Abstract
Purpose
To determine the effects of visible light on development of mouse embryos and the potential of fibroblast cells to overcome deleterious effects of visible light on mouse preimplantation stage embryos.
Methods
Two-cell mouse embryos were randomly allocated to un-exposed group (control) and exposed group receiving 1600 lx visible light for various time lengths. Both exposed and un-exposed embryos were co-cultured with either Mouse Embryonic Fibroblast (MEF) or Human Embryonic Fibroblast (HEF). Developmental rate of embryos at day 3 (morula), 4 (expanded blastocyst) and 5 (hatching or hatched blastocyst) was evaluated.
Results
Exposure of embryos to visible light for 30 min decreased developmental rate significantly (P < 0.01). Developmental rate of exposed embryos co-cultured with MEF (58%; p < 0.05 both at day 4 and 5) and HEF (67%; P < 0.01 both at day 4 and 5) was higher than control.
Conclusions
Visible light adversely affects embryo development in a time-dependent manner. Feeder cells may enhance embryo development particularly when suboptimal conditions are involved.
Keywords: Visible light, Co-culture, Human embryonic fibroblast, Mouse embryonic fibroblast, Mouse embryo
Introduction
Under normal conditions, mammalian oocytes are fertilized in fallopian tube, and the zygotes develop into cleaved embryos in the shielding environment of the female reproductive tract. On the contrary, in vitro produced embryos as well as oocytes are prone to various environmental factors during manipulations in laboratories [1]. The adverse effects of pH fluctuations [2, 3], high and low laboratory temperature [4], change in carbon dioxide and O2 level [5, 6] and light [7, 8] have been investigated to some extent. Among the environmental factors, light is known to exert some harmful effects on developmental competence of mammalian oocytes and embryos [9]. Extent of such harmful effects depends on the strain of animal. An obvious example of sensitivity to light are golden hamster zygotes: their meiosis is affected by the short-wavelength visible light emitted from the cool white fluorescent lamps that are used in laboratories [9]. In contrasts, oocytes of many oviparous animals, such as fish and amphibians, can be exposed to direct sunlight during normal fertilization and development. These oocytes and embryos have mechanisms to protect themselves from harmful effects of light, especially UV irradiation [10]. The nature of light, wavelength and duration of exposure to light are responsible for its adverse effects on mammalian embryos [9]. The most harmful effects of light appear at lower wavelengths (<340 nm) [11]. However, visible light also has some adverse effects on embryos as well as oocytes. The stage of embryo is an important factor that determines the extent of harmful effects of light [12]. Experiments on preimplantation rabbit embryos have shown that after exposure of embryos to 1,600 lx visible light for 8 h the development of day-1 embryos had decreased. In addition, development of hamster 1-cell embryos has also been impaired following exposure to visible light in a time-dependant manner [8]. In contrast, mouse oocytes exposed to 4,000 lx visible light for 1 to 4 h had both normal fertilization and speed of cleavage [13]. A recent study by Takenaka (2007), in which B6D2F1 mouse zygotes were exposed to light from different sources and transferred into surrogate mothers, has shown very short exposure to sunlight and 15 min exposure to cool white fluorescent light reduced the rate of live term fetuses. Photo-mediated changes in DNA are known to be responsible for adverse effects of light on mammalian cells as well as embryos [14, 15].
Some studies have addressed the ways by which deleterious effects of environmental factors on embryos are minimized. i.e., change in the composition of materials and refinement of culture media which are commonly used for gamete handling and embryo cultivation [16, 17], application of well controlled gas mixture in CO2 incubators [4, 5], working at low density illumination, using green filters in the microscopes and utilizing feeder cells to refine in vitro embryo conditions [18, 19].
Although at highly controlled conditions in vitro grown embryos have developmental competence, their quality is lower than in vivo grown embryos at the same developmental stage [4, 20, 21]. Numerous reports have demonstrated that feeder cells may enhance embryo development in vitro [22, 23]. However, controversies exist between reports after co-culture of embryos with various feeder cells in terms of implantation and the rate of take home baby [15, 24]. Some authors propose that feeder cells may enhance embryo development especially when suboptimal conditions are present [4, 25–27]. Since light is one of the physical factors of the embryonic environment with likely harmful effects on embryo development, the present study was designed to investigate the possible impact of visible light on development of mouse embryos and to demonstrate whether or not Human Embryonic Fibroblasts (HEF) and Mouse Embryonic Fibroblasts (MEF) as feeder cells may overcome probable harmful effects of visible light on mouse 2-cell embryos.
Materials and methods
All experiments were approved by Kerman University of Medical Sciences ethics committee for work on animals. A written consent was obtained from the parents to work on the aborted fetus. All chemicals were purchased from Sigma-aldrich Chemical Company (Saint Louis, MO, USA) unless otherwise stated.
Embryos
Six to 10-week-old female National Medical Research Institute (NMRI) mice were superovulated with intra-peritoneal injection of 10 IU PMSG (Folligon, Intervet, Belgium) followed 48 h later by 10 IU hCG (Serono, Italy). Superovulated mice were caged overnight with male mice from the same strain with proven fertility. Pregnant mice were sacrificed with cervical dislocation 46–48 h later. Uterine tube and the distal portion of uterine horn was cut and transferred into drops of Hepes-buffered HTF with 3 mg/mL BSA (Roche, Germany). Two-cell stage embryos were flushed into pre-warmed HTF and after three washes in the same medium, morphologically normal embryos were used for the experiments.
Feeder cells
Two primary fibroblast cells were prepared in the laboratory. Mouse Embryonic Fibroblast (MEF) was prepared from 13-day old NMRI mouse embryos as described elsewhere [28]. Briefly, head and abdominal viscera were removed in the sterile conditions. Cells were released by trypsin/EDTA digestion for 30 min and harvested in 75 cm2 culture flasks (Falcon®) at a density of 3 × 105/ml in MEM-α supplemented with 10% FBS (Gibco, USA), 100 IU/ml Penicilline G and 60 μg/ml streptomycin. Sub-cultures were prepared after the cells reached a confluence of >90% in the same medium. Cells at passages 2–5 were used for experiments.
Human Embryonic Fibroblast (HEF) was prepared using the method described for MEF with minor modifications; briefly, the upper limb of a 13 week old aborted male fetus with normal karyotype was carefully dissected free of the skin. Underlying connective tissues were removed and digested by 0.1% hyaluronidase and 0.25% trypsin in PBS. Other steps were similar to the procedure for MEF preparation. Cells were used at passages 2–7.
Visible light
Light was emitted from a 30 W halogen lamp and was conducted via optic fibers (cool light) above the culture dishes with their lids open, inside a CO2 incubator. The distance between the free end of optic fibers and culture dish was adjusted so that 1,600 lx illumination was applied to the embryos. Daily observation of embryos was carried out under constant minimal light intensity emitted from the light source of microscope (6 A, 30 W lamp). Each Petri-dish containing embryos was examined under an inverted microscope and returned back to the CO2 incubator within 3 min.
Assessment of appropriate time for exposure of embryos to visible light
The appropriate time at which embryos were damaged after exposure to visible light was determined in three consequent experiments. To carry out these experiments, morphologically normal mouse 2-cell embryos were pooled in 30 μl drops of HTF medium [29] under 5 ml light paraffin oil in 60 × 15 mm culture dishes (Falcon®). In experiments 1, embryos in treatment and control groups were cultured in HTF with 3 mg/ml BSA for 5 days. In experiments 2 and 3, embryos in treatment group were exposed to visible light and with their control were randomly divided into two portions and were cultured in either drops of MEM-α with 10% FBS and HTF with 10% FBS for 5 days. Un-exposed embryos served as control in all experimental groups.
In experiment 1, mouse 2-cell embryos were randomly allocated to three treatment groups and one control group. Embryos in treatment groups were exposed to 1,600 lx visible light for 1, 2 and 3 h respectively. In experiment 2, mouse 2-cell embryos were randomly allocated to four treatment groups and one control group. Embryos in treatment groups were exposed to 1,600 lx visible light for 15, 30, 45 and 60 min respectively. In experiment 3, mouse 2-cell embryos were allocated to three treatment groups and one control group. Embryos in treatment groups received 1,600 lx visible light for 10, 20 and 30 min respectively. In experiment 1, at least eight embryos were used in each group and experiments were repeated three times. In experiment 2 and 3, at least six embryos per group were used and experiments were replicated six times. Embryos were examined under an inverted microscope every 24 h and the developmental rate of the embryos were recorded for 5 days (see below; Data collection and statistical analysis).
Co-culture of exposed and un-exposed embryos with MEF and HEF
Both fibroblast monolayers (2 × 105 cell/ml) were prepared in 30 μl drops of MEM-α with 10% FBS under light paraffin oil 48 h prior to experiments. Twenty-four hours before the experiments were carried out, the media was changed with fresh MEM-α supplemented with 10% FBS. Two-cell embryos in drops of HTF were exposed to visible light for 30 min. Exposed embryos were removed and transferred into drops of MEM-α, HEF and MEF. Un-exposed embryos were cultured at the same conditions as control. Experiments were replicated eight times with at least five embryos in each group.
Data collection and statistical analysis
Embryos were observed carefully every 24 h for 5 days under an inverted microscope (Nikon; TS100, Japan). Developmental stage of the embryos was determined daily. Development to morula at day 3, expanded blastocyst at day 4 and hatching or hatched blastocyst at day 5 was recorded. Differences between developmental rates in experimental groups were statistically analyzed by χ2 test. A difference with P ≤ 0.05 was considered statistically significant.
Results
Assessment of the suitable time of exposure of embryos to visible light
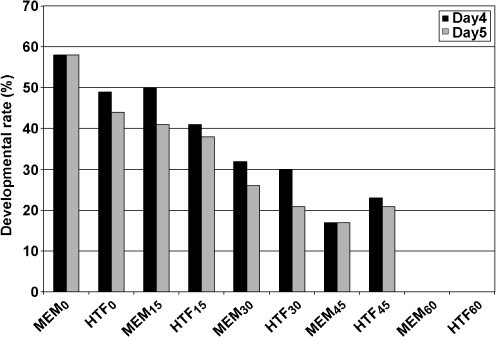
In experiment 1 none of the mouse 2-cell embryos developed beyond eight cell stage after >1 h exposure to visible light and culture in appropriate conditions. In addition, in experiment 2, few embryos developed to the blastocyst stage following 30–60 min exposures to visible light and culture in appropriate conditions (Fig. 1).
Fig. 1.
Effect of various exposure time of visible light on the development of 2-cell mouse embryos. MEM0-60 and HTF0-60 show embryos exposed to the visible light for 0, 15, 30, 45 and 60 min and cultured in MEM-α and HTF media respectively
Effects of visible light exposure for 10, 20 and 30 min on development of mouse 2-cell embryos
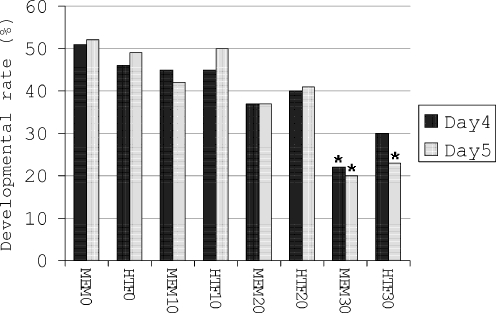
According to the data obtained from experiments 1 and 2, in the next steps, embryos were exposed to 10, 20 and 30 min visible light. At day 4 and 5 a significant (p < 0.01) decrease in developmental rate of embryos was noted when 30 min exposed embryos were cultured in MEM-α (MEM30; Fig. 2) and their developmental rate was compared to un-exposed embryos (MEM0; Fig. 2). In addition, when embryos were exposed to 30 min visible light and cultured in HTF medium (HTF30; Fig. 2), they had significantly lower developmental rate at day 5 compared to un-exposed embryos (HTF0; Fig. 2). No significant difference was detected between developmental rate of embryos in HTF and MEM-α group (Fig. 2).
Fig. 2.
Development of mouse 2-cell embryos after exposure to 1,600 lx visible light for various lengths of time. MEM0-30 and HTF0-30 are embryos exposed to visible light for 0, 10, 20 and 30 min and cultured in MEM-α and HTF media respectively. Bars with * above them are significantly (p < 0.01) different from MEM0 and HTF0
Effect of co-culture on development of un-exposed mouse embryos
One hundred twenty 2-cell embryos (40 embryos in each group) were randomly cultured in MEF, HEF and MEM- α as control. The rate of development was higher (P < 0.05) in both co-culture groups at day 3, 4 and 5 than control (Table 1). Developmental rate of the embryos in MEF and HEF was nearly identical at day 4 and 5 with no significant difference.
Table 1.
Development of mouse 2-cell embryos after exposure to visible light for 30 min followed by co-culture with either MEF or HEF
| Experimental groups | No. of embryos | Development (%) | ||
|---|---|---|---|---|
| Day 3 | Day 4 | Day 5 | ||
| MEM0 | 40 | 24 (60) | 19 (48) | 21(52) |
| MEM30 | 40 | 23 (57) | 9 (22) | 8 (20) |
| HEF0 | 40 | 32 (80) | 30 (75) | 33 (82) |
| HEF30 | 42 | 28 (67) | 28 (67) | 28 (67)** |
| MEF0 | 40 | 35 (87) | 32 (80) | 32 (80) |
| MEF30 | 43 | 29 (67) | 25 (58) | 25 (58)* |
Experiments were replicated eight times. MEM0 is un-exposed embryos cultured in MEM- α; MEM30 is the groups of embryos exposed to visible light for 30 min followed by culture in MEM- α; HEF0 and MEF0 are un-exposed embryos co-cultured with HEF and MEF respectively; HEF30 and MEF30 are the groups of embryos exposed to visible light for 30 min followed by co-culture with HEF and MEF respectively. Day 3, 4 and 5 are days in which embryos developed beyond morula, expanded blastocyst and hatching or hatched blastocyst stage respectively
*P < 0.05 and **P < 0.01 Comparing to MEM30
Effect of co-culture on development of mouse embryos exposed to 1,600 lx visible light for 30 min
One hundred and twenty five embryos were exposed to 1,600 lx visible light for 30 min in HTF supplemented with 10% FBS. The exposed embryos were randomly allocated to MEF, HEF and MEM-α as control. Co-culture of exposed 2-cell embryos with either feeder cells significantly (p < 0.01 for HEF and p < 0.05 for MEF) increased the number of expanded blastocysts and hatching or hatched blastocysts after 4 and 5 days cultivation (Table 1). In addition, developmental rate of exposed embryos co-cultured with MEF and HEF (MEF30 and HEF30; Table 1) at either day studied was not significantly lower than un-exposed embryos cultured in MEF and HEF (MEF0 and HEF0; Table 1).
Comparison of developmental rate of exposed and un-exposed embryos in MEF and HEF
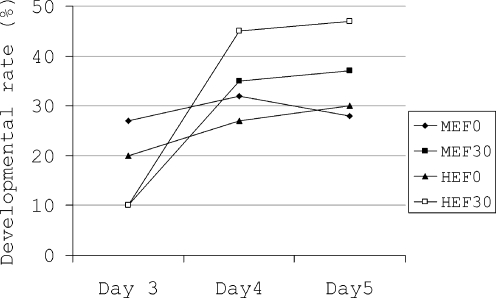
To find out whether any difference in developmental rate of embryos after exposure to visible light and co-culture with either MEF or HEF may exist compared to their control, we subtracted the developmental rate in exposed co-cultured embryos (MEF30 and HEF30; Table 1) from its control (MEM30; Table 1) and un-exposed co-cultured embryos (MEF0 and HEF0; Table 1) from its control (MEM0; Table 1). By referring to Fig. 3 it can be seen that the developmental rate difference in exposed embryos co-cultured with either MEF or HEF was nonsignificantly higher than un-exposed embryos co-cultured with either MEF or HEF.
Fig. 3.
Change in developmental rate when development in co-cultured groups was subtracted from control at the same developmental day. HEF0 and MEF0 are un-exposed embryos and HEF30 and MEF30 are embryos which were exposed to 1,600 lx visible light for 30 min
Discussion
Present study has investigated the potential of two embryonic fibroblast co-culture systems to overcome possible deleterious effects of visible light which may affect embryo development during manipulations in the laboratory. To come closer to the conditions that embryos are prone to during manipulations and cultivation in the laboratory, we exposed 2-cell mouse embryos to 1,600 lx visible light for various lengths of time. Our results showed that mouse 2-cell embryos may not tolerate exposure to visible light longer than 45 min in either simple (HTF) or complex (MEM-α) culture media. Exposure of mouse oocytes to 4,000 lx illumination had no adverse effect on the rate of implantation following IVF and embryo transfer [13]. Similar to mouse oocytes and in contrast to mouse 2-cell embryos (our results), rabbit day 1 embryos are less sensitive to visible light and they may tolerate 8 h exposure to visible light. But rabbit oocytes as well as hamster oocytes are more sensitive to visible light [7]. By comparing the results of different studies on various animals, it can be suggested that the nature of an animal is an influential factor in resistance to visible light. The studies on animal living cells have demonstrated that short-wavelength visible light is more damaging to cultured cells [11]. Human fibroblasts when exposed to fluorescent light underwent degrees of break in chromatin and also more chromatid exchanges were detected [30]. In addition, exposure of bovine trabecullar cells to different wavelength visible light resulted in various degrees of changes in the cell shape, cell metabolism and phagocytic activities [31]. The same conclusion is correct for mouse embryos having been exposed to various types of visible light [9].
Both feeder layers we used in our study could enhance embryo development following 4 and 5 days cultivation in laboratory. In Fig. 3 we have subtracted the developmental rate of un-exposed and exposed embryos from their own controls to show which treatment group was more greatly influenced by feeder cells. Differences between developmental rates of exposed embryos co-cultured with both MEF and HEF were greater than the difference between developmental rate of un-exposed embryos cultured with either MEF or HEF. In other words, under suboptimal conditions co-culture systems had greater positive effects on embryo development. Many studies have reported the benefits of co-culture systems in animal and human studies. However, use of feeder cells in human ART procedures has some limitations. Co-culture systems require both skilled persons and cell culture equipments. Besides, some investigators claim that co-culture systems are time-consuming and may increase the risk of genetic contaminations. They suggest that sequential culture media can be a good candidate for co-culture systems. Even if this argument is correct, it should be noted that in suboptimal conditions, which are most often present in ART laboratories, co-culture systems might eliminate the harmful effects of environmental factors on embryo development. Many studies have reported the beneficial effects of co-cultures on embryo development in vitro in humans [1, 32, 33]; domestic animals [34, 35] and rodents [19, 26, 27]. However, it is not fully elucidated why co-culture of embryos with somatic cells improves embryo development. Some investigators claim that co-culture systems may remove toxic components such as heavy metal divalent cations and metabolic inhibitors from the culture medium; negative conditioning, and discharge embryotrophic components such as peptides into the culture medium; positive conditioning, [36, 37]. In fact, in vivo produced embryos traveling through the fallopian tube towards the uterine cavity benefit from a highly dynamic micro-environment surrounding. In vitro produced and cultured embryos are prone to suboptimal conditions present during embryo cultivation in the laboratory. By the use of feeder cells the embryo environment comes closer to the in vivo conditions. If these conditions are similar to in vivo environments, the results will be comparable to the in vivo embryo development [32, 38, 39]. However, even by using the most controlled conditions for embryo development in the laboratory, still the rate of development and further pregnancy is lower than the expected rate [21]. We may suggest that deleterious effects of visible light may have overcome by the aim of feeder cells. It is not clear which mechanisms in co-culture systems, positive conditioning or negative conditioning interfere with improvement of embryo development. Since the negative conditioning is proposed to remove the reactive oxidative species produced in suboptimal conditions, for example after exposure to light from different sources [9], we speculate that the negative conditioning most likely may have been responsible for reducing the harmful effects of environmental factors on embryo.
In co-culture systems an important challenge has received little attention: the type of a media which can simultaneously support the growth of feeder cells and embryos. Embryos are usually grown in simple culture media with low glucose concentration, EDTA and free of fatty acids. Most of the feeder cells benefit from high concentrations of glucose, vitamins and essential and non-essential amino acids. Our results showed embryo development in HTF medium (a simple culture medium compared to MEM-α) did not significantly change developmental rate to hatching blastocyst. MEM-α is a modified MEM medium with low glucose concentration which in our work supported both growth of feeder cells and development of embryos. To improve embryo development in vitro, Azadbakht et al. (2007) used a rather complicated two step culture system previously suggested by Fong et al. (1998) in which embryos were cultured first in G-1™ ver3 and after 24 h transferred into polarized or non-polarized oviductal cell co-culture system. By this method they reported high developmental rate as well as low aopoptotic blastomeres in the blastocysts. In our study we used MEM-α through the experiments and the results after co-culture are promising and show MEM-α is an appropriate culture media in embryos co-culture procedures. However, further studies, in which different culture media as well as sequential media are used, may demonstrate the appropriate conditions for co-culturing of embryos with feeder layers.
Both established [26, 40] and non-established cells [19, 37, 41] have been employed as feeder cells to improve the quality of embryos and the rate of embryo development and further implantation. Cells from the genital tract, especially fallopian tube epithelium, have been extensively used as feeder cells. However, genital tract epithelial cells production have some limitations [42]. In our experiments we used mouse and human embryonic fibroblasts because fibroblast cells are easily grown in the laboratory and do not require special growth factors and supplements. They secrete various growth factors, and also remain un-changed in passages and their doubling time is relatively high [21, 37, 43]. By culturing fibroblasts from a given species for the co-culture of embryos from the same species, the danger of genetic inter-species contamination is eliminated. In our experiments, the rate of embryo development in both co-culture systems (Human Embryonic Fibroblasts and Mouse Embryonic Fibroblasts) was nearly identical, leading to the assumption that the origin of feeder cells is not an influential factor for embryo co-culture. This conclusion is supported by the study of Miami et al. (1994) who reported the influence of oviductal cells on embryo development is not species-specific and the study of Li (2001) who reported no significant difference in blastocyst transformation after equine oocytes maturation, fertilization, and development in the presence of either oviduct epithelial cells or fetal fibroblast cells.
Conclusions
From the results of our study we may conclude that mouse embryos are sensitive to visible light and may not withstand deleterious effects of visible light when exposed longer than 45 min to 1,600 lx visible light. Embryonic fibroblast feeder cells, irrespective to their origin, improve embryo development especially when embryos encounter sub-optimal conditions such as visible light.
Acknowledgments
Mr. Mohammad-ali Kiani and Saeed Rajabalian are acknowledged for their technical assistance. This work was financially supported by the grant No. 81-09 from Kerman University of Medical Sciences to S.N. Nematollahi-mahani.
Footnotes
Capsule
Mouse embryos were exposed to visible light and co-cultured with feeder layers. Our co-culture systems increased developmental rate especially when suboptimal conditions were present.
References
- 1.Desai N, Abdelhafez F, Bedaiwy MA, Goldfarb J. Live births in poor prognosis IVF patients using a novel non-contact human endometrial co-culture system. Reprod Biomed Online 2008;16(6):869–74. [DOI] [PubMed]
- 2.Dale B, Menezo Y, Cohen J, DiMatteo L, Wilding M. Intracellular pH regulation in the human oocyte. Hum Reprod 1998;13(4):964–70. doi:10.1093/humrep/13.4.964. [DOI] [PubMed]
- 3.Lane M, Baltz JM, Bavister BD. Na+/H+ antiporter activity in hamster embryos is activated during fertilization. Dev Biol 1999;208(1):244–52. doi:10.1006/dbio.1999.9198. [DOI] [PubMed]
- 4.Bavister BD. Interactions between embryos and the culture milieu. Theriogenology 2000;53(2):619–26. doi:10.1016/S0093-691X(99)00262-9. [DOI] [PubMed]
- 5.Jiang JX, Choi RC, Siow NL, Lee HH, Wan DC, Tsim KW. Muscle induces neuronal expression of acetylcholinesterase in neuron-muscle co-culture: transcriptional regulation mediated by cAMP-dependent signaling. J Biol Chem 2003;278(46):45435–44. doi:10.1074/jbc.M306320200. [DOI] [PubMed]
- 6.Parshad R, Taylor WG, Sanford KK, Camalier RF, Gantt R, Tarone RE. Fluorescent light-induced chromosome damage in human IMR-90 fibroblasts. Role of hydrogen peroxide and related free radicals. Mutat Res 1980;73(1):115–24. doi:10.1016/0027-5107(80)90140-2. [DOI] [PubMed]
- 7.Hegele-Hartung C, Schumacher A, Fischer B. Effects of visible light and room temperature on the ultrastructure of preimplantation rabbit embryos: a time course study. Anat Embryol (Berl) 1991;183(6):559–71. doi:10.1007/BF00187905. [DOI] [PubMed]
- 8.TTakahashi M, Saka N, Takahashi H, Kanai Y, Schultz RM, Okano A. Assessment of DNA damage in individual hamster embryos by comet assay. Mol Reprod Dev. 1999;54(1):1–7. doi:10.1002/(SICI)1098-2795(199909)54:1<1::AID-MRD1>3.0.CO;2-0. [DOI] [PubMed]
- 9.Takenaka M, Horiuchi T, Yanagimachi R. Effects of light on development of mammalian zygotes. Proc Natl Acad Sci USA 2007;104(36):14289–93. doi:10.1073/pnas.0706687104. [DOI] [PMC free article] [PubMed]
- 10.Hamdoun A, Epel D. Embryo stability and vulnerability in an always changing world. Proc Natl Acad Sci USA 2007;104(6):1745–50. doi:10.1073/pnas.0610108104. [DOI] [PMC free article] [PubMed]
- 11.Gorgidze LA, Oshemkova SA, Vorobjev IA. Blue light inhibits mitosis in tissue culture cells. Biosci Rep 1998;18(4):215–24. doi:10.1023/A:1020104914726. [DOI] [PubMed]
- 12.Garcia-Garcia RM, Dominguez V, Gonzalez-Bulnes A, Veiga-Lopez A, Cocero MJ. Effect of embryo developmental stage and culture conditions on number and quality of ovine in vitro produced blastocysts. Zygote 2006;14(3):181–7. doi:10.1017/S0967199406003728. [DOI] [PubMed]
- 13.Barlow P, Puissant F, Van der Zwalmen P, Vandromme J, Trigaux P, Leroy F. In vitro fertilization, development, and implantation after exposure of mature mouse oocytes to visible light. Mol Reprod Dev 1992;33(3):297–302. doi:10.1002/mrd.1080330310. [DOI] [PubMed]
- 14.Sangwan VS, Vemuganti GK, Singh S, Balasubramanian D. Successful reconstruction of damaged ocular outer surface in humans using limbal and conjuctival stem cell culture methods. Biosci Rep 2003;23(4):169–74. doi:10.1023/B:BIRE.0000007690.43273.73. [DOI] [PubMed]
- 15.d’Estaing SG, Lornage J, Hadj S, Boulieu D, Salle B, Guerin JF. Comparison of two blastocyst culture systems: coculture on Vero cells and sequential media. Fertil Steril 2001;76(5):1032–5. doi:10.1016/S0015-0282(01)02737-6. [DOI] [PubMed]
- 16.Arechiga CF, Hansen PJ. Response of preimplantation murine embryos to heat shock as modified by developmental stage and glutathione status. In Vitro Cell Dev Biol Anim 1998;34(8):655–9. doi:10.1007/s11626-996-0016-8. [DOI] [PubMed]
- 17.Langley MT, Marek DM, Gardner DK, Doody KM, Doody KJ. Extended embryo culture in human assisted reproduction treatments. Hum Reprod 2001;16(5):902–8. doi:10.1093/humrep/16.5.902. [DOI] [PubMed]
- 18.Nematollahi N, Valojerdi MR. Effect of Vero cell coculture on the development of frozen-thawed two-cell mouse embryos. J Assist Reprod Genet 1999;16(7):380–4. doi:10.1023/A:1020598031275. [DOI] [PMC free article] [PubMed]
- 19.Taniguchi F, Harada T, Nara M, Deura I, Mitsunari M, Terakawa N. Coculture with a human granulosa cell line enhanced the development of murine preimplantation embryos via SCF/c-kit system. J Assist Reprod Genet 2004;21(6):223–8. doi:10.1023/B:JARG.0000040238.61586.86. [DOI] [PMC free article] [PubMed]
- 20.Rizos D, Ward F, Boland MP, Lonergan P. Effect of culture system on the yield and quality of bovine blastocysts as assessed by survival after vitrification. Theriogenology 2001;56(1):1–16. doi:10.1016/S0093-691X(01)00538-6. [DOI] [PubMed]
- 21.Brison DR, Schultz RM. Apoptosis during mouse blastocyst formation: evidence for a role for survival factors including transforming growth factor alpha. Biol Reprod 1997;56(5):1088–96. doi:10.1095/biolreprod56.5.1088. [DOI] [PubMed]
- 22.Goldberg JM, Khalifa EA, Friedman CI, Kim MH. Improvement of in vitro fertilization and early embryo development in mice by coculture with human fallopian tube epithelium. Am J Obstet Gynecol 1991;165(6 Pt 1):1802–5. [DOI] [PubMed]
- 23.Khatir H, Anouassi A, Tibary A. Production of dromedary (Camelus dromedarius) embryos by IVM and IVF and co-culture with oviductal or granulosa cells. Theriogenology 2004;62(7):1175–85. doi:10.1016/j.theriogenology.2004.01.016. [DOI] [PubMed]
- 24.Seta M. Embryo transfer after autologous endometrial coculture improves pregnancy rates. Hum Cell 2001;14(2):135–40. [PubMed]
- 25.Kim YB, Ahn SH, Chang DY, Chung KN, Koh JW. Vero cell co-culture counteracts the detrimental effects of hydrosalpinx fluid on the development of mouse embryos in vitro. J Korean Med Sci 2002;17(2):217–9. [DOI] [PMC free article] [PubMed]
- 26.Noh JH, Chung KN, Kim YB. The effect of Vero cell coculture on the development of mouse embryos exposed to monoclonal antibodies specific for mammalian heat shock protein 60. J Korean Med Sci 2006;21(2):304–8. [DOI] [PMC free article] [PubMed]
- 27.Azadbakht M, Valojerdi MR, Mowla SJ. Development of mouse embryos co-cultured with polarized or non-polarized uterine epithelial cells using sequential culture media. Anim Reprod Sci 2007;100(1–2):141–57. doi:10.1016/j.anireprosci.2006.06.012. [DOI] [PubMed]
- 28.Pelz O, Wu M, Nikolova T, Kamprad M, Ackermann M, Egger D, et al. Duplex polymerase chain reaction quantification of human cells in a murine background. Stem Cells 2005;23(6):828–33. doi:10.1634/stemcells.2004-0206. [DOI] [PubMed]
- 29.Rabinovitch A, Russell T, Mintz DH. Factors from fibroblasts promote pancreatic islet B cell survival in tissue culture. Diabetes 1979;28(12):1108–13. doi:10.2337/diabetes.28.12.1108. [DOI] [PubMed]
- 30.Parshad R, Sanford KK, Jones GM, Tarone RE, Hoffman HA, Grier AH. Susceptibility to fluorescent light-induced chromatid breaks associated with DNA repair deficiency and malignant transformation in culture. Cancer Res 1980;40(12):4415–9. [PubMed]
- 31.Jiang F, Hao F, Wei H, Xu D. Effects of visible light on cultured bovine trabecular cells. J Huazhong Univ Sci Technolog Med Sci 2004;24(2):178–80. , 84. [DOI] [PubMed]
- 32.Johnson JE, Higdon Iii HL, Boone WR. Effect of human granulosa cell co-culture using standard culture media on the maturation and fertilization potential of immature human oocytes. Fertil Steril. 2007. [DOI] [PubMed]
- 33.Rubio C, Simon C, Mercader A, Garcia-Velasco J, Remohi J, Pellicer A. Clinical experience employing co-culture of human embryos with autologous human endometrial epithelial cells. Hum Reprod 2000;15(Suppl 6):31–8. [PubMed]
- 34.Qian Y, Shi WQ, Ding JT, Liu JY, Sha JH, Fan BQ. Effects of type and state of co-culture cells on in-vitro development of porcine oocytes matured and fertilized in vitro. J Assist Reprod Genet 2005;22(6):233–8. doi:10.1007/s10815-005-5145-6. [DOI] [PMC free article] [PubMed]
- 35.Katska-Ksiazkiewicz L, Opiela J, Rynska B. Effects of oocyte quality, semen donor and embryo co-culture system on the efficiency of blastocyst production in goats. Theriogenology 2007;68(5):736–44. doi:10.1016/j.theriogenology.2007.06.016. [DOI] [PubMed]
- 36.Urman B, Balaban B. Is there still a place for co-cultures in the era of sequential media? Reprod Biomed Online 2005;10(4):492–6. [DOI] [PubMed]
- 37.Hatoya S, Sugiyama Y, Torii R, Wijewardana V, Kumagai D, Sugiura K, et al. Effect of co-culturing with embryonic fibroblasts on IVM, IVF and IVC of canine oocytes. Theriogenology 2006;66(5):1083–90. doi:10.1016/j.theriogenology.2005.12.015. [DOI] [PubMed]
- 38.Zhang NY, Hu YL, Sun HX, Wang B, Xu ZP, Chen H. [Establishment of autologous endometrial coculture and sequential system for human early embryo culture]. Zhonghua Nan Ke Xue 2006;12(11):997–9. , 1003. [PubMed]
- 39.Mercader A, Garcia-Velasco JA, Escudero E, Remohi J, Pellicer A, Simon C. Clinical experience and perinatal outcome of blastocyst transfer after coculture of human embryos with human endometrial epithelial cells: a 5-year follow-up study. Fertil Steril 2003;80(5):1162–8. doi:10.1016/S0015-0282(03)01178-6. [DOI] [PubMed]
- 40.Lee YL, Xu JS, Chan ST, Ho PC, Yeung WS. Vero cells, but not oviductal cells, increase the hatching frequency and total cell count of mouse blastocysts partly by changing energy substrate concentrations in culture medium. J Assist Reprod Genet 2001;18(10):566–74. doi:10.1023/A:1011910125079. [DOI] [PMC free article] [PubMed]
- 41.Baghaban Eslami Nejad MR, Rezazadeh Valojerdi M, Kazemi Ashtiani S. A comparison of polarized and non-polarized human endometrial monolayer culture systems on murine embryo development. J Exp Clin Assist Reprod 2005;2(1):7. doi:10.1186/1743-1050-2-7. [DOI] [PMC free article] [PubMed]
- 42.Xu KP, Yadav BR, Rorie RW, Plante L, Betteridge KJ, King WA. Development and viability of bovine embryos derived from oocytes matured and fertilized in vitro and co-cultured with bovine oviducal epithelial cells. J Reprod Fertil 1992;94(1):33–43. doi:10.1530/jrf.0.0940033. [DOI] [PubMed]
- 43.Wetzels AM, Bastiaans BA, Hendriks JC, Goverde HJ, Punt-van der Zalm AP, Verbeet JG, et al. The effects of co-culture with human fibroblasts on human embryo development in vitro and implantation. Hum Reprod 1998;13(5):1325–30. doi:10.1093/humrep/13.5.1325. [DOI] [PubMed]