Abstract
Progression-free survival is an important end point in advanced disease settings. Blinded independent central review (BICR) of progression in randomized clinical trials has been advocated to control bias that might result from errors in progression assessments. However, although BICR lessens some potential biases, it does not remove all biases from evaluations of treatment effectiveness. In fact, as typically conducted, BICRs may introduce bias because of informative censoring, which results from having to censor unconfirmed locally determined progressions. In this article, we discuss the rationale for BICR and different ways of implementing independent review. We discuss the limitations of these approaches and review published trials that report implementing BICR. We demonstrate the existence of informative censoring using data from a randomized phase II trial. We conclude that double-blinded trials with consistent application of measurement criteria are the best means of ensuring unbiased trial results. When such designs are not practical, BICR is not recommended as a general strategy for reducing bias. However, BICR may be useful as an auditing tool to assess the reliability of marginally positive results.
INTRODUCTION
Randomized clinical trials are the gold standard for shaping clinical practice by providing definitive evaluation of treatment efficacy. In oncology, overall survival (OS) is the most objective end point to measure patient benefit. In some settings, however, progression-free survival (PFS) is preferable. For example, effective salvage therapy may reduce the effect of experimental therapy on OS. In these situations, PFS is often used to assess a treatment's inherent efficacy in advanced disease. PFS may also represent clinical benefit on its own.1
Several potential sources of bias must be addressed when PFS is the primary end point. Whereas OS can be assessed objectively and the timing of the event is known, progression assessment has an inherently subjective component and is not assessed continuously. Evaluation-time bias,2 which results from systematic differences in evaluation times according to treatment arm, is not considered here. Another source of bias is attrition bias, in which patient dropout varies by treatment arm. Although attrition bias can be problematic even with OS, it may be more problematic with PFS since, once lost, a patient's progression time is difficult to determine, while time of death can usually be recovered.
In this article we focus on a third source of bias—bias that may be introduced by the potentially subjective components of imaging end point assessment. While important efforts both to minimize subjectivity and to improve consistency of radiologic end point assessment have been made,3,4 end point evaluation still depends to some extent on the individual reviewing the image and the time-point at which s/he reviews it. The potential for subjective elements to affect end point assessment differentially across treatment arms leads to concerns about biased evaluation, particularly in unblinded trials.5 Blinded independent central review (BICR) is one strategy proposed to reduce the potential for this bias.
After discussing the rationale for independent end point assessment, we review different ways to incorporate BICR, describing its limitations and benefits. We argue that, while blinding is a generally desirable means of reducing potential bias, BICR may itself introduce bias into treatment efficacy estimation. The pros and cons of including a BICR for any specific trial will need careful consideration during trial development.
MOTIVATION FOR INDEPENDENT END POINT EVALUATION
A BICR might be used to reduce potential systematic imaging reader bias as well as to decrease measurement variability.
Reducing Bias
The conjecture of imaging reader bias assumes that the reader systematically either over- or underinterprets tumor shrinkage. For example, differences in response rates determined by investigator and independent assessments have been documented in many studies, with the latter frequently reporting lower response rates, especially in single-arm phase II studies.6,7 Such differences may be part of the rationale for recommendations of independent evaluations by the US Food and Drug Administration.8 However, the extent to which these differences result in systematically biased comparisons is not obvious because discordant evaluations do not necessarily imply that estimates of treatment effect are biased.
In the present application, imaging reader bias assumes that the reader's interpretation can be influenced by his/her knowledge of treatment assignment in a way that results in an incorrect assessment of the therapy's effect on PFS. One potential situation in which this could occur is a setting in which an experimental therapy is tested against a control treatment with cross-over to the experimental therapy at the time of progression. It is conceivable that, in some borderline cases in unblinded trials, clinicians may tend to call a patient's disease progression on the control arm earlier than if that patient had been on the experimental arm. In other words, treatment knowledge may predispose the clinician to keep a patient on the experimental treatment as long as possible and to alter therapy for a patient on the control arm at the first suggestion of progression.
As a practical note, with certain cytostatic agents, investigators may wish to administer the agent beyond radiographic progression under the belief that continued administration will result in maximum benefit. This gives incentive for investigators to declare progression later if the protocol requires treatment cessation at progression. Explicit protocol language allowing continued treatment after progression may minimize this source of bias.
When feasible, any potential for knowledge of treatment assignment to influence outcome assessment should be eliminated. In a double-blind trial, the potential for biased end point evaluation is minimized. In nonblinded trials, BICR is one means of ensuring this because assessments are made without knowledge of treatment assignment or patient toxicities. While the rationale for implementing BICRs in trials is clear, there are several limitations, which will be discussed in the next section. First, we discuss measurement variability.
Reducing Measurement Variability
Another purpose of BICR is to reduce measurement variability (measurement error; ie, random discrepancies unrelated to treatment assignment). Even with blinded assessments of images, two readers may disagree on progression status, especially with borderline or complex cases. Further, discrepancies may result from tracking different target lesions or from missing development of a new lesion that is small at its first appearance. Discrepancies alone do not indicate systematic bias in the evaluation of the treatment effect.
Measurement variability makes treatments appear more similar than they really are, and therefore leads to reduced power to detect true treatment effects. Central review by a small number of reviewers with expertise in a specific area may lessen measurement variability. However, even with measurement variability, tests of the null hypothesis (that the treatments are equally effective) will still be valid—type I error will not be inflated. For example, in a recently presented phase III trial9 that implemented a BICR, the random discrepancy rate between two central reviewers blinded to treatment assignment was 34.7%.10,11 Since the two central reviewers were blinded to treatment assignment, these discrepancies reflect measurement variability and not biased end point evaluation. Protection against a type I error will be preserved here but treatments will appear less effective than in reality.
An important consideration in minimizing measurement variability is to ensure that a single reader evaluates the full set of exams for any given patient.12 Further, observers should encounter images in sequence, as reader consistency may be improved when the observer is presented with images in a time-ordered manner. An additional issue is the selection of target lesions. Tracking the same target lesions will reduce the discrepancy rate but not necessarily measurement variability as the central review may identify more relevant target lesions. We note that if the goal of a BICR is to assess local evaluation bias, tracking the same lesions is advocated.
INCORPORATING INDEPENDENT REVIEW INTO TRIAL DESIGN
The results of an independent review can be used in the primary analysis or sensitivity analyses. In either case, there are various options for incorporating it into the trial design.
Option 1. BICR in Real Time
From a trial design perspective, an ideal evaluation of PFS (if a double-blinded trial is infeasible) would be BICR contemporaneous with the treating physician's decision making. Central to this option is that the central review evaluation is the basis for any decision to continue or alter treatment. Although this approach is ideal from a treatment evaluation perspective (as will be clear shortly), it has practical, ethical, and legal hurdles that may prohibit its implementation. First, all sites must have the ability to transfer images to the central review without delay. This requires special technologies and additional effort by the local site, although broad availability of network technology has made this hurdle less problematic. A potentially greater barrier is that the local site must cede the final determination of a patient's status to the central review. The treating physician may resist granting such authority, and legal issues must be considered.
Option 2. Retrospective BICR
Images are reviewed retrospectively by an expert independent reader(s) blinded to treatment assignment. An adjudication process to resolve discrepancies may be considered in some cases. Adjudicator knowledge of the unblinded (local) radiologist's evaluation has the potential to introduce bias into centralized review; however, it may reduce measurement variation. This option could also be implemented only when local end point evaluations indicate a positive trial. This restricts much of the cost of central review to only those trials where central review is most likely to have an impact. In such cases, prospective collection of scans is recommended.
This option is not without complications. Perhaps the greatest problem occurs when the locally determined progression time occurs before the BICR-determined time. This discrepancy presents complexities for analysis that may bias results. In particular, once a patient has progressed according to the local site, s/he will be off protocol and further imaging is unlikely. Treatment could also change. As a result, determination of the BICR progression time may be impossible. The US Food and Drug Administration has suggested censoring such cases8; however, this is problematic. This type of censoring will be informative—patients who progress according to local review (but not according to central review) will be more likely to progress by the next scheduled scan than patients who have not been determined to progress by local review. This is discussed further in the next section.
Option 3. Extra Scans After Local Progression
For all cases (or just cases that are near the boundary of progression), another possibility is to perform at least one additional scan after local progression is called. The presumption is that more reliable documentation of radiologic progression from a central review is likely with an extra scan. This should be required per protocol if this approach is going to be taken, but even then may be difficult to implement.
Option 4. Central Review–Directed Follow-Up
A policy that allows real-time central review to direct follow-up (but that does not guide treatment changes) would alleviate some of the problems of informative censoring discussed under option 2. Specifically, any patient for whom the central review was not confirmed centrally would continue to be followed until progression was documented. Although any change in therapy as a result of the locally determined progression time may confound evaluation of the true treatment effect, the effect of informative censoring is reduced. This approach requires the ability to perform real-time central review, which may not be possible.
Option 5. Blinded Local Review
Blinding the local radiologist to treatment assignment and using her/his report exclusively for progression determination would eliminate the potential for biased end point evaluation. A potential for bias arises when the clinical investigator, with knowledge of treatment assignment, uses radiologic evaluations, but makes the definitive determination of progression. In such cases, one would use the radiologist's assessment for the analysis. Informative censoring is a potential problem here if the patient is not followed after the investigator calls a progression when the radiologist did not. Again, option 3 could mitigate this problem.
PHASE III TRIALS THAT HAVE USED BICR IN THE LITERATURE
We reviewed the literature for studies in breast, colorectal, lung, and renal cell cancer (Table 1). We found that option 2, retrospective BICR, has been implemented in several trials. In all trials, differences between the local and central review did not produce different conclusions about treatment efficacy, in spite of relatively high discrepancy rates.
Table 1.
Trials That Have Used Retrospective Blinded Independent Central Reviews
| Trial | Sample Size | Discrepancy Rate Between Central and Local Progression Times | Per Central Review
|
Per Local Review
|
|||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | P | |||
| Renal cell carcinoma | |||||||
| Sorafenib v placebo*17 | 903 | NR | 0.44 | 0.35 to 0.55 | 0.51 | 0.43 to 0.60 | |
| Sunitinib v interferon alpha18 | 750 | NR | 0.42 | 0.32 to 0.54 | 0.42 | 0.33 to 0.52 | |
| Colorectal cancer | |||||||
| Panitumumab plus best supportive care v best supportive care19 | 463 | NR | 0.54 | 0.44 to 0.66 | 0.39 | 0.32 to 0.48 | |
| Breast cancer | |||||||
| Lapatinib plus capecitabine v capecitabine20 | 324 | Lapatinib plus capecitabine, 40 of 163 = 25%; capecitabine, 40 of 161 = 25% | 0.49 | 0.34 to 0.71 | 0.59 | 0.42 to 0.84 | |
| Bevacizumab plus capecitabine v capecitabine21 | 462 | 105 of 462 = 23%† | 0.98 | 0.77 to 1.25 | NR, value not significantly different from 1 | ||
| Bevacizumab plus paclitaxel v paclitaxel10,11 | 722 | 232 of 649 = 36%‡ | 0.42 | 0.34 to 0.52 | 0.48 | 0.39 to 0.61 | |
| Ixabeplone plus capecitabine v capecitabine,22 months | 752 | NR | NR | NR | |||
| Median PFS | 5.8 v 4.2 | 5.3 v 3.8 | < .0011 | ||||
| 95% CI | 5.45 to 6.97 v | NR | |||||
| 3.81 to 4.50 | |||||||
NOTE. We reviewed the literature and searched PubMed for studies in breast cancer, colorectal cancer, lung cancer and renal cell carcinoma. Search terms included, "progression- free survival" or "time to progression," with filters of "randomized controlled trial" and "published in last five years." This revealed 209 manuscripts, of which only six reported having a central review of progression. The bevacizumab plus paclitaxel trial in breast cancer (last row) was included separately because it generated much discussion during an US Food and Drug Administration Oncologic Drug Advisory Committee meeting on December 5, 2007. All of these trials implemented a retrospective blinded independent central review (option 2). The panitumimab trial11 allowed cross-over at the time of locally determined progression among patients receiving the control treatment. As a result, patients for whom progression was not confirmed centrally continued to be evaluated centrally for progression.
Abbreviations: HR, hazard ratio; NR, not reported, PFS, progression-free survival.
Double-blinded trial.
Seventy-nine of the 105 discrepancies were investigator to determined progressions unconfirmed by central review. The investigators state that the investigator progressions were most commonly called by physical exam. The remaining 26 progressions were called by the independent review before the locally determined time.
The discrepancy rate was estimated amongst the 649 patients for whom images were available for central review. Due to limited data availability, an agreement was counted if dates were within 6 weeks of one another.
Informative Censoring and Central Review
Standard analyses assume that the progression course of censored individuals is the same as those remaining under observation; if not, censoring is informative and will bias results. Under option 2, we noted that central review would lead to informative censoring when a local progression cannot be confirmed centrally. In general, this type of informative censoring will make the survival course of those on a particular treatment appear more favorable than in truth. If censoring occurs similarly across treatment arms, hypothesis testing should be valid (ie, type I error is preserved). However, if informative censoring occurs more frequently in one arm, bias will result. It is reasonable to speculate that, in some studies, more informative censoring will occur in the control arm. In such cases, the biased and more optimistic survival curve of the control arm will attenuate estimates of the effectiveness of the experimental therapy.
To examine the potential for informative censoring in an actual trial, we utilize data from a double-blind randomized phase II trial of bevacizumab in renal cell carcinoma.13 This trial is somewhat unique because patients continued to be followed after local (or, in this case, investigator-assessed) progression calls. One-hundred sixteen patients were randomized to high- or low-dose bevacizumab or placebo. The primary end point was time to progression, which was confirmed according to a BICR. Because patients were followed after investigator-assessed progression was determined, there is a subset of patients for whom centrally reviewed time of progression was allocated to a time point later than that of the local reviewer (n = 116). Data sets with follow-up after local progression are rare in the literature because most trials with central review do not continue to monitor patients after local progression.
Of the 116 patients, 107 had both centrally and locally determined PFS times. Of these, there were 42 patient cases (39%) in which the two reviews disagreed (Table 2). Discrepancies are not necessarily of concern with time-to-event end points, particularly when discrepant event times are not far apart. The majority (33 of 42; 79%) of discrepancies in this trial occurred within one evaluation period of each other. Indeed, despite the high discrepancy rate, the hazard ratios were close under both reads (rows 1 v row 2 of Table 3).
Table 2.
Agreement/Disagreement in Progression Times by Central and Local Reviewers from Yang et al13
| Progression Time | No. of Patients
|
||
|---|---|---|---|
| Placebo | Low Dose | High Dose | |
| Local progression called more than 60 days after central | 3 | 0 | 3 |
| Local progression called 1 to 60* days after central review | 6 | 6 | 8 |
| Local and central progression times equal | 27 | 22 | 16 |
| Central progression called 1 to 60 days after local review | 2 | 5 | 6 |
| Central progression called more than 60 days after local | 2 | 0 | 1 |
| Total | 40 | 33 | 34 |
After a first evaluation, patients were evaluated every 2 months (approximately 60 days) for progression the first year then every 3 months thereafter.
Table 3.
Hazard Ratios from Yang et al13
| Low Dose v Placebo
|
High Dose v Placebo
|
|||
|---|---|---|---|---|
| HR for PFS | 95% CI | HR for PFS | 95% CI | |
| Central review | 0.68 | 0.42 to 1.1 | 0.37 | 0.22 to 0.67 |
| Local review | 0.82 | 0.51 to 1.31 | 0.47 | 0.29 to 0.77 |
| Central review with censoring at unconfirmed local progression* | 0.68 | 0.40 to 1.14 | 0.38 | 0.21 to 0.68 |
NOTE. The original manuscript reports hazard ratios (HRs) based on adjudicated progression times and reports time to progression, not progression-free survival (PFS).
Subjects for whom local progressions were called before the centrally determined time were artificially censored.
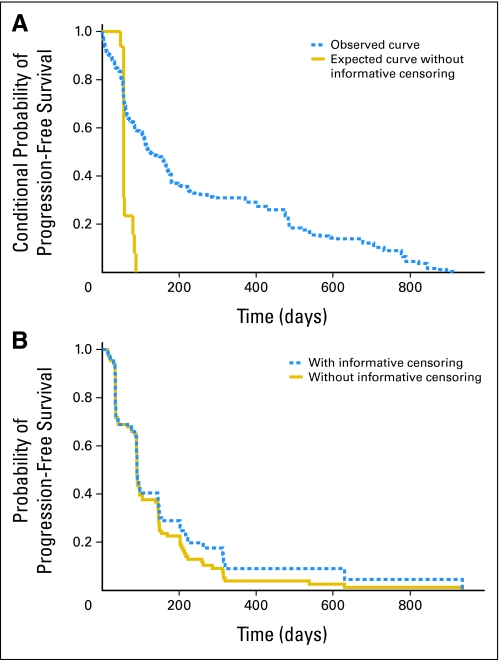
Of the discrepancies, 38% (16 of 42) were cases in which the locally determined progression time was before the centrally reviewed progression time. These are the cases that would be censored in practice when patients are not followed after locally designated progression. Because we have progression times for this subset, we observe that their centrally reviewed progression course after locally designated progression was worse than those not classified as progressed locally (Fig 1A). Figure 1B demonstrates the impact of this informative censoring on the estimate of the PFS curve. It can be observed that the curve with informative censoring is overly optimistic. Although the censoring is informative, if this type of censoring had been present in this trial, the impact on the hazard ratios would have been minimal (row 3 v row 1 of Table 3). The lack of an effect may be due to the large effect size, the small sample size and/or similar informative censoring on all treatment arms in this double-blinded trial.
Fig 1.
(A) Demonstrates the existence of informative censoring via conditional progression-free survival (PFS) curves. The PFS curves are conditional on time of local progression. The “observed curve” plots the centrally determined progression from the time of investigator-assessed progression for the 16 patients for whom investigator-assessed progression was called before the blinded independent central review (BICR) –determined time. The “expected curve without informative censoring” is the curve that would be expected if the censoring was not informative. Curves are constructed as described in the Appendix. Data are from Yang et al.13 (B) Demonstrates the impact of informative censoring shown in 1A on the unconditional PFS curves. The curves plot BICR PFS with and without informative censoring. However, the “with informative censoring” curve censors cases for which BICR progression determined after local progression at the locally designated progression time.
Other Issues: Symptomatic Progression and Comined End Points
We have focused on imaging-determined progression thus far; however, PFS is a combined end point that may include symptomatic progression in addition to radiologic progression and death. Symptomatic deterioration may be a reason to discontinue or alter therapy and may be a protocol-specified end point. Blinded central review of such end points is problematic. Objective verification should be obtained when possible (eg, bone scans documenting bone metastases). However, this may not always be possible, in which case one may (1) consider the patient as lost-to-follow-up (“censored”) at the time of symptomatic deterioration, or (2) consider undocumented symptomatic deterioration as an outcome event. The problems from censoring this patient must be weighed against the potential for biased end point evaluation in making this choice.
Including death in this combined end point is necessary; however, this creates difficulties when a patient is lost to follow-up for a radiologic exam, but time of death is determined at some considerable interval after the last radiologic exam. Progression may have occurred long before the time of death. Central review will exacerbate this problem because unconfirmed local progressions produce a subset of subjects without progression times. Inclusion of late deaths as events will tend to attenuate estimates of treatment effectiveness and reduce power. An appropriate maximum time interval between last scan and death (to include time of death as an event) should be specified to minimize bias. When determining this interval, one should consider both the planned imaging intervals as well as the median PFS time.
DISCUSSION
When PFS is a desired end point, a double-blinded trial is best for minimizing biased end point evaluation. When double-blinded trials are not feasible, BICR is not recommended as a general strategy for reducing bias. Unless it is possible to have patient management follow the blinded central review, the blinded review does not resolve the bias problem. Additional scans after locally designated progression calls may lessen the informative censoring problem and should be encouraged. However, this may not be practical as patients may be lost to additional follow-up after local progression. In some cases, BICR may be considered for measurement-variation reduction, although the benefits must be weighed against the potential bias from informative censoring. Computer-assisted diagnostic techniques to determine changes in tumor size may further improve reader consistency at local sites. However, the benefits of these techniques in the clinical trial setting have yet to be proven.14
Methods for modeling informative censoring have been proposed.15,16 However, these methods cannot conclusively eliminate the potential confounding effects of informative censoring and are intended for sensitivity analyses.
While we do not advocate BICR as an alternative means of definitive analysis, it may be useful as an auditing tool to detect bias in local evaluations. In a situation, for example, with a moderate or marginal positive effect, confirmation of the local results by the BICR strengthens confidence in the trial's conclusions. Note that if the BICR does not agree with the local assessment, this does not necessarily imply that local assessments were biased for the reasons described here. Signs of concern that such bias exists include: 1) differential rates of unconfirmed progression (resulting in potential informative censoring) according to treatment arm, and/or 2) a higher rates of earlier progression calls according to BICR in one treatment arm.
In conclusion, the implementation of BICR cannot be universally recommended, although it might be considered as an auditing tool. RCTs that use PFS should target large treatment effects to be more convincing as large effects are of greater clinical significance and will be more robust to the types of biases discussed here.1
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Lori E. Dodd, Edward L. Korn, Boris Freidlin, Conrade Carl Jaffe, Lawrence Rubinstein, Margaret M. Mooney
Collection and assembly of data: Lori E. Dodd, Edward L. Korn, Boris Freidlin
Data analysis and interpretation: Lori E. Dodd, Edward L. Korn, Boris Freidlin
Manuscript writing: Lori E. Dodd, Edward L. Korn, Boris Freidlin, Conrade Carl Jaffe, Lawrence Rubinstein, Janet E. Dancey, Margaret M. Mooney
Final approval of manuscript: Lori E. Dodd, Edward L. Korn, Boris Freidlin, Conrade Carl Jaffe, Lawrence Rubinstein, Janet E. Dancey, Margaret M. Mooney
Appendix: Calculation of Curves Displayed in Figure 1A
The conditional progression-free survival (PFS) probabilities were estimated using the blinded independent central review (BICR) progression times from Yang et al.13 Sixteen subjects had investigator-assessed (or “local”) progression times called before the BICR time. For this set of 16 subjects (which we shall denote set S), we plot the Kaplan-Meier curve after each subject's investigator-assessed progression call (“observed curve” in Fig 1A). Specifically, for each of these subjects the baseline time was taken as the investigator-determined progression time and the event time was the BICR-determined progression time.
The expected curve without informative censoring was calculated in the following manner. For each of the 16 set S subjects, we first estimated a comparison curve as follows (16 curves in all): For a given set S subject with a local progression time of t, we considered all non–set S patients who had not progressed by time t. We estimated the Kaplan-Meier curve for this subset of non–set S subjects conditional on time t until the BICR-determined progression time. For example, if set S subject #1 was determined by local review to progress at 60 days, but her centrally determined progression time was 100 days, the conditional PFS time was 40 days for use in the “observed curve.” The comparison curve for subject #1 was estimated among all nonsubset individuals who had not progressed by day 60 (say, there are 25 such subjects). The conditional PFS time for each of these 25 subjects contributing to the comparison curve for subject #1 was taken as each subject's BICR-determined progression time minus 60. The comparison curve for subject #1 was the Kaplan-Meier curve using these 25 conditional PFS times. The 16 curves calculated in this manner were then averaged for the figure (“expected curve without informative censoring”).
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Published by the American Society of Clinical Oncology
REFERENCES
- 1.Johnson JR, Williams G, Pazdur R: End points and United States Food and Drug Administration approval of oncology drugs. J Clin Oncol 21:1404-1411, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Freidlin B, Korn EL, Hunsberger S, et al: Proposal for the use of progression-free survival in unblinded randomized trials. J Clin Oncol 25:2122-2126, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Therasse P, Arbuck SG, Eisenhauer EA, et al: New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205-216, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Miller AB, Hoogstraten B, Staguet M, et al: Reporting results of cancer treatment. Cancer 47:207-214, 1981 [DOI] [PubMed] [Google Scholar]
- 5.Juni P, Altman DG, Egger M: Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 323:42-46, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez EA, Lerzo G, Pivot X, et al: Efficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine. J Clin Oncol 25:3407-3414, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Abou-Alfa GK, Schwartz L, Ricci S, et al: Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 24:4293-4300, 2006 [DOI] [PubMed] [Google Scholar]
- 8.US Food and Drug Administration: Guidance for Industry Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics. Washington, DC, US Food and Drug Administration, 2007, pp. 1-19
- 9.Miller K, Wang M, Gralow J, et al: Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357:2666-2676, 2007 [DOI] [PubMed] [Google Scholar]
- 10.US Food and Drug Administration: FDA Briefing Document: Oncology Drug Advisory Committee Meeting BLA STN 125085/91.018 Avastin® (bevacizumab). Washington, DC, US Food and Drug Administration, 2007
- 11.Genetech: Oncology Drugs Advisory Committee Meeting: December 5, 2007. South San Fransisco, CA, Genentech, 2007
- 12.Erasmus JJ, Gladish GW, Broemeling L, et al: Interobserver and intraobserver variability in measurement of non–small-cell carcinoma lung lesions: Implications for assessment of tumor response. J Clin Oncol 21:2574-2582, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Yang JC, Haworth L, Sherry RM, et al: A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349:427-434, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaffe CC: Measures of response: RECIST, WHO, and new alternatives. J Clin Oncol 24:3245-3251, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Scharfstein D, Robins JM, Eddings W, et al: Inference in randomized studies with informative censoring and discrete time-to-event endpoints. Biometrics 57:404-413, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Ruan PK, Gray RJ: Sensitivity analysis of progression-free survival with dependent withdrawal. Stat Med 27:1180-1198, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Escudier B, Eisen T, Stadler WM, et al: Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356:125-134, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ, Hutson TE, Tomczak P, et al: Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356:115-124, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Van Cutsem E, Peeters M, Siena S, et al: Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25:1658-1664, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Geyer CE, Forster J, Lindquist D, et al: Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355:2733-2743, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Miller KD, Chap LI, Holmes FA, et al: Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 23:792-799, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Thomas ES, Gomez HL, Li RK, et al: Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol 25:5210-5217, 2007 [DOI] [PubMed] [Google Scholar]