Abstract
Objective
To test the hypothesis that amphotericin B deoxycholate is less toxic when given by continuous infusion than by conventional rapid infusion.
Design
Randomised, controlled, non-blinded, single centre study.
Setting
University hospital providing tertiary clinical care.
Patients
80 mostly neutropenic patients with refractory fever and suspected or proved invasive fungal infections.
Intervention
Patients were randomised to receive 0.97 mg/kg amphotericin B by continuous infusion over 24 hours or 0.95 mg/kg by rapid infusion over four hours.
Main outcome measures
Patients were evaluated for side effects related to infusion, nephrotoxicity, and mortality up to three months after treatment. Analysis was on an intention to treat basis.
Results
Patients in the continuous infusion group had fewer side effects and significantly reduced nephrotoxicity than those in the rapid infusion group. Overall mortality was higher during treatment and after three months' follow up in the rapid infusion than in the continuous infusion group.
Conclusion
Continuous infusions of amphotericin B reduce nephrotoxicity and side effects related to infusion without increasing mortality.
Introduction
Amphotericin B deoxycholate has remained the mainstay of treatment for life threatening fungal infections in immunocompromised patients because of its broad fungicidal activity and cheapness. Treatment with amphotericin B, however, is associated with acute reactions related to infusion and dose dependent nephrotoxicity. It is recommended that amphotericin B is infused slowly over two to six hours, based on the assumption that the severity and frequency of toxic reactions increase during more rapid infusions.1–4
Incorporation of amphotericin B into liposomal formulations reduces its toxicity, but the reasons for this are unclear.5–11 As liposomes do not specifically target fungal cells it would seem that the reduction in toxicity, at least in part, depends on a slower delivery of amphotericin B to tissues. The question as to whether a slower delivery of amphotericin B from lipid formulations might be reproduced by a slow infusion rate therefore arises. The hypothesis that a continuous infusion of amphotericin B results in reduced toxicity has not been addressed yet in a prospective study. We therefore conducted a randomised, controlled, and open trial to compare the toxicity of amphotericin B given as a continuous infusion with a conventional rapid regimen over four hours.
Patients and methods
Inclusion and exclusion criteria and treatment
All consecutive patients at our tertiary referral centre for adult internal medicine (Zurich University Hospital), including those at the medical intensive care unit, were considered eligible for entry to the study, providing their doctors had decided to start treatment with amphotericin B. Exclusion criteria were a baseline serum creatinine concentration in excess of 300 μmol/l or systemic treatment with amphotericin B within the past seven days.
Patients received either a continuous (24 hours) or a rapid (four hours) infusion of amphotericin B. The drug was given in 500 ml of 5% glucose without any additives through a separate intravenous line. To take into account clinical practice patterns, doctors were free to adjust the drug dose during the study if necessary.
Drugs to prevent chills or fever were prohibited on day 1 of entry to the study.12 To reduce nephrotoxicity from amphotericin B all patients received infusions of saline as standard care.13–15 The protocol gave no other restrictions on the use of any concomitant treatment.
Outcome measures
Chills, rigors, and vomiting were monitored prospectively. Each patient completed a standardised questionnaire daily until the end of the study and was interviewed regularly. We also evaluated nursing charts for any other adverse reaction. Temperature was measured at least three times daily. Fever was defined as a core temperature of at least 39.3°C (core temperature readings are 1°C above the peripheral temperature readings). The concentrations of C reactive protein were determined every 24 hours during the first three days and at least every other day thereafter.
Serum creatinine concentrations were measured daily during treatment. To obtain a variable for nephrotoxicity independent of age and body mass, we used a model of calculated creatinine clearance based on lean body mass.16,17 This model predicts accurately the change in creatinine clearance from serum creatinine values. It works equally for patients with either stable or unstable renal function. Creatinine clearance per lean body mass may be calculated according to the formula ClLBM=E/S+[600×(S1-S2)/(T×S)], where S is the mean of two serum creatinine values S1 and S2, T is the time interval between S1 and S2, and E corresponds to an age dependent urinary creatinine excretion rate in mg/min/50 kg lean body mass.17
Electrolytes, pH, and venous bicarbonate concentrations were measured every other day. Hypokalaemia was defined as a serum potassium concentration less than 2.5 mmol/l and hypomagnesaemia as a serum magnesium concentration less than 0.5 mmol/l, regardless of any enteral or parenteral substitution. Efficacy was monitored for overall mortality, mortality due to invasive fungal infections, and breakthrough fungaemia during treatment.
Statistics and study ethics
Analysis was on an intention to treat basis. The design of our trial was based on a prior power calculation for the calculated serum creatinine clearance at the end of treatment. The possible difference between the study arms was estimated from a retrospective analysis of patients receiving amphotericin B at our centre. We planned to randomise 40 patients to each arm so as to achieve a power of 90% for the detection of an effect size of [difference]/[standard deviation]=0.8 when applying a two sided t test at the 5% level. For the calculated creatinine clearance, this corresponds to a difference of at least 20 ml/min between the treatments, assuming a standard deviation of 25 ml/min within each group.
We present continuous data as the median (range), which were evaluated using the Mann-Whitney U test. The effect of treatment was reported as the difference of medians between study groups. We calculated the differences of medians with the Hodge-Lehmann estimator and the confidence intervals of median differences with the exact distribution of the Mann-Whitney test statistic.18 We present dichotomous data as the number of patients, analysed with Fisher's exact test. We report the effect of amphotericin B treatment for dichotomous data as relative risk. Confidence intervals for relative risks were obtained as described previously.19 We considered differences for which P<0.05 significant.
Study ethics—Our study was approved by the institutional ethics committee of Zurich University Hospital. We obtained written consent from all patients at enrolment.
Assignment
The initial dosage for amphotericin B was chosen before randomisation by the doctors in charge, who were not members of the study team. Eligible patients were then randomised in blocks of 10 by sealed envelope. Treatment started immediately after randomisation.
Results
Patient flow and follow up
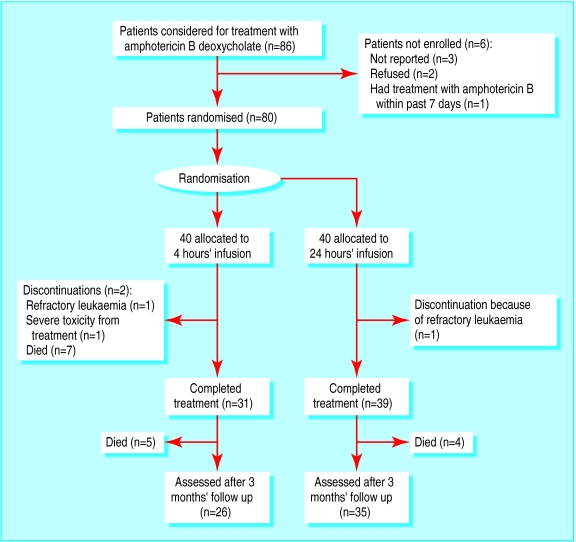
Figure 1 shows the flow of patients through the trial. Patients were followed up three months after completion of treatment or when treatment was discontinued for any reason.
Figure 1.
Trial profile
Analysis
Patients—Overall, 86 consecutive patients received amphotericin B during the study period. Most of them were severely neutropenic, with haematological neoplasias. Of these patients, only three had not been reported to the study team. One patient was not eligible because of amphotericin B treatment within the past seven days, and two did not give their consent. We enrolled 80 patients. The study population also included patients with moderately impaired renal function (baseline serum creatinine concentration less than 300 μmol/l). The groups did not differ significantly with regard to treatment with aminoglycosides, vancomycin, diuretics, and granulocyte colony stimulating factor. No patient received cyclosporin (table 1). At enrolment the two groups did not differ significantly regarding number of patients with refractory fever and probable and proved fungal infections (table 2).
Definitions
Refractory fever
Persistent fever for more than 72 hours despite antibiotics in patients who are neutropenic (less than 500 neutrophils/μl)
Possible fungal infections
Persistent fever, neutropenia, chest symptoms, or the presence of pulmonary infiltrations on chest radiographs
Probable fungal infections
Persistent fever or increased concentration of C reactive protein, neutropenia, and infiltrates characteristic of fungal pneumonia on a computed tomogram
Proved fungal infections
Typical infiltrates on a computed tomogram and detection of moulds in bronchoalveolar lavage or bronchial secretions by culture and microscopy during neutropenia, or presence of pathogenic fungi in usually sterile sites
Table 1.
Dosages of amphotericin B deoxycholate and characteristics of patients receiving rapid (four hours) and continuous (24 hours) infusions of the drug. Values are numbers (percentages) of patients unless stated otherwise
| Infusion rate
|
||
|---|---|---|
| Rapid (n=40) | Continuous (n=40) | |
| Median age (range) | 46 (20-75) | 47 (17-74) |
| Median (range) No of days of treatment | 12 (3-51) | 16 (3-89) |
| Median (range) maximum daily dose (mg/kg) | 0.95 (0.4-1.2) | 0.96 (0.5-1.5) |
| Median (range) cumulative dose (mg/kg) | 10.8 (2.1-42.7) | 14.3 (1.8-89.0) |
| Male | 22 | 27 |
| Diagnosis: | ||
| Acute myeloid leukaemia | 29 (73) | 25 (63) |
| Acute lymphatic leukaemia | 4 (10) | 5 (13) |
| Lymphoma | 4 (10) | 5 (13) |
| Solid tumour | 2 (5) | 2 (5) |
| HIV | 0 | 2 (5) |
| Others | 1 (3) | 1 (3) |
| Neutropenia (<500/μl) | 36 (90) | 37 (93) |
| Concurrent treatment: | ||
| Aminoglycosides | 18 (45) | 22 (55) |
| Vancomycin | 10 (25) | 11 (28) |
| Diuretics | 18 (45) | 16 (40) |
| Granulocyte colony stimulating factor | 13 (33) | 18 (45) |
Table 2.
Indications for treatment with amphotericin B deoxycholate, by rapid (four hours) or continuous (24 hours) infusion. Values are numbers (percentages) of patients
| Infusion rate
|
||
|---|---|---|
| Rapid (n=40) | Continuous (n=40) | |
| Refractory fever | 18 (45) | 12 (30) |
| Possible fungal infection | 12 (30) | 8 (20) |
| Probable fungal infection | 10 (25) | 17 (43) |
| Proved fungal infection | 0 | 3* (8) |
* Cryptococcosis (two patients), pulmonary aspergillosis (one).
Dosage and dose reductions—Overall duration of treatment and cumulative and daily doses did not differ significantly between the groups. There was a non-significant trend towards longer duration of treatment and higher cumulative doses in the continuous infusion group. We observed significantly more dose reductions or infusion interruptions due to side effects in the rapid infusion group (table 3).
Table 3.
Infusion related side effects and drugs to suppress febrile reactions in patients receiving rapid (four hours) or continuous (24 hours) infusions of amphotericin B deoxycholate. Values are numbers (percentages) of patients unless stated otherwise
| Infusion rate
|
P value | Relative risk (95% CI) | ||
|---|---|---|---|---|
| Rapid (n=40) |
Continuous (n=40) | |||
| Reactions on day 1: | ||||
| Fever* | 21 (53) | 10 (25) | 0.021 | 2.1 (1.1 to 3.9) |
| Chills or rigors | 21 (53) | 5 (13) | 0.0003 | 4.2 (1.8 to 10) |
| Vomiting | 14 (35) | 4 (10) | 0.009 | 3.5 (1.3 to 9.7) |
| Headache | 4 (10) | 0 | ||
| Others | 1 (3) | 0 | ||
| Overall reactions: | ||||
| Chills or rigors | 25 (63) | 8 (20) | 0.0001 | 3.1 (1.6 to 6.1) |
| Vomiting | 24 (60) | 11 (28) | 0.004 | 2.2 (1.2 to 3.8) |
| Headache | 11 (28) | 4 (10) | ||
| Others | 8 (20) | 2 (5) | ||
| Drugs after day 1: | ||||
| Meperidine | 20 (50) | 6 (15) | 0.002 | 3.3 (1.5 to 7.4) |
| Steroids | 18 (45) | 3 (8) | 0.0001 | 6 (1.9 to 19) |
| Acetaminophen | 30 (75) | 19 (48) | 0.021 | 1.6 (1.1 to 2.3) |
| Dose reductions or infusion interruption | 11 (28) | 3 (8) | 0.022 | 3.7 (1.1 to 12) |
| Median (range) defervescence† (days) | 2 (1-10) | 1 (1-4) | 0.016 | −1 (−2 to 0)‡ |
>39.3°C core temperature (corresponding to an axillary temperature of 38.3°C).
Fever within 24 hours before treatment was documented for 26 patients in the rapid infusion group and 22 patients in the continuous infusion group.
Median difference.
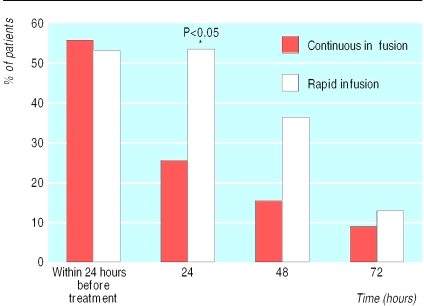
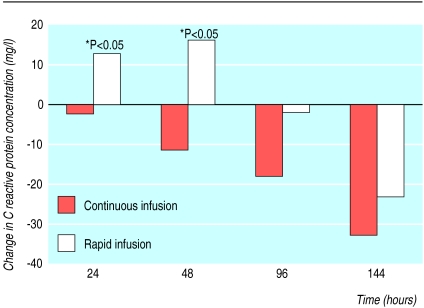
Side effects—The major side effects related to infusion were chills, fever, and vomiting. They occurred mainly during the first three days of treatment. We also observed flushing reactions, rashes, and headaches. Patients receiving continuous infusions had fewer side effects. We found significantly more patients with fever in the rapid infusion group during the first 24 hours after treatment had started (fig 2). For those who had fever at the beginning of treatment there was also a significant difference in the mean time to defervescence (table 3). The concentrations of C reactive protein did not differ between the two groups at entry to the study, but there was a significant increase in the rapid infusion group 24 and 48 hours after the start of treatment. Concentrations gradually decreased from baseline levels in the continuous infusion group (fig 3). Reflecting the reduced frequency of side effects in the continuous infusion group, these patients were less likely to receive drugs directed against febrile reactions or chills after the first treatment day (table 3).
Figure 2.
Percentage of patients with fever during first days of treatment with amphotericin B
Figure 3.
C reactive protein (CRP) concentrations during treatment with amphotericin B compared with baseline levels, expressed as median of ΔCRP (ΔCRP=CRP−CRPbaseline) in mg/l
Nephrotoxicity—The serum creatinine concentration or calculated creatinine clearance did not differ between the two groups at baseline. Comparing the ratio of peak serum creatinine to baseline creatinine concentrations during and at the end of treatment, we found a significantly higher ratio in the rapid infusion group (table 4). The increase in serum creatinine concentration was reversible in all but two patients, both assigned to the rapid infusion group, within three months after treatment had been completed. Comparison of the calculated creatinine clearance ratios between both infusion groups illustrates a significantly less impaired creatinine clearance for patients with continuous infusions during and at the end of treatment (table 4).
Table 4.
Nephrotoxicity in patients receiving rapid (four hours) or continuous (24 hours) infusion of amphotericin B deoxycholate. Values are median (range) unless stated otherwise
| Infusion rate
|
P value | Median difference (95% CI) | ||
|---|---|---|---|---|
| Rapid (n=40) | Continuous (n=40) | |||
| Serum creatinine concentration (μmol/l): | ||||
| Baseline serum creatinine | 78 (51-128) | 78 (54-165) | ||
| Maximal creatinine:baseline | 1.62 (0.94-3.42) | 1.25 (0.91-2.53) | 0.005 | −0.26 (−0.49 to−0.08) |
| Creatinine at end of study:baseline | 1.55 (0.79-3.42) | 1.17 (0.46-2.26) | 0.001 | −0.32 (−0.53 to −0.13) |
| Calculated creatinine clearance*: | ||||
| Minimal clearance:baseline | 0.62 (0.29-1.05) | 0.80 (0.39-1.10) | 0.013 | 0.12 (0.03 to 0.22) |
| Clearance at end of study:baseline | 0.65 (0.29-1.26) | 0.86 (0.44-1.91) | 0.001 | 0.19 (0.09 to 0.29) |
| Electrolyte abnormalities: | ||||
| No (%) with hypokalaemia (<2.5 mmol/l) | 10 (25) | 4 (10) | ||
| No (%) with hypernatraemia (>155 mmol/l) | 3 (8) | 2 (5) | ||
| No (%) with hypomagnesaemia (<0.5 mmol/l) | 19 (48) | 17 (43) | ||
| Venous bicarbonate concentration† | ||||
| Minimal bicarbonate:baseline | 0.77 (0.44-1.00) | 0.84 (0.59-1.37) | 0.015 | 0.09 (0.02 to 0.17) |
Creatinine clearance per 50 kg lean body mass calculated from serum creatinine concentrations.
Data missing for eight (rapid infusion) and five (continuous infusion) patients.
The proportion of patients who had hypokalaemia or hypomagnesaemia did not differ significantly between the two groups. Hypokalaemia was observed in 10 patients in the rapid infusion group and four patients in the continuous infusion group. Hypomagnesaemia occurred in 19 patients in the rapid infusion group and 17 patients in the continuous infusion group (table 4).
Efficacy
All seven deaths during treatment occurred in the rapid infusion group. Necropsy was carried out in six of these seven cases and severe pneumonia was found. Invasive fungi were proved in three cases; in one case Pneumocystis carinii was detected. In two patients no infection was found. Breakthrough fungaemia did not occur in any patient of either group (table 5).
Table 5.
Mortality and frequency of invasive fungal infections in patients receiving rapid (four hours) or continuous (24 hours) infusions of amphotericin B deoxycholate. Values are numbers (percentages) of patients
| Infusion rate
|
||
|---|---|---|
| Rapid (n=40) | Continuous (n=40) | |
| Proved fungal infections | 3* (8) | 7† (18) |
| Breakthrough fungaemia | 0 | 0 |
| Mortality: | ||
| During treatment‡ | 7 (18) | 0 |
| After 3 months' follow up§ | 12 (30) | 4 (10) |
Proved angioinvasive pulmonary mould infection with septated branching hyphae at necropsy.
Cryptococcosis (two patients), systemic candidiasis (one), pulmonary aspergillosis (three), and pulmonary Rhizopus pusillus and Aspergillus fumigatus infection (one).
P=0.012, relative risk is infinity.
P=0.048, relative risk (3.0, 95% confidence interval 1.1 to 8.5).
Treatment was discontinued in two patients assigned to rapid infusion: one because of refractory leukaemia and the other because of severe nephrotoxicity from treatment. Treatment was discontinued in one patient in the continuous infusion group because of refractory leukaemia. None of the patients with refractory disease showed invasive mycosis at necropsy.
After 3 months' follow up the two groups still differed with regard to death (table 5). All patients recovered from neutropenia, with the exception of those who died or where treatment had been discontinued.
Discussion
Continuous infusions of amphotericin B are significantly better tolerated than rapid infusions. Similar advantages of continuous infusions could be sought for other toxic drugs—for example, antineoplastic agents. Continuous applications are, however, not feasible if high peak values are necessary for efficacy (that is, with aminoglycosides). The rapid infusion of amphotericin B over fewer hours has been adopted empirically in clinical practice. Despite a retrospective analysis suggesting fewer side effects from continuous infusions, no controlled trials have compared rapid and continuous infusions of amphotericin B .2
Our study was not blinded for practical reasons. Nevertheless, differences in toxicity between groups were evident regarding C reactive protein and creatinine concentrations, which were assessed at predetermined times and were not biased by the non-blinded study design. The reduction of side effects by continuous infusion of amphotericin B seems comparable to recent reports of liposomal amphotericin B (table 6),8 despite higher daily and cumulative doses being given in our study. Amphotericin B triggers a proinflammatory response by activating different cytokines.20,21 Continuous infusions may be better tolerated because of delayed induction or release of such mediators, as reflected by differences in concentrations of C reactive protein and fever. We also observed a noticeable reduction of nephrotoxicity in the continuous infusion group. The mechanisms involved in amphotericin B nephrotoxicity are not yet fully understood.15 They can be broken down into pretubular and tubular effects. Pretubular effects, which include mainly a decrease in renal blood flow and glomerular filtration rate, are clearly reduced in patients having continuous infusions, as suggested by only a small decrease in calculated creatinine clearance. No noticeable difference was found in tubular effects between the two groups. Hypokalaemia was non-significantly decreased and tubular acidosis significantly decreased in the continuous infusion group. These observations are comparable to those in patients treated with liposomal amphotericin B: Walsh et al8 found no differences in the frequency of hypomagnesaemia between patients treated with liposomal amphotericin B and those receiving amphotericin B. In addition, they found only slight, although significant, differences in the frequency of hypokalaemia. Our study comprised only 80 patients. Nevertheless, hypokalaemia had been observed in 10 patients allocated to the rapid infusion arm compared with four patients in the continuous infusion group. Therefore, as with liposomal amphotericin B, a continuous infusion of amphotericin B primarily reduces pretubular toxicity.
Table 6.
Nephrotoxicity from liposomal amphotericin B8 and continuous infusions of amphotericin B deoxycholate (24 hours) compared with rapid (four hours) and continuous (24 hours) infusions of amphotericin B deoxycholate in present study. Values are numbers (percentages) of patients
| Walsh et al8
|
Infusion rate
|
|||
|---|---|---|---|---|
| Liposomal amphotericin B (n=343) | Amphotericin B deoxycholate (n=344) | Continuous (n=40) | Rapid (n=40) | |
| Creatinine concentration during treatment: | ||||
| 1.5 times baseline value | 99 (29) | 168 (49) | 13 (33) | 23 (58) |
| 2.0 times baseline value | 65 (19) | 117 (34) | 6 (15) | 11 (28) |
| 3.0 times baseline value | 27 (8) | 58 (17) | 0 | 4 (10) |
| Hypokalaemia | 24 (7) | 41 (12) | 4 (10) | 10 (25) |
Indications for amphotericin B in our study were proved fungal infections, probable fungal infections, possible fungal infections, and refractory fever during neutropenia. Given the high mortality of invasive mycoses after a delay in treatment, amphotericin B is often prescribed empirically. A definitive baseline diagnosis of invasive mycosis would require invasive diagnostic procedures that are seldom justified in neutropenic and thrombocytopenic patients. It is therefore scarcely ever possible to identify the true prevalence of invasive mycoses, although clinical and radiological criteria (computed tomography) allow some risk stratification. For patients surviving proved mycoses during aplasia it is also difficult to assess efficacy of treatment. For instance, progressive changes in computed tomgrams of these patients may represent ongoing infection as well as host response after resolution of neutropenia.22 In addition, recovery from neutropenia in itself results in healing of invasive mycoses. As criteria for efficacy we therefore chose mortality, mortality due to invasive fungal infections, and breakthrough fungaemia. Although our study population was small for assessment of efficacy, the outcome was encouraging for continuous infusion. We found a higher overall mortality during amphotericin B in the rapid infusion group. Mortality also remained significantly higher after three months' follow up. Despite a higher number of proved or probable fungal infections in the continuous infusion group, three patients died with proved fungal infections at necropsy in the rapid infusion group whereas none died in the continuous infusion group. Accordingly, the death rate in the rapid infusion group was strongly influenced by the occurrence of invasive mycoses. Consequently, our data support the notion that a continuous infusion of amphotericin B may be at least as effective as daily infusions over four hours. We therefore recommend continuous infusions of amphotericin B , where practical, as an effective and well tolerated alternative to the usual rapid infusions.
What is already known on this topic
Amphotericin B is the cornerstone for treatment of invasive fungal infections, especially in neutropenic patients
Its use is limited by general toxic reactions and nephrotoxicity
What this study adds
By giving amphotericin B as a continuous infusion, both nephrotoxicity and infusion related toxicity can be lowered significantly without loss of efficacy
Acknowledgments
We thank Dr K Barbatti for her help with data acquisition. Parts of this work were presented at the 39th interscience conference on antimicrobial agents and chemotherapy, 1999, San Francisco, California (organised by the American Society of Microbiology).
Footnotes
Funding: None.
Competing interests: UE has been reimbursed by Bristol-Myers Squibb, the manufacturer of amphotericin B deoxycholate (Fungizone), for attending the 39th interscience conference on antimicrobial agents and chemotherapy, 1999, San Francisco, California.
References
- 1.Ellis ME, Al-Hokail AA, Clink HM, Padmos MA, Ernst P, Spence DG, et al. Double-blind randomized study of the effect of infusion rates on toxicity of amphotericin B. Antimicrob Agents Chemother. 1992;36:172–179. doi: 10.1128/aac.36.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chabot GG, Pazdur R, Valeriote FA, Baker LH. Pharmacokinetics and toxicity of continuous infusion amphotericin B in cancer patients. J Pharm Sci. 1989;78:307–310. doi: 10.1002/jps.2600780409. [DOI] [PubMed] [Google Scholar]
- 3.Oldfield EC, Garst PD, Hostettler C, White M, Samuelson D. Randomized, double-blind trial of 1- versus 4-hour amphotericin B infusion durations. Antimicrob Agents Chemother. 1990;34:1402–1408. doi: 10.1128/aac.34.7.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz AM, Peacock JE, Loomer L, Holder LH, Evans GW, Powell BL, et al. Rapid intravenous infusion of amphotericin B: a pilot study. Am J Med. 1992;93:123–130. doi: 10.1016/0002-9343(92)90040-i. [DOI] [PubMed] [Google Scholar]
- 5.Millis W, LiChopra R, Linch DC, Goldstone AH. Liposomal amphotericin B in the treatment of fungal infections in neutropenic patients: a single center experience of 133 episodes in 116 patients. Br J Haematol. 1994;86:754–760. doi: 10.1111/j.1365-2141.1994.tb04825.x. [DOI] [PubMed] [Google Scholar]
- 6.Prentice HG, Hann IM, Herbrecht R, Aoun M, Kvaloy S, Catovsky D, et al. A randomized comparison of liposomal versus conventional amphotericin B for the treatment of pyrexia of unknown origin in neutropenic patients. Br J Haematol. 1997;98:711–718. doi: 10.1046/j.1365-2141.1997.2473063.x. [DOI] [PubMed] [Google Scholar]
- 7.Hiemenz JW, Walsh TJ. Lipid formulations of amphotericin B: recent progress and future directions. Clin Infect Dis. 1996;22(suppl 2):133–44S. doi: 10.1093/clinids/22.supplement_2.s133. [DOI] [PubMed] [Google Scholar]
- 8.Walsh TJ, Finberg RW, Arndt C, Hiemenz J, Schwartz C, Bodensteiner D, et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. N Engl J Med. 1999;340:764–771. doi: 10.1056/NEJM199903113401004. [DOI] [PubMed] [Google Scholar]
- 9.Daneshmend TK, Warnock DW. Clinical pharmacokinetics of systemic antifungal drugs. Clin Pharmacokinet. 1983;8:17–42. doi: 10.2165/00003088-198308010-00002. [DOI] [PubMed] [Google Scholar]
- 10.Schöffski P, Freund M, Wunder R, Petersen D, Köhne CH, Hecker H, et al. Safety and toxicity of amphotericin B in glucose 5% or intralipid 20% in neutropenic patients with pneumonia or fever of unknown origin: a randomised study. BMJ. 1998;317:379–384. doi: 10.1136/bmj.317.7155.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adler-Moore J. AmBisome targeting to fungal infections. Bone Marrow Transplant. 1994;14:S3–S7. [PubMed] [Google Scholar]
- 12.Goodwin DS, Cleary JD, Walawander CA, Taylor JW, Grasela TH. Pretreatment regimens for adverse events related to infusion of amphotericin B. Clin Infect Dis. 1995;20:755–761. doi: 10.1093/clinids/20.4.755. [DOI] [PubMed] [Google Scholar]
- 13.Bernardo JF, Murakami S, Branch RA, Sabra R. Potassium depletion potentiates amphotericin B induced toxicity to renal tubules. Nephron. 1995;70:234–241. doi: 10.1159/000188590. [DOI] [PubMed] [Google Scholar]
- 14.Feely J, Heidemann H, Gerkens J, Roberts LJ, Branch RA. Sodium depletion enhances nephrotoxicity of amphotericin B. Lancet. 1981;i:1422–1423. doi: 10.1016/s0140-6736(81)92597-6. [DOI] [PubMed] [Google Scholar]
- 15.Arning M, Scharf RE. Prevention of amphotericin B-induced nephrotoxicity by loading with sodium chloride: a report of 1291 days of treatment with amphotericin B without renal failure. Klin Wochenschr. 1989;67:1020–1028. doi: 10.1007/BF01727003. [DOI] [PubMed] [Google Scholar]
- 16.Hallynck TH, Soep HH, Thomis J, Boelaert J, Daneels R, Fillastre JP, et al. Prediction of creatinine clearance from serum creatinine concentration based on lean body mass. Clin Pharmacol Ther. 1981;30:414–420. doi: 10.1038/clpt.1981.181. [DOI] [PubMed] [Google Scholar]
- 17.Hallynck TH, Soep HH, Thomis J, Boelaert J, Daneels R, Dettli L. Should clearance be normalised to body surface or to lean body mass? Br J Clin Pharmacol. 1981;11:523–526. doi: 10.1111/j.1365-2125.1981.tb01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell MJ, Gardner MJ. Calculating confidence intervals for some non-parametric analyses. BMJ. 1988;296:1454–1456. doi: 10.1136/bmj.296.6634.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow-up data. Biometrics. 1985;41:55–68. [PubMed] [Google Scholar]
- 20.Rogers PD, Jenkins JK, Chapman SW, Ndebele K, Chapman BA, Cleary JD. Amphotericin B activation of human genes encoding for cytokines. J Infect Dis. 1998;178:1726–1730. doi: 10.1086/314495. [DOI] [PubMed] [Google Scholar]
- 21.Cleary JD, Chapman SW, Nolan RL. Pharmacologic modulation of interleukin-1 expression by amphotericin B-stimulated human mononuclear cells. Antimicrob Agents Chemother. 1992;36:977–981. doi: 10.1128/aac.36.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caillot D, Casanovas O, Bernard A, Couaillier J, Durand C, Cuisenier B, et al. Improved management of invasive pulmonary aspergillosis in neutropenic patients using early thoracic computed tomographic scan and surgery. J Clin Oncol. 1997;15:139–147. doi: 10.1200/JCO.1997.15.1.139. [DOI] [PubMed] [Google Scholar]