Abstract
Rationale: Acute lung injury (ALI) remains an important cause of mortality in intensive care units. Inflammation is controlled by cytokines and eicosanoids derived from the n-6 fatty acid (FA) arachidonic acid (AA). The n-3 FA eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) and mediators derived from EPA and DHA possess reduced inflammatory potency.
Objectives: To determine whether the ability of fat-1 mice to endogenously convert n-6 to n-3 FA, and thus generate an increased ratio of n-3 to n-6 FA, impacts experimental ALI.
Methods: We investigated ALI induced by intratracheal instillation of endotoxin in fat-1 and wild-type (WT) mice, assessing leukocyte numbers, protein concentration, and prostaglandin and cytokine levels in bronchoalveolar lavage fluid, as well as free FA in plasma, and lung ventilator compliance. Body temperature and motor activity of mice—markers of sickness behavior—were also recorded.
Measurements and Main Results: In ALI, fat-1 mice exhibited significantly reduced leukocyte invasion, protein leakage, and macrophage inflammatory protein-2 and thromboxane B2 levels in lavage fluid compared with WT mice. Free AA levels were increased in the plasma of WT mice in response to endotoxin, whereas EPA and DHA were increased in the fat-1 group. Ventilator compliance was significantly improved in fat-1 mice. Body temperature and motor activity were decreased in ALI. fat-1 Mice recovered body temperature and motor activity faster.
Conclusions: fat-1 Mice exhibited reduced features of ALI and sickness behavior. Increasing the availability of n-3 FA may thus be beneficial in critically ill patients with ALI.
Keywords: fat-1 mice, eicosapentaenoic acid, sickness behavior, inflammation, acute lung injury
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
n-3–Containing diets or lipid emulsions were reported to have beneficial effects on the acute lung injury (ALI) in experimental models and patients. Still, uncertainty exists if the supply of n-3 fatty acids (FAs) is of benefit per se or is just counteracting the negative effects of n-6 FAs supplied as controls.
What This Study Adds to the Field
ALI and signs of systemic inflammation as fever were ameliorated in mice endogenously synthesizing n-3 FA without dietary or parenteral nutritional intervention.
Acute respiratory distress syndrome (ARDS) and acute lung injury (ALI) are common clinical disorders, characterized by alveolar epithelial and endothelial injury, leading to the development of pulmonary edema, elevation of pulmonary artery pressure, and finally acute respiratory failure (1, 2). According to recent data, the incidence of ALI or ARDS was 4.5–7.1% of all patients admitted to an intensive care unit (ICU), increasing to 12.5% when only patients treated longer than 24 hours in the ICU were considered (3, 4). The high mortality rate associated with ARDS and ALI has declined to 30–40% in recent randomized trials (1), but there is no proven pharmacologic treatment, despite a multitude of successful strategies in animal models (5, 6). Pathophysiologic features of ALI include a compromised endothelial–alveolar barrier, leading to increased vascular permeability, neutrophil migration into the lung tissue, and formation of proinflammatory mediators, such as cytokines and eicosanoids (e.g., thromboxane [Tx]B2 and prostaglandin [PG] E2) (2, 7).
Lipids, lipid mediators, and inflammation are closely interrelated (8–11). The generation of proinflammatory and antiinflammatory as well as vasoactive eicosanoids (e.g., PGE2 and TxA2) is coupled with the generation of free arachidonic acid (AA) from phospholipids. Eicosanoids are of major interest due to their ability to control inflammation through their proinflammatory and antiinflammatory potencies (10). In Western diet and current nutritional regimes applied in ICUs, linoleic acid is the most prominent n-6 fatty acid (FA), which can be elongated and desaturated to the eicosanoid precursor AA. The n-3 FAs, including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), make up an appreciable part of the fat in cold-water fish and seal meat. The EPA-derived 5-series leukotrienes, generated by the 5-lipoxygenase and the cyclooxygenase product TxA3, possess markedly reduced inflammatory and vasomotor potencies when compared with the AA-derived lipid mediators, and may even exert antagonistic functions (12, 13).
Both n-3 and n-6 polyunsaturated FAs (PUFAs) are essential FAs, because mammals rely on dietary supply due to their inability to synthesize or convert them. Transgenic fat-1 mice, engineered to express the Caenorhabditis elegans fat-1 gene encoding an n-3 FA desaturase, are capable of producing n-3 PUFA from n-6 PUFA, and thereby have a lower ratio of n-6:n-3 FAs in their tissues and organs, without the need for dietary interventions (14). This ratio corresponds more to the n-6:n-3 FA ratio in early evolution, when the human enzyme systems were developed (15).
Different groups have reported a major influence of nutrition, including n-3 FAs, on the morbidity of patients in intensive care. In ARDS, a tailored nutrition with EPA, γ-linoleic acid, and antioxidants was reported to improve oxygenation, to reduce length of mechanical ventilation, to decrease incidence of new organ failure, and to shorten length of stay in the ICU (16–18). There is increasing evidence that including fish oil in parenteral nutritional regimes may decrease length of stay in surgical patients (9, 11, 19–21).
In the present study, we examined the effect of n-3 versus n-6 FAs, independent of possible confounding enteral or parenteral nutrition, in a murine model of ALI comparing transgenic fat-1 to wild-type (WT) mice. We aimed to investigate the difference in the susceptibility to ALI of an organism with a reduced n-6:n-3 ratio compared with one with a “Western diet–style” n-6:n-3 ratio.
Some of these data were presented at the Second Congress of the International Society on Nutrigenetics/Nutrigenomics (22).
METHODS
Reagents
Chemicals of highest purity were obtained from Merck (Darmstadt, Germany). LPS (O111:B4) from Escherichia coli was from Sigma-Aldrich (Dreisenhofen, Germany).
Animals
Local government authorities and university officials responsible for animal protection approved the study. Parent and offspring fat-1 (14) and WT mice on the C57BL/6 background were maintained on a 12-hour day/night cycle under specific pathogen-free conditions. Animals 12- to 16-weeks of age (weight, 23–25 g) of either sex were used for experiments. The n-6:n-3 ratio of the FAs in the diet was 7.3:1.
Murine Model of ALI
Mice were anesthetized, a small catheter was inserted in the trachea, and LPS (10 μg in 50 μl normal saline/mouse) was instilled, as previously described (23). At baseline, 4, 24, or 72 hours after LPS application, mice were killed, bronchoalveolar lavage (BAL) was performed, and the lung was then removed for determination of myeloperoxidase (MPO) (23). Alveolar-recruited leukocytes and leukocytes in peripheral blood were counted using a counting chamber. Differentiation of leukocytes was performed in blinded fashion using differential cell counts of Pappenheim-stained cytocentrifuge preparations (23). Protein in BAL was determined according to Lowry (24). Fixation, embedding, and staining of lungs with hematoxylin and eosin was performed as previously described (25). For immunohistochemical analysis of nuclear factor (NF)-κB expression, the primary NF-κB antibodies were purchased from Cell Signaling (Beverly, MA). Alveolar type II epithelial cells were isolated as previously described (26).
Determination of Lung Compliance by Ventilator
Mice were tracheostomized and ventilated in a volume-driven mode at a positive end-expiratory pressure of 0 kPa, as described previously (27). The respiration rate was set at 20 breaths/minute, and ventilation pressure was recorded while inflating the lung at a Vt of 200 μl. Lung compliance is given and was corrected for animal weight.
ELISA
Tumor necrosis factor (TNF)-α, macrophage inflammatory protein (MIP)-2, PGE2 (all from R&D Systems, Wiesbaden, Germany), and TxB2 (Assay Designs, Ann Arbor, Michigan) from BAL, as well as active NF-κB (Thermo Fisher Scientific, Schwerte, Germany), were determined by ELISA according to the manufacturers' instructions.
Determination of Resolvin E1 and Neuroprotectin D1
Resolvin (Rv) E1 and neuroprotectin (NP) D1 in the reconstituted BAL samples were analyzed by liquid chromatography–tandem mass spectrometry, using D4-PGE2 as the internal standard. Detection was performed on an API-4000 mass spectrometer (Applied Biosystems, Foster City, CA), essentially as described by Arita and colleagues (28).
Measurement of Body Temperature and Locomotor Activity
Abdominal temperature and locomotor activity were measured using a biotelemetry system (Mini-Mitter; Bend, OR). Biotelemetry transmitters were implanted into the abdominal cavity after appropriate anesthesia 1 week before the experiment, as previously described (29). After surgery, animals were housed individually in a climate chamber at a thermoneutral ambient temperature of 30°C and 50% humidity. Artificial light was provided between 7:00 a.m. and 7:00 p.m. Body temperature was continuously recorded at 5-minute intervals. A mean temperature was calculated for each animal every 12 hours (7:00 a.m.–7:00 p.m. and 7:00 p.m.–7:00 a.m.). Activity pulses were counted every 5 minutes, and were added together for 12 hours to yield a cumulative measure of day-time or night-time activity, and were expressed as activity counts per 12 hours.
Determination of Free FAs in Plasma and FAs from Lung Tissue
Plasma was collected by venous puncture directly after death. FAs were determined from plasma and tissue by gas chromatography, as previously described (30, 31).
Statistical Analysis
Data are given as the mean (± SEM). Independent experiments (n = 6–8) were performed per group and time point. Two-way analysis of variance was performed to test for differences between different groups (WT versus fat-1) and time (0, 4, 24, and 72 h). Repeated-measure two-way analysis of variance was used in the cases of activity and temperature to detect differences between groups (WT, WT + LPS, fat-1, fat-1 + LPS) and different time points. Post hoc analysis was performed using Student-Newman-Keul's test. As values of leukocytes, MPO, TNF-α, MIP-2, PGE2, TxB2, and locomotor activity were not normally distributed, log transformation was performed. P values less than 0.05 were considered to indicate statistical significance. Analyses were performed using SigmaStat 3.5 for Windows (Systat Software, San Jose, CA).
RESULTS
Lung Histology of WT and fat-1 Mice Undergoing ALI
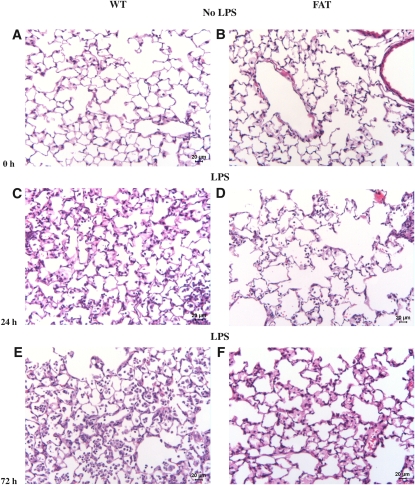
Before challenge, lungs of WT and fat-1 mice displayed a similar histological pattern (Figure 1). At 24 hours after LPS injection, WT mice demonstrated significant invasion of leukocytes into the alveolar space, as well as interstitial edema formation. These features were even more pronounced beyond 72 hours after ALI induction. In contrast, leukocyte invasion and edema formation were ameliorated in fat-1 mice subjected to LPS instillation at both time points.
Figure 1.
Lung histology of wild type (WT) and fat-1 mice before and after induction of acute lung injury (ALI). (A, C, E) Lung histology was performed in WT and (B, D, F) fat-1 mice (A and B) before (0 hours), and (C and D) 24 hours, and (E and F) 72 hours after instillation of 10 μg LPS into the trachea. No difference was found before induction of the injury. After 24 hours, lungs of WT mice displayed a higher amount of amount of leukocytes and interstitial edema as compared with fat-1 mice. Both features were aggravated after 72 hours, with lungs of WT mice still showing a higher degree of interstitial fluid and leukocytes.
Intraalveolar Leukocyte Invasion in ALI
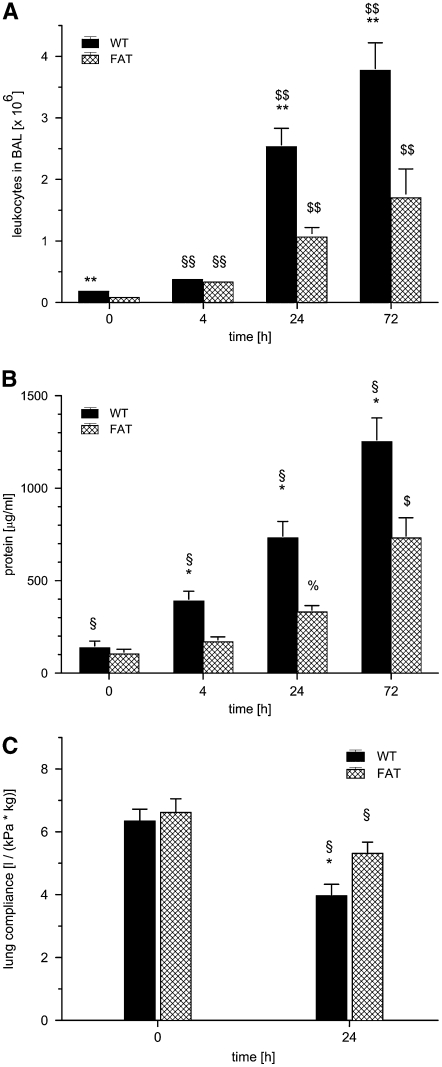
Next, we sought to quantify the amount of leukocyte invasion. Before induction of ALI, 0.18 (± 0.01) × 106 and 0.08 (± 0.01) × 106 leukocytes were detected in the BAL of WT and fat-1 mice, respectively (P < 0.01; Figure 2A). After LPS challenge, leukocyte counts rose over time to 3.77 × 106 and 1.70 × 106 measured in WT and fat-1 mice at 72 hours, respectively. Leukocyte numbers were significantly lower in fat-1 mice at 24 and 72 hours (P < 0.01 for each comparison).
Figure 2.
fat-1 Mice and (A) leukocyte invasion, (B) protein leakage, and (C) lung ventilator compliance in a model of acute lung injury (ALI). Wild-type (WT) and fat-1 (FAT) mice underwent bronchoalveolar lavage (BAL) before (0), and 4, 24, and 72 hours after instillation of 10 μg LPS into the trachea. (A) Leukocytes and (B) protein were determined in BAL fluid. After LPS stimulation, leukocytes increased markedly, and differed significantly from baseline at 4 hours (§§P < 0.01), and from 4 hours and baseline at 24 and 72 hours ($$P < 0.01). Even before LPS stimulation, fewer leukocytes were found in the BAL fluids of fat-1 mice. After LPS challenge, the invasion of leukocytes was markedly reduced in fat-1 mice (**P < 0.01). Protein concentration increased steadily after LPS instillation. The difference in WT mice was significant at all time points (§P < 0.05). In fat-1 mice, the protein concentration after 24 and 72 hours was significantly different from baseline at all time points (%P < 0.05 and $P < 0.05 for both comparisons). fat-1 Mice exhibited lower protein concentrations in BAL fluid in ALI (*P < 0.05 versus WT). (C) Lung ventilator compliance decreased 24 hours after LPS instillation (§P < 0.05 versus baseline). The loss of compliance was of a smaller magnitude in fat-1 mice compared with that observed in WT mice (*P < 0.05). Data are given as mean ± SEM (n = 6–8 independent experiments each). Error bars that appear to be missing are too small to be seen on the scale of the figure.
Under baseline conditions, the percentage of neutrophils in BAL cells was less than 2% in both mouse strains. After 24 and 72 hours, the fraction of neutrophils in BAL cells was 89 (± 2) % and 87 (± 2) % in WT and fat-1 mice, in line with the predominantly neutrophilic invasion in LPS-induced ALI (23). Before injury, 5 (± 1) % of BAL cells were lymphocytes, as determined in WT mice. In contrast, the percentage of lymphocyte was 10 (± 2) % in BAL cells of fat-1 mice (P < 0.05). The WT mice had a fraction of 3 (± 1) % and of 5 (± 1) % lymphocytes after 24 and 72 hours, respectively, with no significant difference compared with fat-1 mice. More than 94% monocytes/macrophages were present in the BAL cells of WT mice at baseline. In contrast, fat-1 mice exhibited only a fraction of 86 (± 3) % of monocytes/macrophages in BAL cells (P < 0.05). However, after injury, the percentage monocytes/macrophages was below 11% in both groups at 24 and 72 hours.
Leukocytes in Peripheral Blood after LPS-induced ALI
Leukocytes counts were 3.29 (± 0.35) × 1012/L and 3.75 (± 0.86) × 1012/L in peripheral blood at baseline (Figure E1A in the online supplement) in WT and fat-1 mice, respectively. Leukocytes increased in fat-1 mice 4 hours after LPS challenge (P < 0.05 versus WT), dropped after 24 hours, and increased to a second peak after 72 hours. The numbers at 24 hours were significantly lower compared with the 4- and 72-hour values (P < 0.05). In the WT group, no significant difference over time was evident.
Lung MPO in ALI
MPO was measured after performing BAL to determine interstitial and intravascular leukocytes in the lung (Figure E1B). At baseline, 1.9 (± 0.3) U/g were detected in WT mice, with comparable values in fat-1 animals. The MPO increased in WT animals, reaching a peak at 24 hours, and dropping at 72 hours. This increase in MPO was clearly blunted in fat-1 mice, and differed significantly from WT animals at 24 hours (P < 0.05). The MPO values at baseline and at 72 hours after LPS instillation were significantly different from all other time points in both groups (P < 0.01).
LPS-induced Protein Extravasation in ALI
Baseline protein concentrations in the BAL fluids were 136 (± 36) μg/ml and 103 (± 26) μg/ml in WT and fat-1 mice, respectively (Figure 2B). After ALI, protein concentration in BAL fluids from WT mice steadily increased as a marker of leakage, and was nearly 10 times higher after 72 hours compared with baseline. In contrast, protein leakage was blunted in fat-1 mice, reaching only 770 (± 110) μg/ml after 72 hours. The difference between both strains was significant at all time points after onset of ALI (4, 24, and 72 h; P < 0.05).
Lung Ventilator Compliance in LPS-induced ALI
Lung ventilator compliance in WT mice without LPS application was 6.14 (± 0.38) L/(kg × kPa) in control animals, without significant variation in fat-1 mice (Figure 2C). LPS induced a significant decrease in compliance after 24 hours in both strains. However, the reduction was significantly less pronounced in fat-1 animals compared with WT mice (P < 0.05).
Cytokine Generation in ALI
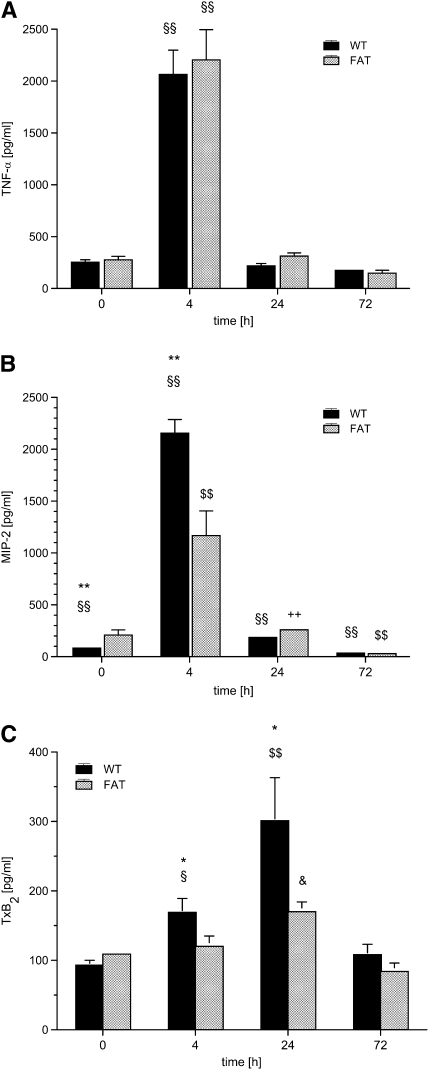
The concentration of TNF-α was determined as 254 (± 23) pg/ml under baseline conditions in WT mice, and did not differ in fat-1 animals (Figure 3A). After LPS challenge, TNF-α peaked at 4 hours in both groups, and returned to baseline after 24 hours. Interestingly, no significant difference between the groups was evident.
Figure 3.
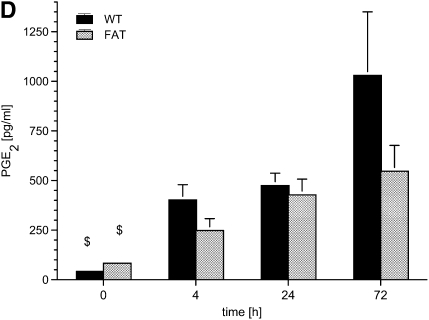
fat-1 Mice and mediator generation in bronchoaveolar lavage (BAL) in a model of acute lung injury. Wild-type (WT) and fat-1 (FAT) mice underwent BAL before (0), and 4, 24, and 72 hours after instillation of 10 μg LPS into the trachea. The (A) tumor necrosis factor (TNF)-α, (B) macrophage inflammatory protein (MIP)-2, (C) thromboxane (Tx) B2, and (D) prostaglandin (PG) E2 levels were determined by ELISA in the BAL fluid. TNF-α levels increased massively after 4 hours (§§P < 0.01 vs. all other time points) and returned to baseline concentrations. The baseline levels of MIP-2 were higher in fat-1 mice as compared with the WT group (**P < 0.01). The MIP-2 concentration also peaked at 4 hours, and steadily decreased to values even below baseline after 72 hours. The differences between all time points were significant in the WT group (§§P < 0.01 for each comparison). In the fat-1 group, MIP-2 levels at 4 and 72 hours differed from all other time points ($$P < 0.01). The concentration of MIP-2 at 24 hours differed from concentrations observed at 4 and 72 hours, but not from values at baseline (++P < 0.01). The increase in MIP-2 after LPS challenge was blunted in fat-1 mice (**P < 0.01 vs. WT). The TxB2 was already increased in WT mice after 4 hours (§P < 0.05 vs. baseline and 72 h). The TxB2 levels peaked at 24 hours ($$P < 0.01 vs. all other time points; &P < 0.05 vs. baseline and 72 h). The elevation was blunted in fat-1 mice in comparison with the WT group (*P < 0.05). PGE2 increased steadily and differed from baseline in both groups ($P < 0.05), in contrast to all other mediators determined. Data are given as mean ± SEM (n = 6–8 independent experiments each). Error bars that appear to be missing are too small to be seen on the scale of the figure.
The MIP-2 concentration in the BAL fluid was 83 (± 10) pg/ml before the induction of ALI in WT mice (Figure 3B). Of note, the MIP-2 level was significantly higher in fat-1 animals before LPS challenge (P < 0.01). In WT mice, MIP-2 levels peaked at 4 hours, and declined steadily to concentrations even below baseline at 72 hours. The course of cytokine release was comparable in fat-1 mice, but the peak concentration only reached 50% of that observed in WT mice (P < 0.01).
NF-κB in ALI
To investigate possibly altered expression of NF-κB—one of the central intracellular mediators of inflammation—immunohistochemical analysis of sections derived from the lungs of WT and fat-1 mice was performed (Figure E2). The NF-κB was expressed primarily in the bronchial epithelium and endothelium in both WT and fat-1 animals. The fat-1 animals exhibited a significantly reduced NF-κB expression compared with WT mice, both before and after LPS challenge.
In addition, isolated alveolar type II epithelial cells were stimulated for 120 minutes with 1 ng/ml TNF-α, and active NF-κB was determined by an ELISA-based assay (Figure E2 [e]). Active NF-κB was already increased 10 minutes after TNF-α application, and declined subsequently. The increase was higher and more prolonged in alveolar epithelial cells derived from WT mice as compared with the fat-1 group (P < 0.05).
Eicosanoid Formation in ALI
Under baseline conditions, the TxB2 concentration was 93 (± 7) pg/ml in WT mice, with comparable concentrations determined in fat-1 animals (Figure 3C). The TxB2 was already increased in WT mice after 4 hours (P < 0.05 versus baseline and 72 h). At 24 hours after induction of ALI in WT mice, TxB2 levels peaked, and were increased more than threefold, reaching baseline levels after 72 hours. In fat-1 mice, the rise in TxB2 concentration was largely blunted, and the peak concentration was less than 60% compared with WT animals (P < 0.05).
Before induction of ALI, we found less than 100 pg/ml PGE2 in the BAL fluids of both groups. After LPS challenge, the PGE2 levels rose steadily, and, after 72 hours, reached 1,030 (± 320) pg/ml and 547 (± 130) pg/ml in WT and fat-1 mice, respectively. However, this difference failed to reach levels of statistical significance.
Rv Generation in ALI
The BAL was either analyzed directly, or pooled for the same treatment group/time point (n = 4 mice for each time point), and evaporated down to 100 μl to enrich the concentrations of Rvs. Despite our ability to detect RvE1 and NPD1 with a detection limit of 0.01 ng/ml, we were not able to detect any Rvs in BAL.
Activity and Temperature in Mice Undergoing ALI
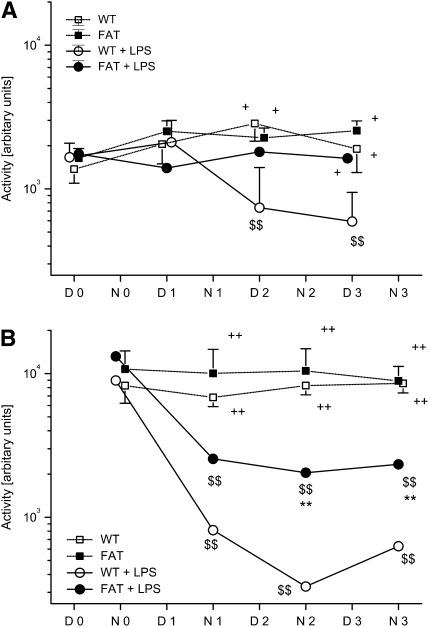
As fat-1 mice seemed to cope better with ALI, we sought to determine the activity and temperature in mice with ALI. All mice exhibited a preferential night activity, and moved less during the day (Figures 4A and 4B). The frequency and pattern of movement were undisturbed, even after instilling normal saline into the trachea (sham injury). After induction of ALI in WT mice, we found an equalization of the day (D) and night (N) pattern, and a dramatic reduction in movements beginning 12 hours after LPS instillation (P < 0.01 versus baseline). fat-1 Mice undergoing ALI also exhibited a reduction in the night activity, beginning 12 hours after the injury (P < 0.01 versus baseline), but this decrease was markedly less pronounced compared with WT mice undergoing ALI. Furthermore, fat-1 mice exhibited no detectable change in daytime activity after initiation of injury. The LPS-exposed fat-1 and WT mice differed significantly beginning with the second night after injury (N2, D3, N3; P < 0.05).
Figure 4.
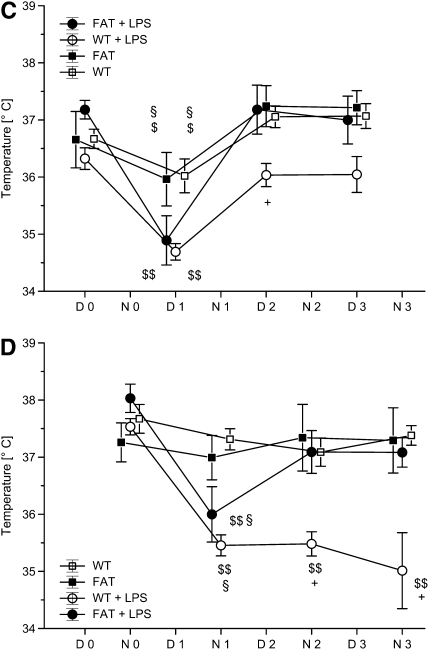
fat-1 Mice and motor activity and body temperature in a model of acute lung injury (ALI). Wild-type (WT) and fat-1 (FAT) mice were housed individually in a climate chamber at a thermoneutral ambient temperature of 30°C and 50% humidity with a 12-hour day/night cycle. (A) Day-time and (B) night-time motor activity, as well as (C and D) temperature, were recorded under baseline conditions (D0 = Day 0; N0 = Night 0) and after instillation of LPS or 0.9% NaCl into the trachea (D1–D3 = Day 1 to Day 3; N1–N3 = night 1 to night 3). Day-time motor activity was depressed in the WT plus LPS group ($$P < 0.01 vs. D0; +P < 0.05 vs. marked other groups). Night-time activity was reduced in both groups undergoing ALI ($$P < 0.01 vs. respective N0; ++P < 0.01 vs. groups without LPS challenge), but the fat-1 plus LPS mice show increased motor activity compared with the WT plus LPS group (**P < 0.01). Body temperature decreased in all groups on D1 ($P < 0.05 and $$P < 0.01, vs. baseline). Both control groups had a higher temperature as compared with the LPS groups (§P < 0.05). Whereas fat-1 mice recovered their day-time body temperature at D2, WT mice differed from all other groups at this time point (+P < 0.05). Mean night-time temperature was decreased in both lung injury groups at N1, but fat-1 mice returned to baseline values ($$P < 0.01 vs. baseline). The difference in body temperature was significant compared with the control groups at N1 (§P < 0.05). The temperature remained depressed in the WT plus LPS group, but not in fat-1 mice with ALI (+P < 0.05 vs. all other groups).
Body temperature was higher during the night compared with the day under baseline conditions in all groups, corresponding to the increased night activity of the mice (Figures 4C and 4D). After instillation of normal saline, the temperature dropped by 0.5°C in both fat-1 and WT mice. Induction of ALI led to a pronounced reduction in body temperature (−2.5°C) in both groups. The groups with normal saline injection differed significantly from mice receiving LPS during the first day (D1; WT or fat-1 versus WT + LPS or fat-1 + LPS; P < 0.05). The decrease in temperature was also significant the first night of injury in both ALI groups (P < 0.05 versus control groups). Both groups with sham injury and the fat-1 group undergoing ALI returned to a preinjury temperature profile beginning with the second day (D2). In contrast, WT mice had a reduced body temperature until the third night after injury (N3), and did not recover.
Free FAs in Plasma
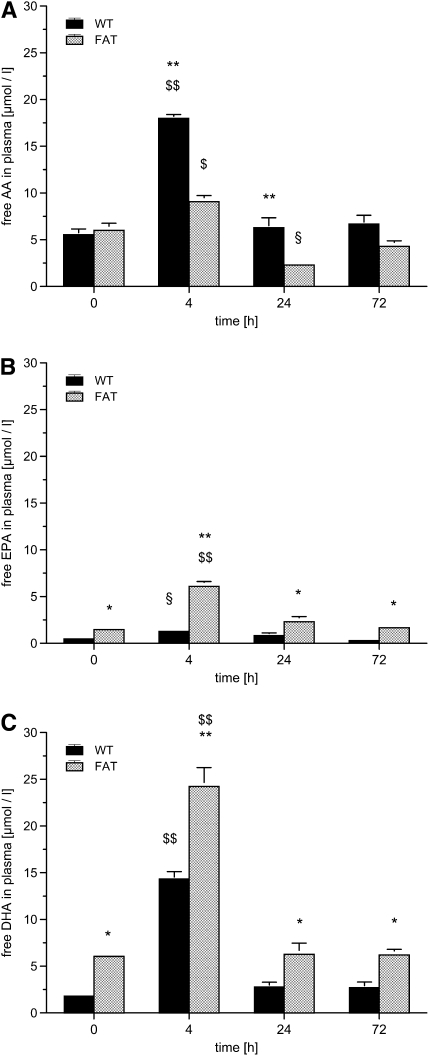
Due to the introduction of the C. elegans fat-1 gene encoding an n-3 FA desaturase, fat-1 mice are capable of converting n-6 into n-3 PUFA. We determined free FAs at baseline, 4, 24, and 72 hours after instillation of LPS in plasma as AA and EPA (Figure 5). The baseline concentration of AA was 5.5 (± 0.6) μmol/L in WT mice, with similar concentrations determined in fat-1 mice. At 4 hours after induction of ALI, free AA was tripled in WT mice, but only increased by 50% in the fat-1 group (P < 0.01). In WT animals, the AA levels returned to baseline after 24 hours. The AA concentration was decreased to 2.3 (± 0.1) μmol/L at 24 hours in fat-1 mice (P < 0.01 versus WT), and returned to baseline after 72 hours.
Figure 5.
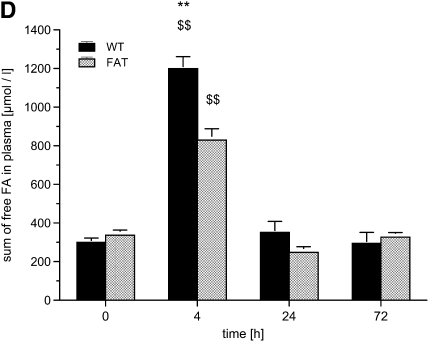
fat-1 Mice and free fatty acids (FAs) in plasma in a model of acute lung injury. Blood was drawn from wild-type (WT) and fat-1 (FAT) mice before (0), and 4, 24, and 72 hours after instillation of 10 μg LPS into the trachea. (A) Free arachidonic acid (AA), (B) eicosapentaenoic acid (EPA), (C) docosahexaenoic acid (DHA), and (D) the sum of free FAs were determined in plasma. The AA concentration increased at 4 hours ($P < 0.05 and $$P < 0.01, vs. all other time points). At 24 hours, the concentration of AA fell below the baseline concentration in fat-1 mice (§P < 0.05). The AA concentration in WT and fat-1 mice differed significantly at 4 and 24 hours (**P < 0.01). The EPA differed significantly between WT and fat-1 at all time points (*P < 0.05 and **P < 0.01), and also peaked at 4 hours in fat-1 ($$P < 0.01 versus all other time points) and in WT mice (§P < 0.05 vs. 72 h). Concentrations of DHA differed at all time points between the WT and fat-1 mice (*P < 0.05 and **P < 0.01). The DHA peaked in both groups at 4 hours (§§P < 0.01 vs. all other time points). The sum of free FAs exhibited nearly the same time profile, with a peak at 4 hours ($$P < 0.01 vs. all other time points). fat-1 Mice had a lower sum of free FAs as WT mice at 4 hours (**P < 0.01). Data are given as mean ± SEM (n ≥ 6 independent experiments each). Error bars that appear to be missing are too small to be seen on the scale of the figure.
fat-1 Mice exhibited higher baseline concentrations of n-3 FAs. Concentrations of EPA and DHA were 1.5 (± 0.2) and 6.1 (± 0.4) μmol/L, respectively, as opposed to 0.5 (± 0.1) and 1.8 (± 0.2) μmol/L in WT mice (P < 0.05). We could determine a massive increase in n-3 FAs in fat-1 mice, reaching 30 μmol/L when combining EPA and DHA levels 4 hours after the injury. This level was nearly double the sum of EPA and DHA in WT mice.
Both FAs returned to baseline concentrations 24 hours after the induction of ALI in both groups. Interestingly, the ratio of AA:(EPA + DHA) was 2.4:1 in WT mice and 1:1.3 in fat-1 animals under baseline conditions. The ratio changed to 1.2:1 in WT mice and 1:3.3 in fat-1 mice 4 hours after the instillation of LPS. In WT mice, EPA was also increased after 4 hours, but continued to decline until 72 hours to concentrations even below baseline.
When measuring the sum of all free FAs under baseline conditions in WT mice, we found a concentration 300.1 (± 21.7) μmol/L in plasma, with similar levels in fat-1 mice. Free FAs increased by a factor of four in WT mice after 4 hours, but less than three times in fat-1 mice (P < 0.01), returning to baseline concentrations after 24 hours.
FAs in Lung Tissue
AA constituted 9.09 (± 0.38) % of all FAs in lung tissue in WT mice (Figure E3). In fat-1 mice, the AA level was significantly lower, as its fraction was only 6.82 (± 0.62) %. After LPS instillation, AA increased over time, and reached 12.08 (± 0.29) % after 72 hours in WT mice. In contrast, the AA levels dropped in fat-1 mice after 4 hours, increasing thereafter to reach baseline levels after 72 hours. Both groups differed significantly at every time point (P < 0.01).
EPA content was 0.07 (± 0.01) % and 0.49 (± 0.08) % in WT and in fat-1 mice, respectively. In both groups, EPA peaked at 4 hours, with an increase of nearly 75% in fat-1 mice, and subsequently declined, reaching near-baseline levels at 72 hours. Again, both groups differed significantly at every time point (P < 0.01).
DHA was nearly twofold higher in fat-1 mice compared with WT animals under baseline conditions. After LPS challenge, DHA steadily increased in both groups. In the WT group, DHA values at 24 and 72 hours were significantly higher compared with the baseline and 4-hour values (P < 0.01). At all time points, DHA levels in fat-1 mice were higher than in WT animals (P < 0.01).
DISCUSSION
We were able to demonstrate a reduction in the severity of LPS-induced ALI in fat-1 mice, compared with WT animals. fat-1 Mice exhibited lower neutrophil invasion, lower protein leakage, and better lung compliance after injury. The generation of the proinflammatory mediators MIP-2, TxB2, and PGE2, but not TNF-α, was reduced in fat-1 mice. fat-1 Mice also exhibited accelerated return to normal motor activity and temperature after lung injury. Under conditions of ALI, we could also demonstrate lower concentrations of free AA and a reduced sum of free FAs, but higher concentrations of EPA and DHA in fat-1 mice.
In our model, the general response of fat-1 mice to endotoxin was intact, as mirrored, for example, by the release of inflammatory eicosanoids and cytokines. In contrast to WT mice, the release in TxB2 and PGE2 was reduced in fat-1 mice. Two mechanisms may have contributed to this effect in fat-1 animals. Reduced availability of AA and increased concentrations of the n-3 FAs, EPA and DHA, as mirrored in the plasma, may have decreased the generation of AA-derived eicosanoids. Furthermore, increases in n-3 lipids in the cell membrane may interfere with lipid-based second messenger formation, such as, for example, generation inositolphosphates (32), phosphatidylinositol 3-kinase–dependent signaling (33), or plasma membrane translocation and activation of protein kinase C (34). Reduced activation of NF-κB by n-3 FAs may also add to the reduction in eicosanoids (35, 36). In line with this observation, we detected decreased staining intensity of NF-κB in histological sections of lungs derived from fat-1 mice compared with WT animals. LPS-induced MIP-2 release into the BAL fluid was also reduced in fat-1 mice. Of note, the baseline concentration of MIP-2 was increased in fat-1 mice, in parallel with a lower leukocyte count in BAL fluids, a feature that might speculatively be caused by an adaption process due to reduced basal migration of leukocytes into the airspace.
Interestingly, we could not show a difference between WT and fat-1 mice in TNF-α generation. When infusing fish oil–based lipid emulsions for 2–5 days, a reduced release of TNF-α in isolated monocytes derived from patients with sepsis (30), in the BAL fluids of mice with ALI (23), or in the plasma of volunteers injected intravenously with LPS (37), has previously been demonstrated. However, when volunteers underwent the same intravenous LPS challenge after long-term oral fish oil supplementation, no significant difference in TNF-α levels in plasma was evident compared with control volunteers (38). We speculate that this may be part of a long-term compensation process, which contrasts the effects of acute intervention with n-3 lipids.
A major feature of ALI is the intrapulmonary invasion of leukocytes. The recruitment of neutrophils through the endothelial–epithelial barrier is a tightly regulated, multistep process (39). The n-3 lipids may interfere with this process at multiple stages, involving reduced presentation of endothelial adhesion molecules (36). Furthermore, the transition from rolling to firm adhesion is facilitated by platelet-activated factor presented by endothelial cells to leukocytes (40), and activation of phosphatidylinositol 3-kinase γ in leukocytes (41), with both features being inhibited by n-3 FAs (33, 42). All these aspects, together with reduced generation of MIP-2—the murine equivalent of the human chemotaxin IL-8—may be responsible for the reduced recruitment of neutrophils into the alveolar space in fat-1 mice. As TNF-α levels were similar in both groups, but NF-κB was lower in fat-1 mice, we speculate that, in our setting, the TNF-α–NF-κB axis was not a critical factor involved in the differential effect of n-3 FAs on adhesion and transmigration. The characteristic of reduced neutrophil infiltration was already demonstrated by infusion of fish oil–based lipid emulsions in mice (23). The idea of reduced transmigration of neutrophils into the interstitial and alveolar compartment is further strengthened by our data demonstrating increased numbers of circulating leukocytes in fat-1 mice 4 and 72 hours after injury, together with reduced MPO activity in lung tissue.
Furthermore, we provide evidence that LPS instillation into the lungs significantly impairs lung compliance within 24 hours after instillation. Determination of compliance represents a recognized means to evaluate lung injury (43), and impairment is found in patients with ALI (44). The reduced loss in lung compliance underscores the fact that ALI is reduced in fat-1 mice.
Increase in free FAs in the plasma is a general response in severe inflammation and infection (45, 46). Due to limited stores of carbohydrates, a change in the utilization and release of substrates occurs. Glucose oxidation is limited, but fat oxidation is increased in severe sepsis (47). Free FAs in the plasma are increased, and mirror the lipids accumulated during long-term nutrition in fat stores (46, 48, 49). Free AA is increased to nearly 300% in WT mice, but only by 50% in the fat-1 group 4 hours after LPS instillation. The sum of EPA and DHA in WT animals rose to 15.6 μmol/L, in contrast to 30.3 μmol/L in fat-1 mice. As nutrition in man is thought to have changed from a higher intake of n-3 to reduced availability in n-3 and increased ingestion of n-6 FAs, in what we now call Western diet, the general response to inflammation may have changed. After induction of ALI, the ratio of AA:(EPA + DHA) was 1.2:1 and 1:3.3 in WT and in fat-1 mice, respectively.
Reduced inflammatory activation, as estimated by reduced leukocyte infiltration, prostanoids, and MIP-2, may have translated into improved vascular and epithelial barrier function, as judged by decreased protein concentration in the BAL fluid. Improvement of capillary leakage and protein extravasation by n-3 lipids were found in models of ALI, and linked to the reduced formation of 4-series leukotrienes derived from AA and generation of less active 5-series leukotrienes originating from EPA (23, 50). Interestingly, generation of either 4-series or 5-series leukotrienes in vitro, and in isolated organs, can be controlled by providing the free precursor FA. After activation of the 5-lipoxygenase, formation of 4-series or 5-series leukotrienes is regulated by the availability of either (endogenous or exogenous) free AA or EPA (51, 52). Furthermore, a shift in levels of precursor FAs may also result in different cyclooxygenase-derived eicosanoids (53), bearing in mind that inhibition of this enzyme in patients with sepsis resulted only in a trend toward improved pulmonary function, and did not impact the mortality rate (54).
Reduced injury and better recovery may also be delineated from the temperature and activity recording in mice subjected to lung injury. fat-1 Mice returned to regular body temperature within 24 hours after instillation of LPS. This is in clear contrast with WT mice, which did not recover regular temperature during the observation time of 72 hours. These data on body temperature are well in line with the recordings of mouse activity. Although we did find an unchanged day-time activity in fat-1 mice, it was reduced in WT animals. Night-time activity was severely decreased in WT mice, and was reduced in the fat-1 group. The thermoregulatory response to inflammation can consist of phases of fever and hypothermia (55). Hypothermia is often regarded as maladaptive, correlating with poor clinical outcome, as the mortality of patients with hypothermia and sepsis has been reported as twice that of febrile patients (56). Under the conditions of a thermoneutral environment, the mice used in this study would have been able to develop fever as well as hypothermia, depending on the kind and severity of a given inflammatory insult (55). The faster recovery of fat-1 mice from the hypothermia induced by ALI is thus indicative of the protective effects of n-3 FAs. In line with this observation is the attenuation of depressed motor activity in fat-1 mice. Suppression of motor activity is a characteristic component of the array of centrally controlled signs of illness, collectively termed “sickness behavior” (57). The fact that this aspect of the sickness syndrome could effectively be antagonized in fat-1 mice clearly demonstrates the adaptive and beneficial effects of n-3 FAs. The faster recovery from hypothermia, and the reduced depression of activity, may speculatively be linked to the formation of Rvs. Generation of this new class of EPA- or DHA-derived lipid mediators has already been demonstrated in fat-1 mice (58) and in human blood (59). Synthesis of Rvs or injection of RvE1 facilitated and accelerated resolution of inflammation (60, 61). In a murine model of asthma, RvE1 or NPD1 facilitated resolution of ovalbumin-induced allergic airway inflammation and hyperresponsiveness (62, 63). This is contrasted by our negative determinations of RvE1 or NPD1 in the BAL in LPS-induced ALI. Given that Levy and colleagues (63) reported NPD1 in whole murine lungs, the BAL may be the wrong compartment in which to examine these highly interesting mediators.
A beneficial impact of a diet rich in n-3 lipids and antioxidants on ventilation time, secondary organ failures, and length of stay in patients with ALI has been shown (16–18). A faster reduction in leukotriene and protein influx in the alveolar space was demonstrated in these patients (64), which is in line with our experimental observations. From the data of the clinical studies and experimental evidence, inclusion of n-3 lipids in the nutrition of patients suffering from ALI should be considered. The choice of the nutritional lipid supplied may influence plasma profile of FAs (46), lipid mediators (52), and lung injury (50).
The use of fat-1 mice to examine the influence of n-3 lipids in ALI combines the advantage of an increased ratio of n-3 to n-6 FAs, without the need to supply additional lipids. Exogenous supply of lipids may be an additional confounding factor, as ingestion of lipids may modulate the inflammatory response via the neuroendocrine axis, the vagus nerve, and acetylcholine receptors on leukocytes (65). The infusion of lipid emulsions may lead to increased generation of precursor FAs for the lipid mediator pathways (46). A limitation of the study is the use of the LPS-induced model. The single application of LPS creates a single-hit injury to induce inflammatory responses. Such a model is clearly different from clinical and experimental ALI induced by bacterial infection.
In conclusion, we were able to show that fat-1 mice develop a less severe lung injury compared with WT animals after LPS instillation. fat-1 Mice were able to increase free n-3 FAs instead of n-6 FAs, and to produce less inflammatory mediators, such as MIP-2 and TxB2. We also found decreased neutrophil invasion and reduced protein influx in the alveolar space, together with improved lung compliance. fat-1 Mice returned faster to normal body temperature, and exhibited a reduced loss of activity compared with WT animals. We demonstrate here, for the first time, that fat-1 mice with a reduced n-6:n-3 ratio are less prone to ALI compared with WT mice with an n-6:n-3 ratio corresponding to our normal Western diet and the nutrition ratio used for patients in intensive care. Nutritional interventions to manipulate the availability of n-3 FAs in patients with ALI in intensive care might have significant implications for their outcome.
Supplementary Material
Acknowledgments
The authors thank Andrea Mohr for skillful technical assistance. This manuscript includes portions of the doctoral thesis of Almuth Kiessling.
Supported by Deutsche Forschungsgemeinschaft, Collaborative Research Center 547 “Cardiopulmonary Vascular System,” Project B4 (K.M.), the Excellence Cluster Cardio-Pulmonary System, Giessen, Germany, by National Institutes of Health grant CA113605 (J.X.K.), and by a start-up grant from the University of Giessen School of Medicine (M.B.S.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200807-1064OC on January 8, 2009
Conflict of Interest Statement: K.M. has received fees for speaking at conferences from Abbott, Baxter, BBraun, Fresenius Kabi, and Nestle. The sum of all speaking fees was equal to or less than €5,000/year in the years 2005–2007. A.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.O. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.B.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. I.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. W.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.X.K. has participated as a speaker in scientific meetings sponsored by a food company (BNL Food), and is also the inventor and coapplicant (with Massachusetts General Hospital, a nonprofit organization) of a U.S. patent (no. 7238851. Massachusetts General Hospital is the owner, but has not received any income so far), which is about “Non-human transgenic mammals expressing an n-3 desaturase gene.”
References
- 1.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 2007;369:1553–1564. [DOI] [PubMed] [Google Scholar]
- 2.Hecker M, Walmrath HD, Seeger W, Mayer K. Clinical aspects of acute lung insufficiency (ALI/TRALI). Transfus Med Hemother 2008;35:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brun-Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, Bion J, Romand JA, Villar J, Thorsteinsson A, et al. Epidemiology and outcome of acute lung injury in European intensive care units: results from the ALIVE study. Intensive Care Med 2004;30:51–61. [DOI] [PubMed] [Google Scholar]
- 4.Esteban A, Anzueto A, Frutos F, Alia I, Brochard L, Stewart TE, Benito S, Epstein SK, Apezteguia C, Nightingale P, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA 2002;287:345–355. [DOI] [PubMed] [Google Scholar]
- 5.Cepkova M, Matthay MA. Pharmacotherapy of acute lung injury and the acute respiratory distress syndrome. J Intensive Care Med 2006;21:119–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calfee CS, Matthay MA. Nonventilatory treatments for acute lung injury and ARDS. Chest 2007;131:913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol 2005;33:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sijben JW, Calder PC. Differential immunomodulation with long-chain n-3 PUFA in health and chronic disease. Proc Nutr Soc 2007;66:237–259. [DOI] [PubMed] [Google Scholar]
- 9.Mayer K, Schaefer MB, Seeger W. Fish oil in the critically ill: from experimental to clinical data. Curr Opin Clin Nutr Metab Care 2006;9:140–148. [DOI] [PubMed] [Google Scholar]
- 10.Heller A, Koch T, Schmeck J, van Ackern K. Lipid mediators in inflammatory disorders. Drugs 1998;55:487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer K, Seeger W. Fish oil in critical illness. Curr Opin Clin Nutr Metab Care 2008;11:121–127. [DOI] [PubMed] [Google Scholar]
- 12.Calder PC. Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids 2007;77:327–335. [DOI] [PubMed] [Google Scholar]
- 13.Yaqoob P, Calder PC. Fatty acids and immune function: new insights into mechanisms. Br J Nutr 2007;98:S41–S45. [DOI] [PubMed] [Google Scholar]
- 14.Kang JX, Wang J, Wu L, Kang ZB. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature 2004;427:504. [DOI] [PubMed] [Google Scholar]
- 15.Simopoulos AP. Evolutionary aspects of omega-3 fatty acids in the food supply. Prostaglandins Leukot Essent Fatty Acids 1999;60:421–429. [DOI] [PubMed] [Google Scholar]
- 16.Gadek JE, DeMichele SJ, Karlstad MD, Pacht ER, Donahoe M, Albertson TE, Van Hoozen C, Wennberg AK, Nelson JL, Noursalehi M. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Enteral Nutrition in ARDS Study Group. Crit Care Med 1999;27:1409–1420. [DOI] [PubMed] [Google Scholar]
- 17.Singer P, Theilla M, Fisher H, Gibstein L, Grozovski E, Cohen J. Benefit of an enteral diet enriched with eicosapentaenoic acid and gamma-linolenic acid in ventilated patients with acute lung injury. Crit Care Med 2006;34:1033–1038. [DOI] [PubMed] [Google Scholar]
- 18.Pontes-Arruda A, Aragao AM, Albuquerque JD. Effects of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crit Care Med 2006;34:2325–2333. [DOI] [PubMed] [Google Scholar]
- 19.Wichmann MW, Thul P, Czarnetzki HD, Morlion BJ, Kemen M, Jauch KW. Evaluation of clinical safety and beneficial effects of a fish oil containing lipid emulsion (Lipoplus, MLF541): data from a prospective, randomized, multicenter trial. Crit Care Med 2007;35:700–706. [DOI] [PubMed] [Google Scholar]
- 20.Tsekos E, Reuter C, Stehle P, Boeden G. Perioperative administration of parenteral fish oil supplements in a routine clinical setting improves patient outcome after major abdominal surgery. Clin Nutr 2004;23:325–330. [DOI] [PubMed] [Google Scholar]
- 21.Berger MM, Tappy L, Revelly JP, Koletzko BV, Gepert J, Corpataux JM, Cayeux MC, Chiolero RL. Fish oil after abdominal aorta aneurysm surgery. Eur J Clin Nutr 2008;62:1116–1122. [DOI] [PubMed] [Google Scholar]
- 22.Mayer K, Kang JX. fat-1 Mice are protected from acute lung injury. J Nutrigenet Nutrigenomics 2008;1:271. [Google Scholar]
- 23.Schaefer MB, Ott J, Mohr A, Bi MH, Grosz A, Weissmann N, Ishii S, Grimminger F, Seeger W, Mayer K. Immunomodulation by n-3– versus n-6–rich lipid emulsions in murine acute lung injury—role of platelet-activating factor receptor. Crit Care Med 2007;35:544–554. [DOI] [PubMed] [Google Scholar]
- 24.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Gunther A, Lubke N, Ermert M, Schermuly RT, Weissmann N, Breithecker A, Markart P, Ruppert C, Quanz K, Ermert L, et al. Prevention of bleomycin-induced lung fibrosis by aerosolization of heparin or urokinase in rabbits. Am J Respir Crit Care Med 2003;168:1358–1365. [DOI] [PubMed] [Google Scholar]
- 26.Rosseau S, Wiechmann K, Moderer S, Selhorst J, Mayer K, Krull M, Hocke A, Slevogt H, Seeger W, Suttorp N, et al. Moraxella catarrhalis–infected alveolar epithelium induced monocyte recruitment and oxidative burst. Am J Respir Cell Mol Biol 2005;32:157–166. [DOI] [PubMed] [Google Scholar]
- 27.Wygrecka M, Markart P, Ruppert C, Petri K, Preissner KT, Seeger W, Guenther A. Cellular origin of pro-coagulant and (anti)-fibrinolytic factors in bleomycin-injured lungs. Eur Respir J 2007;29:1105–1114. [DOI] [PubMed] [Google Scholar]
- 28.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med 2005;201:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hubschle T, Mutze J, Muhlradt PF, Korte S, Gerstberger R, Roth J. Pyrexia, anorexia, adipsia, and depressed motor activity in rats during systemic inflammation induced by the Toll-like receptors-2 and -6 agonists MALP-2 and FSL-1. Am J Physiol Regul Integr Comp Physiol 2006;290:R180–R187. [DOI] [PubMed] [Google Scholar]
- 30.Mayer K, Gokorsch S, Fegbeutel C, Hattar K, Rosseau S, Walmrath D, Seeger W, Grimminger F. Parenteral nutrition with fish oil modulates cytokine response in patients with sepsis. Am J Respir Crit Care Med 2003;167:1321–1328. [DOI] [PubMed] [Google Scholar]
- 31.Mayer K, Meyer S, Reinholz-Muhly M, Maus U, Merfels M, Lohmeyer J, Grimminger F, Seeger W. Short-time infusion of fish oil–based lipid emulsions, approved for parenteral nutrition, reduces monocyte proinflammatory cytokine generation and adhesive interaction with endothelium in humans. J Immunol 2003;171:4837–4843. [DOI] [PubMed] [Google Scholar]
- 32.Sperling RI, Benincaso AI, Knoell CT, Larkin JK, Austen KF, Robinson DR. Dietary omega-3 polyunsaturated fatty acids inhibit phosphoinositide formation and chemotaxis in neutrophils. J Clin Invest 1993;91:651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaefer MB, Wenzel A, Fischer T, Braun-Dullaeus RC, Renner F, Dietrich H, Schaefer CA, Seeger W, Mayer K. Fatty acids differentially influence phosphatidylinositol 3-kinase signal transduction in endothelial cells: impact on adhesion and apoptosis. Atherosclerosis 2008;197:630–637. [DOI] [PubMed] [Google Scholar]
- 34.Denys A, Hichami A, Khan NA. n-3 PUFAs modulate T-cell activation via protein kinase C-alpha and -epsilon and the NF-kappaB signaling pathway. J Lipid Res 2005;46:752–758. [DOI] [PubMed] [Google Scholar]
- 35.Camandola S, Leonarduzzi G, Musso T, Varesio L, Carini R, Scavazza A, Chiarpotto E, Baeuerle PA, Poli G. Nuclear factor κB is activated by arachidonic acid but not by eicosapentaenoic acid. Biochem Biophys Res Commun 1996;229:643–647. [DOI] [PubMed] [Google Scholar]
- 36.Weber C, Erl W, Pietsch A, Danesch U, Weber PC. Docosahexaenoic acid selectively attenuates induction of vascular cell adhesion molecule-1 and subsequent monocytic cell adhesion to human endothelial cells stimulated by tumor necrosis factor-alpha. Arterioscler Thromb Vasc Biol 1995;15:622–628. [DOI] [PubMed] [Google Scholar]
- 37.Pluess TT, Hayoz D, Berger MM, Tappy L, Revelly JP, Michaeli B, Carpentier YA, Chiolero RL. Intravenous fish oil blunts the physiological response to endotoxin in healthy subjects. Intensive Care Med 2007;33:789–797. [DOI] [PubMed] [Google Scholar]
- 38.Michaeli B, Berger MM, Revelly JP, Tappy L, Chiolero R. Effects of fish oil on the neuro-endocrine responses to an endotoxin challenge in healthy volunteers. Clin Nutr 2007;26:70–77. [DOI] [PubMed] [Google Scholar]
- 39.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 2007;7:678–689. [DOI] [PubMed] [Google Scholar]
- 40.Zimmerman GA, McIntyre TM, Mehra M, Prescott SM. Endothelial cell–associated platelet-activating factor: a novel mechanism for signaling intercellular adhesion. J Cell Biol 1990;110:529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith DF, Deem TL, Bruce AC, Reutershan J, Wu D, Ley K. Leukocyte phosphoinositide-3 kinase {gamma} is required for chemokine-induced, sustained adhesion under flow in vivo. J Leukoc Biol 2006;80:1491–1499. [DOI] [PubMed] [Google Scholar]
- 42.Mayer K, Merfels M, Muhly-Reinholz M, Gokorsch S, Rosseau S, Lohmeyer J, Schwarzer N, Krull M, Suttorp N, Grimminger F, et al. Omega-3 fatty acids suppress monocyte adhesion to human endothelial cells: role of endothelial PAF generation. Am J Physiol Heart Circ Physiol 2002;283:H811–H818. [DOI] [PubMed] [Google Scholar]
- 43.Parker JC, Townsley MI. Evaluation of lung injury in rats and mice. Am J Physiol Lung Cell Mol Physiol 2004;286:L231–L246. [DOI] [PubMed] [Google Scholar]
- 44.Esper AM, Martin GS. Evolution of treatments for patients with acute lung injury. Expert Opin Investig Drugs 2005;14:633–645. [DOI] [PubMed] [Google Scholar]
- 45.Weissman C. The metabolic response to stress: an overview and update. Anesthesiology 1990;73:308–327. [DOI] [PubMed] [Google Scholar]
- 46.Mayer K, Fegbeutel C, Hattar K, Sibelius U, Kramer HJ, Heuer KU, Temmesfeld-Wollbruck B, Gokorsch S, Grimminger F, Seeger W. Omega-3 vs. omega-6 lipid emulsions exert differential influence on neutrophils in septic shock patients: impact on plasma fatty acids and lipid mediator generation. Intensive Care Med 2003;29:1472–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stoner HB, Little RA, Frayn KN, Elebute AE, Tresadern J, Gross E. The effect of sepsis on the oxidation of carbohydrate and fat. Br J Surg 1983;70:32–35. [DOI] [PubMed] [Google Scholar]
- 48.Forse RA, Leibel R, Askanazi J, Hirsch J, Kinney JM. Adrenergic control of adipocyte lipolysis in trauma and sepsis. Ann Surg 1987;206:744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spitzer JA, Leach GJ, Palmer MA. Some metabolic and hormonal alterations in adipocytes isolated from septic dogs. Adv Shock Res 1980;4:55–62. [PubMed] [Google Scholar]
- 50.Grimminger F, Wahn H, Mayer K, Kiss L, Walmrath D, Seeger W. Impact of arachidonic versus eicosapentaenoic acid on exotonin-induced lung vascular leakage: relation to 4-series versus 5-series leukotriene generation. Am J Respir Crit Care Med 1997;155:513–519. [DOI] [PubMed] [Google Scholar]
- 51.Grimminger F, Durr U, Seeger W. Ligand-operated synthesis of 4-series and 5-series leukotrienes in human neutrophils: critical dependence on exogenous free fatty acid supply. Mol Pharmacol 1992;41:757–766. [PubMed] [Google Scholar]
- 52.Grimminger F, Mayer K, Kiss L, Walmrath D, Seeger W. PAF-induced synthesis of tetraenoic and pentaenoic leukotrienes in the isolated rabbit lung. Am J Physiol Lung Cell Mol Physiol 2000;278:L268–L275. [DOI] [PubMed] [Google Scholar]
- 53.Grimminger F, Wahn H, Kramer HJ, Stevens J, Mayer K, Walmrath D, Seeger W. Differential influence of arachidonic vs. eicosapentaenoic acid on experimental pulmonary hypertension. Am J Physiol 1995;268:H2252–H2259. [DOI] [PubMed] [Google Scholar]
- 54.Bernard GR, Wheeler AP, Russell JA, Schein R, Summer WR, Steinberg KP, Fulkerson WJ, Wright PE, Christman BW, Dupont WD, et al. The effects of ibuprofen on the physiology and survival of patients with sepsis: the Ibuprofen in Sepsis Study Group. N Engl J Med 1997;336:912–918. [DOI] [PubMed] [Google Scholar]
- 55.Romanovsky AA, Almeida MC, Aronoff DM, Ivanov AI, Konsman JP, Steiner AA, Turek VF. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front Biosci 2005;10:2193–2216. [DOI] [PubMed] [Google Scholar]
- 56.Clemmer TP, Fisher CJ Jr, Bone RC, Slotman GJ, Metz CA, Thomas FO. Hypothermia in the sepsis syndrome and clinical outcome: the Methylprednisolone Severe Sepsis Study Group. Crit Care Med 1992;20:1395–1401. [DOI] [PubMed] [Google Scholar]
- 57.Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci 2001;933:222–234. [DOI] [PubMed] [Google Scholar]
- 58.Hudert CA, Weylandt KH, Lu Y, Wang J, Hong S, Dignass A, Serhan CN, Kang JX. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc Natl Acad Sci USA 2006;103:11276–11281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells: autacoids in anti-inflammation. J Biol Chem 2003;278:14677–14687. [DOI] [PubMed] [Google Scholar]
- 60.Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid–induced colitis. Proc Natl Acad Sci USA 2005;102:7671–7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 2007;447:869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol 2008;9:873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, Israel E, Haley KJ, Serhan CN. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J Immunol 2007;178:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pacht ER, DeMichele SJ, Nelson JL, Hart J, Wennberg AK, Gadek JE. Enteral nutrition with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants reduces alveolar inflammatory mediators and protein influx in patients with acute respiratory distress syndrome. Crit Care Med 2003;31:491–500. [DOI] [PubMed] [Google Scholar]
- 65.Luyer MD, Greve JW, Hadfoune M, Jacobs JA, Dejong CH, Buurman WA. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J Exp Med 2005;202:1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.