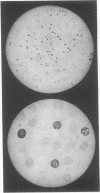
Abstract
An alkaline phosphatase-labeled oligonucleotide DNA probe (CTAP) that was specific for the cholera toxin gene (ctxA) was identified. All cholera toxin-producing strains of Vibrio cholerae, regardless of serotype, hybridized with the CTAP probe, while nontoxigenic strains from either environmental sources or from deletion or substitution mutations did not hybridize. Unlike the whole-gene probes for either ctxA or for the heat-labile toxin or Escherichia coli (eltA), this 23-base sequence did not hybridize with E. coli or with vibrios other than V. cholerae that produce related toxins. By using CTAP to identify colonies grown on nonselective medium, V. cholerae was enumerated at concentrations of 10(3) to 10(7)/g from stool samples of volunteers who had ingested V. cholerae O1 strain 569B. CTAP provides a specific and sensitive tool for diagnosis and environmental monitoring of cholera toxin-producing V. cholerae.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brickman T. J., Boesman-Finkelstein M., Finkelstein R. A., McIntosh M. A. Molecular cloning and nucleotide sequence analysis of cholera toxin genes of the CtxA- Vibrio cholerae strain Texas Star-SR. Infect Immun. 1990 Dec;58(12):4142–4144. doi: 10.1128/iai.58.12.4142-4144.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A. R., Wentz B. A., Hill W. E. Detection of hemolytic Listeria monocytogenes by using DNA colony hybridization. Appl Environ Microbiol. 1987 Sep;53(9):2256–2259. doi: 10.1128/aem.53.9.2256-2259.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge C. W., Sethabutr O., Bodhidatta L., Echeverria P., Robertson D. C., Morris J. G., Jr Use of a synthetic oligonucleotide probe to detect strains of non-serovar O1 Vibrio cholerae carrying the gene for heat-stable enterotoxin (NAG-ST). J Clin Microbiol. 1990 Jun;28(6):1473–1476. doi: 10.1128/jcm.28.6.1473-1476.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Moseley S. L., Falkow S. Molecular characterization of environmental and nontoxigenic strains of Vibrio cholerae. Infect Immun. 1981 May;32(2):661–667. doi: 10.1128/iai.32.2.661-667.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Nataro J. P., Roberts N. C., Siebeling R. J., Bradford H. B. Molecular epidemiology of non-O1 Vibrio cholerae and Vibrio mimicus in the U.S. Gulf Coast region. J Clin Microbiol. 1986 Mar;23(3):652–654. doi: 10.1128/jcm.23.3.652-654.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. T., Peterson J. W., Sarles H. E., Jr, Romanko M., Martin D., Hafkin B. Cholera on the Texas Gulf Coast. JAMA. 1982 Mar 19;247(11):1598–1599. [PubMed] [Google Scholar]
- Levine M. M., Black R. E., Clements M. L., Cisneros L., Saah A., Nalin D. R., Gill D. M., Craig J. P., Young C. R., Ristaino P. The pathogenicity of nonenterotoxigenic Vibrio cholerae serogroup O1 biotype El Tor isolated from sewage water in Brazil. J Infect Dis. 1982 Mar;145(3):296–299. doi: 10.1093/infdis/145.3.296. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Herrington D., Ketley J., Losonsky G., Tacket C. O., Tall B., Cryz S. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet. 1988 Aug 27;2(8609):467–470. doi: 10.1016/s0140-6736(88)90120-1. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Herrington D., Losonsky G., Morris J. G., Clements M. L., Black R. E., Tall B., Hall R. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect Immun. 1988 Jan;56(1):161–167. doi: 10.1128/iai.56.1.161-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Young C. R., Black R. E., Takeda Y., Finkelstein R. A. Enzyme-linked immunosorbent assay to measure antibodies to purified heat-labile enterotoxins from human and porcine strains of Escherichia coli and to cholera toxin: application in serodiagnosis and seroepidemiology. J Clin Microbiol. 1985 Feb;21(2):174–179. doi: 10.1128/jcm.21.2.174-179.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockman H. A., Galen J. E., Kaper J. B. Vibrio cholerae enterotoxin genes: nucleotide sequence analysis of DNA encoding ADP-ribosyltransferase. J Bacteriol. 1984 Sep;159(3):1086–1089. doi: 10.1128/jb.159.3.1086-1089.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockman H., Kaper J. B. Nucleotide sequence analysis of the A2 and B subunits of Vibrio cholerae enterotoxin. J Biol Chem. 1983 Nov 25;258(22):13722–13726. [PubMed] [Google Scholar]
- Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983 Dec 8;306(5943):551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- Minami A., Hashimoto S., Abe H., Arita M., Taniguchi T., Honda T., Miwatani T., Nishibuchi M. Cholera enterotoxin production in Vibrio cholerae O1 strains isolated from the environment and from humans in Japan. Appl Environ Microbiol. 1991 Aug;57(8):2152–2157. doi: 10.1128/aem.57.8.2152-2157.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. G., Jr, Black R. E. Cholera and other vibrioses in the United States. N Engl J Med. 1985 Feb 7;312(6):343–350. doi: 10.1056/NEJM198502073120604. [DOI] [PubMed] [Google Scholar]
- Ogawa A., Kato J., Watanabe H., Nair B. G., Takeda T. Cloning and nucleotide sequence of a heat-stable enterotoxin gene from Vibrio cholerae non-O1 isolated from a patient with traveler's diarrhea. Infect Immun. 1990 Oct;58(10):3325–3329. doi: 10.1128/iai.58.10.3325-3329.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive D. M., Khalik D. A., Sethi S. K. Identification of enterotoxigenic Escherichia coli using alkaline phosphatase-labeled synthetic oligodeoxyribonucleotide probes. Eur J Clin Microbiol Infect Dis. 1988 Apr;7(2):167–171. doi: 10.1007/BF01963071. [DOI] [PubMed] [Google Scholar]
- Ristaino P. A., Levine M. M., Young C. R. Improved GM1-enzyme-linked immunosorbent assay for detection of Escherichia coli heat-labile enterotoxin. J Clin Microbiol. 1983 Oct;18(4):808–815. doi: 10.1128/jcm.18.4.808-815.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira W. M., Fedorka-Cray P. J. Purification of enterotoxins from Vibrio mimicus that appear to be identical to cholera toxin. Infect Immun. 1984 Sep;45(3):679–684. doi: 10.1128/iai.45.3.679-684.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twedt R. M., Madden J. M., Hunt J. M., Francis D. W., Peeler J. T., Duran A. P., Hebert W. O., McCay S. G., Roderick C. N., Spite G. T. Characterization of Vibrio cholerae isolated from oysters. Appl Environ Microbiol. 1981 Jun;41(6):1475–1478. doi: 10.1128/aem.41.6.1475-1478.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Takeda Y., Miwatani T., Craig J. P. Evidence that a non-O1 Vibrio cholerae produces enterotoxin that is similar but not identical to cholera enterotoxin. Infect Immun. 1983 Sep;41(3):896–901. doi: 10.1128/iai.41.3.896-901.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Gojobori T., Yokota T. Evolutionary origin of pathogenic determinants in enterotoxigenic Escherichia coli and Vibrio cholerae O1. J Bacteriol. 1987 Mar;169(3):1352–1357. doi: 10.1128/jb.169.3.1352-1357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]