Abstract
Objective
To study if luteal E2 pre-treatment before GnRH antagonist protocol improves IVF/ICSI outcomes compared with standard long GnRH agonist protocol.
Design
A prospective, randomized and controlled study.
Setting
ART center of a state public hospital
Patient(s)
Two hundred twenty infertile women underwent IVF/ICSI treatments.
Intervention(s)
Participants received oral Estradiol Valerate 4 mg/day preceding the IVF cycle from day 21 until day 2 of next cycle before GnRH antagonist protocol (E2 pre-treatment group n = 109) or received standard long GnRH agonist protocol as control group (n = 111).
Main outcome measure(s)
Number of oocytes collected, MII oocytes, fertilization, implantation, live birth and early pregnancy rate, and hormone profiles.
Result(s)
E2 pre-treatment exerted a significant suppressive effect on FSH but not LH secretion compared with basal FSH and LH levels. In E2 pre-treatment group serum LH level was significantly higher during COH and serum P was also significantly higher on the day of HCG injection compared with control group. Five patients from E2 pre-treatment group had elevated LH at all time (≥10 IU/L) and also a concomitantly high P (>1 ng/mL). Two of the five women achieved pregnancy but had early pregnancy loss. Overall, IVF/ICSI outcomes such as implantation, clinical pregnancy and live birth rates were similar between E2 pre-treatment and control groups.
Conclusion(s)
Luteal E2 pre-treatment before GnRH antagonist protocol significantly increases serum LH level and incidence rate of premature LH but no significant effect is observed on implantation, clinical pregnancy, live birth and early pregnancy loss rates compared with long GnRH agonist protocol. However, more studies in large numbers of cycles are needed to confirm that increased serum LH level by E2 pre-treatment during COH has no negative effect on the IVF/ICSI outcomes.
Keywords: FSH, Estradiol pre-treatment, Controlled ovarian hyperstimulation, IVF
Introduction
Compared with long GnRH agonist protocols, the GnRH antagonist protocols for controlled ovarian hyperstimulation (COH) has offered a greater benefit in reducing the duration of treatment and the consumption of gonadotrophins as well as lowering the physical and psychological burden for patients undergoing pituitary desensitization. However, most studies indicate that there is a slight reduction in the number of retrieved oocytes and in the pregnancy rate in the GnRH antagonist protocols compared with long GnRH agonist protocols. This may be partly attributed to the absence of synchronization of the follicular cohort before ovarian stimulation [1, 2], reducing the number of viable oocytes and embryos and therefore the chance of pregnancy [3–5]. Thus, more attention has been paid to the potential use of steroid in the pre-treatments to program cycles, to modify the hormonal environment in relation to the negative feedback exerted by steroid on endogenous gonadotrophin secretion and therefore to synchronize the follicular cohort before stimulation. The potential benefit of natural estradiol (E2) pre-treatment has been recently assessed in GnRH antagonist cycles. This approach seems to be promising because endogenous estrogen secretion is actually the main factor involved in the negative regulation of FSH secretion during the luteal-follicular transition period. Indeed, previous reports have shown that E2 pre-treatment prevents intercycle FSH rise, improves follicle synchronization effectively within the cohort and enhances the recovery of mature oocytes [5, 6]. However, there is currently no prospective study on whether the use of E2 pre-treatment with GnRH antagonist protocol can significantly improve the IVF/ICSI outcome. We conducted a prospective, randomized and controlled trial in a large number of patients, comparing the IVF/ICSI outcomes between E2 pre-treatment before GnRH antagonist protocol and standard long GnRH agonist protocol.
Materials and methods
Patients
A total of 220 IVF/ICSI cycles were included in this prospective randomized study in our IVF centre between August 2006 and February 2007. The inclusion criteria were (1) age between 25–35 years old; (2) BMI between 18–25 kg/m2; (3) the number of previous IVF cycles <3, and no previous poor response to ovarian stimulation (poor ovarian response was characterized by cancellation of the cycle due to either poor follicular development or ≤4 cumulus-oocyte-complexes collected at oocyte retrieval); (4) normal-ovulatory cycles (25–35 days); (5) both ovaries present and normal uterus; (6) no hormone therapy within the past 3 months; and (7) no current or past diseases affecting ovaries, gonadotrophin, sex steroid secretion, clearance or excretion.
Selected women were randomly assigned to receive E2 pre-treatment with antagonist protocol or long GnRH agonist protocol. Randomization allocation sequence was generated from a table of computer-generated random numbers. This study was not blind. The study was approved by the hospital research and ethnical committee and patients signed consent to participate in the study.
Stimulation protocols
E2 pre-treatment group was administered daily oral Estradiol Valerate 4 mg (Progynava Schering) preceding the IVF cycle from day 21 until day 2 of next cycle. Stimulation was started by daily injection of 225 IU of recombinant FSH (Gonal-F, Serono) from day 3. This dose was maintained constantly for 5 days and then adjusted according to ovarian response. When the leading follicles reached 12–14 mm in diameter, GnRH antagonist (Cetrotide, Serono) 0.25 mg was injected daily until the day of hCG injection.
Control group received daily Troptorelin (Decapeptyl,Ferring) 0.1 mg s.c. preceding the IVF cycle from day 21 until pituitary down regulation (serum E2 < 50 pg/mL, LH < 5 IU/L, P < 0.9 ng/mL, and in the absence of follicular structures larger than 10 mm). When pituitary down regulation was achieved, the Troptorelin dose was reduced to 0.05 mg/d until the day of hCG injection. Stimulation was started by daily injection of 225 IU of recombinant FSH (Gonal-F, Serono) for the first 5 days and then adjusted according to ovarian response.
In both groups, injection of 10000IU hCG (Profasi, Serono) were given when at least three mature (≥18 mm) follicles were obtained and oocytes retrievals were performed 36 h later. Usually two to three embryos were transferred at 72 h after IVF insemination or ICSI injection. Luteal phase was supported by injection of progesterone 80 mg/day (LiZhu, China) starting on the day of oocyte retrieval until the day of pregnancy test. If a pregnancy occurred, progesterone administration was extended up to 10–12 weeks of pregnancy. The early pregnancy loss was defined as spontaneous abortion before 12 weeks.
Hormonal measurements
All blood samples were obtained by vein puncture, performed at 8:00–11:00 a.m. before injection of medication daily. Serum samples were separated and frozen-stored until assessment of FSH, LH, P and E2 using microparticle enzyme immunoassay with Abbott Axsym System (USA). Coefficient of variation (CV) and lowest limit detection for both intra- and inter-assays were: FSH 2.8%, 4.1%, 0.05IU/L; LH 1.8%, 2.5%, 0.5IU/L; P 1.8%, 2.5%, 0.1 ng/mL and E2 3%, 3.1%, 20 pg/mL, respectively. Hormonal assessment was performed at basal serum levels (menses 2–4 days during 3 months previous to COH), at the start of stimulation (D0), on day 6 (D6) and on day 8 (D8) of r-FSH stimulation and on the day of HCG administration.
Ultrasound was performed with a 7.2 MHZ multi frequency transvaginal probe (Aloka 1400, Japan) by one single operator concomitantly with hormonal assessment at each visit.
Statistics
Statistical analysis was performed by using the Statistical Package for the Social Science (SPSS) for Windows 10.0 and PASS. Either t test or chi-square tests were used to detect significant differences (P < 0.05) of all the variables between E2 pre-treatment and control groups.
Results
Baseline characteristics
There was no significant difference in the baseline hormone levels between the two groups (Table 1). 12 COH cycles (6 cycles in each group) were cancelled before embryo transfer (ET). The reasons of cancellation in both E2 pre-treatment and control groups were ovarian hyperstimulation syndrome (OHSS) in three patients from experimental group and 2 from control; the sign of endometrial fluid in 1 and 3; others in 2 and 1, respectively. Overall, ETs were performed in 103 cycles for the E2 pre-treatment group and 105 cycles for the control group.
Table 1.
Summary of clinical characteristics, basal hormonal profiles and ART procedures between E2 pre-treatment and control groups
| Parameters | E2 Pre-treatment group n = 109 | Control group n = 111 | P |
|---|---|---|---|
| Age (range) | 30.3 ± 2.8 (24–35) | 30.2 ± 2.8 (25–35) | NS |
| BMI (range) | 20.7 ± 1.9 (16.9–24.9) | 21.0 ± 1.8 17.7–25) | NS |
| Primary infertility (%) | 35 (32.1%) | 37(33.3%) | NS |
| Secondary infertility (%) | 74(67.9%) | 74(66.7%) | NS |
| Duration of infertility (years) | 6.2 ± 3.5(1–14) | 5.3 ± 3.2(1–14) | NS |
| Indication for treatment | |||
| Tubal factor (%) | 80(73.4%) | 85(76.6%) | NS |
| Endometriosis (%) | 10(9.2%) | 7(6.3%) | NS |
| Male factor (%) | 18(16.5%) | 17(15.3%) | NS |
| Idiopathic (%) | 1(0.9%) | 2(1.8%) | NS |
| Repeated IVF cycles (%) | 4 (93.7%) | 3(2.7%) | NS |
| Basal E2 (pg/mL) | 34.2 ± 15.8 | 32.8 ± 12.9 | NS |
| Basal FSH(Iu/L) | 6.2 ± 1.6 | 6.5 ± 1.3 | NS |
| Basal LH(Iu/L) | 3.4 ± 1.3 | 3.8 ± 2.0 | NS |
| Basal P(ng/mL) | 0.6 ± 0.3 | 0.5 ± 0.2 | NS |
| Antral follicle count(AFC) | 7.7 ± 2.5 | 7.9 ± 2.9 | NS |
| ART procedures (%) | |||
| IVF | 69.4% | 69.4% | NS |
| IVF+ICSI | 22.2% | 17.2% | NS |
| ICSI | 8.3% | 13.5% | NS |
COH and IVF result in two groups
The mean length of E2-treatment was 5.4 ± 1.0 (range 4–9) days and the baseline cycle length of the preceding cycle was not altered by luteal E2 administration. There were no significant differences between the two groups in mean dose of gonadotrophins (Gn); stimulation duration; endometrial thickness on the day of hCG; the numbers and sizes of the follicles in the early, mid, late follicular phases; the mean number of achieved oocytes and MII oocytes; the fertilization rate; and the cleavage rate (Table 2).
Table 2.
Characteristics of COH and IVF/ICSI results between E2 pre-treatment and control groups
| Parameters | E2 Pre-treatment group(n = 109) | Control group (n = 111) | P |
|---|---|---|---|
| Duration of E2/GnRH-agonist down regulation days (range) | 5.4 ± 1.00 (4–9) | 20.7 ± 2.4 (14–27) | NS |
| Doses of r-FSH (Amps) | 31.7 ± 6.7 (18–73) | 31.2 ± 5.8 (17–47) | NS |
| Duration of r-FSH stimulation (days) | 10.4 ± 1.1 (8–14) | 10.4 ± 1.2 (7–14) | NS |
| Endometrial thickness on the day of HCG (mm) | 10.8 ± 1.5 | 10.8 ± 1.5 | NS |
| Follicles 11–14 mm on D6 | 2.3 ± 2.3 | 2.1 ± 2.6 | NS |
| Follicles 11–14 mm on D8 | 6.6 ± 3.3 | 6.9 ± 3.6 | NS |
| Follicles 15–16 mm on D8 | 0.9 ± 1.4 | 0.7 ± 1.5 | NS |
| Follicles≥17 mm on D8 | 0.2 ± 0.6 | 0.3 ± 1.5 | NS |
| Follicles 11–14 mm on the day of HCG | 2.8 ± 2.0 | 2.5 ± 2.1 | NS |
| Follicles 15–16 mm on the day of HCG | 2.3 ± 1.9 | 2.2 ± 1.8 | NS |
| Follicles≥17 mm on the day of HCG | 7.4 ± 3.8 | 8.0 ± 3.5 | NS |
| No. of oocytes retrieved | 12.8 ± 5.7 | 13.8 ± 5.4 | NS |
| No. of MII (%) | 10.7 ± 5.0 (83.5%) | 11.5 ± 5.1 (83.4%) | NS |
| Fertilization rate (%) | 85.5% | 86.6% | NS |
| No. of 2PN oocytes (%) | 8.0 ± 4.2 (74.9%) | 8.8 ± 4.5 (76.4%) | NS |
| No. of embryos(2PN) (%) | 7.9 ± 4.2 (98.3%) | 8.6 ± 4.5 (98.0%) | NS |
| No. of viable embryos (%) | 5.6 ± 3.4 (71.4%) | 6.2 ± 3.4 (71.9%) | NS |
The IVF outcomes in the two groups
The mean number of transferred embryos was similar between the two groups. There were no significant differences in overall IVF/ICSI outcomes between the two groups but there was a trend of lower implantation rate (34.7% vs 37.8%), lower clinical pregnancy rate (52.4% vs 60%), lower live birth rate (34% vs 37.1%), and higher early pregnancy loss rate (22.2% vs 12.7%) in the E2 pre-treatment group compared with control group (Table 3).
Table 3.
Comparison of the outcomes of IVF/ICSI between E2 pre-treatment and control groups
| E2 Pre-treatment | Control | P | |
|---|---|---|---|
| No. of ET cycles | 103 | 105 | |
| No. of transferred embryos | 2.0 ± 0.4 | 2.0 ± 0.2 | NS |
| Implantation rate | 74/213 (34.7%) | 79/209 (37.8%) | NS |
| Clinical pregnancy rate/ET | 54/103 (52.4%) | 63/105 (60%) | NS |
| Early pregnancy loss rate | 12/54 (22.2%) | 8/63 (12.7%) | NS |
| Live birth rate/ET | 35/103 (34%) | 39/105 (37.1%) | NS |
| OHSS rate/stimulation cycle | 3/109 (2.8%) | 2/111 (1.8%) | NS |
Serum hormonal dynamics
Compared with the basal hormonal level, E2 pre-treatment group showed a significant suppressive effect on FSH and P level (P < 0.001) but not LH level after exogenous E2 discontinuation. The serum E2 level after exogenous E2 discontinuation was significantly higher than the basal E2 level (P < 0.001). The control group showed a significant suppressive effect on E2, LH, FSH and P levels after pituitary down regulation (P < 0.001), (Table 4).
Table 4.
Comparison of hormonal profiles between basal and day 0
| Basal | Day 0 | P | |
|---|---|---|---|
| E2 pre-treatment | |||
| E2 (pg/mL) | 34.2 ± 15.8 | 70.1 ± 36.1 | <0.001 |
| LH (IU/L) | 3.4 ± 1.3 | 2.8 ± 6.6 | NS |
| FSH (IU/L) | 6.2 ± 1.6 | 3.9 ± 1.5 | <0.001 |
| P (ng/mL) | 0.6 ± 0.3 | 0.5 ± 0.2 | <0.001 |
| Control | |||
| E2 (pg/mL) | 32.8 ± 12.9 | 17.8 ± 9.0 | <0.001 |
| LH (IU/L) | 3.8 ± 2.0 | 1.5 ± 0.8 | <0.001 |
| FSH (IU/L) | 6.5 ± 1.3 | 2.9 ± 0.8 | <0.001 |
| P (ng/mL) | 0.5 ± 0.2 | 0.5 ± 0.2 | NS |
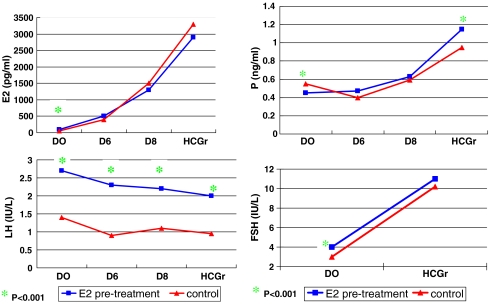
Compared with the control, the E2 pre-treatment group had significantly higher values for serum E2, LH and FSH on D0 (P < 0.001). However, the serum P value was significantly lower (P < 0.001) on D0. The serum LH on D6, D8 and on the day of HCG administration was significantly higher (P < 0.001), and the serum P level on the day of HCG administration was significantly higher than in the control group (p < 0.001, Fig. 1). The ratio of serum LH/P increased: five patients from E2 pre-treatment group had elevated LH at all time (≥10 IU/L) and a concomitantly high P (>1 ng/ml). Two of the five women achieved pregnancy but had early pregnancy loss.
Fig. 1.
Level of E2, LH, P and FSH from D0 to D8 and the time of HCG injection for both E2 pre-treatment and control groups
Discussion
Although complex mechanisms inherent in each follicle act together to determine its sensitivity to FSH and developmental competence, asynchronous multi-follicular growth during COH may be a direct consequence of size heterogeneities of early antral follicles during the early follicular phase. The physiological mechanisms in this process remain poorly understood [7]. A plausible explanation involves the premature, gradient FSH elevation paralleling the corpus luteum demise that occurs during the late luteal phase in the menstrual cycle. During the luteal-follicular transition, FSH preserves early antral follicles from atresia and ensures their subsequent growth [8]. According to the intrinsic sensitivity to this hormone, some follicles are able to respond to lower FSH levels than others and, therefore, start their development earlier, during the late luteal phase [9]. Since the larger follicles are more responsive to FSH, exogenous gonadotrophin administration is likely to intensify further size discrepancies of growing follicles during COH [10]. Hence, according to this hypothesis, suppression of luteal FSH secretion may prevent untimely, uncoordinated development of FSH-sensitive follicles during the luteal-follicular transition and foster follicular growth synchronization during COH.
The long GnRH agonist protocol induces profound suppression of endogenous release of gonadotrophins during the early follicular phase, allowing the early antral follicles to grow coordinately in response to exogenous gonadotrophins to accomplish simultaneous maturation. This leads to an extended widening of the FSH window, increased FSH requirement and at the end, more follicles [2]. In antagonist protocols, more attention has been paid to the potential effect of steroid pre-treatment to program antagonist protocol cycles. Durnerin-Cedrin et al. [11] and Fanchin et al. [7] verified that E2 pre-treatment could suppress FSH levels, reduce the pace of growth, and improve the size homogeneity of antral follicles. After discontinuation of E2 treatment, serum FSH increased progressively. The abrupt FSH rebound after stopping E2 intake with a concomitant increase in follicle size argues for a short wash-out interval of 1 day or 2 days. However, oral contraceptive pill (OCP) and progestogen pre-treatments strongly suppress FSH and LH values for a 5-day wash-out period. Meanwhile, the follicle size inside the cohort remained homogeneous. OCP and progestogen pre-treatments were more suppressive than natural E2 pre-treatment [11]. Steroids may interfere with gonadotrophin secretion not only by the suppressive effect induced by steroid intake but also by the rebound of gonadotrophin secretion after the cessation of administration. The significant FSH rebound is likely to synergize with exogenous gonadotrophins and further promote multi-follicular development.
Estrogens are believed to primarily inhibit FSH secretion. This approach seems to be promising because endogenous estrogen secretion is actually the main factor involved in the negative regulation of FSH secretion during the luteal-follicular transition [12].The potential benefit of natural estradiol pre-treatment has been recently assessed in GnRH antagonist cycles. Our data have demonstrated that serum FSH values were significantly lower than the baseline FSH values after E2 pre-treatment during the mid-luteal phase of the preceding IVF cycle and stimulated by r-FSH, the number and the pace of growth of the follicles were similar to those in the long GnRH agonist protocol. A similar situation was observed in the number of retrieved oocytes, MII oocytes and the serum E2 values on D6, D8 and the day of HCG administration in COH. Thus E2 pre-treatment may produce the same adequate synchronization of follicles as the long GnRH agonist protocol. This supported Fanchin’s [6] report that E2 pre-treatment could improve the coordination of follicles and produce more MII oocytes.
As E2 pre-treatment can slow down the velocity of the follicle development, improve the follicular synchronization and produce more MII oocytes, it is considered to be a potential, more physiologic and selective technique to ameliorate the coordination of follicles. However, it is still not clear whether E2 pre-treatment can overcome the inferior IVF outcome resulting from the GnRH-antagonist protocol compared with the long GnRH agonist protocol.
It is likely that two phenomena play an important role to facilitate optimal IVF results when GnRH analogues are used: stable and low LH and progesterone levels throughout the stimulation phase to achieve optimal conditions for implantation, and sustained low levels of endogenous FSH before the start of stimulation to allow optimal synchronization of the follicular cohort [2]. The changes in follicular dynamics and coordination induced by luteal FSH suppression may offer a plausible explanation for the reported improved outcome of long GnRH agonist protocols, compared with GnRH antagonist protocol [13, 14]. According to this, luteal E2 administration could render an effectiveness of GnRH antagonist equivalent to that of long GnRH agonist protocols. Furthermore, luteal E2 administration presents some advantages over long GnRH agonist protocols, such as simplicity, less side effects and no endogenous gonadotrophin suppression during the early follicular phase of COH [6].
The present study investigated whether luteal E2 pre-treatment before GnRH antagonist protocol achieves the similar IVF outcomes to the luteal GnRH-agonist long protocol. Our study was conducted as a prospective, randomized and controlled trial. Our data shows that there was no significant difference in the mean numbers of retrieved oocytes, MII oocytes, fertilization rate, cleavage rate, available embryo rate and transferred embryos between the two groups. Also there were no significant differences in implantation rates (34.7% vs. 37.8%), clinical pregnancy rate (52.4% vs. 60%), live birth rate (34% vs. 37.1%) and early pregnancy loss rate (22.2% vs.12.7%) between the two groups. However, E2 pre-treatment group appears to have relatively lower implantation, clinical pregnancy and live birth rates and higher early pregnancy loss rate compared with control group. Statistically, to be able to reach significant difference of 8% clinical pregnancy rate between the two groups, we would need about 668 cycles fro each group based on the calculation by two-tailed hypothesis test with β of 0.2 and α of 0.05. Therefore, more study with large numbers of cycles is needed to determine whether E2 pre-treatment with GnRH antagonist protocol is comparable to the long GnRH agonist protocol.
The important significant difference is the different internal hormone secretion environments between the long GnRH agonist protocol and E2 pre-treatment with GnRH antagonist protocol. Our results showed that after E2 administration, serum LH levels had not significantly changed compared with the basal LH levels (P > 0.05). This revealed that E2 pre-treatment is not useful for the LH down-regulation which is in agreement with previous reports by Durnerin IC et al. [11] and Fanchin [6]. Furthermore, the serum LH level after E2pre-treatment was significantly higher than the control group (p < 0.001) and five patients from E2 pre-treatment group had elevated LH at all time (≥10 IU/L) and also a concomitantly high P (>1 ng/mL). Two of the five women achieved pregnancy but had early pregnancy loss. This demonstrated that E2 pre-treatment before GnRH-antagonist protocol, especially before the GnRH antagonist injection, had increased incidence rate of premature LH, which is similar to the previous report by Durnerin IC et al. [11]. This could be clinically relevant because pregnancy rate seems optimal within a certain window of LH secretion in GnRH antagonist cycles [15]. Kolibianakis et al. [16] suggested that the lower the LH levels, the higher probability of pregnancy in GnRH antagonist protocol. Conversely, Rombauts et al. [17] observed decreased implantation rate after OCP pre-treatment with a short wash-out interval of 2 days leading to a profound pituitary suppression. Therefore, the ability of serum LH levels to predict the ART outcome in GnRH antagonist cycles remains questionable. Nevertheless, in cycles without E2 pre-treatment, exposure to high LH levels is associated with a reduced chance of pregnancy [18]. Whether a serum LH level elevated in the subsequent COH cycle could lead to lesser outcomes remains to be determined in a large series of patients.
In conclusion, luteal E2 pre-treatment before GnRH antagonist protocol has a similar effect on improving the synchronization of follicular growth and number of MII oocytes. However, this method has a higher serum LH level in the whole COH and a concomitantly increased incidence rate of premature LH, compared to the long GnRH agonist protocol. Although there were no significant differences in overall IVF/ICSI outcomes, E2 pre-treatment protocol produces relatively low implantation, clinical pregnancy and live birth rates and higher early pregnancy loss rate compared with the long GnRH agonist protocol. More study with large number of subjects is required to determine whether increased LH serum levels by E2 pre-treatment in COH has potential negative effect on the ART outcomes.
Acknowledgement
The authors thank Dr. De Yi Liu from the University of Melbourne, Australia, for revising the final draft of the manuscript.
References
- 1.Al-lnany H, Aboulghar M. GnRH antagonist in assisted reproduction: a Cochcane review. Hum Reprod. 2002;17:874–85. doi:10.1093/humrep/17.4.874. [DOI] [PubMed]
- 2.Huirne JA, Homburg R, Lambalk CB. Are GnRH antagonists comparable to agonists for use in IVF? Hum Reprod. 2007;22:2805–13. doi:10.1093/humrep/dem270. [DOI] [PubMed]
- 3.Devreker F, Pogonici E, De Maertelaer V, Revelard P, Vanden Bergh M, et al. Selection of good embryos for transfer depends on embryo cohort size: implication for the mila ovarian stimulation debate. Hum Reprod. 1999;14:3002–8. doi:10.1093/humrep/14.12.3002. [DOI] [PubMed]
- 4.Opsahl MS, Blauor KL, Black SH, Lincoln SR, Thorell L, Sherins RJ. The number of embryos available for transfer predicts successful pregnancy outcome in women over 39 years with normal ovarian hormonal reserve testing. J Assist Reprod Genet. 2001;18:551–6. doi:10.1023/A:1011906024170. [DOI] [PMC free article] [PubMed]
- 5.Fanchin R, Cunha-Filho JS, Schonauer LM, Kadoch IJ, Cohen-Bacri P, Frydman R. Coordination of early antral follicles by luteal estradiol administration provides a basis for alternative controlled ovarian hyperstimulation. Fertil Steril. 2003;79:316–21. doi:10.1016/S0015-0282(02)04574-0. [DOI] [PubMed]
- 6.Fanchin R, Salomon L, Castelo-Branco A, Olivennes F, Frydman N, Frydman R. Luteal estradiol pre-treatment coordinates folliclar growth during controlled ovarian hyperstimulation with antagonist. Hum Reprod. 2003;18:2698–703. doi:10.1093/humrep/deg516. [DOI] [PubMed]
- 7.Fanchin R, Cunha-Filho JS, Schonauer LM, Righini C, de Ziegler D, Frydman R. Luteal estradiol administration strengthens the relationship between day 3 follicle-stimulations hormone and inhibin B levels and ovarian follicular status. Fertil Steril. 2003;79:585–9. doi:10.1016/S0015-0282(02)04757-X. [DOI] [PubMed]
- 8.Chun SY, Eisenhauer KM, Minami S, Billig H, Perlas E, Hsueh AJ. Hormonal regulation of apoptosis in early antral follicles: follicle-stimulating hormone sa a major survival factor. Endocrinology. 1996;137:1447–56. doi:10.1210/en.137.4.1447. [DOI] [PubMed]
- 9.Klein NA, Battaglia DE, Fujimoto VY, Davis GS, Bremner WJ, Soules MR. Reproductive aging: accelerated ovarian follicular development associated with a monotropic follicle-stimulating hormone rise in normal older women. J Clin Endocrinol Metab. 1996;81:1038–45. doi:10.1210/jc.81.3.1038. [DOI] [PubMed]
- 10.Hillier SG, Van den Boogaard AM, Reichert LE Jr, Van Hall EV. Intraovarian sex steroia hormme interaction and the regulation of follicular maturation: aromatization of androgens by human granulose cells in vitro. J Clin Endocrinol Metab. 1980;50(4):640–7. [DOI] [PubMed]
- 11.Durnerin-Cedrin I, Bstandig B, Parneix L, et al. Effects of oral comfraceptive, synthetic progestogen or natural estrogen pre-treatments on the hormonal profile and the antral follicle cohort before GnRH antagonist protocol. Hum Reprod. 2007;22:109–16. doi:10.1093/humrep/del340. [DOI] [PubMed]
- 12.Laholu N, Chabbert-Buffer N, Christin-Maitre S, Le Nestour E, Roger M, Bouchard P. Main inhibitor of follicle stimulating hormone in the luteal-follicular transition: inhibin A, Destradios, or inhibin B? Hum Reprod. 1999;14:1190–3. doi:10.1093/humrep/14.5.1190. [DOI] [PubMed]
- 13.Borm G, Mannaerts B. Treatment with the gonadotrophin-releasing hormone antagonist ganirelix in women undergoing ovarian stimulation with recombinant follicle stimulating hormone in effective, safe and convenient: results of a controlled, randomize, multi-centre trial. The European Orgalutran Study Group. Hum Reprod. 2000;15:1490–8. doi:10.1093/humrep/15.7.1490. [DOI] [PubMed]
- 14.Al-Inany H, Aboulghar M. GnRH antagonist in assisted reproduction: a Cochrane review. Hum Reprod. 2002;7:874–85. [DOI] [PubMed]
- 15.Huime JAF, van Loenen ACD, Schats R, McDonnell J, Hompes PGA, Schoemaker J, et al. Dose-finding study of daily GnRH antagonist for the prevention of premature LHsurges in IVF/ICSI patients: optimal changes in LH and progesterone for clinical pregnancy. Hum Reprod. 2005;20:359–67. [DOI] [PubMed]
- 16.Kolibianakis EM, Zikopoulous K, Schiettecatte J, Smita J, Tournaye H, Camus M, et al. Profound LH suppression after GnRH antagonist administration is associated with a significantly higher ongoing pregnancy rate in IVF. Hum Reprod. 2004;19:2490–6. doi:10.1093/humrep/deh471. [DOI] [PubMed]
- 17.Rombauts L, Healy D, Norman RJ. A comparative randomized trial to assess the impact of oral contraceptive pretreatment on follicular growth and hormone profiles in GnRH antagonist-treated patients. On behalf of the Orgalutran Scheduling Study Group. Hum Reprod. 2006;21:95–103. doi:10.1093/humrep/dei302. [DOI] [PubMed]
- 18.Kolibianakis EM, Albano C, Kahn J, Camus M, Tournaye H, Van Steirteghem AC, et al. Exposure to high levels of luteinizing hormone and estradiol in the early follicular phase of gonadotropin-releasing hormone antagonist cycles is hv+mcnkjjassociatoal with a reduced chance of pregnancy. Fertil Steril. 2003;79:873–80. doi:10.1016/S0015-0282(02)04920-8. [DOI] [PubMed]