Abstract
"Intra-abdominal hypertension", the presence of elevated intra-abdominal pressure, and "abdominal compartment syndrome", the development of pressure-induced organ-dysfunction and failure, have been increasingly recognized over the past decade as causes of significant morbidity and mortality among critically ill surgical and medical patients. Elevated intra-abdominal pressure can cause significant impairment of cardiac, pulmonary, renal, gastrointestinal, hepatic, and central nervous system function. The significant prognostic value of elevated intra-abdominal pressure has prompted many intensive care units to adopt measurement of this physiologic parameter as a routine vital sign in patients at risk. A thorough understanding of the pathophysiologic implications of elevated intra-abdominal pressure is fundamental to 1) recognizing the presence of intra-abdominal hypertension and abdominal compartment syndrome, 2) effectively resuscitating patients afflicted by these potentially life-threatening diseases, and 3) preventing the development of intra-abdominal pressure-induced end-organ dysfunction and failure. The currently accepted consensus definitions surrounding the diagnosis and treatment of intra-abdominal hypertension and abdominal compartment syndrome are presented.
Review
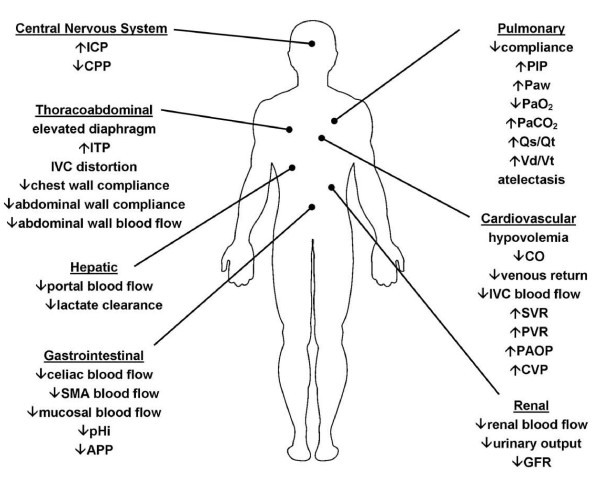
Although initially recognized over 150 years ago, the pathophysiologic implications of elevated intra-abdominal pressure (IAP) have essentially been rediscovered only within the past two decades [1-3]. An explosion of scientific investigation and accumulation of clinical experience has confirmed the significant detrimental impact of both "intra-abdominal hypertension" (IAH) (see figure 1), the presence of elevated intra-abdominal pressure, and "abdominal compartment syndrome" (ACS), the development of IAH-induced organ-dysfunction and failure, among the critically ill [4,5]. IAH has been identified as a continuum of pathophysiologic changes beginning with regional blood flow disturbances and culminating in frank end-organ failure and the development of ACS. ACS has been identified to be a cause of significant morbidity and mortality among critically ill surgical, medical, and pediatric patients. Previously present, but significantly under-appreciated, IAH and ACS are now recognized as common occurrences in the intensive care unit (ICU) setting [6-16]. Elevated IAP has been identified as an independent predictor of mortality during critical illness and likely plays a major role in the development of multiple system organ failure, a syndrome which has plagued ICU patients and physicians for decades [8,17,18].
Figure 1.
Pathophysiologic Implications of Intra-abdominal Hypertension. The effects of intra-abdominal hypertension are not limited just to the intra-abdominal organs, but rather have an impact either directly or indirectly on every organ system in the body. ICP – intracranial pressure; CPP – cerebral perfusion pressure; ITP – intrathoracic pressure; IVC – inferior vena cava; SMA – superior mesenteric artery; pHi – gastric intramuscosal pH; APP – abdominal perfusion pressure; PIP- peak inspiratory pressure; Paw – mean airway pressure; PaO2 – oxygen tension; PaCO2 – carbon dioxide tension; Qs/Qt – intrapulmonary shunt; Vd/Vt – pulmonary dead space ; CO – cardiac output; SVR – systemic vascular resistance; PVR – pulmonary vascular resistance; PAOP – pulmonary artery occlusion pressure; CVP – central venous pressure; GFR – glomerular filtration rate.
Recently, evidence-based consensus definitions and recommendations for the resuscitation and rehabilitation of patients with IAH and ACS have been published [19,20]. Central to this evolving strategy are the use of early serial IAP measurements to detect the presence of IAH, application of comprehensive medical management strategies to reduce elevated IAP and restore end-organ perfusion, timely surgical abdominal decompression for refractory organ dysfunction, and early attempts at fascial closure once physiologically appropriate [21,22]. Such a strategy has been demonstrated to significantly improve patient survival, reduce complications (such as enteroatmospheric fistula), and decrease resource utilization [23,24]. The following review addresses both the pathophysiologic impact of elevated IAP on the various organ systems as well as the currently accepted definitions surrounding IAH and ACS. The diagnosis, prevention, and treatment of IAH/ACS have been addressed in a number of recent publications [6,10,12,13,19-22,24-29].
History
The impact of elevated IAP upon respiratory function was first documented by Marey in 1863 and subsequently by Burt in 1870 [30]. In 1890, Henricius identified in an animal model that an IAP between 27 and 46 cm H2O significantly impaired diaphragmatic excursion leading to elevated intrathoracic pressure, respiratory failure, and death [30]. The theory that respiratory failure is the cause of death in severe IAH persisted until 1911 when Emerson demonstrated in cat, dog, and rabbit models that elevated IAP causes death by cardiovascular collapse rather than by respiratory failure [30]. The detrimental effect of elevated IAP on renal function and urinary output was first identified by Wendt in 1876 and the restoration of urinary output through abdominal decompression by Thorington and Schmidt in 1923 [31-33]. Overholt extensively studied the properties of the abdominal wall and confirmed that normal IAP is subatmospheric and that procedures which restrict movement of the abdominal wall or distention of the stomach or colon all result in an increase in IAP [34]. He postulated that IAP is governed by both the pressure induced by the abdominal contents and the "flexibility" (compliance) of the abdominal wall. Investigation into the physiologic effects of IAP on renal function in humans essentially began in 1947 with the work of Bradley [35]. The experiences of surgeons treating infants with gastroschisis or omphalocele further contributed to our understanding of both the concept of "loss of abdominal domain" as well as the life-threatening cardiac, pulmonary, and gastrointestinal complications which can occur when abdomens are primarily closed without consideration of elevated IAP [36-39]. Gross, in 1948, first described the use of a "staged abdominal repair" in the management of such infants unknowingly pioneering the open abdomen techniques which have now become standard in the treatment of IAH and ACS [36].
Although surrogate measurement of IAP via measurement of intravesicular, intragastric, and intracolonic pressure in animal models was commonplace in the 1920's and 1930's, it was Söderberg who, in 1970, first described the strong correlation between IAP and intravesicular pressure during laparoscopy in humans [40]. The landmark work of Harman, Kron, and Richards in the early 1980's "rediscovered" IAH as a cause of unexplained oliguria and subsequent renal failure in post-operative patients with abdominal distention [32,41,42]. They further reported the benefits of open abdominal decompression in restoring renal function and improving patient outcome in patients with an IAP in excess of 25 mmHg [32,41]. The introduction of laparoscopic techniques into mainstream surgical practice in the late 1980's and early 1990's led to numerous experimental and clinical studies which further advanced our understanding of the injurious effects of elevated IAP on cardiac, pulmonary, renal, gastrointestinal, hepatic, and cerebral function. Increased appreciation of these effects by both anesthesiologists and surgeons set the stage for recognition of both IAH and ACS in the critically ill patient population.
Pathophysiology
An increasing body of literature has identified the significant physiologic derangements that occur as a result of elevated IAP. The effects of IAH are not limited just to the intra-abdominal organs, but rather have an impact either directly or indirectly on every organ system in the body. As a result, patients with prolonged, untreated IAH commonly manifest significant malperfusion and subsequent organ failure. Pre-existing comorbidities, such as chronic renal failure, pulmonary disease, or cardiomyopathy, play an important role in aggravating the effects of elevated IAP and may reduce the threshold of IAH that causes the clinical manifestations of ACS. The etiology for the patient's IAH is similarly of vital importance and may be determined as being either intra-abdominal, as occurs in surgical or trauma patients following damage control laparotomy, or extra-abdominal, as occurs in medical patients with sepsis or burn patients who require aggressive fluid resuscitation [6,7,43-46].
Cardiovascular
As originally described over 80 years ago by Emerson, rising IAP increases intrathoracic pressure through cephalad deviation of the diaphragm [30]. Increased intrathoracic pressure significantly reduces venous return resulting in reduced cardiac output [33,47-57]. Such reductions have been demonstrated to occur at an IAP of only 10 mmHg [18,57]. Hypovolemic patients appear to sustain reductions in cardiac output at lower levels of IAP than do normovolemic patients [50,53]. Hypervolemic patients demonstrate increased venous return in the presence of mild to moderate elevations in IAP suggesting that volume resuscitation may have a protective effect [53]. Diaphragmatic elevation and increased intrathoracic pressure have also been postulated to cause direct cardiac compression reducing ventricular compliance and contractility [49]. Systemic vascular resistance (afterload) is increased through compression of both the aorta and systemic vasculature and pulmonary vascular resistance through compression of the pulmonary parenchyma [33,48,51-56,58]. As a result, in the absence of severe IAH, mean arterial pressure typically remains stable despite a decrease in venous return and cardiac output. Such increases in afterload may be poorly tolerated by those with marginal cardiac contractility or inadequate intravascular volume. Preload augmentation through volume administration appears to ameliorate, at least partially, the injurious effects of IAH-induced increases in afterload [18,33,48,53,56,58,59].
Paradoxically, intracardiac filling pressures such as pulmonary artery occlusion ("wedge") pressure (PAOP) and central venous pressure (CVP) typically increase with rising IAP despite the reduced venous return and cardiac output [47-49,51,53,56,57,59-64]. This apparent deviation from Starling's Law of the heart is due to the fact that both PAOP and CVP are measured relative to atmospheric pressure and are actually the sum of both intravascular pressure and intrathoracic pressure [63,64]. In the presence of IAH-induced elevations in intrathoracic pressure, PAOP and CVP tend to be erroneously elevated and no longer reflective of true intravascular volume status [47-49,57,59-61,63,64]. Such alterations in PAOP and CVP have been demonstrated with an IAP of only 10 mmHg [57]. Attempts to correct for this measurement error through use of transmural pressures (i.e., PAOP minus intrathoracic pressure) has confirmed that transmural PAOP decreases with rising IAP correctly reflecting the decreased venous return and cardiac preload [59]. Several studies have demonstrated that volumetric parameters, such as right ventricular end-diastolic volume (RVEDV), global end-diastolic volume (GEDV), or stroke volume variation (SVV) are superior predictors of intravascular volume status whose accuracy is unaffected by changes in intrathoracic pressure [63-66]. When traditional intracardiac filling pressures must be used, transmural pressures may be estimated as follows [63,64]:
| Transmural PAOP = PAOP - 0.5*IAP |
| Transmural CVP = CVP - 0.5*IAP |
IAH also reduces venous return from the lower extremities functionally obstructing inferior vena caval blood flow by two mechanisms. First, inferior vena caval pressure increases significantly in the presence of IAH and has been demonstrated to parallel changes in IAP [18,33,53,56]. Second, cephalad deviation of the diaphragm causes a mechanical narrowing of the vena cava at the diaphragmatic crura further reducing venous return to the heart [54,67]. Femoral vein pressures are markedly increased and venous blood flow and pulsatility dramatically reduced [68,69]. The resulting increases in extremity venous hydrostatic pressure promote the formation of peripheral edema. These changes place the patient with IAH at risk for development of deep venous thrombosis [69-71]. Reduction of IAP restores femoral venous blood flow, but has anecdotally been reported to result in pulmonary embolism [71].
Pulmonary
The pulmonary effects of elevated IAP have been recognized for many years [30,33,49,51,59,68,72-74]. IAP is transmitted to the thorax both directly and through cephalad deviation of the diaphragm. This significantly increases intrathoracic pressure resulting in extrinsic compression of the pulmonary parenchyma and development of pulmonary dysfunction [18,47,48,57,59,68]. Compression of the pulmonary parenchyma appears to begin with an IAP of 16–30 mmHg and is accentuated by the presence of hemorrhagic shock and hypotension [57,75]. Parenchymal compression results in alveolar atelectasis, decreased oxygen transport across the pulmonary capillary membrane, and an increased intrapulmonary shunt fraction (Qsp/Qt). IAH-induced atelectasis has been demonstrated to cause an increase in the rate of pulmonary infection [76]. Parenchymal compression also reduces pulmonary capillary blood flow leading to decreased carbon dioxide excretion and an increased alveolar dead space (Vd/Vt) [57]. Both peak inspiratory and mean airway pressures are significantly increased and may result in alveolar volutrauma [57,75]. Spontaneous tidal volumes and dynamic pulmonary compliance are reduced resulting in further ventilation-perfusion mismatching [57,75]. In combination, these effects lead to the arterial hypoxemia and hypercarbia that, in part, characterize ACS [18,33,48,51,59,73].
Renal
IAH-induced reductions in renal blood flow and function have been demonstrated in both animal and human models [33,35,42,51,77]. These changes occur in direct response to increasing IAP with oliguria developing at an IAP of 15 mmHg and anuria at 30 mmHg [32,33,42]. Renal artery blood flow has been demonstrated to be preferentially diminished in comparison to both celiac and superior mesenteric artery blood flow [68]. Renal vein pressure and renal vascular resistance are both significantly elevated [35,42,48]. All of these changes shunt blood away from the renal cortex and functioning glomeruli leading to impaired glomerular and tubular function and significant reductions in urinary output [32,33,35,41,42,48,49,51,73,77-80].
Several mechanisms have been proposed as the etiology for IAH-induced renal dysfunction and failure. Harman et al. negated direct ureteral compression as a cause through studies utilizing ureteral stents [42]. Other authors have suggested that direct parenchymal compression and development of a "renal compartment syndrome" results in renal ischemia and subsequent failure [70,81]. Stone demonstrated in traumatically injured patients that incising the renal capsule could reverse renal failure if performed early and prior to development of severe renal dysfunction [81]. Recent studies suggest that compression of the renal vein likely plays the primary role in the development of renal dysfunction with reduced cardiac output playing a secondary role [32,33,48,81].
IAH decreases glomerular filtration rate causing a rise in both blood urea nitrogen and serum creatinine and a reduction in creatinine clearance [33,35,42,48,51,79]. Osmolar clearance is similarly decreased and fractional excretion of sodium increased [79]. Urinary sodium and chloride concentrations decrease and urinary potassium concentrations increase [33]. Plasma renin activity and aldosterone levels increase significantly [33,48]. Antidiuretic hormone levels have been demonstrated to increase to more than twice basal levels [82]. All of these pathophysiologic changes appear to be potentially reversible if the patient's IAH is recognized and treated appropriately before significant organ dysfunction has developed [32,48].
Gastrointestinal
Of all the organ systems, the gut appears to be one of the most sensitive to elevations in IAP. Such reductions in mesenteric blood flow may appear with an IAP of only 10 mmHg [83]. Caldwell et al. has demonstrated decreased blood flow to virtually all intra-abdominal and retroperitoneal organs as a result of elevated IAP [56]. The sole exception was adrenal blood flow which appears to be preserved and has been postulated to be a survival mechanism by which to support catecholamine release in the face of ongoing shock [56]. Celiac artery blood flow is reduced by up to 43% and superior mesenteric artery blood flow by as much as 69% in the presence of intra-abdominal pressures of 40 mmHg [68,83,84]. The negative effects of IAP on mesenteric perfusion are augmented by the presence of hypovolemia or hemorrhage [8,50,68,83,85]. Reintam et al. have recently validated a grading system for predicting mortality due to gastrointestinal dysfunction among patients with IAH/ACS [86].
In addition to reducing arterial blood flow, IAP compresses thin walled mesenteric veins promoting venous hypertension and intestinal edema. Visceral swelling further increases IAP initiating a vicious cycle which results in worsening malperfusion, bowel ischemia, decreased intramucosal pH, feeding intolerance, systemic metabolic acidosis, and significantly increased patient mortality [8,13,50,86,87]. Intestinal mucosal perfusion is diminished by levels of IAP as low as 20 mmHg as demonstrated using gastric or colonic tonometry and by laser flow probe [8,50,84,87]. Sugrue et al. found that patients with IAH were over 11 times more likely to have abnormal gastric intramucosal pH measurements than were those without IAH [87]. Djavani et al have recently reported a similar significant correlation between abnormal colonic intramucosal pH and IAH [85]. They have further confirmed a high risk of colonic ischemia in post-abdominal aortic aneurysmectomy patients with IAP > 20 mmHg [88]. Malperfusion of the gut as a result of elevated IAP has been speculated as a possible mechanism for loss of the mucosal barrier and subsequent development of bacterial translocation, sepsis, and multiple system organ failure [84,89,90]. Gargiulo et al. demonstrated bacterial translocation to mesenteric lymph nodes in the presence of hemorrhage and an IAP of only 10 mmHg [90].
Hepatic
Hepatic artery, hepatic vein, and portal vein blood flow are all reduced by the presence of IAH [50,52,54,77,91]. Hepatic artery flow is directly affected by decreases in cardiac output. Hepatic and portal venous flow are diminished as a result of both extrinsic compression of the liver as well as anatomic narrowing of the hepatic veins as they pass through the diaphragm [67]. Increased hepatic vein pressures have been demonstrated to result in increased azygos vein blood flow suggesting a compensatory increase in gastroesophageal collateral blood flow in response to hepatic venous congestion [54]. On a microscopic level, hepatic microcirculatory blood flow is decreased resulting in a reduction in hepatic mitochondrial function and production of energy substrates [50,91]. Lactic acid clearance by the liver appears to be compromised potentially confounding its use as a marker of resuscitation adequacy [92]. Of particular importance is that these changes have been documented with IAP elevations of only 10 mmHg and in the presence of both normal cardiac output and mean arterial blood pressure [50].
Central Nervous System
Cerebral perfusion and function are also directly affected by the presence of IAH. According to the Monroe-Kellie doctrine, the brain consists of four discrete compartments: parenchymal, vascular, osseous, and cerebrospinal fluid. An increase in the pressure within one compartment results in a reciprocal increase in the pressure within each of the other non-osseous compartments. Whereas chronic, slowly developing increases in intracranial pressure (ICP) may allow time for compensation, the acute increases in ICP characteristic of both traumatic injury and acute illness commonly result in rapidly escalating intracranial pressures. Elevations in intra-abdominal and intrathoracic pressure may also directly impact the pressures within the cranium. Coughing, defecating, emesis, and other common causes of increased intra-abdominal and intrathoracic pressure are well known to transiently increase ICP [48,93,94]. IAH can induce similar increases in ICP, but these elevations are sustained as long as the IAH is present and can result in significant reductions in cerebral perfusion pressure (CPP) [47,48,61,94-96]. The mechanism by which IAH causes elevations in ICP has long been a subject of debate [47,48,94,97,98]. Proposed mechanisms have included decreased lumbar venous plexus blood flow (leading to increased CSF pressure), increased PaCO2 (resulting in increased cerebral blood flow), and decreased cerebral venous outflow [47,48,94,97,98]. Luce et al. in a series of animal experiments and Bloomfield et al. in clinical studies involving humans have confirmed that increased intrathoracic pressure impairs venous return from the cranium and decreases cerebral venous blood flow [48,97]. This increases intracranial venous blood volume in a manner similar to that encountered with the use of both PEEP and military anti-shock trousers [97-99]. Intracerebral venous pooling can markedly worsen pre-existing cerebral perfusion abnormalities due to trauma, chronic intracranial hypertension, or other causes of decreased cerebral compliance [96,98]. Sugerman et al. have demonstrated that normal cerebral compliance appears to be protective against intrathoracic pressure-induced increases in ICP [96]. Decreased pulmonary compliance as a result of severe pulmonary dysfunction, as occurs in IAH, also appears to have a protective effect on ICP [61,95]. Hypovolemia, on the other hand, may worsen already marginal cerebral perfusion [79,95].
Abdominal wall
Although commonly overlooked, the abdominal wall is also subject to the effects of elevated IAP. Visceral edema, abdominal packs, and free intraperitoneal fluid all distend the abdomen and reduce abdominal wall compliance [67,100]. Abdominal wall edema secondary to shock and fluid resuscitation also decreases abdominal compliance. Previous pregnancy, morbid obesity, cirrhosis, and other conditions associated with increased abdominal wall compliance all appear to be protective, to an extent, against the development of IAH [87,96]. Diebel et al. have demonstrated that IAH dramatically reduces abdominal wall blood flow [101]. Rectus sheath blood flow decreases to 58% of baseline at an IAP of only 10 mmHg and to 20% of baseline at 40 mmHg [101]. These findings may explain the impaired wound healing, high rate of fascial dehiscence, and predilection to development of necrotizing fasciitis identified in patients whose abdomens are closed under tension [70,101].
Definitions
In 2004, a consensus conference was convened by the World Society of the Abdominal Compartment Syndrome (WSACS) http://www.wsacs.org consisting of European, Australasian, and North American surgical, trauma, and medical critical care specialists. Recognizing the lack of accepted definitions, and the resulting confusion and difficulty in comparing studies published in this area, the WSACS tasked these specialists to create evidence-based definitions for IAH and ACS. After extensively reviewing the existing literature, the authors suggested a conceptual framework for standardizing the definitions of IAH and ACS as well as a general technique for IAP monitoring based upon the current understanding of the pathophysiology of these two syndromes [19]. A brief summary of these definitions follows (Table 1).
Table 1.
Definitions
| Definition 1 | IAP is the steady-state pressure concealed within the abdominal cavity. |
| Definition 2 | APP = MAP - IAP |
| Definition 3 | FG = GFP - PTP = MAP - 2 * IAP |
| Definition 4 | IAP should be expressed in mmHg and measured at end-expiration in the complete supine position after ensuring that abdominal muscle contractions are absent and with the transducer zeroed at the level of the mid-axillary line. |
| Definition 5 | The reference standard for intermittent IAP measurement is via the bladder with a maximal instillation volume of 25 mL of sterile saline. |
| Definition 6 | Normal IAP is approximately 5–7 mmHg in critically ill adults. |
| Definition 7 | IAH is defined by a sustained or repeated pathologic elevation of IAP ≥ 12 mmHg. |
| Definition 8 | IAH is graded as follows: |
| • Grade I: IAP 12–15 mmHg | |
| • Grade II: IAP 16–20 mmHg | |
| • Grade III: IAP 21–25 mmHg | |
| • Grade IV: IAP > 25 mmHg | |
| Definition 9 | ACS is defined as a sustained IAP > 20 mmHg (with or without an APP < 60 mmHg) that is associated with new organ dysfunction/failure. |
| Definition 10 | Primary ACS is a condition associated with injury or disease in the abdomino-pelvic region that frequently requires early surgical or interventional radiological intervention. |
| Definition 11 | Secondary ACS refers to conditions that do not originate from the abdomino-pelvic region. |
| Definition 12 | Recurrent ACS refers to the condition in which ACS redevelops following previous surgical or medical treatment of primary or secondary ACS. |
Consensus definitions as proposed by the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome.
Intra-abdominal pressure (IAP)
The abdomen may be considered as a closed box with walls that are either rigid (costal arch, spine, and pelvis) or flexible (abdominal wall and diaphragm). The compliance of these walls and the volume of the organs contained within determine the pressure within the abdomen at any given time [102-104] IAP is defined as the steady-state pressure concealed within the abdominal cavity, increasing with inspiration (diaphragmatic contraction) and decreasing with expiration (diaphragmatic relaxation). IAP is directly affected by the volume of the solid organs or hollow viscera (which may be either empty or filled with air, liquid or fecal matter), the presence of ascites, blood or other space-occupying lesions (such as tumors or a gravid uterus), and the presence of conditions that limit expansion of the abdominal wall (such as burn eschars or third-space edema) [19].
Abdominal perfusion pressure (APP)
Analogous to the widely utilized concept of cerebral perfusion pressure, abdominal perfusion pressure (APP), defined as MAP minus IAP, has been demonstrated to be an accurate predictor of visceral perfusion and an endpoint for resuscitation [64,105,106]. APP, by considering both arterial inflow (MAP) and restrictions to venous outflow (IAP), is statistically superior to either parameter alone in predicting patient survival from IAH and ACS [64,105,106]. APP is also superior to other common resuscitation endpoints such as arterial pH, base deficit, arterial lactate, and hourly urinary output. Failure to maintain an APP of at least 60 mmHg by day 3 of critical illness has been demonstrated to predict survival from IAH and ACS [64,105,106]. APP thus figures prominently in the resuscitation strategy recommended by the WSACS.
Filtration Gradient
As described above, oliguria is one of the first visible signs of IAH. Inadequate renal perfusion pressure and renal filtration gradient (FG) have been proposed as key factors in the development of IAP-induced renal failure [107,108]. The FG is the mechanical force across the glomerulus and equals the difference between the glomerular filtration pressure (GFP) and the proximal tubular pressure (PTP). In the presence of IAH, GFP may be approximated as MAP minus IAP (or APP) while PTP may be assumed to equal IAP. The FG is thus defined as MAP minus two times the IAP, illustrating that changes in IAP have a greater impact upon renal function and urine production than do changes in MAP.
IAP measurement
The sensitivity of both clinical judgement and physical examination have been demonstrated to be very poor in predicting a patient's IAP [109,110]. Early, serial IAP measurements are therefore essential to both diagnosing the presence of IAH as well as guiding resuscitative therapy [111]. While a variety of methods for IAP measurement have been described, intravesicular or "bladder" pressure has achieved the most widespread adoption worldwide due to its simplicity, minimal cost, and low risk of complications [103,112-115]. Several key points must be considered to ensure accurate and reproducible IAP measurements. Early IAH studies utilized water manometers to determine IAP with results reported in cm H2O while subsequent studies using electronic pressure transducers reported IAP in mmHg (1 mmHg = 1.36 cm H2O). This led to confusion and difficulty in comparing studies. A point of further confusion has been the appropriate zero reference point for the abdomen. Changes in body position (i.e., supine, prone, head of bed elevated) can have a significant impact upon the measured IAP. While head of bed elevation is now commonly performed to reduce the incidence of ventilator-associated pneumonia, the clinical studies that determined the threshold IAP values that lead to organ dysfunction were determined in the supine position. Further, the presence of both abdominal and bladder detrusor muscle contractions have been demonstrated to impact the accuracy of IAP measurements. Perhaps the greatest point of contention has been the proper priming-volume to be instilled into the bladder to ensure a conductive fluid column between bladder wall and transducer. Large instillation volumes, as commonly utilized in years past, have been demonstrated to result in artificial increases in IAP that could lead to inappropriate therapy. In an attempt to address these issues and ensure both the accuracy and reproducibility of IAP measurements, the WSACS has recommended that IAP be expressed in mmHg and measured at end-expiration in the complete supine position after ensuring that abdominal muscle contractions are absent and with the transducer zeroed at the level of the mid-axillary line [20]. Further, IAP should be measured via the bladder with a maximal instillation volume of 25 mL of sterile saline [20].
Normal and Pathologic IAP values
Normal IAP ranges from sub-atmospheric to zero mmHg [109,113,116]. In the typical intensive care unit patient, however, IAP is commonly elevated to a range of 5–7 mmHg while patients with recent abdominal surgery, sepsis, organ failure, or need for volume resuscitation may demonstrate IAPs of 10–20 mmHg [11,15]. Prolonged elevation in IAP to such levels can result in organ dysfunction and failure while pressures above 25 mmHg are associated with significant potential mortality [65,80,105].
Intra-Abdominal Hypertension (IAH)
Pathological IAP is a continuum ranging from mild IAP elevations without clinically significant adverse effects to substantial increases in IAP with grave consequences to virtually all organ systems in the body. The exact IAP that defines IAH has long been debated. Burch et al. defined an early grading system for IAH (in cm H2O) as follows: Grade I, 7.5–11 mmHg (10–15 cm H20); Grade II, 11–18 mmHg (15–25 cm H20); Grade III, 18–25 mmHg (25–35 cm H20); and Grade IV, > 25 mmHg (> 35 cm H20) [117]. Burch suggested that most patients with Grade III and all patients with Grade IV should undergo abdominal decompression. The deleterious effects of elevated IAP on renal, cardiac, and gastrointestinal function, however, may be witnessed at IAP levels as low as 10–15 mmHg which would be classified as Grade I in the Burch system [11,44,87,104,118-124]. In recognition of the pathophysiologic impact of these lower levels of IAP, the WSACS has defined IAH as a sustained or repeated pathologic elevation of IAP ≥ 12 mmHg. The WSACS has also modified the Burch system to increase its clinical sensitivity as follows: Grade I: IAP 12–15 mmHg; Grade II: IAP 16–20 mmHg; Grade III: IAP 21–25 mmHg; and Grade IV: IAP > 25 mmHg [19,20]. In this scenario, medical intervention is appropriate for any grade of IAH while surgical decompression is typically reserved for Grade IV IAH.
Abdominal compartment syndrome (ACS)
Among the majority of patients, critical IAP appears to be 10–15 mmHg. It is at this pressure that reductions in microcirculatory blood flow occur and the initial signs of organ dysfunction and failure are witnessed. ACS is the natural progression of these pressure-induced end-organ changes and develops if IAH is not recognized and treated in a timely manner. Failure to recognize and appropriately treat ACS is commonly fatal while prevention and/or timely intervention is associated with marked improvements in organ function and patient survival [8,11,23,44,125-127].
In contrast to IAH, ACS is not graded, but rather considered an "all or nothing" phenomenon. The WSACS defines ACS as a sustained IAP > 20 mmHg (with or without an APP < 60 mmHg) that is associated with new organ dysfunction or failure (Appendix 1) [19,20]. ACS may be further classified as either primary, secondary, or recurrent based upon the duration and etiology of the patient's IAH. Primary ACS is characterized by IAH of relatively brief duration occurring as a result of an intra-abdominal etiology such as abdominal trauma, ruptured abdominal aortic aneurysm, hemoperitoneum, acute pancreatitis, secondary peritonitis, retroperitoneal haemorrhage, or liver transplantation. Primary ACS is therefore defined as a condition associated with injury or disease in the abdomino-pelvic region that frequently requires early surgical or interventional radiological intervention. It is most commonly encountered in the traumatically injured or post-operative surgical patient. Secondary ACS is characterized by IAH that develops as a result of an extra-abdominal etiology such as sepsis, capillary leak, major burns, or other conditions requiring massive fluid resuscitation. It is most commonly encountered in the medical or burn patient [43,104,128,129]. Recurrent ACS represents a redevelopment of ACS symptoms following resolution of an earlier episode of either primary or secondary ACS. It is most commonly associated with the development of acute IAH in a patient who is recovering from IAH/ACS and therefore represents a "second-hit" phenomenon. It may occur despite the presence of an open abdomen or as a new ACS episode following definitive closure of the abdominal wall. Recurrent ACS, due to the patient's current or recent critical illness, is associated with significant morbidity and mortality.
Conclusion
Elevated IAP commonly causes marked deficits in both regional and global perfusion that, when unrecognized, result in significant organ failure and patient morbidity and mortality. Significant progress has been made over the past decade with regard to understanding the etiology of IAH and ACS as well as implementing appropriate resuscitative therapy. Routine measurement of IAP in patients at risk is essential to both recognizing the presence of IAH/ACS and guiding effective treatment. Adoption of the proposed consensus definitions and recommendations has been demonstrated to significantly improve patient survival from IAH/ACS and will facilitate future investigation in this area.
Abbreviations
IAP: intra-abdominal pressure; IAH: intra-abdominal hypertension; ACS: abdominal compartment syndrome; MAP: mean arterial pressure; APP: abdominal perfusion pressure; FG: filtration gradient; GFP: glomerular filtration pressure; PTP: proximal tubular pressure; PIP: peak inspiratory pressure; FiO2: fraction of inspired oxygen; PEEP: positive end-expiratory pressure; ICP: intracranial pressure; PAOP: pulmonary artery occlusion pressure; CVP: central venous pressure.
Competing interests
Financial competing interests
• Dr. Cheatham has served as a consultant for Kinetic Concepts, Inc., Wolfe-Tory Medical, Inc., and Bard Medical, Inc.
Non-financial competing interests
• Dr. Cheatham is a member of the World Society of the Abdominal Compartment Syndrome Executive Committee.
Authors' contributions
MLC is the sole contributor to this manuscript.
Appendix 1 – Signs of Abdominal Compartment Syndrome
Abdominal distention
Elevated IAP
Oliguria refractory to volume administration
Elevated PIP
Hypercarbia
Hypoxemia refractory to increasing FiO2 and PEEP
Refractory metabolic acidosis
Elevated ICP
Legend: These represent the most common organ dysfunctions associated with the development of severe intra-abdominal hypertension and a diagnosis of abdominal compartment syndrome.
References
- Schein M. Abdominal Compartment Syndrome:Historical Background. In: Ivatury R, Cheatham M, Malbrain M, Sugrue M, editor. Abdominal Compartment Syndrome. Georgetown, Texas: Landes Biosciences; 2006. pp. 1–7. [Google Scholar]
- Van Hee R. Historical highlights in concept and treatment of abdominal compartment syndrome. Acta Clin Belg Suppl. 2007:9–15. [PubMed] [Google Scholar]
- Cheatham ML. Intra-abdominal hypertension and abdominal compartment syndrome. New Horizons. 1999;7:96–115. [Google Scholar]
- Cheatham M, Ivatury R, Malbrain M, Sugrue M. Epilogue: Options and Challenges for the Future. In: Ivatury R, Cheatham M, Malbrain M, Sugrue M, editor. Abdominal Compartment Syndrome. Georgetown, Texas: Landes Biosciences; 2006. pp. 297–302. [Google Scholar]
- Malbrain ML. Abdominal compartment syndrome: it is time. Acta Clin Belg Suppl. 2007;62:1–5. [PubMed] [Google Scholar]
- Ball CG, Kirkpatrick AW, McBeth P. The secondary abdominal compartment syndrome: not just another post-traumatic complication. Can J Surg. 2008;51:399–405. [PMC free article] [PubMed] [Google Scholar]
- Daugherty EL, Hongyan L, Taichman D, Hansen-Flaschen J, Fuchs BD. Abdominal compartment syndrome is common in medical intensive care unit patients receiving large-volume resuscitation. J Intensive Care Med. 2007;22:294–299. doi: 10.1177/0885066607305247. [DOI] [PubMed] [Google Scholar]
- Ivatury RR, Porter JM, Simon RJ, Islam S, John R, Stahl WM. Intra-abdominal hypertension after life-threatening penetrating abdominal trauma: prophylaxis, incidence, and clinical relevance to gastric mucosal pH and abdominal compartment syndrome. J Trauma. 1998;44:1016–1021. doi: 10.1097/00005373-199806000-00014. [DOI] [PubMed] [Google Scholar]
- Kimball EJ, Kim W, Cheatham ML, Malbrain ML. Clinical awareness of intra-abdominal hypertension and abdominal compartment syndrome in 2007. Acta Clin Belg Suppl. 2007;1:66–73. doi: 10.1179/acb.2007.62.s1.009. [DOI] [PubMed] [Google Scholar]
- Madigan MC, Kemp CD, Johnson JC, Cotton BA. Secondary abdominal compartment syndrome after severe extremity injury: are early, aggressive fluid resuscitation strategies to blame? J Trauma. 2008;64:280–285. doi: 10.1097/TA.0b013e3181622bb6. [DOI] [PubMed] [Google Scholar]
- Malbrain ML, Chiumello D, Pelosi P, Wilmer A, Brienza N, Malcangi V, et al. Prevalence of intra-abdominal hypertension in critically ill patients: a multicentre epidemiological study. Intensive Care Med. 2004;30:822–829. doi: 10.1007/s00134-004-2169-9. [DOI] [PubMed] [Google Scholar]
- Parsak CK, Seydaoglu G, Sakman G, Acarturk TO, Karakoc E, Hanta I, et al. Abdominal compartment syndrome: current problems and new strategies. World J Surg. 2008;32:13–19. doi: 10.1007/s00268-007-9286-x. [DOI] [PubMed] [Google Scholar]
- Reintam A, Parm P, Kitus R, Kern H, Starkopf J. Primary and secondary intra-abdominal hypertension – different impact on ICU outcome. Intensive Care Med. 2008;34:1624–1631. doi: 10.1007/s00134-008-1134-4. [DOI] [PubMed] [Google Scholar]
- Serpytis M, Ivaskevicius J. The influence of fluid balance on intra-abdominal pressure after major abdominal surgery. Medicina (Kaunas) 2008;44:421–427. [PubMed] [Google Scholar]
- Malbrain ML, Chiumello D, Pelosi P, Bihari D, Innes R, Ranieri VM, et al. Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: a multiple-center epidemiological study. Crit Care Med. 2005;33:315–322. doi: 10.1097/01.CCM.0000153408.09806.1B. [DOI] [PubMed] [Google Scholar]
- Deeren D, Malbrain M. Prevalence and incidence of intra-abdominal hypertension. In: Ivatury R, Cheatham M, Malbrain M, Sugrue M, editor. Abdominal Compartment Syndrome. Georgetown, Texas: Landes Biosciences; 2006. pp. 82–88. [Google Scholar]
- Schein M, Ivatury R. Intra-abdominal hypertension and the abdominal compartment syndrome. Br J Surg. 1998;85:1027–1028. doi: 10.1046/j.1365-2168.1998.00831.x. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Trinkle JK. Hemodynamic and respiratory alterations with increased intra-abdominal pressure. J Surg Res. 1976;20:401–404. doi: 10.1016/0022-4804(76)90112-8. [DOI] [PubMed] [Google Scholar]
- Malbrain ML, De laet I, Cheatham M. Consensus conference definitions and recommendations on intra-abdominal hypertension (IAH) and the abdominal compartment syndrome (ACS) – the long road to the final publications, how did we get there? Acta Clin Belg Suppl. 2007:44–59. doi: 10.1179/acb.2007.62.s1.007. [DOI] [PubMed] [Google Scholar]
- Cheatham ML, Malbrain ML, Kirkpatrick A, Sugrue M, Parr M, De Waele JJ, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. II. Recommendations. Intensive Care Med. 2007;33:951–962. doi: 10.1007/s00134-007-0592-4. [DOI] [PubMed] [Google Scholar]
- Cheatham ML. Non-operative management of intra-abdominal hypertension and abdominal compartment syndrome. World J Surg. 2009.
- Cheatham ML. Abdominal Compartment Syndrome. Curr Opin Crit Care. 2009. [DOI] [PubMed]
- Cheatham ML, Safcsak K. Is the evolving management of IAH/ACS improving survival? Acta Clinica Belgica. 2007;62:268. Abstract. [Google Scholar]
- Ennis JL, Chung KK, Renz EM, Barillo DJ, Albrecht MC, Jones JA, et al. Joint Theater Trauma System implementation of burn resuscitation guidelines improves outcomes in severely burned military casualties. J Trauma. 2008;64:S146–S151. doi: 10.1097/TA.0b013e318160b44c. [DOI] [PubMed] [Google Scholar]
- Chen H, Li F, Sun JB, Jia JG. Abdominal compartment syndrome in patients with severe acute pancreatitis in early stage. World J Gastroenterol. 2008;14:3541–3548. doi: 10.3748/wjg.14.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De I, Malbrain M. Current insights in intra-abdominal hypertension and abdominal compartment syndrome. Med Intensiva. 2007;31:88–99. doi: 10.1016/S0210-5691(07)74781-2. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick AW, Balogh Z, Ball CG, Ahmed N, Chun R, McBeth P, et al. The secondary abdominal compartment syndrome: iatrogenic or unavoidable? J Am Coll Surg. 2006;202:668–679. doi: 10.1016/j.jamcollsurg.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Maerz L, Kaplan LJ. Abdominal compartment syndrome. Crit Care Med. 2008;36:S212–S215. doi: 10.1097/CCM.0b013e318168e333. [DOI] [PubMed] [Google Scholar]
- Cheatham ML. Intraabdominal pressure monitoring during fluid resuscitation. Curr Opin Crit Care. 2008;14:327–333. doi: 10.1097/MCC.0b013e3282fce783. [DOI] [PubMed] [Google Scholar]
- Coombs H. The mechanism of the regulation of intra-abdominal pressure. Am J Physiol. 1922;61:159–170. [Google Scholar]
- Diebel LN, Dulchavsky SA, Wilson RF. Effect of increased intra-abdominal pressure on mesenteric arterial and intestinal mucosal blood flow. J Trauma. 1992;33:45–48. doi: 10.1097/00005373-199207000-00010. [DOI] [PubMed] [Google Scholar]
- Richards WO, Scovill W, Shin B, Reed W. Acute renal failure associated with increased intra-abdominal pressure. Ann Surg. 1983;197:183–187. doi: 10.1097/00000658-198302000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenasky JH. The renal hemodynamic and functional effects of external counterpressure. Surg Gynecol Obstet. 1972;134:253–258. [PubMed] [Google Scholar]
- Overholt R. Intraperitoneal pressure. Arch Surg. 1931;22:691–703. [Google Scholar]
- Bradley S, Bradley G. The effect of increased intra-abdominal pressure on renal function in man. J Clin Invest. 1947;26:1010–1015. doi: 10.1172/JCI101867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R. A new method for surgical treatment of large omphaloceles. Surgery. 1948;24:277–292. [PubMed] [Google Scholar]
- Lacey SR, Carris LA, Beyer AJ, III, Azizkhan RG. Bladder pressure monitoring significantly enhances care of infants with abdominal wall defects: a prospective clinical study. J Pediatr Surg. 1993;28:1370–1374. doi: 10.1016/S0022-3468(05)80329-X. [DOI] [PubMed] [Google Scholar]
- Gongaware RD, Marino BL, Smith RM, Sacks LM, Morrison JV., Jr Management of gastroschisis. Am Surg. 1987;53:468–471. [PubMed] [Google Scholar]
- Schwartz MZ, Tyson KR, Milliorn K, Lobe TE. Staged reduction using a Silastic sac is the treatment of choice for large congenital abdominal wall defects. J Pediatr Surg. 1983;18:713–719. doi: 10.1016/S0022-3468(83)80010-4. [DOI] [PubMed] [Google Scholar]
- Soderberg G, Westin B. Transmission of rapid pressure increase from the peritoneal cavity to the bladder. Scand J Urol Nephrol. 1970;4:155–156. doi: 10.3109/00365597009137590. [DOI] [PubMed] [Google Scholar]
- Kron IL, Harman PK, Nolan SP. The measurement of intra-abdominal pressure as a criterion for abdominal re-exploration. Ann Surg. 1984;199:28–30. doi: 10.1097/00000658-198401000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman PK, Kron IL, McLachlan HD, Freedlender AE, Nolan SP. Elevated intra-abdominal pressure and renal function. Ann Surg. 1982;196:594–597. doi: 10.1097/00000658-198211000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick AW, Balogh Z, Ball CG, Ahmed N, Chun R, McBeth P, et al. The secondary abdominal compartment syndrome: iatrogenic or unavoidable? J Am Coll Surg. 2006;202:668–679. doi: 10.1016/j.jamcollsurg.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Balogh Z, McKinley BA, Holcomb JB, Miller CC, Cocanour CS, Kozar RA, et al. Both primary and secondary abdominal compartment syndrome can be predicted early and are harbingers of multiple organ failure. J Trauma. 2003;54:848–859. doi: 10.1097/01.TA.0000070166.29649.F3. [DOI] [PubMed] [Google Scholar]
- Balogh Z, McKinley BA, Cocanour CS, Kozar RA, Holcomb JB, Ware DN, et al. Secondary abdominal compartment syndrome is an elusive early complication of traumatic shock resuscitation. Am J Surg. 2002;184:538–543. doi: 10.1016/S0002-9610(02)01050-4. [DOI] [PubMed] [Google Scholar]
- Ivy ME, Atweh NA, Palmer J, Possenti PP, Pineau M, D'Aiuto M. Intra-abdominal hypertension and abdominal compartment syndrome in burn patients. J Trauma. 2000;49:387–391. doi: 10.1097/00005373-200009000-00001. [DOI] [PubMed] [Google Scholar]
- Bloomfield GL, Ridings PC, Blocher CR, Marmarou A, Sugerman HJ. Effects of increased intra-abdominal pressure upon intracranial and cerebral perfusion pressure before and after volume expansion. J Trauma. 1996;40:936–941. doi: 10.1097/00005373-199606000-00012. [DOI] [PubMed] [Google Scholar]
- Bloomfield GL, Blocher CR, Fakhry IF, Sica DA, Sugerman HJ. Elevated intra-abdominal pressure increases plasma renin activity and aldosterone levels. J Trauma. 1997;42:997–1004. doi: 10.1097/00005373-199706000-00002. [DOI] [PubMed] [Google Scholar]
- Cullen DJ, Coyle JP, Teplick R, Long MC. Cardiovascular, pulmonary, and renal effects of massively increased intra-abdominal pressure in critically ill patients. Crit Care Med. 1989;17:118–121. doi: 10.1097/00003246-198902000-00002. [DOI] [PubMed] [Google Scholar]
- Diebel LN, Wilson RF, Dulchavsky SA, Saxe J. Effect of increased intra-abdominal pressure on hepatic arterial, portal venous, and hepatic microcirculatory blood flow. J Trauma. 1992;33:279–282. doi: 10.1097/00005373-199208000-00019. [DOI] [PubMed] [Google Scholar]
- Iberti TJ, Lieber CE, Benjamin E. Determination of intra-abdominal pressure using a transurethral bladder catheter: clinical validation of the technique. Anesthesiology. 1989;70:47–50. doi: 10.1097/00000542-198901000-00011. [DOI] [PubMed] [Google Scholar]
- Ishizaki Y, Bandai Y, Shimomura K, Abe H, Ohtomo Y, Idezuki Y. Safe intraabdominal pressure of carbon dioxide pneumoperitoneum during laparoscopic surgery. Surgery. 1993;114:549–554. [PubMed] [Google Scholar]
- Kashtan J, Green JF, Parsons EQ, Holcroft JW. Hemodynamic effect of increased abdominal pressure. J Surg Res. 1981;30:249–255. doi: 10.1016/0022-4804(81)90156-6. [DOI] [PubMed] [Google Scholar]
- Luca A, Cirera I, Garcia-Pagan JC, Feu F, Pizcueta P, Bosch J, et al. Hemodynamic effects of acute changes in intra-abdominal pressure in patients with cirrhosis. Gastroenterology. 1993;104:222–227. doi: 10.1016/0016-5085(93)90855-7. [DOI] [PubMed] [Google Scholar]
- Westerband A, Van De WJ, Amzallag M, Lebowitz PW, Nwasokwa ON, Chardavoyne R, et al. Cardiovascular changes during laparoscopic cholecystectomy. Surg Gynecol Obstet. 1992;175:535–538. [PubMed] [Google Scholar]
- Caldwell CB, Ricotta JJ. Changes in visceral blood flow with elevated intraabdominal pressure. J Surg Res. 1987;43:14–20. doi: 10.1016/0022-4804(87)90041-2. [DOI] [PubMed] [Google Scholar]
- Simon RJ, Friedlander MH, Ivatury RR, DiRaimo R, Machiedo GW. Hemorrhage lowers the threshold for intra-abdominal hypertension-induced pulmonary dysfunction. J Trauma. 1997;42:398–403. doi: 10.1097/00005373-199703000-00006. [DOI] [PubMed] [Google Scholar]
- Smith PK, Tyson GS, Jr, Hammon JW, Jr, Olsen CO, Hopkins RA, Maier GW, et al. Cardiovascular effects of ventilation with positive expiratory airway pressure. Ann Surg. 1982;195:121–130. doi: 10.1097/00000658-198202000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridings PC, Bloomfield GL, Blocher CR, Sugerman HJ. Cardiopulmonary effects of raised intra-abdominal pressure before and after intravascular volume expansion. J Trauma. 1995;39:1071–1075. doi: 10.1097/00005373-199512000-00010. [DOI] [PubMed] [Google Scholar]
- Bendahan J, Coetzee CJ, Papagianopoulos C, Muller R. Abdominal compartment syndrome. J Trauma. 1995;38:152–153. doi: 10.1097/00005373-199501000-00034. [DOI] [PubMed] [Google Scholar]
- Bloomfield GL, Dalton JM, Sugerman HJ, Ridings PC, DeMaria EJ, Bullock R. Treatment of increasing intracranial pressure secondary to the acute abdominal compartment syndrome in a patient with combined abdominal and head trauma. J Trauma. 1995;39:1168–1170. doi: 10.1097/00005373-199512000-00028. [DOI] [PubMed] [Google Scholar]
- Diamant M, Benumof JL, Saidman LJ. Hemodynamics of increased intra-abdominal pressure: Interaction with hypovolemia and halothane anesthesia. Anesthesiology. 1978;48:23–27. doi: 10.1097/00000542-197801000-00005. [DOI] [PubMed] [Google Scholar]
- Cheatham M, Malbrain M. Intra-abdominal hypetension and the cardiovascular system. In: Ivatury R, Cheatham M, Malbrain M, Sugrue M, editor. Abdominal Compartment Syndrome. Georgetown, Texas: Landes Biosciences; 2006. pp. 89–104. [Google Scholar]
- Cheatham M, Malbrain M. Cardiovascular implications of abdominal compartment syndrome. Acta Clin Belg Suppl. 2007;1:98–112. [PubMed] [Google Scholar]
- Cheatham ML, Safcsak K, Block EF, Nelson LD. Preload assessment in patients with an open abdomen. J Trauma. 1999;46:16–22. doi: 10.1097/00005373-199901000-00004. [DOI] [PubMed] [Google Scholar]
- Malbrain ML, De laet I. Functional haemodynamics during intra-abdominal hypertension: what to use and what not use. Acta Anaesthesiol Scand. 2008;52:576–577. doi: 10.1111/j.1399-6576.2007.01567.x. [DOI] [PubMed] [Google Scholar]
- Schein M, Wittmann DH, Aprahamian CC, Condon RE. The abdominal compartment syndrome: the physiological and clinical consequences of elevated intra-abdominal pressure. J Am Coll Surg. 1995;180:745–753. [PubMed] [Google Scholar]
- Barnes GE, Laine GA, Giam PY, Smith EE, Granger HJ. Cardiovascular responses to elevation of intra-abdominal hydrostatic pressure. Am J Physiol. 1985;248:R208–R213. doi: 10.1152/ajpregu.1985.248.2.R208. [DOI] [PubMed] [Google Scholar]
- Goodale RL, Beebe DS, McNevin MP, Boyle M, Letourneau JG, Abrams JH, et al. Hemodynamic, respiratory, and metabolic effects of laparoscopic cholecystectomy. Am J Surg. 1993;166:533–537. doi: 10.1016/S0002-9610(05)81148-1. [DOI] [PubMed] [Google Scholar]
- Watson RA, Howdieshell TR. Abdominal compartment syndrome. South Med J. 1998;91:326–332. doi: 10.1097/00007611-199804000-00002. [DOI] [PubMed] [Google Scholar]
- MacDonnell SP, Lalude OA, Davidson AC. The abdominal compartment syndrome: the physiological and clinical consequences of elevated intra-abdominal pressure. J Am Coll Surg. 1996;183:419–420. [PubMed] [Google Scholar]
- Baxter JN, O'Dwyer PJ. Pathophysiology of laparoscopy. Br J Surg. 1995;82:1–2. doi: 10.1002/bjs.1800820102. [DOI] [PubMed] [Google Scholar]
- Fietsam R, Jr, Villalba M, Glover JL, Clark K. Intra-abdominal compartment syndrome as a complication of ruptured abdominal aortic aneurysm repair. Am Surg. 1989;55:396–402. [PubMed] [Google Scholar]
- Mertens zur Borg I, Verbrugge S, Olvera C. Intra-abdominal hypertension and the respiratory system. In: Ivatury R, Cheatham M, Malbrain M, Sugrue M, editor. Abdominal Compartment Syndrome. Georgetown, Texas: Landes Biosciences; 2006. pp. 105–118. [Google Scholar]
- Obeid F, Saba A, Fath J, Guslits B, Chung R, Sorensen V, et al. Increases in intra-abdominal pressure affect pulmonary compliance. Arch Surg. 1995;130:544–547. doi: 10.1001/archsurg.1995.01430050094016. [DOI] [PubMed] [Google Scholar]
- Aprahamian C, Wittmann DH, Bergstein JM, Quebbeman EJ. Temporary abdominal closure (TAC) for planned relaparotomy (etappenlavage) in trauma. J Trauma. 1990;30:719–723. doi: 10.1097/00005373-199006000-00011. [DOI] [PubMed] [Google Scholar]
- Cade R, Wagemaker H, Vogel S, Mars D, Hood-Lewis D, Privette M, et al. Hepatorenal syndrome. Studies of the effect of vascular volume and intraperitoneal pressure on renal and hepatic function. Am J Med. 1987;82:427–438. doi: 10.1016/0002-9343(87)90442-6. [DOI] [PubMed] [Google Scholar]
- Platell CF, Hall J, Clarke G, Lawrence-Brown M. Intra-abdominal pressure and renal function after surgery to the abdominal aorta. Aust N Z J Surg. 1990;60:213–216. doi: 10.1111/j.1445-2197.1990.tb07407.x. [DOI] [PubMed] [Google Scholar]
- Savino JA, Cerabona T, Agarwal N, Byrne D. Manipulation of ascitic fluid pressure in cirrhotics to optimize hemodynamic and renal function. Ann Surg. 1988;208:504–511. doi: 10.1097/00000658-198810000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue M, Buist MD, Hourihan F, Deane S, Bauman A, Hillman K. Prospective study of intra-abdominal hypertension and renal function after laparotomy. Br J Surg. 1995;82:235–238. doi: 10.1002/bjs.1800820234. [DOI] [PubMed] [Google Scholar]
- Stone HH, Fulenwider JT. Renal decapsulation in the prevention of post-ischemic oliguria. Ann Surg. 1977;186:343–355. doi: 10.1097/00000658-197709000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roith D, Bark H, Nyska M, Glick SM. The effect of abdominal pressure on plasma antidiuretic hormone levels in the dog. J Surg Res. 1982;32:65–69. doi: 10.1016/0022-4804(82)90186-X. [DOI] [PubMed] [Google Scholar]
- Friedlander MH, Simon RJ, Ivatury R, DiRaimo R, Machiedo GW. Effect of hemorrhage on superior mesenteric artery flow during increased intra-abdominal pressures. J Trauma. 1998;45:433–489. doi: 10.1097/00005373-199809000-00002. [DOI] [PubMed] [Google Scholar]
- Diebel LN, Myers T, Dulchavsky S. Effects of increasing airway pressure and PEEP on the assessment of cardiac preload. J Trauma. 1997;42:585–590. doi: 10.1097/00005373-199704000-00002. [DOI] [PubMed] [Google Scholar]
- Djavani K, Wanhainen A, Valtysson J, Bjorck M. Colonic ischemia and intra-abdominal hypertension following open surgery for ruptured abdominal aortic aneurysm: A prospective study. Br J Surg. 2009. [DOI] [PubMed]
- Reintam A, Parm P, Kitus R, Starkopf J, Kern H. Gastrointestinal Failure score in critically ill patients: a prospective observational study. Crit Care. 2008;12:R90. doi: 10.1186/cc6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue M, Jones F, Janjua KJ, Deane SA, Bristow P, Hillman K. Temporary abdominal closure: a prospective evaluation of its effects on renal and respiratory physiology. J Trauma. 1998;45:914–921. doi: 10.1097/00005373-199811000-00013. [DOI] [PubMed] [Google Scholar]
- Djavani K, Wanhainen A, Bjorck M. Intra-abdominal hypertension and abdominal compartment syndrome following surgery for ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2006;31:581–584. doi: 10.1016/j.ejvs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Diebel LN, Dulchavsky SA, Brown WJ. Splanchnic ischemia and bacterial translocation in the abdominal compartment syndrome. J Trauma. 1997;43:852–855. doi: 10.1097/00005373-199711000-00019. [DOI] [PubMed] [Google Scholar]
- Gargiulo NJ, III, Simon RJ, Leon W, Machiedo GW. Hemorrhage exacerbates bacterial translocation at low levels of intra-abdominal pressure. Arch Surg. 1998;133:1351–1355. doi: 10.1001/archsurg.133.12.1351. [DOI] [PubMed] [Google Scholar]
- Nakatani T, Sakamoto Y, Kaneko I, Ando H, Kobayashi K. Effects of intra-abdominal hypertension on hepatic energy metabolism in a rabbit model. J Trauma. 1998;44:446–453. doi: 10.1097/00005373-199803000-00004. [DOI] [PubMed] [Google Scholar]
- Burchard KW, Ciombor DM, McLeod MK, Slothman GJ, Gann DS. Positive end expiratory pressure with increased intra-abdominal pressure. Surg Gynecol Obstet. 1985;161:313–318. [PubMed] [Google Scholar]
- Hopgood P, Moody P, Nelson RA, Edwards P. The abdominal compartment syndrome: the physiological and clinical consequences of elevated intra-abdominal pressure. J Am Coll Surg. 1996;183:420–422. [PubMed] [Google Scholar]
- Josephs LG, Este-McDonald JR, Birkett DH, Hirsch EF. Diagnostic laparoscopy increases intracranial pressure. J Trauma. 1994;36:815–818. doi: 10.1097/00005373-199406000-00011. [DOI] [PubMed] [Google Scholar]
- Irgau I, Koyfman Y, Tikellis JI. Elective intraoperative intracranial pressure monitoring during laparoscopic cholecystectomy. Arch Surg. 1995;130:1011–1013. doi: 10.1001/archsurg.1995.01430090097028. [DOI] [PubMed] [Google Scholar]
- Sugerman HJ, DeMaria EJ, Felton WL, III, Nakatsuka M, Sismanis A. Increased intra-abdominal pressure and cardiac filling pressures in obesity-associated pseudotumor cerebri. Neurology. 1997;49:507–511. doi: 10.1212/wnl.49.2.507. [DOI] [PubMed] [Google Scholar]
- Luce JM, Huseby JS, Kirk W, Butler J. Mechanism by which positive end-expiratory pressure increases cerebrospinal fluid pressure in dogs. J Appl Physiol. 1982;52:231–235. doi: 10.1152/jappl.1982.52.1.231. [DOI] [PubMed] [Google Scholar]
- Burchiel KJ, Steege TD, Wyler AR. Intracranial pressure changes in brain-injured patients requiring positive end-expiratory pressure ventilation. Neurosurgery. 1981;8:443–449. doi: 10.1097/00006123-198104000-00007. [DOI] [PubMed] [Google Scholar]
- Gardner SR, Maull KI, Swensson EE, Ward JD. The effects of the pneumatic antishock garment on intracranial pressure in man: a prospective study of 12 patients with severe head injury. J Trauma. 1984;24:896–900. doi: 10.1097/00005373-198410000-00005. [DOI] [PubMed] [Google Scholar]
- Mutoh T, Lamm WJ, Embree LJ, Hildebrandt J, Albert RK. Volume infusion produces abdominal distension, lung compression, and chest wall stiffening in pigs. J Appl Physiol. 1992;72:575–582. doi: 10.1063/1.351835. [DOI] [PubMed] [Google Scholar]
- Diebel L, Saxe J, Dulchavsky S. Effect of intra-abdominal pressure on abdominal wall blood flow. Am Surg. 1992;58:573–575. [PubMed] [Google Scholar]
- Ivatury RR, Sugerman HJ, Peitzman AB. Abdominal compartment syndrome: recognition and management. Adv Surg. 2001;35:251–269. [PubMed] [Google Scholar]
- Malbrain ML. Different techniques to measure intra-abdominal pressure (IAP): time for a critical re-appraisal. Intensive Care Med. 2004;30:357–371. doi: 10.1007/s00134-003-2107-2. [DOI] [PubMed] [Google Scholar]
- Malbrain ML. Is it wise not to think about intraabdominal hypertension in the ICU? Curr Opin Crit Care. 2004;10:132–145. doi: 10.1097/00075198-200404000-00010. [DOI] [PubMed] [Google Scholar]
- Cheatham ML, White MW, Sagraves SG, Johnson JL, Block EF. Abdominal perfusion pressure: a superior parameter in the assessment of intra-abdominal hypertension. J Trauma. 2000;49:621–626. doi: 10.1097/00005373-200010000-00008. [DOI] [PubMed] [Google Scholar]
- Cheatham M, Malbrain M. Abdominal perfusion pressure. In: Ivatury R, Cheatham M, Malbrain M, Sugrue M, editor. Abdominal Compartment Syndrome. Georgetown, Texas: Landes Biosciences; 2006. pp. 69–81. [Google Scholar]
- Sugrue M, Hallal A, D'Amours S. Intra-abdominal hypertension and the kidney. In: Ivatury RR, Cheatham ML, Malbrain MLNG, Sugrue M, editor. Abdominal Compartment Syndrome. Georgetown, Texas: Landes Biosciences; 2006. pp. 119–128. [Google Scholar]
- De laet I, Malbrain M, Jadoul J, Rogiers P, Sugrue M. Renal implications of increased intra-abdominal pressure: Are the kidneys the canary for abdominal hypertension? Acta Clin Belg Suppl. 2007;1:119–130. doi: 10.1179/acb.2007.62.s1.015. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick AW, Brenneman FD, McLean RF, Rapanos T, Boulanger BR. Is clinical examination an accurate indicator of raised intra-abdominal pressure in critically injured patients? Can J Surg. 2000;43:207–211. [PMC free article] [PubMed] [Google Scholar]
- Sugrue M, Bauman A, Jones F, Bishop G, Flabouris A, Parr M, et al. Clinical examination is an inaccurate predictor of intraabdominal pressure. World J Surg. 2002;26:1428–1431. doi: 10.1007/s00268-002-6411-8. [DOI] [PubMed] [Google Scholar]
- Cheatham ML. Resuscitation end points in severe sepsis: central venous pressure, mean arterial pressure, mixed venous oxygen saturation, and. intra-abdominal pressure. Crit Care Med. 2008;36:1012–1014. doi: 10.1097/CCM.0B013E318165FBF5. [DOI] [PubMed] [Google Scholar]
- Cheatham ML, Safcsak K. Intraabdominal pressure: a revised method for measurement. J Am Coll Surg. 1998;186:594–595. doi: 10.1016/S1072-7515(98)00122-7. [DOI] [PubMed] [Google Scholar]
- De Waele JJ, De l I, Malbrain ML. Rational intraabdominal pressure monitoring: how to do it? Acta Clin Belg Suppl. 2007:16–25. doi: 10.1179/acb.2007.62.s1.004. [DOI] [PubMed] [Google Scholar]
- Malbrain ML, Jones F. Intra-abdominal pressure monitoring techniques. In: Ivatury RR, Cheatham ML, Malbrain MLNG, Sugrue M, editor. Abdominal Compartment Syndrome. Georgetown, Texas: Landes Biosciences; 2006. pp. 19–68. [Google Scholar]
- Cheatham ML, Sagraves SG, Johnson JL, White MW. Intravesicular pressure monitoring does not cause urinary tract infection. Intensive Care Med. 2006;32:1640–1643. doi: 10.1007/s00134-006-0350-z. [DOI] [PubMed] [Google Scholar]
- Sanchez NC, Tenofsky PL, Dort JM, Shen LY, Helmer SD, Smith RS. What is normal intra-abdominal pressure? Am Surg. 2001;67:243–248. [PubMed] [Google Scholar]
- Burch JM, Moore EE, Moore FA, Franciose R. The abdominal compartment syndrome. Surg Clin North Am. 1996;76:833–842. doi: 10.1016/S0039-6109(05)70483-7. [DOI] [PubMed] [Google Scholar]
- Hong JJ, Cohn SM, Perez JM, Dolich MO, Brown M, McKenney MG. Prospective study of the incidence and outcome of intra-abdominal hypertension and the abdominal compartment syndrome. Br J Surg. 2002;89:591–596. doi: 10.1046/j.1365-2168.2002.02072.x. [DOI] [PubMed] [Google Scholar]
- Ivy ME, Possenti PP, Kepros J, Atweh NA, D'Aiuto M, Palmer J, et al. Abdominal compartment syndrome in patients with burns. J Burn Care Rehabil. 1999;20:351–353. doi: 10.1097/00004630-199909000-00003. [DOI] [PubMed] [Google Scholar]
- Loftus IM, Thompson MM. The abdominal compartment syndrome following aortic surgery. Eur J Vasc Endovasc Surg. 2003;25:97–109. doi: 10.1053/ejvs.2002.1828. [DOI] [PubMed] [Google Scholar]
- McNelis J, Marini CP, Jurkiewicz A, Fields S, Caplin D, Stein D, et al. Predictive factors associated with the development of abdominal compartment syndrome in the surgical intensive care unit. Arch Surg. 2002;137:133–136. doi: 10.1001/archsurg.137.2.133. [DOI] [PubMed] [Google Scholar]
- Meldrum DR, Moore FA, Moore EE, Franciose RJ, Sauaia A, Burch JM. Prospective characterization and selective management of the abdominal compartment syndrome. Am J Surg. 1997;174:667–672. doi: 10.1016/S0002-9610(97)00201-8. [DOI] [PubMed] [Google Scholar]
- Offner PJ, de Souza AL, Moore EE, Biffl WL, Franciose RJ, Johnson JL, et al. Avoidance of abdominal compartment syndrome in damage-control laparotomy after trauma. Arch Surg. 2001;136:676–681. doi: 10.1001/archsurg.136.6.676. [DOI] [PubMed] [Google Scholar]
- Raeburn CD, Moore EE, Biffl WL, Johnson JL, Meldrum DR, Offner PJ, et al. The abdominal compartment syndrome is a morbid complication of postinjury damage control surgery. Am J Surg. 2001;182:542–546. doi: 10.1016/S0002-9610(01)00821-2. [DOI] [PubMed] [Google Scholar]
- Balogh Z, McKinley BA, Cocanour CS, Kozar RA, Valdivia A, Sailors RM, et al. Supranormal trauma resuscitation causes more cases of abdominal compartment syndrome. Arch Surg. 2003;138:637–642. doi: 10.1001/archsurg.138.6.637. [DOI] [PubMed] [Google Scholar]
- Cheatham ML, Safcsak K, Llerena LE, Morrow CE, Jr, Block EF. Long-term physical, mental, and functional consequences of abdominal decompression. J Trauma. 2004;56:237–241. doi: 10.1097/01.TA.0000109858.55483.86. [DOI] [PubMed] [Google Scholar]
- Cheatham ML, Safcsak K. Longterm impact of abdominal decompression: a prospective comparative analysis. J Am Coll Surg. 2008;207:573–579. doi: 10.1016/j.jamcollsurg.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Malbrain ML, Deeren D, De Potter TJ. Intra-abdominal hypertension in the critically ill: it is time to pay attention. Curr Opin Crit Care. 2005;11:156–171. doi: 10.1097/01.ccx.0000155355.86241.1b. [DOI] [PubMed] [Google Scholar]
- Sugrue M. Abdominal compartment syndrome. Curr Opin Crit Care. 2005;11:333–338. doi: 10.1097/01.ccx.0000170505.53657.48. [DOI] [PubMed] [Google Scholar]