Abstract
Non-malignant fibrosing tumors in the pediatric hand or juvenile fibromatoses are clinically challenging because of their relatively infrequent occurrence and because of the variety of names associated with these diseases. We conducted a review of a personal case series of pediatric patients with these tumors and discuss here the more common histologic types and clinical characteristics of the disease spectrum in the context of the available published literature. All histologic samples were reviewed by a single pathologist. Infantile myofibromatosis, fibrous hamartoma of infancy, juvenile aponeurotic fibromatosis, palmar fibromatosis (Dupuytren’s type), infantile digital fibromatosis (Reye’s tumor), fibroma of the tendon sheath, and melorheostosis represent the encountered lesions.
Keywords: Juvenile, Pediatric, Non-malignant, Fibrosing, Tumors, Fibromatoses
Introduction
Stout coined the collective term “juvenile fibromatosis” more than 50 years ago to incorporate the host of non-malignant fibrosing tumors that occur in childhood [26], and the nomenclature has been confusing ever since. Some of the earlier names for these lesions, such as aggressive fibromatosis, recurrent digital fibrous tumor, and congenital fibromatosis, were merely descriptive and conveyed no clinicopathologic meaning [2]. Certain histologic types and pathologic diagnoses, however, are characteristic, as are the clinical properties that distinguish one tumor type from another. Using individual case studies from a personal series of cases, we evaluated 12 children, together with their pathologic subtypes, who presented for treatment of fibrosing tumors of the hands and fingers. Cases representative of each subtype were chosen.
Because of its diagnostic importance, histologic examination of suspected juvenile fibromatosis lesions should, in addition to routine hematoxylin and eosin staining, include Masson Trichrome, immunohistochemical markers for smooth muscle (MSA, HHS 35, Dako), and histiocytic (CD68, PG-M1, Dako) markers as needed. The correct diagnosis can usually be established by attention to the combination of clinical characteristics—age at onset, site of involvement, and appearance—and specific histological features. Knowledge of the typical course of these lesions guides the approach to treatment.
Clinical and photographic records of all patients had been maintained. All patients were operated upon by a single surgeon, and tissue samples were reviewed by one pathologist with a special interest in fibromatoses of children. A review of available published literature was performed.
Clinical Features and Treatment
The juvenile fibromatosis lesions represented in our series of pediatric hand patients are infantile myofibromatosis, fibrous hamartoma of infancy, juvenile aponeurotic fibromatosis, palmar fibromatosis (Dupuytren’s type), infantile digital fibromatosis (Reye’s tumor), fibroma of the tendon sheath, and melorheostosis. Some specific characteristics of these commonly accepted subtypes of juvenile fibromatosis are discussed below (Table 1).
Table 1.
Contrasting characteristics of juvenile fibromatoses.
| Age of onset | Most common location | Characteristic clinical appearance | Histologic characterization | Multiple or viscerala | Potential for spontaneous regression | Role of surgery | |
|---|---|---|---|---|---|---|---|
| Infantile myofibromatosis | Birth to 2 years (60% at birth or neonate) | Head and neck upper extremity | Most often solitary mass. May involve deep subcutaneous tissue and muscle with deep “scarring” and ulceration | Peripheral spindle cells, “zoning”, myofibroblasts present that stain positively for actin | May be multicentric and visceral | Yes | Excision for functional deformity. Avoid mutilating surgery to accomplish complete excision |
| Fibrous hamartoma of infancy | Birth to 2 years | Head and neck, shoulder, groin. Uncommon in hands (except lipofibromatosis) | Solitary mass, which may reach large proportions | 3 cell types: trabeculae, immature fibroblasts, mature adipose cells | Solitary | No | Wide excision |
| Juvenile aponeurotic fibromatosis | Older children | Fingers and palm | Firm mass. May occur around tendons and cause functional stiffness. May have pain | Calcifications in the midst of fibrosis | Solitary | No | Wide excision |
| Palmar fibromatosis (Dupuytren’s) | Rare in children | Palm and fingers can occur on dorsum of hand and wrist | Finger contractures and palmar dimpling | Bland dense collagen deposition | No. May have diffuse involvement like Dupuytren’s in adult | No | Excision of diseased cords only. Wide radical resection unnecessary |
| Infantile digital fibromatosis | Infants and young children | Finger tips and toes—not thumb or great toe | Painless enlarging mass. Adherent overlying dermis—“kissing” lesions | Fibroblastic proliferation in a collagen matrix. Eosinophilic intracytoplasmic inclusion bodies | Yes | Yes | Observe initially, then wide local excision if no involution |
| Fibroma of tendon sheath | Older children and adolescents | Digits and hand. Most commonly thumb | Solitary small mass attached to tendon or tendon sheath. Overlying skin not adherent | Dense fibrous stroma and slit-like vascular channels. Scattered multinucleate giant cells. No “foamy” macrophages that occur in giant cell tumors | No | No | Local excision |
| Melorheostosis | Older children and adolescents | Usually affects long bones with lower extremity more commonly affected than upper. Hand is an uncommon site | Initial periarticular mass and stiffness. Characteristic radiographic changes develop, may have pain. | Bland fibrosis | In-continuity sclerotomal involvement | No | Incisional biopsy |
aMyofibromatosis is the only variety that may have a multicentric form with visceral involvement
Infantile myofibromatosis, a term coined by Chung and Enzinger, exclusively affects infants and young children, with 60% of cases noted at birth or shortly thereafter and more than 80% occurring before the age of 2 years [6]. Infantile myofibromatosis is one of the most commonly occurring tumors of the neonatal period [14, 28], presenting in both solitary and multicentric forms.
Most solitary forms occur in the head and neck region; the upper extremity is the next most common site [6]. Solitary lesions are usually cutaneous, involving the dermis and extending to subcutaneous tissue, muscle, and bone. Our series included three patients with solitary lesions affecting the hand. One type of the multicentric form of infantile myofibromatosis includes visceral involvement and one type does not. The visceral type is associated with significant morbidity, sometimes even mortality, and its presence requires clinical evaluation for other potential multicentric tumors. Spontaneous regression is generally the natural history of those infantile myofibromatosis lesions without visceral involvement [1, 14].
A histologic zoning phenomenon is present in infantile myofibromatosis lesions. Peripheral spindle-shaped cells arranged in bundles blend centrally into less differentiated round or polygonal cells arranged in sheets [22, 29]. The spindle cells have the ultrastructural and immunohistochemical characteristics of myofibroblasts [1, 7], staining positive for vimentin and alpha-smooth-muscle actin but negative for desmin. The cells are also negative for s-100 protein, differentiating them from more immature histiocytes [3]. Progressive cell differentiation is a possible explanation for the spontaneous regression of the solitary lesions [6, 29].
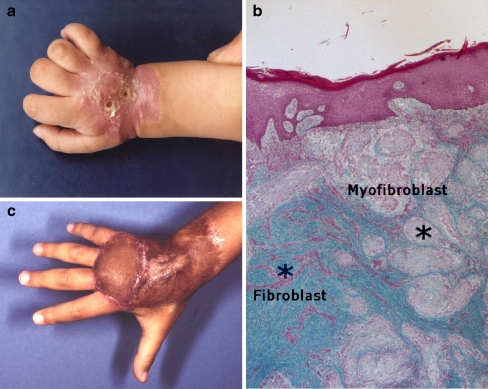
This child was first seen at 12 months of age with a densely indurated, ulcerating lesion, present since birth, on the dorsum of the right hand. No other lesions were present. A diagnosis of infantile myofibromatosis had been established based on tissue biopsy. The child had extension contractures of the wrist and MP joints (Fig. 1a). Histology showed the two cell types characteristic of infantile myofibromatosis, myofibroblasts and fibroblasts, and the zonation effect in which smooth-muscle-appearing cells on the periphery of the lesion give way to central fibromatosis cells (Fig. 1b).
Fig. 1.
a One-year-old child with ulcerated lesion over dorsum of the right hand and extension contractures of the hand and wrist. b Infantile myofibromatosis. Note nodules of myofibroblasts at the periphery of the tumor mass separated by normal dermal connective tissue. The nodules show increased cellularity at the periphery and hyalinization toward the center (Masson Trichrome, ×100). c Finger and wrist extension following flap and skin graft reconstruction.
Magnetic resonance imaging (MRI) demonstrated a large lesion deeply insinuated between the metacarpals. Operation revealed extensor tendons densely encased by tumor. The tendons were dissected out and spared. Because of the lesion’s potential for spontaneous regression, we knowingly left behind tumor deep to the extensor tendons and between the metacarpals to avoid an excessively mutilating extirpative surgery. Immediate soft tissue coverage required a combination of full thickness skin grafting and a pedicle groin flap. The patient underwent a total of four surgical procedures over 5 years, including the original tumor biopsy, tumor resection and soft tissue coverage, scar excisions, and tenolysis.
Figure 1c shows late follow-up of the hand. Both hand and wrist function well. Residual tumor left behind at the original resection has involuted; deep tissue biopsy at the time of tenolysis showed scar tissue only and no tumor recurrence.
Enzinger named fibrous hamartoma of infancy, which occurs in the first 2 years of life and may even be present at birth [9]. These tumors are usually solitary and have previously been thought to occur only rarely in the hands and feet [16, 18]. Fibrous hamartoma of infancy does not spontaneously regress and may recur following surgical resection. Microscopic features include an admixture of mature adipose cells, nodular aggregates of immature mesenchymal elements with interlacing bands of fibrocollagenous tissue, and spindled fibroblastic cells.
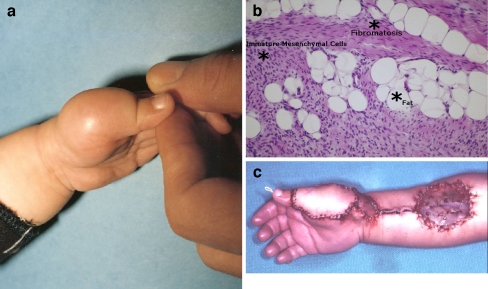
This 1-year-old underwent resection of a tumor on the dorsum of the left thumb at an outside facility and presented to our facility with a recurrent mass that was ill-defined on MRI 10 months later (Fig. 2a). The diagnosis of fibrous hamartoma of infancy was made histologically based on the simultaneous presence of three tissue types, well-defined bundles, or trabeculae of dense fibrocollagenous tissue; loosely textured cellular areas of immature fibroblasts, mesenchymal elements in a myxoid matrix; and mature adipose cells interposed between the other two elements. An organoid growth pattern justified the term hamartoma (Fig. 2b).
Fig. 2.
a One-year-old child with dorsal thumb lesion. b Fibrous hamartoma of infancy. Note fibrous trabeculae and mature adipose cells (hematoxylin and eosin staining, ×200). c Reconstruction immediately following tumor resection with tendon grafting and reverse-flow pedicle radial forearm flap coverage.
The child had a second radical resection, which included skin, a segmental resection of the extensor tendon, and the dorsal capsule of the thumb MP joint. The tendon was immediately reconstructed with a palmaris longus free tendon graft, and soft tissue coverage was achieved with a reverse-flow pedicle radial forearm flap (Fig. 2c). He underwent further surgery for scar and flap revision 3 years later; deep tissue biopsy at that time was free of tumor.
Some argue for a separate categorization of lipofibromatosis, saying that cases of infantile fibrous hamartoma have been previously miscategorized [13]. Lipofibromatosis is said to have a predilection for hands and feet, unlike infantile fibrous hamartoma, which more commonly occurs in the proximal upper and lower limb girdles. Additionally, while both types of lesions have mature adipocytes, the characterizing feature of infantile fibrous hamartoma is primitive organoid nests of mesenchymal cells in a loose myxoid matrix. Lesions of the hands may actually thus be lipofibromatoses, although this separate categorization has not been universally accepted. Certainly, lesions of lipofibromatosis that have had long-term follow-up and although incompletely excised have not progressed but equally have not necessarily regressed. This has been postulated to be the result of lesional maturation [13]. However, local recurrence is common, and complete removal is preferable.
Juvenile aponeurotic fibromatosis was first described by Keasbey [19]. The lesion tends to occur in older children with a relatively wide age range (2.5–9 years) reported and even occasionally in adults [15]. Our patient presented at 14 years of age. Although this lesion was originally called juvenile aponeurotic fibroma, a number of other terms have been used to describe it. The most commonly accepted name is juvenile aponeurotic fibromatosis [19] or, since the lesion may occasionally occur in adults, calcifying aponeurotic fibroma [10].
The histologic hallmark is calcifications seen in conjunction with an otherwise unremarkable fibrosing disease. Stippled calcification may be visible radiographically. Nuclei are large and darkly staining. Calcification may not necessarily occur in younger infants [15]. These lesions classically occur on the fingers and palm but may arise in relation to tendons. Although presentation is reported to be painless [17], our patient presented with a painful lesion. A recurrence rate of more than 50% is reported after resection [8].
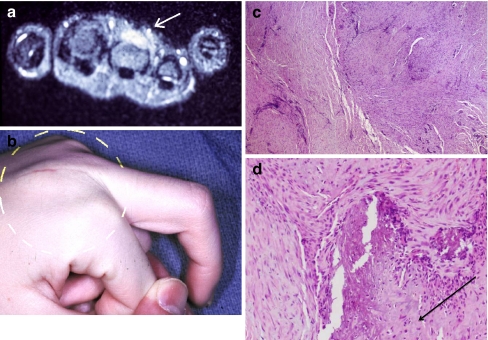
A 14-year-old patient experienced sudden, painful swelling over the dorsum of her right middle finger MP joint not associated with trauma. She presented with MP joint flexion restricted to 45°. Flexion of all other joints was normal. Sedimentation rate, rheumatoid factor, and antinuclear antibody serum studies were negative. MRI showed a mass deep to the extensor mechanism with dorsoradial metacarpal cortical erosion (Fig. 3a,b).
Fig. 3.
a Fourteen-year-old child with painful swelling over dorsum of middle finger MP joint. MRI shows the lesion (arrow). b Reduced flexion at MP joint, with diffuse dorsal swelling. c Juvenile aponeurotic fibromatosis. Note cells containing large, dark-staining nuclei (hematoxylin and eosin staining, ×40). d High-power histology showing calcifications (hematoxylin and eosin staining, ×100).
Incisional biopsy of the mass revealed a pathologic diagnosis of juvenile aponeurotic fibromatosis. The predominant cell has large, dark-staining nuclei on hematoxylin and eosin preparation (Fig. 3c). The characteristic calcifications of this disease are seen against a background of unremarkable fibrosis (Fig. 3d). Radical excision included the metacarpal periosteum and dorsal MP joint capsule and the radial sagittal band of the extensor mechanism. Immediate reconstruction of the sagittal band was done with a free tendon graft weave. The patient had no evidence of recurrence 7 months later with middle finger MP joint flexion improved to 90°.
Palmar fibromatosis (Dupuytren’s type) is rare in children; only two of the 139 patients described by Stout in his original article on juvenile fibromatoses had Dupuytren’s contracture [26]. Subsequently, there have been isolated reports of its occurrence in children [20, 27]. Progressive finger contracture can be confused with camptodactyly or congenital ulnar drift [27]. The histology of this lesion is microscopically bland, showing dense collagen with little or no mitotic activity. While this condition most often occurs in slightly older children, it has occasionally been noted in the very young [27].
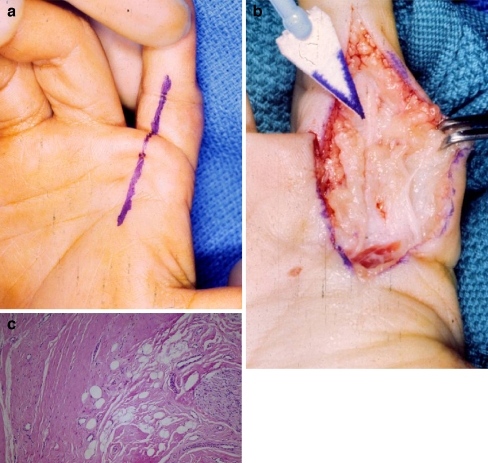
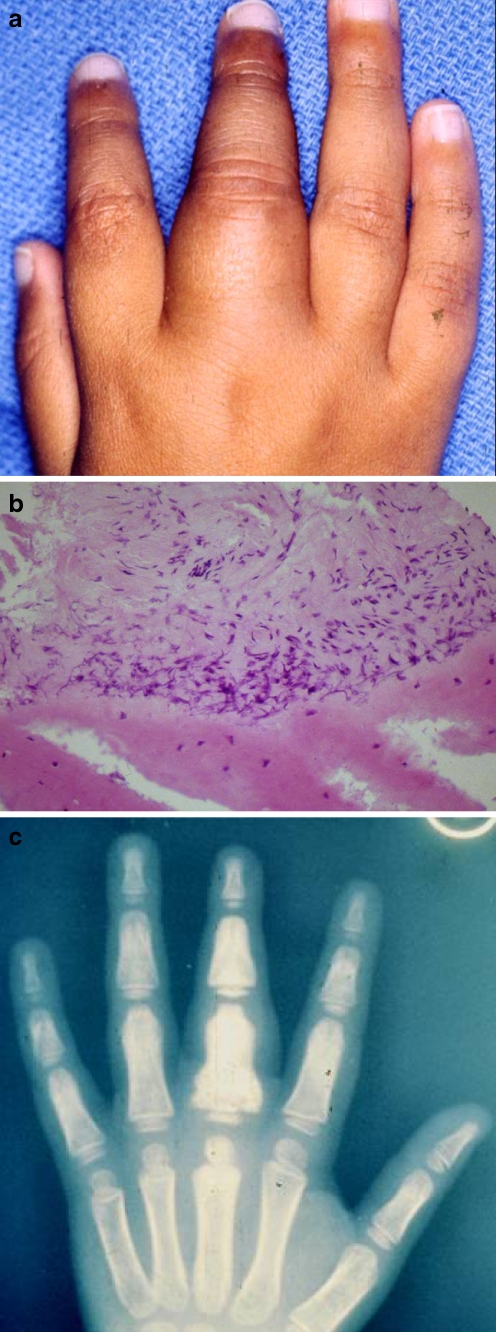
This 5-year-old was referred with a presumptive diagnosis of camptodactyly of the left index finger. The PIP flexion contracture was 60°, and he had extension contractures of both the MP joint of the index finger and of the wrist. This problem was noted by his mother at 9 months of age and had progressively worsened. No palpable masses were present, although the palmar skin was thickened with dimpling and pitting (Fig. 4a). MRI suggested a fibrosing condition involving the left index finger, dorsal wrist, and palmar aponeurosis.
Fig. 4.
a Five-year-old with palmar skin dimpling and flexion and extension contractures of fingers and wrist. b Gross appearance at surgery. Lesion presents as a Dupuytren’s cord. c Palmar fibromatosis, Dupuytren’s type. Note dense collagen deposition (hematoxylin and eosin staining, ×100).
Surgical exploration of the volar index finger and palm revealed longitudinal thickening of the palmar fascia. Thick, longitudinal bands similar to those seen in Dupuytren’s disease were also discovered on the dorsum of the hand and wrist (Fig. 4b). Release of the joint contracture was accomplished by excision of diseased tissue. Extensor tenolysis was required several months later to achieve total restoration of function.
Tumor histology was characteristically bland with dense collagen deposition and little or no mitotic activity in the absence of a clinical history suggestive of wounding or scarring (Fig. 4c). Pathologic examination revealed identical histology on both the palmar and the dorsal aspects of the hand. The scar excised at the secondary surgery showed no histologic evidence of tumor recurrence.
Numerous terms have been used to describe infantile digital fibromatosis (Reye’s tumor), including fibroma durum multiplex, infantile dermal fibromatosis, recurrent digital fibroma, and juvenile dermatofibroma. Reye first described infantile digital fibromatosis in 1955 [24]. Eight percent of infantile digital fibromatoses occur in the first year of life [2]. The condition may occur as a single nodule or in multiple lesions on the fingers or toes, and the thumb and great toe are always spared [11]. Characteristic “kissing” lesions may occur on adjacent surfaces of digits. These lesions may be associated with joint deformities and functional impairment. Recurrence occurs relatively commonly after surgical excision; wide excision reduces the likelihood of recurrence. Lesions may resolve spontaneously, and so, a period of observation before excision is justified. However, frequently, the clinical diagnosis is not appreciated, and the unwitting surgeon may underestimate the significance of a relatively innocuous appearing lesion and so perform inadequate excision.
The histology of infantile digital fibromatosis is characterized by fibroblastic proliferation within a collagenous matrix. Mitotic figures are scant. Eosinophilic, intracytoplasmic inclusion bodies are pathognomonic. Immunohistochemically, the inclusion bodies do not stain with antibodies to vimentin, keratin, or desmin and are thought to be actin contractile filaments [2, 12].
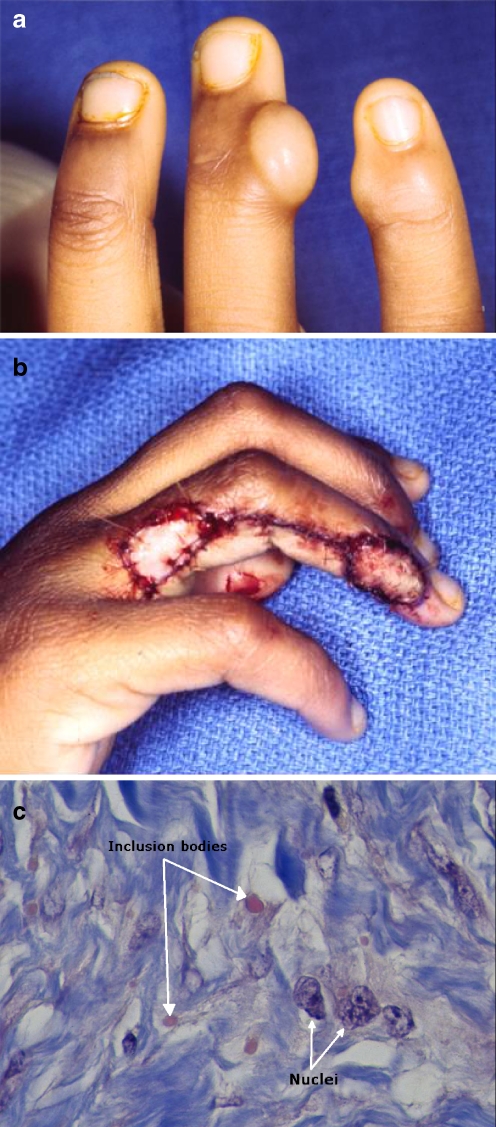
A 6-year-old had undergone excision of a lesion from the ulnar side of the ring finger at the DIP joint 6 months previously with a pathologic diagnosis of infantile digital fibromatosis. She presented to us with recurrence of the ring finger lesion and a new kissing lesion on the radial aspect of the little finger (Fig. 5a). Wide re-excision of the former lesion included resection of the ulnar collateral ligament of the DIP joint; the new lesion was excised. Ring finger wound reconstruction was accomplished with a reverse-flow homodigital island flap and little finger reconstruction with a full thickness skin graft (Fig. 5b). Pathology of the excised lesions showed the characteristics of infantile fibromatosis, the hallmark being intracytoplasmic inclusion bodies within proliferating spindle cells (Fig. 5c).
Fig. 5.
a Six-year-old child with classic kissing lesions of fingers, one new and the other a recurrence. b Wide excision on ring finger reconstructed with homodigital island flap and tumor excision on little finger repaired with full thickness skin graft. c Infantile digital fibromatosis (Reye’s tumor). Note characteristic intracytoplasmic (eosinophilic) inclusion bodies (arrow) (Masson Trichrome, ×1,000).
Fibroma of the tendon sheath occurs in both adults and children, with 20% of cases occurring in children and adolescents [5]. These lesions most commonly affect the fingers, hand, and wrist, with thumb involvement in as many as 41% of patients [5]. Deeply attached to the tendon or tendon sheath, these tumors are usually solitary, with a characteristic lobulated external appearance and usually small, 1–2 cm in diameter. The skin overlying a tendon sheath fibroma, unlike infantile digital fibromatosis lesions, is generally not adherent since the dermis is seldom involved.
Although fibromas of the tendon sheath can be confused with giant cell tumors of the tendon sheath, the giant cell tumor occurs in older patients and contains characteristic macrophages and siderophages, which are not present in the tendon sheath fibroma. Histologically, fibromas of the tendon sheath are composed of a proliferation of dense fibrous stroma with slit-like vascular channels. Fibroblast spindles are stellate-shaped. Cells may be arranged in fascicles. Other histologic features include myxoid change, osteoid change, hyalinization, and scattered multinucleated giant cells [5].
A 12-year-old presented with a 4-month history of a firm, painless, progressively enlarging nodular mass on the volar aspect of the proximal phalanx of the right thumb. The 1.5-cm mass had a lobulated appearance and was non-adherent to the overlying skin but was adherent deeply. Histologic examination on excision showed fibroblastic proliferation with scattered multinucleate giant cells. No foamy macrophages were identified in this lesion. The diagnosis of fibroma of tendon sheath was made.
Melorheostosis or periarticular fibrosis was first described in 1922 [25]. In this lesion, hyperostosis is associated with fibrosis of adjacent soft tissues leading to stiffness and loss of function. It is a condition of mixed clinical and histologic diagnosis. Surgery does not improve function and has little role other than that of diagnostic biopsy.
A 2-year-old child presented with pain and swelling of the proximal phalanx of the middle finger. Because of significant pain and a parent-described inability to move the finger, she was thought to have a “hairline fracture”. When first seen in our facility several weeks later, the child had significant swelling of the digit, minimal residual pain, and obvious stiffness of the finger with limited flexion (Fig. 6a). Radiographs suggested increased sclerosis of the proximal phalanx, but the characteristic radiologic diagnosis was not yet apparent.
Fig. 6.
a Two-year-old with pain and swelling of the middle finger. b Incisional biopsy reveals a bland fibrosis (hematoxylin and eosin staining, ×200). c Melorheostosis or periarticular fibrosis. Note hyperostotic changes and dripping candle wax appearance on plain radiographs.
Incisional biopsy of the mass down to bone revealed a fibrosing tumor of soft tissue (Fig. 6b). Repeat radiographs over the ensuing few months made the diagnosis of melorheostosis more obvious, demonstrating hyperostotic changes of the proximal and middle phalanges and the appearance of dripping candle wax (Fig. 6c). The patient has been followed up for 4 years. Swelling has subsided but was not resolved, and flexion has improved. Radiographs, while still abnormal, show improvement of the middle and proximal phalanges.
While melorheostosis is classified with the sclerosing bone dysplasias, we believe that it warrants inclusion in the clinical differentiation of the fibromatoses. The swelling and periarticular fibrosis may precede the classical bony sclerosis and “dripping candle wax” appearance of radiographs [15]. The fibrosis may involve all layers of soft tissue, extending to the dermis and giving a linear scleroderma-like clinical picture [21].
Discussion
Most of the characteristic juvenile fibromatoses are included in this clinicopathologic synopsis provided by our patient series. While many older terms for the various juvenile fibromatoses or non-malignant fibrosing tumors of the pediatric hand have been discarded, certain histologic types and pathologic diagnoses are characteristic and have stood the test of time. We found examples of most of these juvenile fibromatosis subtypes affecting the hand and fingers in our group of pediatric patients. Each subtype has both a characteristic histologic appearance and clinical features that distinguish one tumor type from another. For example, infantile digital fibromatosis occurs most commonly in young infants and involves fingers and toes; the great toe and thumb are never affected. Fibroma of the tendon sheath, in contrast, occurs in older children and usually involves the thumb.
Lesions of infantile digital fibromatosis probably involute with age. Because these lesions are often nodular finger lesions that appear innocuous, surgeons may underestimate the aggressiveness of their actual clinical behavior, resulting in a high rate of recurrence. Our series of patients included children who developed recurrences after limited local excision. With adequate resection, however, often with a skin graft or local flap for wound closure, the recurrence rate following surgical excision for infantile digital fibromatosis should be low [12].
Infantile myofibromatosis may occur in very young infants and may be quite aggressive; solitary infantile myofibromatosis lesions may, however, eventually involute. Lesions of fibrous hamartoma of infancy tend to occur far less commonly in the hand than in the more proximal limb and in the head and neck [16]. This lesion seldom regresses spontaneously, and post-surgical recurrence is common.
One can usually establish the correct diagnosis through a combination of clinical characteristics—age at onset, site of involvement, and clinical appearance—and specific histological features. Knowledge of the natural history of these lesions guides the development of the optimum treatment strategy. Soft tissue tumors represent 25% of all neoplastic diseases occurring in infants; 85% of these are benign [23]. Infantile fibrosarcoma is one of the most common malignant fibrous tumors of infancy, and it usually occurs on the extremities [23]. It can be present at birth, and patients with infantile fibrosarcoma tend to be 5 years or younger. Infantile fibrosarcomas have a more favorable prognosis compared with adult lesions and are associated with 80% 5-year survival rate, although metastases have been reported up to 17 years after the initial surgical procedure [4]. Incisional biopsy of a suspected juvenile fibromatosis lesion may be necessary to establish the correct diagnosis and rule out malignancy. Surgery is required when non-malignant fibrosing conditions in a child compromise hand function and cause joint contractures.
Clinically, infantile fibrosarcoma presents as a painless, rapidly growing mass. Most of the juvenile fibromatoses present as painless masses. However, two of our patients had pain as a presenting feature—namely juvenile aponeurotic fibroma and fibromatosis associated with melorheostosis. Lesions of infantile fibrous hamartoma can reach particularly large size relative to other fibromatoses. While magnetic resonance imaging is often performed, it is not always helpful in detailing the precise margins of the tumor. Indeed, one of our patients with a palpable lesion in the base of the first webspace had a reportedly “normal” MRI scan.
Given the fact that these young infants may often require sedation to perform an MRI, magnetic resonance scanning is not always recommended. Imaging may already have been done by the time the child is referred. However, where the diagnosis is not clinically obvious and especially for larger lesions, MRI may help in identifying the extent of the lesion and should certainly be done if infantile fibrosarcoma is suspected. However, one cannot absolutely rely on MRI for precise definition of tumor margins since the tumor “blends” into surrounding normal tissue. For the same reason, frozen section is not helpful, and complete resection may be difficult to achieve because it is hard to identify intraoperatively the periphery of the tumor from normal tissue.
Our case series has been very helpful in clarifying clinicopathologic features of juvenile fibromatoses, which can sometimes be a confusing group of “tumors”. We have also tried to rationalize treatment options based on known clinical behavior. It is important to draw the distinction from infantile fibrosarcoma.
Footnotes
Disclaimer: The author had no association or financial support for the research presented in this manuscript and has no conflict of interest to declare.
References
- 1.Baerg J, Murphy JJ, Magee JF. Fibromatosis: clinical and pathological features suggestive of recurrence. J Pediatr Surg 1999;34:1112–4. doi:10.1016/S0022-3468(99)90578-X. [DOI] [PubMed]
- 2.Beckett JH, Jacobs AH. Recurring digital fibrous tumors of childhood. Rev Pediatr 1977;59:401–6. [PubMed]
- 3.Braẽko M, Cindro L, Golouh R. Familial occurrence of infantile myofibromatosis. Cancer 1992;69:1294–9. [DOI] [PubMed]
- 4.Chinyama CN, Roblin P, Watson SJ, et al. Fibromatoses and related tumors of the hand in children. Hand Clin 2000;16:625–35. [PubMed]
- 5.Chung EB, Enzinger FM. Fibroma of tendon sheath. Cancer 1979;44:1945–54. doi:10.1002/1097-0142(197911)44:5<1945::AID-CNCR2820440558>3.0.CO;2-T. [DOI] [PubMed]
- 6.Chung EB, Enzinger FM. Infantile myofibromatosis. Cancer 1981;48:1807–18. doi:10.1002/1097-0142(19811015)48:8<1807::AID-CNCR2820480818>3.0.CO;2-G. [DOI] [PubMed]
- 7.Davies RS, Carty H, Pierro A. Infantile fibromatosis: a review. Br J Radiol 1994;67:619–23. [DOI] [PubMed]
- 8.DeSimone RS, Zielinski CJ. Calcifying aponeurotic fibroma of the hand: a case report. J Bone Joint Surg 2001;83A:586–8. [DOI] [PubMed]
- 9.Enzinger FM. Fibrous hamartoma of infancy. Cancer 1965;18:241–8. doi:10.1002/1097-0142(196502)18:2<241::AID-CNCR2820180216>3.0.CO;2-C. [DOI] [PubMed]
- 10.Enzinger FM, Weiss SW. Soft tissue tumors. 2nd ed. St. Louis: C.V. Mosely; 1988. p. 190–5.
- 11.Enzinger FM, Weiss SW. Fibrous tumors of infancy and childhood. In: Enzinger FM, Weiss SW, editors. Soft tissue tumors. 3rd ed. St. Louis: Mosby; 1995. p. 231.
- 12.Falco NA, Upton J. Infantile digital fibromas. J Hand Surg 1995;20A:1014–20. [DOI] [PubMed]
- 13.Fetsch JF, Miettinen M, Laskin WB, et al. A clinicopathologic study of 45 pediatric soft tissue tumors with an admixture of adipose tissue and fibroblastic elements, and a proposal for classification as lipofibromatosis. Am J Surg Pathol 2000;24:1491–500. doi:10.1097/00000478-200011000-00004. [DOI] [PubMed]
- 14.Goldberg NS, Bauer BS, Kraus H, et al. Infantile myofibromatosis: a review of clinicopathology with perspectives on new treatment choices. Pediatr Dermatol 1988;5:37–46. doi:10.1111/j.1525-1470.1988.tb00882.x. [DOI] [PubMed]
- 15.Goldman RL. The cartilage analogue of fibromatosis (aponeurotic fibroma): further observations based on seven new cases. Cancer 1970;26:1325–31. doi:10.1002/1097-0142(197012)26:6<1325::AID-CNCR2820260620>3.0.CO;2-M. [DOI] [PubMed]
- 16.Goldstein SA, Imbriglia JE. Fibrous hamartoma of the wrist in infancy. J Hand Surg 1986;11A:847–9. [DOI] [PubMed]
- 17.Ishii N, Matsui K, Ichiyama S, et al. A case of infantile digital fibromatosis showing spontaneous regression. Br J Dermatol 1989;121:129–33. doi:10.1111/j.1365-2133.1989.tb01409.x. [DOI] [PubMed]
- 18.Jebson PJ, Louis DS. Fibrous hamartoma of infancy in the hand: a case report. J Hand Surg 1997;22A:740–2. [DOI] [PubMed]
- 19.Keasbey LE. Juvenile aponeurotic fibroma (calcifying fibroma). A distinctive tumor arising in the palms and soles of young children. Cancer 1953;6:338–46. doi:10.1002/1097-0142(195303)6:2<338::AID-CNCR2820060218>3.0.CO;2-M. [DOI] [PubMed]
- 20.Lane JG, Hankin FM. Dupuytren’s contracture in an adolescent. AFP 1988;37:133–6. [PubMed]
- 21.Moreno Alvarez MJ, Lazaro MA, Espado G, et al. Linear scleroderma and melorheostosis: case presentation and literature review. Clin Rheumatol 1996;15:389–93. doi:10.1007/BF02230364. [DOI] [PubMed]
- 22.Netscher DT, Eladoumikdachi F, Popek EJ. Infantile myofibromatosis: case report of a solitary hand lesion with emphasis on differential diagnosis and management. Ann Plast Surg 2001;46:62–7. doi:10.1097/00000637-200101000-00014. [DOI] [PubMed]
- 23.Palumbo JS, Zwerdling T. Soft tissue sarcomas of infancy. Semin Perinatol 1999;23:299–309. doi:10.1016/S0146-0005(99)80038-X. [DOI] [PubMed]
- 24.Reye RDK. Recurrent digital fibrous tumors of childhood. Arch Pathol 1965;80:228–31. [PubMed]
- 25.Sharma R, Burke FD. Melorheostosis of the hand. J Hand Surg 1996;21B:413–5. [DOI] [PubMed]
- 26.Stout PA. Juvenile fibromatoses. Cancer 1954;7:953–78. doi:10.1002/1097-0142(195409)7:5<953::AID-CNCR2820070520>3.0.CO;2-W. [DOI] [PubMed]
- 27.Urban M, Feldberg L, Janssen A, Elliot D. Dupuytren’s disease in children. J Hand Surg 1996;21B:112–6. [DOI] [PubMed]
- 28.Wada H, Akiyama H, Suki H, et al. Spinal canal involvement in infantile myofibromatosis: case report and review of the literature. J Pediatr Hematol Oncol 1998;20:353–6. doi:10.1097/00043426-199807000-00015. [DOI] [PubMed]
- 29.Wiswell TE, Davis J, Cunningham BE, et al. Infantile myofibromatosis: the most common fibrous tumor of infancy. J Pediatr Surg 1988;23:314–8. doi:10.1016/S0022-3468(88)80196-9. [DOI] [PubMed]