Abstract
Purpose
To compare aneuploidy rates in first trimester pregnancy losses following IVF ± ICSI.
Methods
A retrospective cohort analysis of karyotypes of abortuses following conventional IVF (n = 159) and ICSI (n = 196).
Results
50.1% of losses were found to be cytogenetically abnormal among all patients undergoing IVF ± ICSI. A significant increase in fetal aneuploidy rate was noted with increasing maternal age (<30 years = 26.1% vs. 31 to 34 years. = 38.2% vs. 35 to 39 years. = 51.3% vs. >39 years. = 65.9%). Aneuploidy rates were similar in the ICSI vs. conventional IVF groups (52.6% vs. 47.2% [p 0.31, RR 1.11, 95% CI 0.90, 1.38]). More sex chromosome anomalies were noted in the ICSI group.
Conclusions
The aneuploidy rate in first trimester abortuses significantly increases with increasing maternal age. ICSI was not shown to significantly increase the aneuploidy rate. However, more sex chromosome anomalies were found among pregnancies resulting from ICSI.
Keywords: Aneuploidy, ICSI, IVF, Karyotype, Maternal age, Pregnancy loss
Introduction
First trimester miscarriage is the most common complication of human reproduction with an incidence ranging between 50% and 70% of all conceptions. Of all clinically recognized pregnancies, 10% to 15% end in miscarriage. Aneuploidy is found in the majority of first trimester miscarriages; multiple cytogenetic studies have demonstrated aneuploidy rates ranging from 50% to 80% in various populations [1–4]. Autosomal trisomies are the most frequent karyotypic abnormalities; however, polyploidies, sex chromosome monosomies, and structural rearrangements account for a substantial number of miscarriages [2]. Several studies have demonstrated increasing miscarriage and aneuploidy rates with increasing maternal age [5–7].
The literature on pregnancy outcomes after intracytoplasmic sperm injection (ICSI) is limited and inconclusive concerning the risk of miscarriage and aneuploidy. ICSI bypasses natural selection mechanisms and could potentially lead to higher first trimester aneuploidy rates [7]. The theoretical procedure-dependent risks include (i) physical or biochemical disturbance of ooplasm or the meiotic spindle, (ii) injection of biochemical contaminants, (iii) injection of sperm-associated exogenous DNA. Procedure-independent risks include (iv) injection of sperm carrying a chromosomal anomaly, (v) transmission of genetic defect, which may be related to the underlying male factor infertility, (vi) male gamete structural defect, (vii) anomalies of sperm activating factors, (viii) potential for incorporating sperm mitochondrial DNA, and (ix) female gamete anomalies [8].
A recent follow up study of 150 children conceived through ICSI found that major congenital malformations were significantly more frequent when compared to children who were spontaneously conceived; however, no major delays in motor and mental functions were identified [9]. Likewise, a large prospective, controlled, multicenter, nationwide German cohort study found a significantly higher major congenital malformation rate among ICSI offspring [10]. A recent review concluded that chromosomal and genetic abnormalities are increased in ICSI offspring as a direct result of underlying parental risk [11]. Furthermore, higher incidences of de novo sex chromosomal aberrations [12–14], inheritance of CF mutations and Y microdeletions[15, 16] and spermatozoal aneuploidy [17] have been reported following ICSI procedures. ICSI has also been linked to greater embryo fragmentation and lower embryo grade; however, this did not negatively affect implantation or pregnancy rates [18].
Currently no consensus exists on preconception diagnostic evaluation of infertile males. Studies of sperm from infertile men have uncovered an increased frequency of aneuploid sperm, particularly in patients with abnormal semen parameters [19–21]. A recent review found that the use of ICSI is increasing for indication other than male factor infertility [22].
Several studies with small sample sizes have examined cytogenetic results of missed abortions following IVF and ICSI. The results from these studies have been contradictory. Lathi et al. found a significantly higher aneuploidy rate among 21 pregnancies resulting from ICSI [23]. Ma et al. found similar rates of aneuploidy among 34 conventional IVF and 46 ICSI pregnancies [24]. Another study examined the frequency of numerical chromosome anomalies in paternal-derived pronuclei after ICSI and IVF, and found no increase in the rate of autosomal aneuploidy but a higher incidence of sex-chromosome aneuploidy was seen in the ICSI group. Furthermore, this study found no correlation between aneuploidy and severity of male factor infertility [25].
Multiple studies have examined spermatozoa from infertile men as well as karyotypes of fetuses conceived through ICSI. However, very little data exists regarding miscarriage following ICSI. One recent study found similar miscarriage rates following conventional IVF and ICSI; however, the authors did not attempt to account for the cause of the miscarriages, particularly aneuploidy rates [26]. The aim of this retrospective study is to examine karyotypes of missed abortions following conventional IVF and ICSI to determine if there is a significant difference in aneuploidy rates.
Materials and methods
Population
From a retrospective cohort analysis of all autologous oocyte IVF cycles ending in a clinical first trimester abortion with a subsequent dilation and curettage performed at Reproductive Medicine Associates of New Jersey from January 2000 to December 2006, 504 patients were identified. From these 355 patients with a first trimester pregnancy loss followed by a dilatation and curettage and successful cytogenetic analysis of the abortus were identified.
Experimental design
This is a retrospective cohort analysis comparing the cytogenetic analysis results obtained in first trimester losses between IVF and IVF with ICSI ART cycles. The main outcome measure was aneuploidy rate. IRB approval was obtained from the Western Institutional Review Board, Olympia, WA.
Patients underwent stimulation with a variety of stimulation protocols including GnRH-a (Lupron; TAP Pharmaceuticals, Deerfield, IL) down regulation followed by stimulation with exogenous gonadotropins or stimulation with exogenous gonadotropins followed by use of a GnRH antagonist. When ultrasonographic criteria for follicular maturity were met, a single 10,000 IU dose of human chorionic gonadotropin (hCG) was administered. Transvaginal follicular aspiration was performed approximately 36h after hCG administration. Embryos were transferred 72 to 120h after follicular aspiration.
Documentation of normal uterine cavity anatomy was established by saline sonohysterography or hysteroscopy within 12months prior to the initiation of the IVF cycle.
Sperm preparation and fertilization
Fresh ejaculated samples were evaluated by standard andrological screening (volume, count, motility, progression and Kruger morphology) [27]. Following this analysis, the semen samples were prepared for conventional or ICSI insemination by density gradient separation followed by a swim up purification step. Specimens with less than 2 million motile spermatozoa per ejaculate and/or abnormal morphology greater than 95% were considered insufficient for standard conventional in vitro fertilization, and were treated with ICSI. No testicular biopsy or sperm aspiration procedures were included.
For conventional insemination, oocytes were exposed to motile sperm 4 to 6h post oocyte retrieval. For ICSI, oocytes were denuded of cumulus-coronal cells by exposure to hyaluronidase and gentle aspiration of oocytes with a finely pulled pipette to remove residual cumulus-coronal cells and were then injected 6 to 8h post oocyte retrieval. Fertilization was confirmed 16–18h post insemination by the confirmation of two distinct pronulei and polar bodies and the embryos were placed into culture according to established laboratory protocol [28]. Embryonic division and morphology was evaluated every 24h thereafter until embryos were deemed suitable for embryo transfer or cryopreservation.
Statistical analysis
Sample-size determination was based on a presumed aneuploidy rate of 50% in the conventional IVF group and 70% in the ICSI group. A sample size of 208 patients (104 patients in each group) was targeted to be able to detect a difference of at least 20% between the groups, with α (type I error) set at 0.05 and 80% power.
Cytogenetic results were compared with respect to the type of ART procedure (IVF ± ICSI) to assess the primary outcome measure of aneuploidy rate in the abortus. The aneuploidy rates were then calculated for each treatment group. Differences in aneuploidy rates were analyzed using a Chi-square or two-tailed Fisher’s exact test where appropriate.
Data were analyzed by using a t-test for continuous distributions and a Mann-Whitney Rank Sum Test for non-continuous distributions. An alpha error of 0.05 was considered significant for all comparisons. All data are reported as means with their associated standard deviations. Relative risk and 95% confidence intervals are shown where appropriate.
Results
We reviewed the electronic charts of all the patients who underwent IVF treatment with autologous oocytes and subsequently had a first trimester loss followed by evacuation of the pregnancy and karyotyping of the abortus. There were 355 pregnancies identified having a first trimester loss followed by a dilation and curettage and successful cytogenetic analysis. Patients were grouped by type of treatment (Conventional IVF n = 159 and ICSI n = 196) and by cytogenetic results (normal n = 177 and abnormal n = 178).
Patients were further subdivided by maternal age into the following groups: (<30 years, 31 to 34 years, 35 to 39 years, and >39 years). Cytogenetic results were further subdivided into normal female, normal male, 45 XO, autosomal trisomy, 48 chromosomes and other aneuploidy.
The mean age of the female spouse was 36.6 ± 4.1years (range 26 to 46 years). The mean age of the male spouse was 38.3 ± 5.4 years (range 26 to 71 years). The patient demographics, semen parameter results for the specimen used for fertilization on the day of oocyte retrieval, number of embryos transferred, as well as initial β-hCG on days16 and 18 after hCG administration are shown in Table 1. The study population’s demographics were similar for both groups evaluated. Semen parameters as expected varied significantly between the conventional IVF and ICSI groups.
Table 1.
Patient demographics and semen parameters
| Entire population | ICSI | Conventional IVF | P | |
|---|---|---|---|---|
| N | 355 | 196 | 159 | |
| Female spouse age | 36.6 ± 4.1 | 36.8 ± 4.1 | 36.2 ± 4.0 | 0.12 |
| Male spouse age | 38.3 ± 5.4 | 38.7 ± 5.3 | 37.8 ± 5.5 | 0.08 |
| Volume (mL) | 1.6 ± 0.7 | 1.7 ± 0.6 | 1.4 ± 0.8 | <0.001b |
| aMorphology (%) | 6.9 ± 11.0 | 4.4 ± 8.2 | 10.9 ± 13.6 | <0.001b |
| Motility (%) | 83.5 ± 27.7 | 74.5 ± 31.7 | 98.3 ± 3.9 | <0.001b |
| Number of Embryos Transferred | 2.8 ± 1.1 | 2.8 ± 1.1 | 2.7 ± 1.1 | 0.57 |
| β-hCG Day 16 | 105 ± 75 | 106 ± 82 | 103 ± 67 | 0.72 |
| β-hCG Day 18 | 239 ± 180 | 247 ± 196 | 228 ± 156 | 0.99 |
Values are means ± SD
aMorphology assessed using Kruger strict criteria
bValues with different superscripts are significantly different (p < 0.05)
Overall, 50.1% of the miscarriages were found to be cytogenetically abnormal among all patients undergoing IVF. A significant increase in fetal aneuploidy rate was noted with increasing maternal age (<30 years = 26.1% vs. 31 to 34 years. = 38.2% vs. 35 to 39 years. = 51.3% vs. >39 years. = 65.9%). Table 2 summarizes the distribution of cytogenetic results for all of the pregnancies examined in the study. The distribution of cytogenetic results was similar in the two groups; however, six sex chromosome anomalies were noted in the ICSI group vs. none in the conventional IVF group.
Table 2.
Distribution of cytogenetic results
| Cytogenetic Result | ICSI | Conventional IVF | RR | 95% CI |
|---|---|---|---|---|
| 46 XX | 70 (35.7%) | 63 (39.6%) | 0.90 | 0.69, 1.18 |
| 46 XY | 23 (11.7%) | 21 (13.2%) | 0.89 | 0.51, 1.55 |
| Autosomal Trisomy | 77 (39.3%) | 62 (39.0%) | 1.01 | 0.78, 1.31 |
| 45 XO | 6 (3.1%) | 0 (0%) | ||
| 48 Chromosomes | 8 (4.1%) | 4 (2.5%) | 1.62 | 0.50, 5.29 |
| Other | 12 (6.1%) | 9 (5.7%) | 1.08 | 0.47, 2.50 |
| TOTAL Euploid | 93 (47.4%) | 84 (52.8%) | 0.90 | 0.73, 1.11 |
| TOTAL Aneuploid | 103 (52.6%) | 75 (47.2%) | 1.11 | 0.90, 1.38 |
Values are number of patients and (percent) with given cytogenetic result
RR relative risk
95% CI - 95% Confidence interval
Aneuploidy rates were similar in the ICSI and conventional IVF groups (52.6% vs. 47.2%) (p 0.31, RR 1.11, 95% CI 0.90, 1.38). Autosomal trisomy was the most common aneuploidy. Among the miscarriages with normal cytogenetic results, 75% were found to be normal male and 25% were found to be normal female karyotypes. Among the miscarriages with cytogenetic abnormalities, 50.5% were found to be abnormal female while 46.1% were found to be abnormal male and 3.4% had sex chromosome aneuploidy.
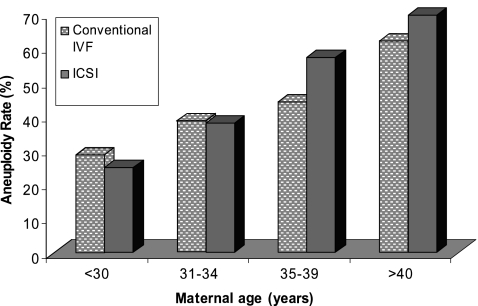
Figure 1 reveals aneuploidy rates in the ICSI and the conventional IVF groups subdivided by maternal age group. There was no statistically significant difference in the aneuploidy rates between the two groups in any of the maternal age categories.
Fig. 1.
Aneuploidy Rates by Oocyte Age for Conventional IVF and ICSI groups
Discussion
Our data did not reveal a statistical difference in aneuploidy rates between conventional IVF and ICSI groups. However, more sex chromosome anomalies were found among pregnancies resulting from ICSI; this finding confirms previous studies [12–14]. The most common abnormal karyotype encountered was autosomal trisomy in both groups. It is critically important to consider maternal age when performing an analysis of first trimester pregnancy loss. Our data confirms previous studies in demonstrating a dramatic increase in aneuploidy rates with increasing maternal age in both the conventional IVF and ISCI groups [5–7].
Among normal cytogenetic results, normal female karyotype was found three times more frequently than normal male karyotype. This finding may represent predominance of maternal decidual cells in culture or absences of products of conception in the D&C sample. However, the sex ratio among abortuses with aneuploidy was equal. This group should not be affected by maternal cell contamination and represents the actual sex ratio in the abortuses. It is possible to eliminate the false negative results due to maternal cell contamination by sending maternal blood to the cytogenetics laboratory along with products of conception for comparison analysis.
Interestingly, the aneuploidy rate in females younger then 30 years was only 26.1%, indicating that other mechanisms account for pregnancy loss in this population. While aneuploidy accounts for the majority of miscarriages in older females, first trimester pregnancy loss remains poorly understood in young patients. Our population underwent extensive testing and treatment prior to undergoing IVF to improve likelihood of a favorable pregnancy outcome. This included optimization of the uterine cavity and correction of major medical problems. The cause of miscarriage in young women remains elusive and is an area for future research.
ICSI is an effective modality for treatment of couples with male factor infertility. The data presented here support that aneuploidy rates following ICSI are similar to those following conventional IVF. However, more sex chromosome anomalies were found among pregnancies resulting from ICSI.
Footnotes
Capsule
First trimester abortuses following conventional IVF and ICSI had similar aneuploidy rates. More sex chromosome anomalies were found among pregnancy losses resulting from ICSI.
References
- 1.Simpson JL, Jauniaux E. Fetal wastage. In: Gabbe SG, Niebyl JR, Simpson JL, editors. Obstetrics: Normal and Problem Pregnancies. New York: Churchill Livingstone; 2007. Chapter 24.
- 2.Simpson JL. Causes of fetal wastage. Clin Obstet Gynecol. 2007;50(1):10–30. doi:10.1097/GRF.0b013e31802f11f6. [DOI] [PubMed]
- 3.Hassold TJ. A cytogenetic study of repeated spontaneous abortions. Am J Hum Genet. 1980;32:723–30. [PMC free article] [PubMed]
- 4.Simpson JL, Bombard AT. Chromosomal abnormalities in spontaneous abortion: frequency, pathology and genetic counseling. In: Edmonds KBMJ, editor. Spontaneous Abortion. London: Blackwell; 1987. p. 51–76.
- 5.Benadiva CA, Kligman I, Munne S. Aneuploidy 16 in human embryos increases significantly with maternal age. Fertil Steril. 1996;66:248–55. [PubMed]
- 6.Angell RR. Aneuploidy in older women. Higher rates of aneuploidy in oocytes from older women. Hum Reprod. 1994;9:1199–2000. [DOI] [PubMed]
- 7.Munne S, Alikani M, Tomkin G, Grifo J, Cohen J. Embryo morphology, developmental rates, and maternal age are correlated with chromosome abnormalities. Fertil Steril. 1995;64:382–91. [PubMed]
- 8.Bonduelle M, Van Assche E, Joris H, Keymolen K, Devroey P, Van Steirteghem A, et al. Prenatal testing in ICSI pregnancies: incidence of chromosomal anomalies in 1586 karyotypes and relation to sperm parameters. Hum Reprod. 2002;17(10):2600–14. doi:10.1093/humrep/17.10.2600. [DOI] [PubMed]
- 9.Belva F, Henriet S, Liebaers I, Van Steirteghem A, Celestin-Westreich S, Bonduelle M. Medical outcome of 8-year-old singleton ICSI children (born >or=32 weeks' gestation) and a spontaneously conceived comparison group. Hum Reprod. 2007;22(2):506–15. doi:10.1093/humrep/del372. [DOI] [PubMed]
- 10.Katalinic A, Rösch C, Ludwig M. German ICSI Follow-Up Study Group. Pregnancy course and outcome after intracytoplasmic sperm injection: a controlled, prospective cohort study. Fertil Steril. 2004;81(6):1604–16. doi:10.1016/j.fertnstert.2003.10.053. [DOI] [PubMed]
- 11.Retzloff MG, Hornstein MD. Is intracytoplasmic sperm injection safe? Fertil Steril. 2003;80(4):851–9. doi:10.1016/S0015-0282(03)01014-8. [DOI] [PubMed]
- 12.Bonduelle M, Camus M, DeVos A, et al. Seven years of intracytoplasmic sperm injection and follow-up of 1987 subsequent children. Hum Reprod. 1999;14(Suppl 1):243–64. [DOI] [PubMed]
- 13.Herve C, Moutel G. Sex chromosome abnormalities after intracytoplasmic sperm injection. Lancet. 1995;346(8982):1096–7. [PubMed]
- 14.Meschede D, Horst J. Sex chromosomal anomalies in pregnancies conceived through intracytoplasmic sperm injection: a case for genetic counselling. Hum Reprod. 1997;12(6):1125–7. doi:10.1093/humrep/12.6.1125. [DOI] [PubMed]
- 15.Van derVen K, Messer L, van derVen H, et al. Cystic fibrosis mutation screening in healthy men with reduced sperm quality. Hum Reprod. 1996;11:513–7. [DOI] [PubMed]
- 16.Pryor JL, Kent-First M, Muallem A, et al. Microdeletions in the Y chromosome of infertile men. N Engl J Med. 1997;336:534–9. doi:10.1056/NEJM199702203360802. [DOI] [PubMed]
- 17.Martin RH. The risk of chromosomal abnormalities following ICSI. Hum Reprod. 1996;11:924–5. [DOI] [PubMed]
- 18.Frattarelli JL, Leondires MP, Miller BT, Segars JH. Intracytoplasmic sperm injection increases embryo fragmentation without affecting clinical outcome. J Assist Reprod Genet. 2000;17(4):207–12. doi:10.1023/A:1009439800398. [DOI] [PMC free article] [PubMed]
- 19.Martin RH. Genetics of Human Sperm. J Assist Reprod Genet. 1998;15(5):240–5. doi:10.1023/A:1022528007564. [DOI] [PMC free article] [PubMed]
- 20.Calogero AE, Burrello N, De Palma A, Barone N, D'Agata R. Vicari. Sperm aneuploidy in infertile men. Reprod Biomed Online. 2003;6(3):310–7. [DOI] [PubMed]
- 21.Pang MG, Hoegerman SF, Cuticchia AJ, Moon SY, Doncel GF, Acosta AA, et al. Detection of aneuploidy for chromosomes 4, 6, 7, 8, 9, 10, 11, 12, 13, 17, 18, 21, X and Y by fluorescence in-situ hybridization in spermatozoa from nine patients with oligoasthenoteratozoospermia undergoing intracytoplasmic sperm injection. Hum Reprod. 1999;14(5):1266–73. doi:10.1093/humrep/14.5.1266. [DOI] [PubMed]
- 22.Jain T, Gupta RS. Trends in the use of intracytoplasmic sperm injection in the United States. N Engl J Med. 2007;357(3):251–7. doi:10.1056/NEJMsa070707. [DOI] [PubMed]
- 23.Lathi RB, Milki AA. Rate of aneuploidy in miscarriages following in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 2004;81(5):1270–2. doi:10.1016/j.fertnstert.2003.09.065. [DOI] [PubMed]
- 24.Ma S, Philipp T, Zhao Y, Stetten G, Robinson WP, Kalousek D. Frequency of chromosomal abnormalities in spontaneous abortions derived from intracytoplasmic sperm injection compared with those from in vitro fertilization. Fertil Steril. 2006;85(1):236–9. doi:10.1016/j.fertnstert.2005.06.041. [DOI] [PubMed]
- 25.Macas E, Zweifel C, Imthurn B. Numerical chromosome anomalies detected in paternally derived pronuclei of tripronuclear zygotes after intracytoplasmic sperm injection. Fertil Steril. 2006;85(6):1753–60. doi:10.1016/j.fertnstert.2005.11.062. [DOI] [PubMed]
- 26.Buckett WM, Chian RC, Dean NL, Sylvestre C, Holzer HE, et al. Pregnancy loss in pregnancies conceived after in vitro oocyte maturation, conventional in vitro fertilization, and intracytoplasmic sperm injection. Fertil Steril. 2007; In press. [DOI] [PubMed]
- 27.Baker G, Lui DY, Bourne H. In Handbook of In vitro Fertilization, 2nd ed, Assessment of the male and preparation of sperm for ARTs. 2000, CRC Press.
- 28.Miller KA, Elkind-Hirsch KE, Levy B, Graubert M, Ross S, Scott RT. Pregnancy after cryopreservation of donor oocytes and preimplantation genetic diagnosis of the embryos in a patient with ovarian failure. Fertil Steril. 2004;82:211–4. doi:10.1016/j.fertnstert.2003.12.031. [DOI] [PubMed]