Abstract
Rationale: Sepsis-related mortality results in part from immunodeficiency secondary to profound lymphoid apoptosis. The biological mechanisms responsible are not understood.
Objectives: Because recent evidence shows that platelets are involved in microvascular inflammation and that they accumulate in lymphoid microvasculature in sepsis, we hypothesized a direct role for platelets in sepsis-related lymphoid apoptosis.
Methods: We studied megakaryocytes and platelets from a murine-induced sepsis model, with validation in septic children, which showed induction of the cytotoxic serine protease granzyme B.
Measurements and Main Results: Platelets from septic mice induced marked apoptosis of healthy splenocytes ex vivo. Platelets from septic granzyme B null (−/−) mice showed no lymphotoxicity.
Conclusions: Our findings establish a conceptual advance in sepsis: Septic megakaryocytes produce platelets with acutely altered mRNA profiles, and these platelets mediate lymphotoxicity via granzyme B. Given the contribution of lymphoid apoptosis to sepsis-related mortality, modulation of platelet granzyme B becomes an important new target for investigation and therapy.
Keywords: blood platelets, sepsis, granzyme B, apoptosis
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
No studies have shown acute changes in platelet mRNA pools as a function of a systemic stimulus, such as experimental or clinical sepsis.
What This Study Adds to the Field
We determined that sepsis induces changes in the megakaryocyte-platelet transcriptional axis and show that the resulting platelets are strongly lymphotoxic. In addition, using platelets from a murine-induced sepsis model, we identified the potent cytotoxic serine protease, granzyme B, as the cause of this lymphotoxicity.
Despite several decades of advances in antimicrobials, critical care, and organ support modalities (1, 2), mortality rates from sepsis have remained largely unchanged at about 40% (3). Sepsis is responsible for 215,000 deaths annually in the United States, which is akin to mortality from acute myocardial infarction (3), making it the 10th leading cause of death (4). A recent paradigm shift indicates that sepsis-related mortality results in part from immunodeficiency secondary to profound lymphoid apoptosis (5). This apoptosis is considered a diagnostic hallmark of progressive sepsis and multiple organ dysfunction. However, the etiology of the apoptosis is unknown.
Studies of sepsis have demonstrated the accumulation of platelets in spleen and other end organs (6, 7). Furthermore, activated platelet-derived microparticles have cytotoxic activity toward vascular endothelium (8–10) and smooth muscle (10). However, platelets are anucleate, having only cytoplasmic components imparted by megakaryocytes residing in the bone marrow, and are incapable of de novo gene transcription. Thus, these previous studies assumed that changes in platelet function were at the posttranscriptional level. Platelets do contain reservoirs of mRNA, and a number of studies have reported the transcriptome of human platelets using mRNA profiling (11–14). It has also been established that platelets regulate translation of their transcriptome in response to external stimuli (15–17). However, no studies have shown acute changes in platelet mRNA pools as a function of a systemic stimulus, such as experimental or clinical sepsis. Therefore, we hypothesized that responses of platelets to systemic perturbations in sepsis could lead to changes in mRNA expression of cell death–associated genes and that this de novo transcriptome would be imparted to platelets by megakaryocytes residing in bone marrow.
Our experiments to test this hypothesis led to two main findings. First, we characterized sepsis-induced changes in the megakaryocyte–platelet transcriptional axis and present a novel finding that the resulting platelets are strongly lymphotoxic. Second, using platelets from a murine-induced sepsis model, we identified the potent cytotoxic serine protease granzyme B as the cause of this lymphotoxicity. Some of the results of these studies have been previously reported in the form of abstracts (18, 19).
METHODS
Animals
Mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed and bred in a conventional animal facility. All experiments were approved by our Institutional Animal Care and Use Committee. Cecal ligation and puncture was performed on male 8- to 12-week-old mice at time = 0 hours, as previously described (20). Briefly, under isoflurane anesthesia with spontaneous ventilation, the cecum was exposed through a 1-cm-long midline abdominal incision, ligated loosely with 4-0 silk ties (Ethicon, Cornelia, GA), and punctured twice proximally with an 18-gauge needle. Fecal material was expressed, and the bowel was replaced in the abdomen. The incision was closed with 4-0 nylon sutures. Mice were resuscitated with 4 ml/100 g of body weight of subcutaneous saline.
Platelet Isolation
Intracardiac blood was drawn directly into sodium citrate (Becton-Dickinson, Franklin Lakes, NJ) and immediately centrifuged at 770 rpm for 10 minutes at 25°C. Platelets were isolated from platelet-rich plasma by a single high-speed centrifugation over Ficoll-Paque Plus (GE Healthcare Bio-Sciences Corporation, Piscataway, NJ). Microscopy of smears of platelet isolates showed >90% platelet purity. Platelets intended for mRNA studies were immediately placed in Trizol (Invitrogen, Carlsbad, CA). Platelets intended for functional studies were filtered through a 10-ml sepharose 2B gel column to remove extraneous proteins as described by Vollmar and colleagues (21). Platelet concentrations were measured using a manual hemocytometer, and concentrations were equalized between samples by diluting with PBS.
Megakaryocyte Isolation
Murine megakaryocytes were isolated from mouse tibial and femoral bone marrow by flushing with Iscove's modified Dulbecco's medium. The resulting marrow suspension was treated and passed through StemSep magnetic gravity columns (StemCell Technologies, Vancouver, BC, Canada) according to the manufacturer's protocol using biotin-labeled anti-CD42d antibodies for positive selection. Purity was confirmed by light microscopy with Wright's stain (Sigma-Aldrich, St. Louis, MO). mRNA was isolated as described for platelets.
Splenectomy
Healthy control spleens were removed and immediately ground through a 40-μm mesh cell strainer. Splenocytes were centrifuged, washed, and layered over Ficoll-Paque Plus (GE Healthcare Bio-Sciences). CD4+ cells were isolated using StemSep magnetic gravity columns according to the manufacturer's protocol.
Expression Profiling
Expression values were calculated using the dChip difference model probe set algorithm (http://biosun1.harvard.edu/complab/dchip/) and Probe Logarithmic Intensity Error Estimation (PLIER) (Affymetrix, Santa Clara, CA) algorithm. dChip and PLIER signals were imported into Hierarchical Clustering Explorer (22), and the resulting unsupervised clusters were examined visually for appropriate grouping of profiles. The signals from the algorithm with the most appropriate profile grouping were used for all subsequent analyses within each species (i.e., murine = dChip, human = PLIER) and imported into GeneSpring GX (Agilent Technologies, Santa Clara, CA). The murine dataset (NCBI GEO Record #GSE10343) and human dataset (NCBI GEO Record #GSE 10,361) were normalized within each chip to the 50th percentile and per gene to control chips. Using the cross-gene error model without multiple testing corrections, one-way ANOVA (P ≤ 0.001) generated a list of differentially expressed probe sets over time.
Quantitative Reverse Transcriptase Polymerase Chain Reaction
cDNA was synthesized using the SuperScript III First-Strand Synthesis System (Invitrogen) per the manufacturer's protocol. DNA primers (Invitrogen) were designed according to known gene sequences as follows: granzyme A (forward) 5′-GAA CCA CTG CTA CTC GGC ATC TGG [FAM]TC-3′; granzyme A (reverse) 5′-CAG AAA TGT GGC TAT CCT TCA CC-3′; granzyme B (forward) 5′-GAC GAT CCT GCT CTG ATT ACC CAT CG[FAM] C-3′; granzyme B (reverse) 5′-TCA GAT CCT GCC ACC TGT CCT A-3′. GAPDH-containing wells served as positive controls, and polymerase-free wells served as as negative controls. Reactions were run using an ABI PRISM 7900HT polymerase chain reaction instrument (Applied Biosystems, Foster City, CA), and relative gene expression levels were calculated using Sequence Detection System 2.2 Software (Applied Biosystems). Expression values were normalized relative to sample GAPDH mRNA expression.
Detection of Apoptosis
CD4+ splenocytes from healthy control mice were coincubated with platelets isolated from control or septic mice for 90 minutes at 37°C and 5% CO2 with or without platelet pretreatment with 10 ng/mL of recombinant tumor necrosis factor (TNF)-α (Sigma-Aldrich) for 90 minutes. Splenocyte apoptosis was evaluated by TiterTACS (Trevigen, Gaithersburg, MDA), a quantitative colorimetric assay for in situ detection of DNA fragmentation. All samples were run in triplicate according to the manufacturer's protocol, with data normalized to negative and nuclease-induced positive controls.
Statistical Analysis
Data were maintained in Microsoft Excel 2007 (Microsoft, Redmond, WA). Statistical significance was tested with SPSS 15 (SPSS, Chicago, IL) using paired or unpaired t tests. Results are reported as mean ± SEM) unless otherwise specified.
RESULTS
Sepsis Induces Platelet Cell Death Gene Expression
All mice that underwent cecal ligation and puncture (CLP) developed signs and symptoms consistent with peritoneal sepsis, including decreased grooming, lethargy, and gross pathologic peritonitis at time of death. These mice developed significant weight loss over 48 hours (mean ± SEM0h versus 48h: −14.8 ± 1.6%; P < 0.0001). Fourteen out of the 96 mice studied (14.6%) died between 6 and 48 hours status after CLP and were not included in the final analyses.
Expression profiles (Mouse 430 plus 2.0 GeneChips; Affymetrix) of platelet mRNA pooled from five mice at each time point (0-naive, 24-, and 48-h status after CLP) showed 59 probe sets, representing 56 unique genes, that were differentially regulated over the time interval studied (Table 1). These genes were primarily related to gene ontology biological process groups previously well described in the response to sepsis: cell adhesion, cell growth regulation, chemotaxis, inflammatory and immune responses, proteolysis, and signal transduction. Of these, six probe sets belonged to the gene ontology molecular function group for cell death (GO:0008219). In particular, between 0- and 48-hour granzymes A and B, potent cytotoxic serine proteases were > 100-fold up-regulated (fold change, 549.6 and 141.3, respectively).
TABLE 1.
DIFFERENTIALLY REGULATED PROBE SETS (N = 59) BETWEEN 0-HOUR CONTROL MICE AND SEPTIC MICE AT 24- AND 48-HOUR STATUS AFTER CECAL LIGATION AND PUNCTURE
| Affymetrix Probe Set ID | 24-h Fold Change | 48-h Fold Change | Genbank ID | Gene Symbol | Gene Name |
|---|---|---|---|---|---|
| 1427747_a_at | 1593.0 | 540.1 | X14607 | Lcn2 | lipocalin 2 |
| 1440865_at | 276.4 | 202.3 | BB193024 | Ifitm6 | interferon-induced transmembrane protein 6 |
| 1419764_at | 189.6 | 181.6 | NM_009892 | Chi3l3 | chitinase 3-like 3 |
| 1442339_at | 185.7 | 606.5 | BB667930 | MGI:3524944 | stefin A2 like 1 |
| 1417898_a_at* | 156.7 | 549.6 | NM_010370 | Gzma | granzyme A |
| 1418809_at | 153.0 | 530.7 | NM_011087 | Pira1 | paired-Ig-like receptor A1 |
| 1449984_at | 137.5 | 206.3 | NM_009140 | Cxcl2 | chemokine (C-X-C motif) ligand 2 |
| 1451563_at | 128.4 | 1831.0 | AF396935 | Emr4 | EGF-like module containing, mucin-like, hormone receptor-like sequence 4 |
| 1456250_x_at | 126.3 | 324.6 | BB533460 | Tgfbi | transforming growth factor, beta induced |
| 1422013_at | 120.3 | 759.8 | NM_011999 | Clec4a2 | C-type lectin domain family 4, member a2 |
| 1436530_at | 109.6 | 287.0 | AA666504 | CDNA clone MGC:107680 IMAGE:6766535 | |
| 1450826_a_at | 104.9 | 524.0 | NM_011315 | Saa3 | serum amyloid A 3 |
| 1419394_s_at | 104.6 | 48.6 | NM_013650 | S100a8 | S100 calcium binding protein A8 (calgranulin A) |
| 1424254_at | 98.9 | 86.1 | BC027285 | Ifitm1 | interferon-induced transmembrane protein 1 |
| 1442798_x_at | 93.7 | 104.1 | BB324660 | Hk3 | hexokinase 3 |
| 1456223_at | 93.2 | 246.8 | BF322016 | Transcribed locus | |
| 1416635_at | 83.0 | 683.2 | NM_020561 | Smpdl3a | sphingomyelin phosphodiesterase, acid-like 3A |
| 1437478_s_at | 81.2 | 200.3 | AA409309 | Efhd2 | EF hand domain containing 2 |
| 1422953_at | 77.8 | 62.0 | NM_008039 | Fpr-rs2 | formyl peptide receptor, related sequence 2 |
| 1436202_at | 76.1 | 160.7 | AI853644 | Malat1 | metastasis associated lung adenocarcinoma transcript 1 (non-coding RNA) |
| 1419709_at | 71.8 | 314.9 | NM_025288 | Stfa3 | stefin A3 |
| 1450808_at | 68.2 | 125.5 | NM_013521 | Fpr1 | formyl peptide receptor 1 |
| 1430700_a_at | 67.7 | 309.5 | AK005158 | Pla2g7 | phospholipase A2, group VII (platelet-activating factor acetylhydrolase, plasma) |
| 1448756_at | 67.5 | 23.5 | NM_009114 | S100a9 | S100 calcium binding protein A9 (calgranulin B) |
| 1420331_at | 65.8 | 251.8 | NM_019948 | Clec4e | C-type lectin domain family 4, member e |
| 1420330_at | 64.9 | 220.2 | NM_019948 | Clec4e | C-type lectin domain family 4, member e |
| 1423346_at | 63.2 | 272.6 | AV286991 | Degs1 | degenerative spermatocyte homolog 1 (Drosophila) |
| 1418722_at | 62.4 | 28.6 | NM_008694 | Ngp | neutrophilic granule protein |
| 1429900_at | 62.0 | 286.4 | BM241296 | 5330406M23Rik | RIKEN cDNA 5330406M23 gene |
| 1434773_a_at | 57.7 | 137.9 | BM207588 | Slc2a1 | solute carrier family 2 (facilitated glucose transporter), member 1 |
| 1420671_x_at | 57.0 | 413.4 | NM_029499 | Ms4a4c | membrane-spanning 4-domains, subfamily A, member 4C |
| 1419598_at | 55.4 | 276.6 | NM_026835 | Ms4a6d | membrane-spanning 4-domains, subfamily A, member 6D |
| 1421392_a_at | 53.8 | 140.5 | NM_007464 | Birc3 | baculoviral IAP repeat-containing 3 |
| 1418189_s_at | 53.2 | 197.8 | AF146523 | Malat1 | metastasis associated lung adenocarcinoma transcript 1 (noncoding RNA) |
| 1435761_at | 51.2 | 322.8 | AW146083 | Stfa3 | stefin A3 |
| 1419599_s_at | 49.6 | 362.9 | NM_026835 | Ms4a11 | membrane-spanning 4-domains, subfamily A, member 11 |
| 1421408_at | 49.3 | 246.7 | NM_030691 | Igsf6 | immunoglobulin superfamily, member 6 |
| 1418204_s_at | 46.1 | 282.2 | NM_019467 | Aif1 | allograft inflammatory factor 1 |
| 1420394_s_at | 40.3 | 89.0 | U05264 | Gp49a; Lilrb4 | glycoprotein 49 A; leukocyte immunoglobulin-like receptor, subfamily B, member 4 |
| 1416530_a_at | 39.0 | 168.2 | BC003788 | Pnp | purine-nucleoside phosphorylase |
| 1437584_at | 38.8 | 158.8 | BE685667 | Transcribed locus | |
| 1419647_a_at | 38.6 | 109.6 | NM_133662 | Ier3 | immediate early response 3 |
| 1419060_at | 35.2 | 141.3 | NM_013542 | Gzmb | granzyme B |
| 1448123_s_at | 33.9 | 129.3 | NM_009369 | Tgfbi | transforming growth factor, beta induced |
| 1429954_at | 28.7 | 245.8 | AK014135 | Clec4a3 | C-type lectin domain family 4, member a3 |
| 1448061_at | 27.9 | 204.0 | AA183642 | Msr1 | macrophage scavenger receptor 1 |
| 1438943_x_at | 27.7 | 136.2 | AV308148 | Rpn1 | ribophorin I |
| 1439057_x_at | 23.3 | 292.2 | BB143557 | Zdhhc6 | zinc finger, DHHC domain containing 6 |
| 1448620_at | 22.2 | 77.9 | NM_010188 | Fcgr3 | Fc receptor, IgG, low affinity III |
| 1455899_x_at | 21.4 | 88.3 | BB241535 | Socs3 | suppressor of cytokine signaling 3 |
| 1447277_s_at | 20.9 | 630.1 | BB785407 | Pcyox1 | prenylcysteine oxidase 1 |
| 1419209_at | 20.5 | 407.7 | NM_008176 | Cxcl1 | chemokine (C-X-C motif) ligand 1 |
| 1433699_at | 17.7 | 58.8 | BM241351 | Tnfaip3 | tumor necrosis factor, alpha-induced protein 3 |
| 1455908_a_at | 16.3 | 212.3 | AV102733 | Scpep1 | serine carboxypeptidase 1 |
| 1457666_s_at | 14.8 | 67.8 | AV229143 | Ifi202b | interferon activated gene 202B |
| 1427076_at | 12.9 | 91.1 | L20315 | Mpeg1 | macrophage expressed gene 1 |
| 1420249_s_at | 8.8 | 94.7 | AV084904 | Ccl6 | chemokine (C-C motif) ligand 6 |
| 1416382_at | 6.1 | 101.0 | NM_009982 | Ctsc | cathepsin C |
| 1449193_at | 2.5 | 66.9 | NM_009690 | Cd5l | CD5 antigen-like |
Cell death (GO:0008219) genes (n = 6) are noted in bold.
We explored expression of these cell death genes in human sepsis in an Institutional Review Board–approved study of septic children (n = 17) between the ages of 1 and 18 years (8.8 ± 1.3 years). Nine participants (53%) were male. The diagnosis of sepsis was made using criteria adapted for pediatrics from the consensus definitions for sepsis (23–25). We collected clinical and laboratory data (i.e., the most extreme value in the previous 24 hours) over 72 hours. Relative clinical severity was determined by unsupervised clustering of all raw clinical and laboratory data in Hierarchical Clustering Explorer (http://www.cs.umd.edu/hcil/hce/) (Figure 1). The participants clearly clustered into two groups by clinical and laboratory variables. Group 1 (n = 6) was designated “severe” because it had significantly higher severity of illness scores (i.e., mean PRISM III (26) score [17.0 ± 2.7 vs. 4.5 ± 1.1; P < 0.001] and longer hospital length of stay [45.5 ± 10.6 vs. 13.7 ± 2.8 d; P = 0.029]). Group 2 (n = 11) was designated “moderate” and was not significantly different from the severe group for other analyzed outcome variables, including mortality and presence of shock.
Figure 1.
Classification of sepsis severity via unsupervised clustering of comprehensive clinical and laboratory data. Data collected over 72 hours on children and adolescents (n = 17) admitted to our tertiary care pediatric ICU with a presumed diagnosis of sepsis were input into Hierarchical Clustering Explorer. Variables input included the following at 0, 24, 48, and 72 hours: temperature; heart rate; respiratory rate; systolic, diastolic, and mean arterial blood pressure; Glasgow Coma Score; blood pH, Pco2, O2, and base excess; white blood cell count; absolute neutrophil, lymphocyte, and monocytes counts; blood hemoglobin and platelet count; prothrombin and activated partial thromboplastin times; serum sodium, potassium, chloride, glucose, creatinine; and blood urea nitrogen. Similarities between these phenotypes are reflected in the cluster shown with shorter bars representing more similarity. These results were used to classify the septic participants as severe (n = 6) and moderate (n = 7) as shown by the overlaid boxes.
As preliminary validation of the murine data, platelet mRNA from one exemplary severe and one exemplary moderate septic human subject was profiled using Human U133A GeneChips (Affymetrix) and compared with platelet gene expression in three healthy young adult control subjects. There was no intent to conduct a statistically robust genome-wide assessment on this small group of samples; rather, we focused on a cross-species screening for the six cell death genes identified in the murine study. Of those, only granzyme B was differentially regulated over 72 h (2.9-fold increase) in the severe subject. None of the other cell death genes studied showed differential expression in either group.
Validation of Sepsis-Induced Changes in the Megakaryocyte–Platelet Transcriptional Axis
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) was used to validate the murine platelet granzyme A and B up-regulation detected by microarray. We studied only the first 24 hours after induction of sepsis because the bulk of granzyme up-regulation seen by microarray occurred during this period. In an independent cohort of septic mice (n = 12; three mice per time point, nonpooled), granzyme B mRNA expression significantly increased from 0 to 24 hours (mean ± SE0h versus 24h: 0.77 ± 0.61 vs. 11.94 ± 3.65; P = 0.04) (Figure 2). The expression of granzyme A mRNA was not significantly increased over that same time (mean ± SE0h versus 24h: 1.57 ± 2.73 vs. 2.61 ± 4.53; P = 0.11).
Figure 2.
Platelet granzyme B mRNA expression reflects megakaryocyte expression in septic mice. Platelets do not have transcriptional machinery; therefore, changes in platelet granzyme B mRNA expression in septic mice (n = 12) were measured simultaneously in autologous megakaryocytes. Results of this quantitative reverse transcriptase polymerase chain reaction analysis show good correlation between increasing megakaryocyte and platelet granzyme B mRNA expression over time.
Because platelets are anucleate and lack transcriptional machinery, we hypothesized that increased platelet granzyme B mRNA expression in sepsis could be further validated by simultaneous measurement in autologous megakaryocytes. Using qRT-PCR, we measured platelet granzyme B mRNA expression in bone marrow megakaryocytes simultaneously acquired from the mice used in the platelet qRT-PCR validation step. Megakaryocyte granzyme B mRNA relative expression increased significantly by 24 hours (mean ± SE0h versus 24h: 2.88 ± 0.27 vs. 8.25 ± 0.52; P = 0.05). Platelet granzyme B mRNA expression over time closely followed that of megakaryocytes (Figure 2). Megakaryocyte granzyme A mRNA expression did not change (mean ± SEM0h versus 24h: 3.18 ± 0.54 vs. 2.99 ± 0.12; P = 0.42).
Sepsis Induces Platelet Granzyme B Protein Expression
To determine if granzyme B mRNA up-regulation translates to increased granzyme B protein expression, additional citrated whole blood was collected from septic and control mice. The blood was fixed with 1% paraformaldehyde, permeabilized, and intracellularly stained with anti-granzyme B (clone 16G6; eBioscience, San Diego, CA) using appropriate isotype and negative (unlabeled) control mice. Flow cytometry data were generated on a FACSCalibur System (BD Biosciences, San Jose, CA), gating on CD61+ (clone 2C9.G2; BD) platelets, and analyzed using FlowJo 7.2 (Tree Star, Inc., Ashland, OR). Platelets from septic mice (n = 9) showed an increase in intracellular granzyme B protein expression after 24 hours (mean ± SEM0h versus 24h: 4.4 ± 1.3 vs. 19.6 ± 6.3%; P = 0.039). Additional platelet activation with TNF-α did not alter intracellular granzyme B (data not shown).
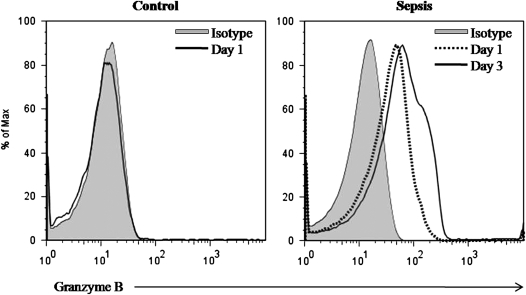
In a cross-species validation step, citrated whole blood from septic and healthy children was studied in a similar manner. In this case, flow cytometry data were generated on CD61+ (clone VI-PL2; BD) platelets stained for intracellular granzyme B (clone GB11; BD). Granzyme B was measured in one “severe” and in three “moderate” subjects 1 and 3 days after subjects were admitted for sepsis and compared with similarly aged healthy control children (n = 10) having blood drawn for routine testing. Platelets from the severe subject expressed intracellular granzyme B at Day 1 (49.7%) and Day 3 (44.3%) (Figure 3). Only one of the moderate septic subjects expressed granzyme B and only at Day 3 (24.0%). There was no measurable intracellular granzyme B in platelets from the control subjects. Platelet activation state (i.e., CD62P+) did not affect granzyme B expression. Furthermore, we did not detect surface expression of other apoptosis inducing proteins (i.e., Fas ligand, IL1β, TNF-α, and TNF-related apoptosis-inducing ligand) on platelets from the septic children.
Figure 3.
Flow cytometric measurement of intracellular granzyme B expression in platelets from septic and healthy children. Citrated whole blood was gated on CD61+ platelets. Intracellular granzyme B was measured in healthy children (n = 10) and septic children, whom we classified as severe (n = 1) and moderate (n = 3) 1 and 3 days after admission. Shown are results from the child with severe disease showing platelet granzyme B expression at Day 1 (49.7%) and Day 3 (44.3%) compared with the isotype control. Only one of the moderate septic subjects expressed granzyme B and only at Day 3 (24.0%). There was no measurable intracellular granzyme B in platelets from the control subjects.
Platelets Are Lymphotoxic Effectors in Sepsis via Granzyme B
Our finding of granzyme B in platelets from septic mice and humans led us to hypothesize that platelets could be lymphotoxic in this scenario. To study this question, platelets from mice 18 hours after CLP were coincubated with CD4+ splenocytes isolated from healthy control mice. Platelets from septic wild-type (i.e., C57BL6) mice induced marked splenocyte apoptosis compared with platelets from sham wild-type mice (rate of apoptosis, 26.0 ± 3.4 vs. 3.9 ± 3.4%; P = 0.007) (Figure 4). This coincubation experiment was repeated with platelets from septic granzyme B null (−/−) mice (i.e., B6.129S2-Gzmbtm1Ley). In this case, there was a complete lack of induced splenocyte apoptosis by septic platelets. Wild-type platelets further activated by TNF-α had no more lymphotoxicity (4.5 ± 1.3%; P = 0.88) than nonactivated control platelets (Figure 4).
Figure 4.
Platelets harvested from septic mice induce apoptosis in control CD4+ splenocytes except in the absence of granzyme B. Percent apoptosis was significantly higher in splenocytes coincubated with platelets harvested from septic wild-type (WT) (i.e., C57BL6) mice than with platelets from healthy wild-type mice and splenocytes without platelets. Repeat experiments with platelets from septic granzyme B null (−/−) mice (i.e., B6.129S2-Gzmbtm1Ley) showed a lack of induced splenocyte apoptosis. Further platelet activation with recombinant tumor necrosis factor–α under any of these conditions did not alter lymphotoxic capacity.
DISCUSSION
Sepsis-related mortality results in part from immunodeficiency secondary to profound lymphoid apoptosis (1, 2, 27–30). The biological mechanisms responsible for this extensive lymphocyte cell death are not understood but have been attributed in part to direct pathogen signaling through toll-like receptors and MyD88 (31). In these studies, we explored the possibility that platelets play a direct role in this process by conducting time series studies in a murine experimental model of sepsis. Microarrays were used as an initial screening tool to hypothesize that responses of platelets to systemic perturbations in sepsis could lead to changes in mRNA expression of cell death-associated genes. This model was then tested through a series of mouse and human studies. Our experiments led us to characterize sepsis-induced changes in the megakaryocyte–platelet transcriptional axis and present a novel finding that the resulting platelets are strongly lymphotoxic. Second, using platelets from a murine-induced sepsis model, we identified the serine protease, granzyme B, as the cause of this lymphotoxicity.
The granzymes are a group of cytotoxic serine proteases that are most commonly secreted within cytotoxic granules by natural killer and cytotoxic T lymphocytes (32). Granzyme B is the most well characterized of these proteases (the other human granzymes include A, H, K, and M) and has multiple known caspase targets and a growing list of caspase-independent substrates, including poly(ADP-ribose) polymerase (33) and fibroblast growth factor receptor-1 (34). Granzyme B typically enters target cells through a channel of co-released perforin (35) but can also enter independently (36–38). Once in the target cell cytoplasm granzyme B cleaves several intracellular proapoptotic cysteine proteases, the most prominent and best-studied being caspase 3 (35). Alternatively, granzyme B has been shown to induce apoptosis via Bid-induced mitochondrial damage (39–41). Granzyme B has been shown to induce cell death by caspase- and noncaspase-mediated mechanisms simultaneously (34, 42). In addition, Wong and colleagues showed that granzyme B is among the transcripts up-regulated in whole blood from pediatric septic shock nonsurvivors compared with survivors (43).
Our experiments showed that platelets are strongly lymphotoxic due to granzyme B in sepsis. Our results build upon previous research demonstrating significant interregulatory interactions between platelets and lymphocytes in a variety of inflammatory disease states, particularly with respect to adaptive immunity. For instance, platelet CD40 has been shown to bind to T lymphocyte CD40 ligand, thereby inducing platelet release of CCL5, which further activates T lymphocytes and thus amplifies the immune response (44). In particular in sepsis, platelet-derived microparticles have been shown to be cytotoxic against vascular endothelium (8–10) and smooth muscle (10). To our knowledge, ours is the first study to examine acute changes in the platelet transcriptome in response to a disease insult. We found that megakaryocytes in the bone marrow respond to systemic sepsis and alter the transcriptome of platelets to include granzyme B.
The presence of granzyme B in platelets in sepsis raises intriguing questions, especially in light of the fact that platelet activation does not seem to affect its expression, implying there is no posttranscriptional regulation. First, it is possible that granzyme B serves a role in megakaryocyte caspase activation, which is critical for normal platelet formation (45). If so, it is possible that in the hyperthrombopoiesis of sepsis megakaryocyte up-regulation of granzyme B mRNA results in inclusion of this transcript in platelets. An alternative is that platelet granzyme B represented an evolutionary advantage at some point. Granzyme B's ability to induce apoptosis through a wide variety of mechanisms makes it a likely mechanism to circumvent the immune evasion strategies of intracellular pathogens. In fact, there is evidence that granzyme B from cytotoxic T cells may play a role in defense against Toxoplasma gondii and Plasmodium species (46, 47).
We acknowledge the following limitations to this study. First, the use of pooled platelets for the murine GeneChips and the use of a single GeneChip for each murine and human phenotype may be questioned. We argue that as a screening tool, these microarray experiments were useful in hypothesizing granzyme B's up-regulation, which then required extensive validation. Therefore, we conducted repeated validation steps for granzyme B, including platelet and megakaryocyte qRT-PCR and cross-species protein level measurement via flow cytometry. These validations make a false-positive microarray result for granzyme B extremely unlikely. Second, it is reasonable to question the effect of thrombocytopenia on platelet lymphotoxicity in sepsis. Thrombocytopenia is often a complication of sepsis and is associated with poor outcomes (48). Logically, fewer circulating platelets would lead to less induced apoptosis. However, sepsis-associated thrombocytopenia occurs in large part as a result of intense platelet activation leading to increased platelet–endothelium interactions. Thus, platelet sequestration in the microvascular endothelium, although associated with circulating thrombocytopenia, results in increased inflammatory interactions with immune cells and endothelium (48). This places them in close proximity to splenic lymphoid tissue, in which the bulk of lymphoid apoptosis occurs.
In summary, we conclude that platelets up-regulate granzyme B in murine and human sepsis. We show that platelets from septic mice induced marked apoptosis of healthy splenocytes ex vivo via granzyme B action. Our findings establish a conceptual advance in sepsis: Septic megakaryocytes produce platelets with acutely altered mRNA profiles, and these platelets mediate lymphotoxicity via granzyme B. Given the contribution of lymphoid apoptosis to sepsis-related mortality, modulation of platelet granzyme B becomes an important new target for investigation and therapy.
Acknowledgments
The authors acknowledge the advice of Kanneboyina Nagaraju, Ph.D., D.V.M.; Robert Munford, M.S., M.D.; James M. Chamberlain, M.D.; and Stephen J. Teach, M.D., M.P.H. during the preparation of this manuscript.
Supported by grants K23RR020069, R24HD050846 (Integrated Molecular Core for Rehabilitation Medicine), and M01RR020359–010049 from the National Institutes of Health and from the Board of Visitors and Research Advisory Council of Children's National Medical Center.
Originally Published in Press as DOI: 10.1164/rccm.200807-1085OC on January 8, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003;348:138–150. [DOI] [PubMed] [Google Scholar]
- 2.Russell JA. Management of sepsis. N Engl J Med 2006;355:1699–1713. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–1310. [DOI] [PubMed] [Google Scholar]
- 4.Kochanek KD, Smith BL. Deaths: preliminary data for 2002. Natl Vital Stat Rep 2004;52:1–47. [PubMed] [Google Scholar]
- 5.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol 2006;6:813–822. [DOI] [PubMed] [Google Scholar]
- 6.Shibazaki M, Nakamura M, Endo Y. Biphasic, organ-specific, and strain-specific accumulation of platelets induced in mice by a lipopolysaccharide from Escherichia coli and its possible involvement in shock. Infect Immun 1996;64:5290–5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drake TA, Cheng J, Chang A, Taylor FB Jr. Expression of tissue factor, thrombomodulin, and e-selectin in baboons with lethal escherichia coli sepsis. Am J Pathol 1993;142:1458–1470 (published erratum appears in Am J Pathol 1993;143:649). [PMC free article] [PubMed] [Google Scholar]
- 8.Azevedo LC, Janiszewski M, Soriano FG, Laurindo FR. Redox mechanisms of vascular cell dysfunction in sepsis. Endocr Metab Immune Disord Drug Targets 2006;6:159–164. [DOI] [PubMed] [Google Scholar]
- 9.Gambim MH, Carmo AO, Marti L, Verissimo-Filho S, Lopes LR, Janiszewski M. Platelet-derived exosomes induce endothelial cell apoptosis through peroxynitrite generation: experimental evidence for a novel mechanism of septic vascular dysfunction. Crit Care 2007;11:R107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janiszewski M, Do Carmo AO, Pedro MA, Silva E, Knobel E, Laurindo FR. Platelet-derived exosomes of septic individuals possess proapoptotic NAD(P)H oxidase activity: a novel vascular redox pathway. Crit Care Med 2004;32:818–825. [DOI] [PubMed] [Google Scholar]
- 11.Raghavachari N, Xu X, Harris A, Villagra J, Logun C, Barb J, Solomon MA, Suffredini AF, Danner RL, Kato G, et al. Amplified expression profiling of platelet transcriptome reveals changes in arginine metabolic pathways in patients with sickle cell disease. Circulation 2007;115:1551–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dittrich M, Birschmann I, Pfrang J, Herterich S, Smolenski A, Walter U, Dandekar T. Analysis of sage data in human platelets: features of the transcriptome in an anucleate cell. Thromb Haemost 2006;95:643–651. [PubMed] [Google Scholar]
- 13.Hillmann AG, Harmon S, Park SD, O'Brien J, Shields DC, Kenny D. Comparative RNA expression analyses from small-scale, single-donor platelet samples. J Thromb Haemost 2006;4:349–356. [DOI] [PubMed] [Google Scholar]
- 14.Ouwehand WH. Platelet genomics and the risk of atherothrombosis. J Thromb Haemost 2007;5:188–195. [DOI] [PubMed] [Google Scholar]
- 15.Weyrich AS, Denis MM, Schwertz H, Tolley ND, Foulks J, Spencer E, Kraiss LW, Albertine KH, McIntyre TM, Zimmerman GA. mTOR-dependent synthesis of Bcl-3 controls the retraction of fibrin clots by activated human platelets. Blood 2007;109:1975–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weyrich AS, Dixon DA, Pabla R, Elstad MR, McIntyre TM, Prescott SM, Zimmerman GA. Signal-dependent translation of a regulatory protein, Bcl-3, in activated human platelets. Proc Natl Acad Sci USA 1998;95:5556–5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmerman GA, Weyrich AS. Signal-dependent protein synthesis by activated platelets: new pathways to altered phenotype and function. Arterioscler Thromb Vasc Biol 2008;28:S17–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freishtat RJ, Natale J, Cohen JS, Mojgani B, Benton AS, Ngor WM, Bradbury M, Bunaj C, Hoffman EP. Platelets as cytotoxic mediators in sepsis: platelet granzyme B induces lymphocyte apoptosis. J Investig Med 2008;56:807–808. [Google Scholar]
- 19.Freishtat RJ, Natale J, Cohen JS, Sachdeva R, De Biase LM, Ngor E, Degnan A, Benton AS, Chow M, Kristosturyan E, et al. Platelet granzyme B expression is increased in sepsis: a potential mechanism for sepsis-associated endothelial apoptosis. FASEB J 2007;21:A1125. [Google Scholar]
- 20.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock: a review of laboratory models and a proposal. J Surg Res 1980;29:189–201. [DOI] [PubMed] [Google Scholar]
- 21.Vollmar B, Slotta JE, Nickels RM, Wenzel E, Menger MD. Comparative analysis of platelet isolation techniques for the in vivo study of the microcirculation. Microcirculation 2003;10:143–152. [DOI] [PubMed] [Google Scholar]
- 22.Seo J, Bakay M, Chen YW, Hilmer S, Shneiderman B, Hoffman EP. Interactively optimizing signal-to-noise ratios in expression profiling: project-specific algorithm selection and detection p-value weighting in affymetrix microarrays. Bioinformatics 2004;20:2534–2544. [DOI] [PubMed] [Google Scholar]
- 23.Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest 1992;101:1481–1483. [DOI] [PubMed] [Google Scholar]
- 24.Proulx F, Fayon M, Farrell CA, Lacroix J, Gauthier M. Epidemiology of sepsis and multiple organ dysfunction syndrome in children. Chest 1996;109:1033–1037. [DOI] [PubMed] [Google Scholar]
- 25.Proulx F, Gauthier M, Nadeau D, Lacroix J, Farrell CA. Timing and predictors of death in pediatric patients with multiple organ system failure. Crit Care Med 1994;22:1025–1031. [DOI] [PubMed] [Google Scholar]
- 26.Pollack MM, Patel KM, Ruttimann UE. Prism III: an updated pediatric risk of mortality score. Crit Care Med 1996;24:743–752. [DOI] [PubMed] [Google Scholar]
- 27.Hotchkiss RS, Tinsley KW, Karl IE. Role of apoptotic cell death in sepsis. Scand J Infect Dis 2003;35:585–592. [DOI] [PubMed] [Google Scholar]
- 28.Groesdonk HV, Wagner F, Hoffarth B, Georgieff M, Senftleben U. Enhancement of nf-{kappa}b activation in lymphocytes prevents t cell apoptosis and improves survival in murine sepsis. J Immunol 2007;179:8083–8089. [DOI] [PubMed] [Google Scholar]
- 29.Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol 2005;174:5110–5118. [DOI] [PubMed] [Google Scholar]
- 30.Wesche DE, Lomas-Neira JL, Perl M, Chung CS, Ayala A. Leukocyte apoptosis and its significance in sepsis and shock. J Leukoc Biol 2005;78:325–337. [DOI] [PubMed] [Google Scholar]
- 31.Peck-Palmer OM, Unsinger J, Chang KC, Davis CG, McDunn JE, Hotchkiss RS. Deletion of MyD88 markedly attenuates sepsis-induced T and B lymphocyte apoptosis but worsens survival. J Leukoc Biol 2008:83:1009–1018. [DOI] [PubMed] [Google Scholar]
- 32.Masson D, Tschopp J. A family of serine esterases in lytic granules of cytolytic T lymphocytes. Cell 1987;49:679–685. [DOI] [PubMed] [Google Scholar]
- 33.Froelich CJ, Hanna WL, Poirier GG, Duriez PJ, D'Amours D, Salvesen GS, Alnemri ES, Earnshaw WC, Shah GM. Granzyme B/perforin-mediated apoptosis of Jurkat cells results in cleavage of poly(ADP-ribose) polymerase to the 89-kDa apoptotic fragment and less abundant 64-kda fragment. Biochem Biophys Res Commun 1996;227:658–665. [DOI] [PubMed] [Google Scholar]
- 34.Loeb CR, Harris JL, Craik CS. Granzyme B proteolyzes receptors important to proliferation and survival, tipping the balance toward apoptosis. J Biol Chem 2006;281:28326–28335. [DOI] [PubMed] [Google Scholar]
- 35.Trapani JA, Jans DA, Jans PJ, Smyth MJ, Browne KA, Sutton VR. Efficient nuclear targeting of granzyme B and the nuclear consequences of apoptosis induced by granzyme B and perforin are caspase-dependent, but cell death is caspase-independent. J Biol Chem 1998;273:27934–27938. [DOI] [PubMed] [Google Scholar]
- 36.Choy JC, Hung VHY, Hunter AL, Cheung PK, Motyka B, Goping IS, Sawchuk T, Bleackley RC, Podor TJ, McManus BM, et al. Granzyme B induces smooth muscle cell apoptosis in the absence of perforin: involvement of extracellular matrix degradation. Arterioscler Thromb Vasc Biol 2004;24:2245–2250. [DOI] [PubMed] [Google Scholar]
- 37.Florian CK, Martin K, Edward F, Klaus D, Rainer R, Hauke L, Dieter EJ. Killing of target cells by redirected granzyme B in the absence of perforin. FEBS Lett 2004;562:87–92. [DOI] [PubMed] [Google Scholar]
- 38.Gondek DC, Lu L-F, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by cd4+cd25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol 2005;174:1783–1786. [DOI] [PubMed] [Google Scholar]
- 39.Waterhouse NJ, Sedelies KA, Browne KA, Wowk ME, Newbold A, Sutton VR, Clarke CJ, Oliaro J, Lindemann RK, Bird PI, et al. A central role for bid in granzyme B-induced apoptosis. J Biol Chem 2005;280:4476–4482. [DOI] [PubMed] [Google Scholar]
- 40.Waterhouse NJ, Sedelies KA, Sutton VR, Pinkoski MJ, Thia KY, Johnstone R, Bird PI, Green DR, Trapani JA. Functional dissociation of deltapsim and cytochrome C release defines the contribution of mitochondria upstream of caspase activation during granzyme B-induced apoptosis. Cell Death Differ 2006;13:607–618. [DOI] [PubMed] [Google Scholar]
- 41.Waterhouse NJ, Sedelies KA, Trapani JA. Role of bid-induced mitochondrial outer membrane permeabilization in granzyme B-induced apoptosis. Immunol Cell Biol 2006;84:72–78. [DOI] [PubMed] [Google Scholar]
- 42.Bredemeyer AJ, Carrigan PE, Fehniger TA, Smith DF, Ley TJ. Hop cleavage and function in granzyme B-induced apoptosis. J Biol Chem 2006;281:37130–37141. [DOI] [PubMed] [Google Scholar]
- 43.Wong HR, Shanley TP, Sakthivel B, Cvijanovich N, Lin R, Allen GL, Thomas NJ, Doctor A, Kalyanaraman M, Tofil NM, et al. Genome-level expression profiles in pediatric septic shock indicate a role for altered zinc homeostasis in poor outcome. Physiol Genomics 2007;30:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Danese S, de la Motte C, Reyes BM, Sans M, Levine AD, Fiocchi C. Cutting edge: T cells trigger cd40-dependent platelet activation and granular rantes release: a novel pathway for immune response amplification. J Immunol 2004;172:2011–2015. [DOI] [PubMed] [Google Scholar]
- 45.Clarke MCH, Savill J, Jones DB, Noble BS, Brown SB. Compartmentalized megakaryocyte death generates functional platelets committed to caspase-independent death. J Cell Biol 2003;160:577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hurd H, Carter V. The role of programmed cell death in plasmodium-mosquito interactions. Int J Parasitol 2004;34:1459–1472. [DOI] [PubMed] [Google Scholar]
- 47.Gavrilescu LC, Denkers EY. Apoptosis and the balance of homeostatic and pathologic responses to protozoan infection. Infect Immun 2003;71:6109–6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warkentin TE, Aird WC, Rand JH. Platelet-endothelial interactions: sepsis, HIT, and antiphospholipid syndrome. Hematology 2003;497–519. [DOI] [PubMed]