Abstract
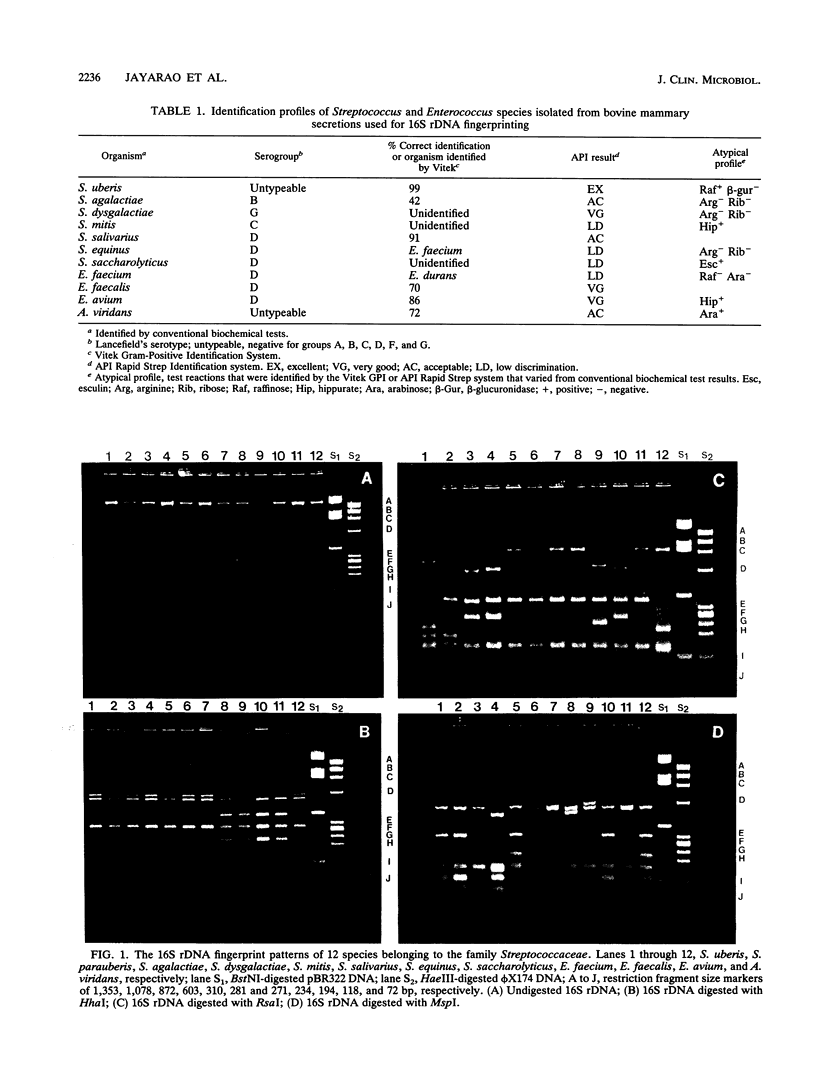
Twelve bacterial species including Streptococcus uberis, S. parauberis, S. agalactiae, S. dysgalactiae, S. bovis, S. mitis, S. salivarius, S. saccharolyticus, Enterococcus faecium, E. faecalis, E. avium, and Aerococcus viridans were examined for their 16S ribosomal DNA fingerprint patterns. Oligonucleotide primers complementary to 16S rRNA genes were used to amplify by the polymerase chain reaction 16S ribosomal gene fragments from genomic DNAs. The molecular sizes of the amplified 16S ribosomal DNA (rDNA) fragments from the 12 species examined ranged from 1,400 to 1,500 bp. Restriction fragment length polymorphism analysis of 16S rDNA was performed with 11 different restriction endonucleases. All 12 species examined could be differentiated on the basis of characteristic 16S rDNA fingerprint patterns by using the restriction endonucleases HhaI, RsaI, and MspI. A scheme for the differentiation of the 12 species is presented. Eleven isolates representing 11 species were obtained from cows with intramammary infections and were examined by 16S rDNA fingerprinting. All 11 species isolated from cows were differentiated by using HhaI, RsaI, and MspI restriction endonucleases. The results of this study demonstrate the potential application of 16S rDNA fingerprinting for the identification and differentiation of bacterial species.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum P. C., Chaurushiya P. S., Jacobs M. R., Duffett A. Evaluation of the rapid strep system for species identification of streptococci. J Clin Microbiol. 1984 May;19(5):588–591. doi: 10.1128/jcm.19.5.588-591.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley R. W., Leigh J. A., Collins M. D. Intrageneric structure of Streptococcus based on comparative analysis of small-subunit rRNA sequences. Int J Syst Bacteriol. 1991 Oct;41(4):487–494. doi: 10.1099/00207713-41-4-487. [DOI] [PubMed] [Google Scholar]
- Bramley A. J., Dodd F. H. Reviews of the progress of dairy science: mastitis control--progress and prospects. J Dairy Res. 1984 Aug;51(3):481–512. doi: 10.1017/s0022029900023797. [DOI] [PubMed] [Google Scholar]
- Böddinghaus B., Rogall T., Flohr T., Blöcker H., Böttger E. C. Detection and identification of mycobacteria by amplification of rRNA. J Clin Microbiol. 1990 Aug;28(8):1751–1759. doi: 10.1128/jcm.28.8.1751-1759.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Neimark H., Rumore P., Steinman C. R. Broad range DNA probes for detecting and amplifying eubacterial nucleic acids. FEMS Microbiol Lett. 1989 Jan 1;48(1):19–24. doi: 10.1016/0378-1097(89)90139-0. [DOI] [PubMed] [Google Scholar]
- Facklam R. R., Rhoden D. L., Smith P. B. Evaluation of the Rapid Strep system for the identification of clinical isolates of Streptococcus species. J Clin Microbiol. 1984 Nov;20(5):894–898. doi: 10.1128/jcm.20.5.894-898.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R., Bosley G. S., Rhoden D., Franklin A. R., Weaver N., Schulman R. Comparative evaluation of the API 20S and AutoMicrobic gram-positive identification systems for non-beta-hemolytic streptococci and aerococci. J Clin Microbiol. 1985 Apr;21(4):535–541. doi: 10.1128/jcm.21.4.535-541.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimont F., Grimont P. A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Göbel U. B., Geiser A., Stanbridge E. J. Oligonucleotide probes complementary to variable regions of ribosomal RNA discriminate between Mycoplasma species. J Gen Microbiol. 1987 Jul;133(7):1969–1974. doi: 10.1099/00221287-133-7-1969. [DOI] [PubMed] [Google Scholar]
- Jayarao B. M., Doré J. J., Jr, Baumbach G. A., Matthews K. R., Oliver S. P. Differentiation of Streptococcus uberis from Streptococcus parauberis by polymerase chain reaction and restriction fragment length polymorphism analysis of 16S ribosomal DNA. J Clin Microbiol. 1991 Dec;29(12):2774–2778. doi: 10.1128/jcm.29.12.2774-2778.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayarao B. M., Oliver S. P., Matthews K. R., King S. H. Comparative evaluation of Vitek gram-positive identification system and API Rapid Strep system for identification of Streptococcus species of bovine origin. Vet Microbiol. 1991 Feb 1;26(3):301–308. doi: 10.1016/0378-1135(91)90023-9. [DOI] [PubMed] [Google Scholar]
- Joshi A. K., Baichwal V., Ames G. F. Rapid polymerase chain reaction amplification using intact bacterial cells. Biotechniques. 1991 Jan;10(1):42, 44-5. [PubMed] [Google Scholar]
- McDonald T. J., McDonald J. S. Streptococci isolated from bovine intramammary infections. Am J Vet Res. 1976 Apr;37(4):377–381. [PubMed] [Google Scholar]
- Nachamkin I., Lynch J. R., Dalton H. P. Evaluation of a rapid system for species identification of alpha-hemolytic streptococci. J Clin Microbiol. 1982 Sep;16(3):521–524. doi: 10.1128/jcm.16.3.521-524.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S. P. Frequency of isolation of environmental mastitis-causing pathogens and incidence of new intramammary infection during the nonlactating period. Am J Vet Res. 1988 Nov;49(11):1789–1793. [PubMed] [Google Scholar]
- Oliver S. P., Mitchell B. A. Prevalence of mastitis pathogens in herds participating in a mastitis control program. J Dairy Sci. 1984 Oct;67(10):2436–2440. doi: 10.3168/jds.S0022-0302(84)81592-1. [DOI] [PubMed] [Google Scholar]
- Poutrel B., Ryniewicz H. Z. Evaluation of the API 20 Strep system for species identification of streptococci isolated from bovine mastitis. J Clin Microbiol. 1984 Feb;19(2):213–214. doi: 10.1128/jcm.19.2.213-214.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pustell J., Kafatos F. C. A high speed, high capacity homology matrix: zooming through SV40 and polyoma. Nucleic Acids Res. 1982 Aug 11;10(15):4765–4782. doi: 10.1093/nar/10.15.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues U., Collins M. D. Phylogenetic analysis of Streptococcus saccharolyticus based on 16S rRNA sequencing. FEMS Microbiol Lett. 1990 Sep 1;59(1-2):231–234. doi: 10.1016/0378-1097(90)90062-u. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Smith K. L., Todhunter D. A., Schoenberger P. S. Environmental mastitis: cause, prevalence, prevention. J Dairy Sci. 1985 Jun;68(6):1531–1553. doi: 10.3168/jds.S0022-0302(85)80993-0. [DOI] [PubMed] [Google Scholar]
- Watts J. L. Characterization and identification of streptococci isolated from bovine mammary glands. J Dairy Sci. 1988 Jun;71(6):1616–1624. doi: 10.3168/jds.S0022-0302(88)79725-8. [DOI] [PubMed] [Google Scholar]
- Watts J. L. Evaluation of the Rapid Strep system for identification of gram-positive, catalase-negative cocci isolated from bovine intramammary infections. J Dairy Sci. 1989 Oct;72(10):2728–2732. doi: 10.3168/jds.S0022-0302(89)79416-9. [DOI] [PubMed] [Google Scholar]
- Williams A. M., Collins M. D. Molecular taxonomic studies on Streptococcus uberis types I and II. Description of Streptococcus parauberis sp. nov. J Appl Bacteriol. 1990 May;68(5):485–490. doi: 10.1111/j.1365-2672.1990.tb02900.x. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]