processing of an anti-B-cell lymphoma monoclonal antibody, LL2. Int J Cancer 56:538-545, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Carnahan J, Wang P, Kendall R, et al: Epratuzumab, a humanized monoclonal antibody targeting CD22: Characterization of in vitro properties. Clin Cancer Res 9:3982S-90S, 2003 [PubMed] [Google Scholar]
- 9.Carnahan J, Stein R, Qu Z, et al: Epratuzumab, a CD22-targeting recombinant humanized antibody with a different mode of action from rituximab. Mol Immunol 44:1331-1341, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Leonard JP, Coleman M, Ketas J, et al: Combination antibody therapy with epratuzumab and rituximab in relapsed or refractory non-Hodgkin's lymphoma. J Clin Oncol 23:5044-5051, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Leonard JP, Coleman M, Ketas JC, et al: Phase I/II trial of epratuzumab (humanized anti-CD22 antibody) in indolent non-Hodgkin's lymphoma. J Clin Oncol 21:3051-3059, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Leonard JP, Coleman M, Ketas JC, et al: Epratuzumab, a humanized anti-CD22 antibody, in aggressive non-Hodgkin's lymphoma: Phase I/II clinical trial results. Clin Cancer Res 10:5327-5334, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Strauss SJ, Morschhauser F, Rech J, et al: Multicenter phase II trial of immunotherapy with the humanized anti-CD22 antibody, epratuzumab, in combination with rituximab, in refractory or recurrent non-Hodgkin's lymphoma. J Clin Oncol 24:3880-3886, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Micallef IN, Kahl BS, Maurer MJ, et al: A pilot study of epratuzumab and rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy in patients with previously untreated, diffuse large B-cell lymphoma. Cancer 107:2826-2832, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Steinfeld SD, Tant L, Burmester GR, et al: Epratuzumab (humanised anti-CD22 antibody) in primary Sjogren's syndrome: An open-label phase I/II study. Arthritis Res Ther 8:R129, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dörner T, Kaufmann J, Wegener WA, et al: Initial clinical trial of epratuzumab (humanized anti-CD22 antibody) for immunotherapy of systemic lupus erythematosus. Arthritis Res Ther 8:R74, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldenberg DM: Epratuzumab in the therapy of oncological and immunological diseases. Expert Rev Anticancer Ther 6:1341-1353, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Coiffier B, Lepage E, Briere J, et al: CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346:235-242, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Borowitz MJ, Pullen DJ, Shuster JJ, et al: Minimal residual disease detection in childhood precursor-B-cell acute lymphoblastic leukemia: Relation to other risk factors—A Children's Oncology Group study. Leukemia 17:1566-1572, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Anderson BD, Adamson PC, Weiner SL, et al: Tissue collection for correlative studies in childhood cancer clinical trials: Ethical considerations and special imperatives. J Clin Oncol 22:4846-4850, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Stein R, Belisle E, Hansen HJ, et al: Epitope specificity of the anti-(B cell lymphoma) monoclonal antibody, LL2. Cancer Immunol Immunother 37:293-298, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tedder TF, Tuscano J, Sato S, et al: CD22, a B lymphocyte-specific adhesion molecule that regulates antigen receptor signaling. Annu Rev Immunol 15:481-504, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Gaynon PS: Childhood acute lymphoblastic leukaemia and relapse. Br J Haematol 131:579-587, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Chessells JM, Veys P, Kempski H, et al: Long-term follow-up of relapsed childhood acute lymphoblastic leukaemia. Br J Haematol 123:396-405, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Gaynon PS, Qu RP, Chappell RJ, et al: Survival after relapse in childhood acute lymphoblastic leukemia: Impact of site and time to first relapse—The Children's Cancer Group Experience. Cancer 82:1387-1395, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Roy A, Cargill A, Love S, et al: Outcome after first relapse in childhood acute lymphoblastic leukaemia: Lessons from the United Kingdom R2 trial. Br J Haematol 130:67-75, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Raetz EA, Borowitz MJ, Devidas M, et al: A re-induction platform for children with first marrow relapse of acute lymphoblastic leukemia: Results from Children's Oncology Group study AALL01P2. J Clin Oncol (in press) [DOI] [PMC free article] [PubMed]
- 28.Buchanan GR, Rivera GK, Boyett JM, et al: Reinduction therapy in 297 children with acute lymphoblastic leukemia in first bone marrow relapse: A Pediatric Oncology Group Study. Blood 72:1286-1292, 1988 [PubMed] [Google Scholar]
- 29.Feig SA, Ames MM, Sather HN, et al: Comparison of idarubicin to daunomycin in a randomized multidrug treatment of childhood acute lymphoblastic leukemia at first bone marrow relapse: A report from the Children's Cancer Group. Med Pediatr Oncol 27:505-514, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Sadowitz PD, Smith SD, Shuster J, et al: Treatment of late bone marrow relapse in children with acute lymphoblastic leukemia: A Pediatric Oncology Group study. Blood 81:602-609, 1993 [PubMed] [Google Scholar]
- 31.Testi AM, Del Giudice I, Arcese W, et al: A single high dose of idarubicin combined with high-dose ARA-C for treatment of first relapse in childhood ‘high-risk’ acute lymphoblastic leukaemia: A study of the AIEOP group. Br J Haematol 118:741-747, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Crooks GM, Sato JK: Ifosfamide and etoposide in recurrent childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol 17:34-38, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Kolb EA, Steinherz PG: A new multidrug reinduction protocol with topotecan, vinorelbine, thiotepa, dexamethasone, and gemcitabine for relapsed or refractory acute leukemia. Leukemia 17:1967-1972, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Wells RJ, Feusner J, Devney R, et al: Sequential high-dose cytosine arabinoside-asparaginase treatment in advanced childhood leukemia. J Clin Oncol 3:998-1004, 1985 [DOI] [PubMed] [Google Scholar]
- 35.Leonard JP, Goldenberg DM: Preclinical and clinical evaluation of epratuzumab (anti-CD22 IgG) in B-cell malignancies. Oncogene 26:3704-3713, 2007 [DOI] [PubMed] [Google Scholar]