Abstract
Introduction
Preimplantation genetic diagnosis (PGD) is widely used for women heterozygous for a Robertsonian translocation. Preconceptional diagnosis (PCD), performed before fertilization, may be an alternative to PGD, especially in countries where PGD is restricted or prohibited, as in France. It could also give different information and clarify the influence of reproductive and obstetric history.
Methods
In our study, translocation was diagnosed before ICSI in five cases (group A), and after newborn or fetal aneuploidy or miscarriage in two cases, (group B).
Results
First polar body (PB1) analysis using acrocentric centromeric probes was done for 85 PB1s, and aneuploidy rate was at 42.4%. Oocyte aneuploidy rate differed (p < 0.0001) between groups A and B (30% vs 84%). Despite the small group sizes, we demonstrate a correlation (p = 0.0358) of aneuploidy rate in polar bodies after 2 or more attempts. Three live births were recorded, all in group A.
Discussion
PCD could thus be an alternative to PGD. This pilot study also provides new prognostic information taking into account the women’s natural history, but further confirmation is required.
Keywords: First polar body biopsy, Robertsonian translocation, Previous aneuploidy, Preconceptional diagnosis, FISH
Introduction
In the general population, the estimated rates of abnormal karyotypes and reciprocal translocation are close to 1% and 0.2 to 0.1%, respectively [1]. For women heterozygous for Robertsonian and reciprocal translocations, preimplantation genetic diagnosis (PGD) is an alternative to prenatal diagnosis and termination of pregnancy, through selection of chromosomally normal embryos for replacement [2]. The two main groups involved in this study were (1) women who need assisted reproductive techniques (ART) to become pregnant, usually due to male infertility, and (2) women who do not need ART to become pregnant, but who have experienced several miscarriages and/or aneuploidy.
Blastomere analysis is probably the most widely used method to investigate translocation in female meiosis [3]. However, PGD is restricted or limited in some countries including France, where the practice is confined to three centers, subject to considerable delays (more than 12 months) and associated with very strict inclusion criteria (age below 35 years; FSH level below 10 IU/L, etc.). Therefore, PB1 diagnosis may be a good alternative for patients qualified for ICSI when a karyotype abnormality is diagnosed, so as to reduce the wait for ICSI, as they can rapidly undergo PCD, and women who respond poorly can be given an opportunity, even if few oocytes are retrieved.
We evaluated PCD as an alternative to PGD in a pilot cohort in women heterozygous for a Robertsonian translocation in two situations: a fortuitous translocation diagnosis, or diagnosis following previous fetal aneuploidy or miscarriages.
Materials and methods
Our polar body evaluation protocol was accepted by our local ethics committee and by the French health authorities. It was proposed to couples after genetic counseling, and informed consent was collected afterwards.
Patients PB1 analysis, before ICSI, was proposed to 7 women, and 18 attempts were made. Patients opted for a PCD cycle to reduce ICSI delay (4 cases) and to avoid long delay in PGD or after exclusion from a French PGD center (3 cases) because of probable low ovarian response to gonadotropins (more than 35 years of age, high FSH level, reduced follicle count). In group A (patients 1 to 5), karyotyping was performed systematically before ICSI for sperm abnormalities. In group B (patients 6 and 7), karyotyping was performed following recurrent miscarriages (patient 6), after medical pregnancy termination because of fetal aneuploidy (patients 6 and 7), and because of a child with Down syndrome (46,XX,rob(14;21)(q10;q10),+21) (patient 7). PCD was proposed in order to increase the chance of normal pregnancy and to evaluate the aneuploidy rate in oocytes. Patient characteristics are listed in Table 1.
Table 1.
Patient characteristics
| Group | Patient | Translocation | ART indication | Age (years) | Previous ART | Medical history |
|---|---|---|---|---|---|---|
| A | 1 | 45,XX,der(13;14)(q10;q10) | Sperm alteration | 27.1 | 0 | |
| 2 | 45,XX,der(13;14)(q10;q10) | Sperm alteration | 23.9 | 0 | ||
| 3 | 45,XX,der(13;14)(q10;q10) | Sperm alteration | 33.3 | 2 | Endometriosis | |
| 4 | 45,XX,der(13;14)(q10;q10) | Sperm alteration | 36.9 | 0 | ||
| 5 | 45,XX,der(13;14)(q10;q10) | Sperm alteration | 26.5 | 0 | ||
| B | 6 | 45,XX,der(13;14)( q10;q10) | AR | 29.7 | 0 | 2 TOP for aneuploidy and 2 miscarriages |
| 7 | 45,XX,der(14;21)( q10;q10) | AR | 42.1 | 0 | One down syndrome child and 1 TOP for double aneuploidy |
AR aneuploidy recurrence, TOP termination of pregnancy
Multiple follicular growth was induced by exogenous gonadotropins following a desensitization protocol with GnRH analogues, and oocytes were retrieved approximately 36 hours after hCG administration.
Polar body biopsy Polar body biopsy was carried out using the Zilos TK laser (Hamilton Thorne Biosciences) as previously described [4] after 3 or 4 zona pellucida laser impacts (180 mW, 0.5 ms pulse). Polar body was then extracted with a biopsy micropipette (Humagen) and placed in 0.5 μl of water on a siliconized slide. This water drop was air-dried and 2 drops of Carnoy solution were added.
FISH procedure The slide was then passed through different solutions: 5 min in methyl alcohol at room temperature (RT), 10 min in 2SSC at 37°C, 10 min in a 1% paraformaldehyde solution at RT, 5 min in PBS at RT, 10 min in 0.1 N HCl with 30 μl/40 ml of 10% active pepsin solution, 5 min in PBS at RT, and 2 min each in 70%, 85% and 100% ethyl alcohol for dehydration. A 3 μl drop of probe solution was placed on each polar body. Codenaturation and hybridization were automatically performed in the Hybrite (Vysis) (73°C for 4 min and 37°C for 4 h).
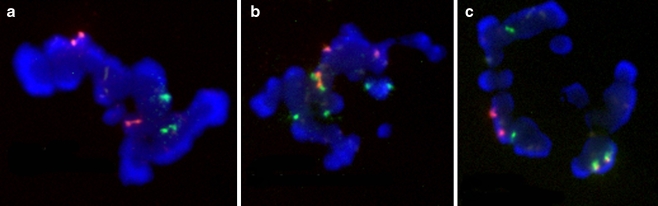
The slide was then washed in 2 solutions: 1 min 45 s in a 0.7SSC / 0.3% NP40 solution, 15 s in a 2SSC / 0.1% NP40 solution, and counterstained with an antifade solution and analyzed with a five-filter fluorescence microscope (Olympus BX60) and the PathVysion imaging system (Fig. 1).
Fig. 1.
a Normal PB1 without der(13;14). b Abnormal PB1 with der(13;14) and +13 or 21. c Normal PB1 with der(13;14)
To screen the translocation and the trisomy 21 incidence in a single step, we used centromeric probes (Cytcocell Ltd, Cambridge, UK) for chromosomes 13–21 (alpha satellite D13Z1-D21Z1) labeled with FITC, and 14–22 (alpha satellite D14Z1-D22Z1) with Texas Red. As acrocentric probes were polymorphic, to assess the efficiency of probe mixtures, we first tested them on lymphocytes for each woman.
Scoring criteria Each polar body chromosome normally consists of two chromatids (Fig. 1). Centromeric probes usually give strong signals for one chromosome or a scarcely separated doublet signal. Acrocentric centromeric probes normally showed 4 spots (4 chromatids), corresponding to two chromosomes (13/21 or 14/22) because of cross-hybridization. All other results were considered as abnormal.
ICSI procedure Oocytes with normal FISH results were microinjected as previously described. For patients 1 to 5, oocytes lacking FISH analysis because of technical failure or immaturity (the biopsy was impossible in the morning but the polar body was expelled before ICSI in the afternoon) were also microinjected.
Statistical analysis The Statview program was used, and differences were considered significant if p < 0.05.
Results (Table 2)
Table 2.
FISH and ICSI results
| Group | Patient | Attempts | Oocytes retrieved | Translocation study | Embryos transferred | Pregnancy | |
|---|---|---|---|---|---|---|---|
| Oocytes analyzed | Normal oocytes | ||||||
| A | 1 | 3 | 22 | 14 | 9 (64%) | 7 | 1 |
| 2 | 4 | 26 | 23 | 17 (74%) | 14 | 2 and 1 ectopic | |
| 3 | 4 | 20 | 19 | 12 (63%) | 6 | 0 | |
| 4 | 2 | 6 | 4 | 4 (100%) | 1 | 0 | |
| 5 | 2 | 9 | 6 | 4 (67%) | 4 | 0 | |
| B | 6 | 2 | 17 | 15 | 3 (20%) | 0 | 0 |
| 7 | 1 | 4 | 4 | 0 (0%) | 0 | 0 | |
| Total | 18 | 104 | 85 | 49 (57.6%) | 32 | 3 | |
Of 104 retrieved oocytes showing the first polar body (mean 5.9 per cycle (2–9)), 96% (99) could be biopsied, and, of these, 85.9% (85) were analyzed by FISH (81.7% of the mature oocytes). Sperm were injected into 62 oocytes, 48 with normal diagnosis by FISH and 14 without FISH diagnosis, all from patients 1 to 5. Fertilization rate was 61.3% and cleavage rate 92.1%. Pregnancies were obtained in group A (one ectopic pregnancy and 3 healthy infants), but not in group B. Pregnancy rate was then 22.2% per cycle, and 26.7% (4 out 15) in group A.
For translocation screening, 36 of 85 (42.4%) PB1s analyzed were abnormal. Considering the history of aneuploidy or miscarriage, aneuploidy rate was 30% (20 abnormal and 46 normal oocytes) for group A, and 84% (16 abnormal and only 3 normal oocytes) for group B (p < 0.0001).
Five out of 7 patients had more than 2 attempts with more than 3 oocytes. This allowed analysis of the correlation between the results of two subsequent attempts (1st / 2nd and 3rd / 4th). Although this sample is small (n = 5), the Z score was 2.088 and the correlation coefficient 0.857, which was statistically significant using the Spearman test (p = 0.0358).
Discussion
In this series, fertilization rates and cleavage rates were in agreement with the French FIVNAT registry data [5]. This suggests that PB1 biopsy had no deleterious effect on the ART results as previously described [6]. The pregnancy rate per transfer was 22.2%, a value similar to previous preconceptional screening (PCS) findings (21.9%, [2]; 22.5%, [7]) and ESHRE PGD Consortium data (24.7% pregnancy rate; 328 transfers) [3]. Even though this is a small cohort, we confirmed the ability of PB1 diagnosis to screen for Robertsonian translocation. It can therefore be helpful in countries where PGD is restricted or prohibited.
We offered PB1 screening to women heterozygous for a Robertsonian translocation in order to help them to become pregnant, but also to evaluate the oocyte aneuploidy rate related to the translocation. Its incidence in PB1 was much lower (p < 0.0001) for women with no particular reproductive history except their partner’s infertility (group A) (30%, 20/66) than for those with previous fetal aneuploidies (group B) (84%, 16/19). Even though this pilot series is small, and more women must be included to confirm the findings, these are the first results to differentiate the two groups, and confirm the variation in prognosis for women heterozygous for a Robertsonian translocation [8]. Further studies are necessary to identify genetic predisposition explaining this heterogeneity. Different mechanisms of Robertsonian translocation exist [9], and variation in breakpoints could affect aneuploidy.
Previous studies, such as analysis of the EHSRE PGD database [3], did not report indication for PGD in cases of translocation. A few studies of PGD in translocation carriers using blastomeres have been published, reporting a high aneuploidy rate in patients with previous miscarriage or termination of pregnancy (for example, 72% abnormality rate for Robertsonian translocation and 82% for reciprocal translocation [10, 11]). Equivalent high aneuploidy rates (for example, 69.0% [12] and 65.5%, [13], respectively) are also obtained by PB1 analysis. For women with no particular obstetric history, included because of male infertility, few cases have been reported and no conclusion has been reached [14].
We noticed stability (p = 0.0358) of aneuploidy rate in 1st polar bodies of two different cohorts from the same patient in this study (r = 0.857). This confirmed the prognostic value of a 1st PCD attempt to evaluate segregation pattern, a result in accordance with those obtained for aneuploidy screening [15, 16], where stability of aneuploidy rate was observed in two different oocyte cohorts from the same patient. These findings may have a predictive value for women heterozygous for a translocation [17], and inform genetic counseling for those patients. Unlike PGD, the PCD evaluation of aneuploidy incidence was based on a large oocyte fraction (81.7% of the matured oocytes) and not only on the cleaved ones.
Even though our series is small and needs confirmatory investigations, it seems that patients at risk of a high oocyte aneuploidy rate should be identified and informed of the limited expectation of a successful pregnancy and counseled regarding possible oocyte donation or adoption.
Furthermore, PCD could also reduce miscarriage frequency, which has recently been reported to be increased and close to 30% for women heterozygous for a Robertsonian translocation [18].
For women requiring ICSI because of male infertility, PCD may be indicated, as previously proposed for PGD [19]. For fertile women, PCD can be proposed after several miscarriages, or fetal aneuploidy, even if the cumulative success rate is identical for PGD and the natural cycle [20]: close to 70% after 2 years. The strategy that we propose may permit identification of women with a particularly high incidence of oocyte aneuploidy, as shown by our pilot series, in a population known to be associated with a variable risk. The 30% miscarriage frequency for women heterozygous for a Robertsonian translocation [18] is probably the combination of two opposite situations: women with low or high risk of chromosome malsegregation.
Conclusion
PCD could thus be an alternative to PGD in countries where PGD is restricted or prohibited. Furthermore, in view of the literature and our results, we argue that classic genetic counseling for Robertsonian translocation, predicated upon a mean incidence of aneuploidy, is not realistic for couples in certain risk categories (15% risk of miscarriage [21] and 2% risk of aneuploid fetus [22]), although our series is small and needs confirmatory investigations. The obstetric natural history (previous miscarriage or fetal/child aneuploidy) must be taken into account. PCD or PGD should be proposed to improve pregnancy rate for women with a Robertsonian translocation combined with either (i) previous miscarriage or fetal/child aneuploidy, or (ii) inclusion in an ICSI program. But, PCD or PGD does not seem to be mandatory for fertile women with no obstetric history. Further studies are necessary to confirm these hypotheses.
References
- 1.Nielsen J, Wohlert M. Chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus. Denmark. Hum Genet 1991;87(1):81–3. doi:10.1007/BF01213097. [DOI] [PubMed]
- 2.Verlinsky Y, Tur-kaspa I, Cieslak J, Bernal A, Morris R, Taranissi M, et al. Preimplantation testing for chromosomal disorders improves reproductive outcome of poor-prognosis patients. Reprod Biomed Online 2005;11(2):219–25. [DOI] [PubMed]
- 3.Goossens V, Harton G, Moutou C, Scriven PN, Traeger-Synoclino J, Sermon K, et al. ESHRE PGD Consortium data collection VIII: cycles from January to December 2005 with pregnancy follow-up to October 2006. Hum Reprod, 2008. [DOI] [PubMed]
- 4.Vialard F, Hammoud I, Molina GD, Wainer R, Bergere M, Albert M, et al. Gamete cytogenetic study in couples with implantation failure: aneuploidy rate is increased in both couple members. J Assist Reprod Genet 2008;25(11–12):539–45. doi:10.1007/s10815-008-9258-6. [DOI] [PMC free article] [PubMed]
- 5.http://perso.wanadoo.fr/fivnat.fr/.
- 6.Vialard F, Lombroso R, Bergere M, Molina GD, Hammoud I, Bailly M, et al. Oocyte aneuploidy mechanisms are different in two situations of increased chromosomal risk: older patients and patients with recurrent implantation failure after in vitro fertilization. Fertil Steril 2007;87(6):1333–9. doi:10.1016/j.fertnstert.2006.11.042. [DOI] [PubMed]
- 7.Montag M, van der Ven K, Dorn C, van der Ven H, et al. Outcome of laser-assisted polar body biopsy and aneuploidy testing. Reprod Biomed Online 2004;9(4):425–9. [DOI] [PubMed]
- 8.Moradkhani K, Puechberty J, Bhatt S, Lespinasse J, Vag P, Lefort G, et al. Rare Robertsonian translocations and meiotic behaviour: sperm FISH analysis of t(13;15) and t(14;15) translocations: a case report. Hum Reprod 2006;21(12):3193–8. doi:10.1093/humrep/del314. [DOI] [PubMed]
- 9.Page SL, Shin JC, Han Jy, Choo KH, Shaffer LG et al. Breakpoint diversity illustrates distinct mechanisms for Robertsonian translocation formation. Hum Mol Genet 1996;5(9):1279–88. doi:10.1093/hmg/5.9.1279. [DOI] [PubMed]
- 10.Munne S. Analysis of chromosome segregation during preimplantation genetic diagnosis in both male and female translocation heterozygotes. Cytogenet Genome Res 2005;111(3–4):305–9. doi:10.1159/000086904. [DOI] [PubMed]
- 11.Scriven PN, Flinter FA, Braude PR, Ogilvie CM, et al. Robertsonian translocations–reproductive risks and indications for preimplantation genetic diagnosis. Hum Reprod 2001;16(11):2267–73. doi:10.1093/humrep/16.11.2267. [DOI] [PubMed]
- 12.Durban M, Benet J, Boada M, Fernandez E, Calafell JM, Lailla JM, et al. PGD in female carriers of balanced Robertsonian and reciprocal translocations by first polar body analysis. Hum Reprod Update 2001;7(6):591–602. doi:10.1093/humupd/7.6.591. [DOI] [PubMed]
- 13.Pujol A, Durban M, Benet J, Boiso I, Calafell JM, Egozcue J, Navarro J, et al. Multiple aneuploidies in the oocytes of balanced translocation carriers: a preimplantation genetic diagnosis study using first polar body. Reproduction 2003;126(6):701–11. doi:10.1530/rep.0.1260701. [DOI] [PubMed]
- 14.Alves C, Sousa M, Silva J, Barros A, et al. Preimplantation genetic diagnosis using FISH for carriers of Robertsonian translocations: the Portuguese experience. Prenat Diagn 2002;22(12):1153–62. doi:10.1002/pd.503. [DOI] [PubMed]
- 15.Vialard F, Gomes DM, Hammoud I, Bergere M, Wainer R, Bailly M, et al. Stability of aneuploidy rate in polar bodies in two cohorts from the same patient. Reprod Biomed Online 2008;17(2):213–9. [DOI] [PubMed]
- 16.Ferraretti AP, Magli MC, Kopcow L, Gianaroli L, et al. Prognostic role of preimplantation genetic diagnosis for aneuploidy in assisted reproductive technology outcome.. Hum Reprod 2004;19(3):694–9. doi:10.1093/humrep/deh121. [DOI] [PubMed]
- 17.Munne S, Escudero T, Colls P, Xuezhong Z, Oter M, Garrisi M, et al. Predictability of preimplantation genetic diagnosis of aneuploidy and translocations on prospective attempts. Reprod Biomed Online 2004;9(6):645–51. [DOI] [PubMed]
- 18.Engels H, Eggermann T, Caliebe A, Jelska A, Schubert R, Schuler HM, et al. Genetic counseling in Robertsonian translocations der(13;14): frequencies of reproductive outcomes and infertility in 101 pedigrees. Am J Med Genet A 2008;146A(20):2611–6. doi:10.1002/ajmg.a.32500. [DOI] [PubMed]
- 19.Otani T, et al. Preimplantation genetic diagnosis significantly improves the pregnancy outcome of translocation carriers with a history of recurrent miscarriage and unsuccessful pregnancies. Reprod Biomed Online 2006;13(6):869–74. [DOI] [PubMed]
- 20.Sugiura-Ogasawara M, Suzumori K. Can preimplantation genetic diagnosis improve success rates in recurrent aborters with translocations? Hum Reprod 2005;20(12):3267–70. doi:10.1093/humrep/dei259. [DOI] [PubMed]
- 21.Harris DJ, Hankins L, Begleiter ML. Reproductive risk of t(13q14q) carriers: case report and review. Am J Med Genet 1979;3(2):175–81. doi:10.1002/ajmg.1320030208. [DOI] [PubMed]
- 22.Boue A, Gallano P. A collaborative study of the segregation of inherited chromosome structural rearrangements in 1356 prenatal diagnoses. Prenat Diagn 1984;4(Spec No):45–67. doi:10.1002/pd.1970040705. [DOI] [PubMed]