Abstract
Rationale: Nitric oxide (NO) plays an important role in lung development and perinatal lung function, and pulmonary NO synthases (NOS) are decreased in bronchopulmonary dysplasia (BPD) following preterm birth. Fetal estradiol levels increase during late gestation and estradiol up-regulates NOS, suggesting that after preterm birth estradiol deprivation causes attenuated lung NOS resulting in impaired pulmonary function.
Objective: To test the effects of postnatal estradiol administration in a primate model of BPD over 14 days after delivery at 125 days of gestation (term = 185 d).
Methods: Cardiopulmonary function was assessed by echocardiography and whole body plethysmography. Lung morphometric and histopathologic analyses were performed, and NOS enzymatic activity and abundance were measured.
Measurements and Main Results: Estradiol caused an increase in blood pressure and ductus arteriosus closure. Expiratory resistance and lung compliance were also improved, and this occurred before spontaneous ductal closure. Furthermore, both oxygenation and ventilation indices were improved with estradiol, and the changes in lung function and ventilatory support requirements persisted throughout the study period. Whereas estradiol had negligible effect on indicators of lung inflammation and on lung structure assessed after the initial 14 days of ventilatory support, it caused an increase in lung neuronal and endothelial NOS enzymatic activity.
Conclusions: In a primate model of BPD, postnatal estradiol treatment had favorable cardiovascular impact, enhanced pulmonary function, and lowered requirements for ventilatory support in association with an up-regulation of lung NOS. Estradiol may be an efficacious postnatal therapy to improve lung function and outcome in preterm infants.
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Postnatal therapies to prevent bronchopulmonary dysplasia (BPD) in preterm infants are limited.
What This Study Adds to the Field
These experiments in a primate model indicate that postnatal estradiol treatment has favorable cardiovascular impact, enhances pulmonary function, and lowers requirements for ventilatory support in BPD. These effects occur in association with an up-regulation of lung nitric oxide synthases. Estradiol may be an efficacious postnatal therapy to improve lung function and outcome in preterm infants.
The signaling molecule nitric oxide (NO), generated by nitric oxide synthase (NOS), plays a key role in multiple processes in the mature lung (1, 2). In the developing lung, NO participates in pulmonary vascularization, alveolarization, and airway branching, and also counteracts apoptosis in multiple lung cell types (3–6). In the perinatal period, epithelium-derived NO is critically involved in the regulation of lung liquid production and of peripheral contractile elements (7, 8), and it also mediates pulmonary vasomotor tone (9). In studies of lungs from fetal baboons, we showed that all three NOS isoforms, neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS), are principally expressed in proximal respiratory epithelium, and that there are maturational increases in their abundance and in NO production during the early third trimester (10). Thus, pulmonary NOS expression is up-regulated during fetal development in the primate, and this process may be critical to lung structural development and airway, parenchymal, and pulmonary vascular function in the early postnatal period.
Bronchopulmonary dysplasia (BPD) is a devastating primary complication of premature birth that develops in the preterm human lung following ventilatory and oxygen support. This disorder results in disrupted lung maturation and postnatal pulmonary maladaptation. We previously determined whether there are alterations in NOS in proximal lung and accompanying changes in NO production in a model of BPD in baboon fetuses delivered at 125 days of gestation (term = 185 d) and ventilated for 14 days. This model best exemplifies the current form of BPD observed in extremely preterm human infants (11). In contrast to the normal 73% increase in NOS activity that occurs over the same developmental period in utero, there was an 83% decline in activity due to decreases in nNOS and eNOS expression. In addition, exhaled NO levels at the time of preterm birth at 125 days of gestation were one-third those observed at birth later in the third trimester, and they remained depressed until the 11th day of life (12). In addition, the administration of inhaled NO gas (iNO) in the same model improved early pulmonary function. However, with iNO these improvements were transient and there was negligible impact on ventilatory support requirements (13). Studies of NOS and iNO in the primate and other animal models of BPD led to trials of iNO therapy in preterm human infants. Collectively, these important trials have indicated that certain subsets of preterm infants benefit from iNO. However, others do not, and new postnatal strategies to improve lung function and prevent BPD continue to be needed (14–17).
The hormone estrogen up-regulates nNOS and eNOS and activates NOS enzymatic activity in diverse tissues and cell types (18–20), including ovine fetal lung in vivo, cultured bronchial epithelial cells, and pulmonary artery endothelial cells (20, 21). Fetal plasma estradiol (E2) levels increase progressively during late gestation, they rise further with the onset of parturition at term, and they fall in the early postnatal period due to the loss of the placentally derived hormone (22, 23). These cumulative observations suggest that there is relative estrogen deprivation following preterm birth that may adversely impact lung NOS and thereby impair pulmonary development and function.
In the present investigation we determined the effects of postnatal E2 administration in the baboon model of BPD that mirrors the current form of the disease in extremely preterm human infants (11). The baboons were born by Cesarean section at 0.67 gestation, which is comparable to 27 weeks postconceptual age in humans, and E2 was administered by subcutaneous pellet beginning at 1 hour of age. We tested the hypothesis that E2 improves the pulmonary dysfunction that is the result of prematurity and the development of BPD, and that this is associated with an up-regulation of pulmonary NOS. Because estrogen has numerous cardiovascular actions (24), we also determined the effects of postnatal E2 administration on pulmonary and systemic circulation and on the status of the ductus arteriosus. In addition, because the current form of BPD is characterized by abnormal elastin deposition and fewer and larger alveoli (25, 26), we evaluated the effect of E2 on pulmonary growth and structure. Furthermore, because estrogen modulates inflammatory responses (27), we assessed the effect of E2 on markers of lung inflammation.
METHODS
Animal Model
Fetal baboons were delivered at 125 ± 2 days gestation (term = 185 d) by Cesarean section. At birth the baboons were weighed, sedated, intubated, given 4 cc/kg of surfactant (Survanta, courtesy of Ross Laboratories, Columbus, OH), and ventilator support was provided for 14 days. Animals were randomly assigned to either the control group, which received routine care and control treatment or to the estrogen group, which received routine care plus E2. Detail on the management of the animals is provided in the online supplement.
Echocardiography and Pulmonary Function Testing
Echocardiographic studies were performed at 1 and 6 hours of age and at 24-hour intervals thereafter. The echocardiograms were done by one of the authors (DM) coincident to the pulmonary function tests, which were performed using the VT1000 body plethysmograph (Vitaltrends Technology, New York, NY) (13). Dynamic lung compliance and resistance measurements were made during controlled mechanical breaths, and they were of the respiratory system as a whole. Because tidal volume can impact measurements of compliance, tidal volume was controlled at 4 to 6 ml/kg, and measured tidal volumes did not differ between study groups. For data analysis, compliance was corrected for body weight.
Postmortem Pressure-Volume Measurements
Immediately before killing, the baboons breathed 100% oxygen for 5 minutes and the lungs were degassed by clamping the trachea at end of expiration for 2 minutes. Following the removal of the lungs from the thoracic cavity en bloc, postmortem quasi-static inflation pressure-volume measurements were performed. Detail on the method used is provided in the online supplement.
Morphometric-histopathologic Analyses and Assessments of Lung Inflammation
Analyses were performed using the methods previously reported (13, 28). Details are provided in the online supplement.
Pulmonary Surfactant Analysis
At the end of study, a bronchoalveolar lavage (BAL) was done on the left lower lobe with 25 ml of normal saline. Cells were removed by centrifugation and the supernatant was centrifuged (27,000 × g, for 60 min) to yield a large aggregate surfactant pellet and supernatant, and pulmonary surfactant analysis was performed. Details are provided in the online supplement.
NOS Enzymatic Activity and Expression
NOS enzymatic activity and expression were evaluated in lung parenchymal samples taken from the proximal third of the respiratory tree. This approach was taken because the three NOS isoforms are primarily expressed in the respiratory epithelium of the proximal airways of the developing primate (10). Details are provided in the online supplement.
Estrogen Receptor Expression
Using previously described methods (29), immunoblot analysis was performed to evaluate ERα and ERβ expression in the proximal lung of 125-day and 140-day gestational control group and in proximal lung from control- and E2-treated groups. Positive controls consisted of lysates of COS-7 cells transfected with cDNA for either human ERα or human ERβ.
Statistical Analysis
Differences between gestational control groups were compared by one-way analysis of variance (ANOVA) followed by Newman-Keuls post-hoc testing. Longitudinal between-group differences in pulmonary and cardiac function parameters over the full course of study were compared by two-way ANOVA. Repeated measure analysis was not performed for these endpoints because values for individual baboons were occasionally unobtainable due to technical difficulties or unavailability of the echocardiographer. The inflation and deflation limbs of the postmortem pressure-volume curves were assessed by separate two-way repeated measures ANOVA. Oxygenation index (OI) and ventilation index (VI) were assessed by repeated measures ANOVA. Single comparisons between two groups were performed with nonpaired Student's t tests or Mann-Whitney (nonparametric) for continuous data and by Fisher's exact test for categorical data. Significance was accepted at the 0.05 level of probability. All results are expressed as mean ± SEM.
RESULTS
Study Groups
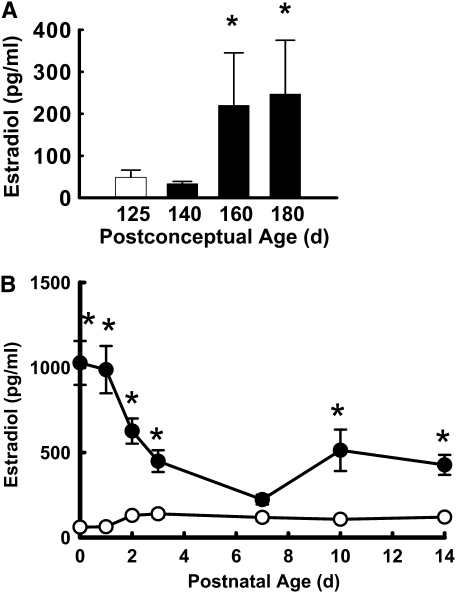
To first evaluate developmental changes in fetal serum E2 concentrations during the third trimester in the baboon, levels were initially measured in additional fetal baboons at 125 days, 140 days, 160 days, or 180 days gestation upon killing immediately at delivery. E2 levels were determined by radioimmunoassay as previously described (30). Fetal serum E2 concentrations rose fivefold between 125 to 140 days gestation and 160 to 180 days gestation to achieve mean levels of 250 pg/ml (Figure 1A). Seeking to attain the upper range of the concentrations observed in the latter third trimester, in the studies of postnatal E2 administration the hormone was provided by placement of a 0.5 mg, 21 days extended release pellet subcutaneously in the left axilla at 1 hour of life. Control animals received a placebo pellet, and a second E2 or control pellet was placed subcutaneously in the right axilla on Day 7 of life. The subcutaneous route was selected for E2 administration due to favorable stability and metabolic fate compared with intravenous or oral forms of estrogen (31, 32).
Figure 1.
(A) Fetal estradiol (E2) levels increase in the latter half of the third trimester of primate pregnancy. Serum E2 was measured in fetal baboons at 125 days, 140 days, 160 days, or 180 days gestation upon killing immediately at delivery. Values are mean ± SEM, n = 6/group. *P < 0.05 versus 125 days gestation. (B) Subcutaneous E2 administration raises serum levels in the immediate postnatal period. The hormone was provided postnatally to preterm baboons delivered by Cesarean section at 125 days gestation by placement of a 0.5 mg, 21-day extended release pellet subcutaneously in the left axilla at 1 hour of life. Control animals received a placebo pellet, and a second E2 or control pellet was placed subcutaneously in the right axilla on Day 7 of life. Serum levels were determined at 6 hours of life and at 1, 2, 3, 7, 10 and 14 days of age. Open circles = control group; Closed circles = estradiol group. Values are mean ± SEM, n = 8 and 9 for control and E2-groups, respectively. *P < 0.05 versus control.
Nine baboons were randomized to the control group and 10 to the E2 treatment group. There was one death in the control group, which occurred at 10 days of age, and there was one death in E2-treated animals at 11 days of age, and these were related to coagulase negative staph sepsis. These animals were excluded from longitudinal analyses. Six of eight animals in the control group and five of the nine in the E2-treated group were born following prenatal betamethasone treatment, and the remaining were born after prenatal dexamethasone. The birthweights, gestational ages at delivery, and number of males versus females were similar in the control and E2-treated groups (Table 1). There were no differences between groups for daily fluid intake, daily urine output, or daily weights over the course of the study (data not shown).
TABLE 1.
STUDY POPULATIONS
| Control | Estradiol | |
|---|---|---|
| Birthweight, g | 387 ± 48 | 370 ± 39 |
| Gestation, d | 125 ± 1 | 124 ± 1 |
| Sex, M/F | 7/1 | 6/3 |
Serum E2 levels achieved in the control and E2-treated groups are shown in Figure 1B. With E2 administration the initial E2 levels were 1,000 pg/ml at 6 hours of life, and they fell to 600 pg/ml on Day 2. By Day 7 of life in the treated animals, E2 levels were 230 pg/ml and a second E2 pellet was placed. E2 then rose to concentrations of 400 to 500 pg/ml during the second week of life. At all time points, except Day 7, E2 levels were greater in the treated group than in the control group.
Effect of E2 on Systemic and Pulmonary Hemodynamics
The administration of E2 immediately after birth and continuously throughout the study period caused an increase in mean systemic BP (Figure 2A). Whereas systolic BP did not differ between study groups, diastolic BP was greater with E2. This led to a lower requirement for pressor support in the estrogen group in which only two of nine animals required therapy versus seven of eight animals in the control group (P = 0.01). There were no demonstrable changes in the ratio of the estimated pulmonary artery pressure to systemic BP or the ratio of pulmonary to systemic blood flow with E2 administration (see Figures E1A and B, respectively, in the online supplement).
Figure 2.
Postnatal estradiol (E2) administration increases systemic blood pressure (BP) and causes closure of the ductus arteriosus. (A) Systemic mean, systolic and diastolic blood pressures were measured via an arterial catheter. (B) Ductal patency was determined by echocardiography. Green indicates an open ductus, red indicates a closed ductus, and white indicates that an echocardiogram was not performed. Values are mean ± SEM, n = 8 and 9 for control and E2-groups, respectively. Open circles = control group; Closed circles = estradiol group. Statistical comparisons of BP between groups were made by repeated measures analysis of variance, and ductal patency was compared by two-way analysis of variance. P < 0.05 for mean BP, diastolic BP and ductal patency.
E2 also altered the incidence of patent ductus arteriosus. Whereas none in the control group had spontaneous ductal closure during the 14-day study period, 4 of 9 in the E2-treated group had spontaneous ductal closure by echocardiography (Figure 2B). In one of these animals closure was apparent at 4 days of age, and in the other three animals ductal closure was found at 8 to 11 days of age. The increases in systemic BP with E2 occurred before the time of ductal closure (Figure 2A), indicating that the change in BP was due to processes other than the loss of left to right shunting across the ductus with its spontaneous closure.
Effect of E2 on Pulmonary Function
The effect of E2 on pulmonary function is shown in Figure 3. Expiratory resistance was lower across the study period in the E2 group (Figure 3A), and dynamic lung compliance was increased with E2 (Figure 3B). To provide an additional assessment of pulmonary function, postmortem pressure–volume (PV) curves were performed. During the procedure, air leaks occurred in the lungs of three control animals and of four E2-treated animals, and complete analysis was therefore available in lungs from five animals per study group. There was a directional change in both the inflation and deflation limbs of the PV curves, with shift upward and to the left with E2, and p values were 0.10 and 0.21, respectively (see Figure E2). The increase in lung volume at 35 cm H2O with E2 also approached, but did not achieve, statistical significance (34 ± 4 vs. 48 ± 6 ml/kg for lungs from control and E2 treatment groups, respectively, P = 0.07).
Figure 3.
Postnatal estradiol (E2) administration causes improvements in pulmonary function. (A) Expiratory resistance (cm H2O/ml/s) and (B) compliance (ml/cm H2O/kg) were measured by whole body plethysmography. Open circles = control group; Closed circles = estradiol group. Reported values are for the respiratory system as a whole. Values are mean ± SEM, n = 8 and 9 for control and E2 groups, respectively. Statistical comparisons were made between groups by two-way ANOVA. P < 0.05 for expiratory resistance and for compliance. Exp. Res. = expiratory resistance.
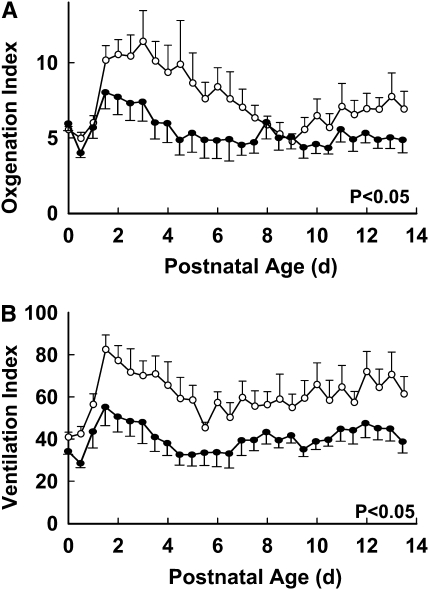
Effect of E2 on Ventilatory Support Requirements
The requirements for ventilatory support are shown in Figure 4. Oxygenation index over the course of the 14-day study was decreased in the E2-treated animals compared with controls (Figure 4A), frequently by 30 to 50%. As impressively, the ventilation index was also lowered throughout the postnatal period by E2 administration, typically by 40% or more (Figure 4B).
Figure 4.
Postnatal estradiol (E2) administration causes improvements in (A) oxygenation index and (B) ventilation index. Open circles = control group; Closed circles = estradiol group. Values are mean ± SEM, n = 8 and 9 for control and E2 groups, respectively. Statistical comparisons were made between groups by two-way analysis of variance (P < 0.05 for both indices).
Effect of E2 on Lung Weight, Structure, and Inflammation
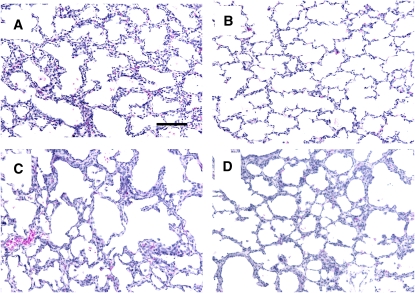
The weights of the lungs from control and E2-treated animals at the end of the study were similar (3.6 ± 0.2 and 3.8 ± 0.2% of body weight, respectively). The wet-to-dry weight ratios were also similar, being 5.13 ± 0.16 and 4.96 ± 0.30, respectively. Representative lungs from 125 days of gestation, 140 days of gestation, and control- and E2-treated animals are shown in Figure 5. With in utero development between 125 days and 140 days of gestation (Figure 5A and 5B), there was thinning of alveolar walls and increased septation. Compared with fetal lungs at 140 days of gestation, which is at the same postconceptual age (Figure 5B), lungs from control-treated animals on ventilatory support for 14 days had areas with thickened alveolar walls and simplification of the alveolar structures (Figure 5C). E2 administration did not alter overall lung histology compared with control treatment (Figure 5D). Pulmonary morphology was quantitatively compared in the two treatment groups by skeletonization of alveolar structures and computer-assisted measurements. E2 did not alter alveolar surface area, the number or length of end segments indicative of secondary crests, or other determined parameters (Table 2). E2 also did not alter lung vascularization evaluated by the distribution of PECAM-1 immunostaining of endothelial cells or PECAM-1 abundance, and VEGF expression or distribution was unchanged (Table E1) (data not shown).
Figure 5.
Representative lungs from (A) 125 days gestation, (B) 140 days gestation (C) control group and (D) E2-treated group. Original magnification, ×20, bar = 100 μm.
TABLE 2.
MORPHOMETRIC ANALYSIS OF ALVEOLAR STRUCTURE
| Control | Estradiol | |
|---|---|---|
| Lung volume, cm3 | 6.6 ± 0.6 | 7.5 ± 0.7 |
| Mean length of primary septa, μm | 11.2 ± 0.5 | 13.3 ± 0.6 |
| Number of branch points, n/mm2 | 1232 ± 115 | 865 ± 107 |
| Surface density of primary septa, cm2/cm3 | 269 ± 17 | 865 ± 107 |
| Total surface area of primary septa, cm2 | 1736 ± 127 | 1645 ± 194 |
| Mean length of end segments, μm | 6.7 ± 0.2 | 7.0 ± 0.2 |
| Number of end segments, n/mm2 | 200 ± 17 | 198 ± 13 |
| Surface density of end segments, cm2/cm3 | 26.3 ± 1.8 | 27.5 ± 1.3 |
| Total surface area of end segments, cm2 | 170 ± 13 | 206 ± 18 |
| End segment/internodal segment, n/mm | 15.0 ± 1.2 | 18.3 ± 1.1 |
| Length ratio of internodal/end segment, mm | 10.5 ± 0.9 | 8.0 ± 0.5 |
| Total alveolar surface area, cm2 | 1905 ± 132 | 1851 ± 208 |
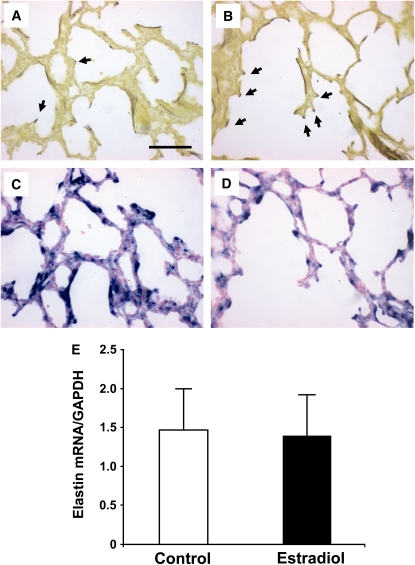
Elastin distribution and levels of expression were evaluated by multiple strategies. Hart's staining indicated that elastic fibers were localized to both alveolar walls and septal tips in lungs of control-treated animals (Figure 6A) and primarily to emerging septal tips in E2-treated lungs (Figure 6B). In situ hybridization on serial sections detected often intense elastin mRNA expression within alveolar walls and at emerging septae in the control-treated group (Figure 6C), and expression was localized primarily to emerging septae in E2-treated group (Figure 6D). However, quantitative reverse transcriptase polymerase chain reaction (RT-PCR) revealed equal total elastin mRNA abundance in control and E2-treated lungs (Figure 6E). Thus, E2 had a modest impact on elastin distribution favoring emerging septae, but did not alter its overall expression in the lung.
Figure 6.
Impact of postnatal estradiol (E2) administration on elastin distribution and expression. Hart's staining indicated that elastic fibers were localized to both alveolar walls and septal tips (arrows) in lungs of (A) control-treated animals and primarily to emerging septal tips in (B) E2-treated lungs. In situ hybridization on serial sections similarly detected often intense elastin mRNA expression within alveolar walls and at emerging septae in (C) control group, and expression was localized primarily to emerging septae in (D) E2-treated animals. Findings in A–D are representative of those in four to five animals per group, original magnification, ×200; bar = 50 μm. (E) Elastin mRNA expression was evaluated by quantitative reverse transcriptase–polymerase chain reaction (RT-PCR). GAPDH = glyceraldehyde 3-phosphate dehydrogenase. Values are mean ± SEM; n = 6/group.
The impact of E2 on lung inflammation was also assessed. Transforming growth factor (TGF)-β1 abundance in tracheal aspirates and terminal BAL samples did not change over the course of development of BPD in the primate, and it was also similar between control and E2 treatment groups (see Figure E3). In addition, levels of mRNA expression of genes that regulate inflammation in the lung and are altered in the primate BPD model (28) were not affected by E2 treatment (see Table E1).
Effect of E2 on Pulmonary Surfactant
In an effort to understand the basis for changes in pulmonary function with E2, surfactant-related parameters were measured in terminal bronchoalveolar lavage samples (see Figure E4). The total protein and phospholipid contents of the surfactant pellet were similar in the control and E2 groups (Figures E4A and E4B, respectively). Mean values for minimal surface tension were also similar, but there was a directional change suggesting improved surface tension properties in surfactant from the E2-treated animals, with surfactant from 44% of the animals achieving a normal minimal surface tension of less than 5 mN/m versus 25% in the control group (Figure E4C). Surfactant protein (SP)-A, SP-B and SP-C content in the surfactant pellet were unaffected by E2 administration (Figure E4D).
Effect of E2 on Pulmonary NOS and ER
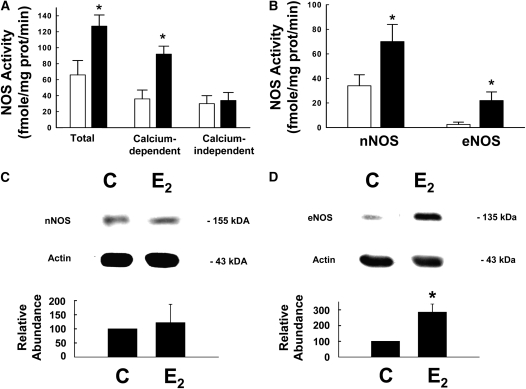
The impact of E2 on pulmonary NOS was first evaluated by determinations of total, calcium-dependent and calcium-independent NOS enzymatic activity in samples of proximal lung. Total NOS activity was greater in lungs from the E2-treated group versus controls, and this was entirely due to greater calcium-dependent activity whereas calcium-independent activity was unchanged (Figure 7A). nNOS- and eNOS-derived activity were determined, and nNOS activity was increased twofold with E2 and eNOS activity was increased by over sevenfold (Figure 7B). Whereas iNOS protein was not detected by immunoblot analysis in lungs from either study group (data not shown), nNOS protein was detected and was similar in abundance in lungs from control and E2-treated groups (Figure 7C). In contrast, eNOS protein expression was increased by more than threefold with E2 treatment (Figure 7D), and eNOS mRNA abundance was also increased by E2 (see Table E1).
Figure 7.
Postnatal estradiol (E2) administration up-regulates lung neuronal nitric oxide synthase (nNOS) and endothelial nitric oxide synthase (eNOS) enzymatic activity. Using arginine-to-citrulline conversion, total, calcium-dependent and calcium-independent NOS activity was measured in (A) proximal lung. (B) nNOS-derived and eNOS-derived enzymatic activity was also quantified. (C) nNOS and (D) eNOS protein abundance was evaluate by immunoblot analysis. Open circles = control group; Closed circles = estradiol group. In (C) and (D), upper panels display representative immunoblots for NOS and actin, and lower panels show the cumulative findings for NOS abundance relative to actin in lungs from six animals per group. C = control group; E2 = estradiol group. Values are mean ± SEM, *P < 0.05 versus control.
Having previously shown that estrogen up-regulation of eNOS expression is ER dependent (33), and that estrogen modifies ERα and ERβ abundance in primary fetal pulmonary artery endothelial cells (34), the impact of E2 on lung estrogen receptor expression was assessed by immunoblot analysis (see Figure E5). ERβ protein was detected in 125 days gestation control lung, and the level of expression did not change between 125 days and 140 days gestation; ERα protein was not detected (data not shown). ERβ was also readily detected in lungs from both control and E2-treated animals (Figure E5) and ERα protein was not (data not shown), and E2 administration did not modify ERβ abundance.
DISCUSSION
With preterm birth early in the third trimester, there is an obligatory decline in circulating E2 levels that otherwise would increase dramatically during the latter part of gestation in utero. Because pulmonary NOS is deficient following preterm birth, because E2 up-regulates NOS expression and activity in diverse tissues and cell types, and because NO plays an important role in lung development and perinatal lung function, we determined the impact of postnatal E2 administration on early postnatal pulmonary status in preterm baboons. We found that the provision of E2 enhanced pulmonary function and caused a persistent decrease in ventilatory support requirements. These benefits were associated with an up-regulation of both nNOS- and eNOS-derived NOS enzyme activity in the lung. Thus, postnatal E2 administration has a potent positive impact on pulmonary status following preterm birth in the primate.
Along with the pulmonary studies, the effect of E2 on systemic hemodynamic status was evaluated. Postnatal E2 administration caused a persistent elevation in mean systemic BP related to increased diastolic BP. The E2-treated animals also required pressor support less frequently than controls, and the disparities in BP between the two groups remained apparent despite the differences in pressor support. Both endogenous and exogenous estrogens stimulate the hepatic synthesis of angiotensin that raises aldosterone via activation of the renin-angiotensin system, and aldosterone causes renal sodium resorption, and these mechanisms may underlie the hypertension that can occur with oral contraceptive use (35, 36). However, these processes are unlikely in the present study in which first-pass hepatic metabolism of E2 was avoided with cutaneous delivery of the hormone (36), and daily weights and urine output were not affected by E2 treatment. Preterm human infants are at significant risk of hypotension and this is mimicked in the preterm baboon (37). Although the underlying mechanism is yet to be elucidated, the increase in systemic BP and the lowered requirements for pressor support observed with E2 in the present study would be of considerable potential clinical benefit.
An additional cardiovascular response observed with postnatal E2 administration was an increase in the rate of spontaneous closure of the ductus arteriosus. The increases in systemic BP with E2 occurred considerably earlier than the ductal closure evaluated daily by echocardiography, indicating that differences in ductal shunting between study groups are most likely not the cause for the disparities in systemic BP. Although a genetic polymorphism of ERα has been associated with a lower likelihood of patent ductus arteriosus in preterm male infants (38), further in-depth investigation will be required to elucidate the mechanisms by which E2 and ER influence ductal patency. The beneficial impact of E2 on ductal patency would decrease the need for pharmacologic or surgical closure of the ductus and also the risk of the multiple significant potential complications that can accompany these interventions (39).
The primary physiologic effects of E2 on the lung were to cause improvements in both dynamic lung compliance and expiratory resistance in the early postnatal period. The degrees and durations of these improvements surpass those obtained previously in the preterm baboon model with interventions including the administration of a superoxide dismutase mimetic, a modulator of prolyl hydrolase that impacts HIF-related processes, and inhaled NO gas (13, 40, 41). Although attempts to generate postmortem PV curves were complicated by air leaks in the lungs of some animals, the available data provide further evidence of improved lung function with postnatal E2 administration.
The improvements in pulmonary function caused by postnatal E2 administration likely underlie the decline in ventilatory support requirements reflected by long-term diminutions in both the oxygenation and ventilation indices. It is notable that this beneficial impact of postnatal E2 was apparent in the setting of maternal prenatal steroid treatment, which is the current clinical strategy in routine use that best optimizes the pulmonary status of the preterm infant (42).
E2 had negligible impact on pulmonary morphology, specifically secondary crest/end segment formation and vascularization. However, this may be due to the timing of the assessment of these parameters at 2 weeks of life, which is early in the postnatal period. Later impact on lung structure is possible because there were modest but consistent E2-induced changes in elastin distribution favoring localization to emerging septae at 2 weeks of age. Studies in postnatal rats and mice indicate that E2 modulates alveolar formation and regeneration (43, 44), and ER and progesterone receptor blockade during late gestation in piglets causes impaired alveolar formation and fluid clearance (45). However, aromatase inhibition during the latter half of baboon pregnancy that lowered umbilical venous E2 levels by 95% did not alter fetal lung growth or alveolarization (46). Considering the improvements in pulmonary function and support requirements that we observed in the first 2 weeks with E2 treatment and the benefits on lung structure that can be found after 28 days in this model if ventilatory support is lessened (11, 47), additional studies including long-term assessments of lung morphology are now warranted to determine the ultimate impact of postnatal E2 administration on the developing lung.
Lung inflammation was also evaluated to determine the potential basis for the observed effects of E2 on lung function. TGF-β1 levels in tracheal aspirates and terminal BAL were unchanged during the course of BPD development in the primate and were also unaffected by E2, and there were no effects of E2 on the expression of genes regulating lung inflammation. Surfactant-related parameters were also assessed in terminal BAL, and no changes were apparent with E2. The latter findings are consistent with previous observations that aromatase inhibition during the latter half of baboon pregnancy did not alter lung SP-A or SP-B expression (46). Thus, there were no observed changes in lung inflammation or surfactant status with postnatal E2 treatment.
Because E2 up-regulates NOS expression and enzymatic activity in numerous tissues and cell types (18–20), changes in lung NOS activity and expression that would be favorable to pulmonary function were anticipated. Increases in calcium-dependent nNOS-derived enzymatic activity were found in the lungs of E2-treated animals, and there were even greater increases in eNOS-derived activity. In contrast, iNOS-derived, calcium-independent activity was unaltered. There was no demonstrable change in nNOS protein expression with E2, and this may reflect the greater sensitivity of the enzyme activity assay to discern alterations in enzyme abundance. However, eNOS protein and mRNA expression were increased by E2 paralleling the rise in eNOS-derived activity, and this mirrors the known capacity of the hormone to up-regulate eNOS gene expression in numerous model systems (20). Thus, the beneficial changes in lung function induced by E2 were associated with up-regulation of NOS enzyme activity, and the pleiotropic functions of NO in pulmonary cells may be operative in this intervention. From a therapeutic standpoint, the ability to up-regulate endogenous pulmonary NOS may be more favorable than the provision of exogenous NO, which benefits only certain subpopulations of preterm infants at risk for BPD (14–17). Additional potential mechanisms of action of E2, including the nongenomic activation of eNOS that is independent in changes in enzyme abundance and other processes that do not involve NO (20), should be queried in future experiments.
BPD occurs in over 20% of the more than 50,000 preterm infants born in the U.S. each year with birthweights less than 1500 g (48), causing considerable morbidity and mortality, and additional strategies are needed to combat the disorder. In the animal model that best exemplifies the human condition, we have found that postnatal transcutaneous E2 administration following preterm birth caused persistent improvements in pulmonary function and a decrease in ventilatory support requirements in association with lung NOS up-regulation. In a recently reported small trial by Trotter and colleagues, intravenous E2 and progesterone treatment in preterm infants tended to decrease the incidence of BPD. Furthermore, their work to date suggests potential added benefits on bone mineralization, retinopathy of prematurity, and neurologic outcome (49–51), lending further credence to the concept of postnatal estrogen treatment. In our model there were also favorable impacts on hypotension and patent ductus arteriosus that are also key complications of prematurity. As such, estrogen-based therapies for BPD and other complications of prematurity should be further developed.
As future studies of postnatal E2 treatment for BPD are contemplated either in animal models or in humans, full consideration must be given to the possible effects of estrogen on nonpulmonary development including that related to reproductive health (52). Fortunately, in the studies of E2 and progesterone replacement in preterm infants, Trotter and coworkers found that changes in vaginal cytology and mammary and uterine growth were no greater than those that would have occurred in utero, and they ceased when replacement was discontinued (53). Furthermore, if necessary, systemic actions of estrogen can potentially be obviated by the use of E2 in aerosolized form (54). Thus, after decades of consideration of estrogen treatment to prevent diseases in the postmenopausal period, the hormone now has the potential to ameliorate a devastating condition at the extreme opposite end of the age spectrum.
Supplementary Material
Acknowledgments
The authors are indebted to Dr. J. Coalson and V. Winter without whom the studies could not have been performed. The authors also thank all the personnel that support the BPD Resource Center: the animal husbandry group led by Drs. D. Carey and M. Leland, the NICU staff (H. Martin, S. Ali, D. Correll, L. Kalisky, L. Nicley, R. Degan, S. Gamez), the Wilford Hall Medical Center neonatal fellows and D. Catland, NNP, who assist in the care of the animals, and the UTHSCSA pathology staff (L. Buchanan, H. Dixon, A. Schreiner) who perform necropsies and obtain biological specimens. The authors also thank M. Dixon for assistance in the preparation of the manuscript.
Supported by National Institutes of Health (NIH) grants HL63399 and HD30276 (P.W.S.), HL63387 (R.A.P.), HL63397 (J.D.C.), and HL46691 and HL56061 (R.I.C.). Additional support was provided by NIH grants HL52636 (to the Bronchopulmonary Dysplasia Resource Center) and P51RR13986 for facility support.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200805-794OC on January 16, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Barnes PJ. Nitric oxide and airway disease. Ann Med 1995;27:389–393. [DOI] [PubMed] [Google Scholar]
- 2.Gaston B, Drazen JM, Loscalzo J, Stamler JS. The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med 1994;149:538–551. [DOI] [PubMed] [Google Scholar]
- 3.Balasubramaniam V, Tang JR, Maxey A, Plopper CG, Abman SH. Mild hypoxia impairs alveolarization in the endothelial nitric oxide synthase (eNOS) deficient mouse. Am J Physiol Lung Cell Mol Physiol 2003;284:L964–L971. [DOI] [PubMed] [Google Scholar]
- 4.Young SL, Evans K, Eu JP. Nitric oxide modulates branching morphogenesis in fetal rat lung explants. Am J Physiol Lung Cell Mol Physiol 2002;282:L379–L385. [DOI] [PubMed] [Google Scholar]
- 5.Tang ZL, Wasserloos KJ, Liu X, Stitt MS, Reynolds IJ, Pitt BR, St Croix CM. Nitric oxide decreases the sensitivity of pulmonary endothelial cells to LPS-induced apoptosis in a zinc-dependent fashion. Mol Cell Biochem 2002;234–235:211–217. [PubMed] [Google Scholar]
- 6.Edwards YS, Sutherland LM, Murray AW. NO protects alveolar type II cells from stretch-induced apoptosis. A novel role for macrophages in the lung. Am J Physiol Lung Cell Mol Physiol 2000;279:L1236–L1242. [DOI] [PubMed] [Google Scholar]
- 7.Cummings JJ. Nitric oxide decreases lung liquid production in fetal lambs. J Appl Physiol 1997;83:1538–1544. [DOI] [PubMed] [Google Scholar]
- 8.Khassawneh MY, Dreshaj IA, Liu S, Chang CH, Haxhiu MA, Martin RJ. Endogenous nitric oxide modulates responses of tissue and airway resistance to vagal stimulation in piglets. J Appl Physiol 2002;93:450–456. [DOI] [PubMed] [Google Scholar]
- 9.Shaul PW. Nitric oxide in the developing lung. Adv Pediatr 1995;42:367–414. [PubMed] [Google Scholar]
- 10.Shaul PW, Afshar S, Gibson LL, Sherman TS, Kerecman JD, Grubb PH, Yoder BA, McCurnin DC. Developmental changes in nitric oxide synthase isoform expression and nitric oxide production in fetal baboon lung. Am J Physiol Lung Cell Mol Physiol 2002;283:L1192–L1199. [DOI] [PubMed] [Google Scholar]
- 11.Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA. Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med 1999;160:1333–1346. [DOI] [PubMed] [Google Scholar]
- 12.Afshar S, Gibson LL, Yuhanna IS, Sherman TS, Kerecman JD, Grubb PH, Yoder BA, McCurnin DC, Shaul PW. Pulmonary NO synthase expression is attenuated in a fetal baboon model of chronic lung disease. Am J Physiol Lung Cell Mol Physiol 2003;284:L749–L758. [DOI] [PubMed] [Google Scholar]
- 13.McCurnin DC, Pierce RA, Chang LY, Gibson LL, Osborne-Lawrence S, Yoder BA, Kerecman JD, Albertine KH, Winter VT, Coalson JJ, et al. Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. Am J Physiol Lung Cell Mol Physiol 2005;288:L450–L459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med 2003;349:2099–2107. [DOI] [PubMed] [Google Scholar]
- 15.Van Meurs KP, Wright LL, Ehrenkranz RA, Lemons JA, Ball MB, Poole WK, Perritt R, Higgins RD, Oh W, Hudak ML, et al. Inhaled nitric oxide for premature infants with severe respiratory failure. N Engl J Med 2005;353:13–22. [DOI] [PubMed] [Google Scholar]
- 16.Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, Sekar KC, Auten RL, Bhutani VK, Gerdes JS, et al. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med 2006;355:354–364. [DOI] [PubMed] [Google Scholar]
- 17.Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, Walsh MC, Durand DJ, Mayock DE, Eichenwald EC, et al. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med 2006;355:343–353. [DOI] [PubMed] [Google Scholar]
- 18.Gingerich S, Krukoff TL. Estrogen modulates endothelial and neuronal nitric oxide synthase expression via an estrogen receptor beta-dependent mechanism in hypothalamic slice cultures. Endocrinology 2005;146:2933–2941. [DOI] [PubMed] [Google Scholar]
- 19.Han G, Ma H, Chintala R, Miyake K, Fulton DJ, Barman SA, White RE. Nongenomic, endothelium-independent effects of estrogen on human coronary smooth muscle are mediated by type I (neuronal) NOS and PI3-kinase-Akt signaling. Am J Physiol Heart Circ Physiol 2007;293:H314–H321. [DOI] [PubMed] [Google Scholar]
- 20.Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev 2002;23:665–686. [DOI] [PubMed] [Google Scholar]
- 21.Parker TA, Kinsella JP, Galan HL, Le Cras TD, Richter GT, Markham NE, Abman SH. Prolonged infusions of estradiol dilate the ovine fetal pulmonary circulation. Pediatr Res 2000;47:89–96. [DOI] [PubMed] [Google Scholar]
- 22.Robertson HA, Dwyer RJ, King GJ. Oestrogens in fetal and maternal fluids throughout pregnancy in the pig and comparisons with the ewe and cow. J Endocrinol 1985;106:355–360. [DOI] [PubMed] [Google Scholar]
- 23.Gelly C, Sumida C, Gulino A, Pasqualini JR. Concentrations of oestradiol and oestrone in plasma, uterus and other tissues of fetal guinea pigs: their relationship to uptakeand specific binding of [3H] oestradiol. J Endocrinol 1981;89:71–77. [DOI] [PubMed] [Google Scholar]
- 24.Klouche M. Estrogens in human vascular diseases. Ann N Y Acad Sci 2006;1089:431–443. [DOI] [PubMed] [Google Scholar]
- 25.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol 1998;29:710–717. [DOI] [PubMed] [Google Scholar]
- 26.Thibeault DW, Mabry SM, Ekekezie II, Truog WE. Lung elastic tissue maturation and perturbations during the evolution of chronic lung disease. Pediatrics 2000;106:1452–1459. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson BO. Modulation of the inflammatory response by estrogens with focus on the endothelium and its interactions with leukocytes. Inflamm Res 2007;56:269–273. [DOI] [PubMed] [Google Scholar]
- 28.McCurnin D, Seidner S, Chang LY, Waleh N, Ikegami M, Petershack J, Yoder B, Giavedoni L, Albertine KH, Dahl MJ, et al. Ibuprofen-induced patent ductus arteriosus closure: physiologic, histologic, and biochemical effects on the premature lung. Pediatrics 2008;121:945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW. ERbeta has nongenomic action in caveolae. Mol Endocrinol 2002;16:938–946. [DOI] [PubMed] [Google Scholar]
- 30.Albrecht ED, Aberdeen GW, Pepe GJ. The role of estrogen in the maintenance of primate pregnancy. Am J Obstet Gynecol 2000;182:432–438. [DOI] [PubMed] [Google Scholar]
- 31.Koh KK, Yoon BK. Controversies regarding hormone therapy: insights from inflammation and hemostasis. Cardiovasc Res 2006;70:22–30. [DOI] [PubMed] [Google Scholar]
- 32.Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 8 Suppl 2005;1:3–63. [DOI] [PubMed] [Google Scholar]
- 33.MacRitchie AN, Jun SS, Chen Z, German Z, Yuhanna IS, Sherman TS, Shaul PW. Estrogen up-regulates endothelial nitric oxide synthase gene expression in fetal pulmonary artery endothelium. Circ Res 1997;81:355–362. [DOI] [PubMed] [Google Scholar]
- 34.Ihionkhan CE, Chambliss KL, Gibson LL, Hahner LD, Mendelsohn ME, Shaul PW. Estrogen causes dynamic alterations in endothelial estrogen receptor expression. Circ Res 2002;91:814–820. [DOI] [PubMed] [Google Scholar]
- 35.ESHRE Capri Workshop Group. Hormones and cardiovascular health in women. Hum Reprod Update 2006;12:483–497. [DOI] [PubMed] [Google Scholar]
- 36.Ashraf MS, Vongpatanasin W. Estrogen and hypertension. Curr Hypertens Rep 2006;8:368–376. [DOI] [PubMed] [Google Scholar]
- 37.Yoder B, Martin H, McCurnin DC, Coalson JJ. Impaired urinary cortisol excretion and early cardiopulmonary dysfunction in immature baboons. Pediatr Res 2002;51:426–432. [DOI] [PubMed] [Google Scholar]
- 38.Derzbach L, Treszl A, Balogh A, Vasarhelyi B, Tulassay T, Rigo JJ. Gender dependent association between perinatal morbidity and estrogen receptor-alpha Pvull polymorphism. J Perinat Med 2005;33:461–462. [DOI] [PubMed] [Google Scholar]
- 39.Chorne N, Leonard C, Piecuch R, Clyman RI. Patent ductus arteriosus and its treatment as risk factors for neonatal and neurodevelopmental morbidity. Pediatrics 2007;119:1165–1174. [DOI] [PubMed] [Google Scholar]
- 40.Chang LY, Subramaniam M, Yoder BA, Day BJ, Ellison MC, Sunday ME, Crapo JD. A catalytic antioxidant attenuates alveolar structural remodeling in bronchopulmonary dysplasia. Am J Respir Crit Care Med 2003;167:57–64. [DOI] [PubMed] [Google Scholar]
- 41.Asikainen TM, Chang LY, Coalson JJ, Schneider BK, Waleh NS, Ikegami M, Shannon JM, Winter VT, Grubb P, Clyman RI, et al. Improved lung growth and function through hypoxia-inducible factor in primate chronic lung disease of prematurity. FASEB J 2006;20:1698–1700. [DOI] [PubMed] [Google Scholar]
- 42.Jobe AH. Indications for and questions about antenatal steroids. Adv Pediatr 2002;49:227–243. [PubMed] [Google Scholar]
- 43.Massaro D, Clerch LB, Massaro GD. Estrogen receptor-alpha regulates pulmonary alveolar loss and regeneration in female mice: morphometric and gene expression studies. Am J Physiol Lung Cell Mol Physiol 2007;293:L222–L228. [DOI] [PubMed] [Google Scholar]
- 44.Morani A, Barros RP, Imamov O, Hultenby K, Arner A, Warner M, Gustafsson JA. Lung dysfunction causes systemic hypoxia in estrogen receptor beta knockout (ERbeta−/−) mice. Proc Natl Acad Sci USA 2006;103:7165–7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trotter A, Ebsen M, Kiossis E, Meggle S, Kueppers E, Beyer C, Pohlandt F, Maier L, Thome UH. Prenatal estrogen and progesterone deprivation impairs alveolar formation and fluid clearance in newborn piglets. Pediatr Res 2006;60:60–64. [DOI] [PubMed] [Google Scholar]
- 46.Pepe GJ, Ballard PL, Albrecht ED. Fetal lung maturation in estrogen-deprived baboons. J Clin Endocrinol Metab 2003;88:471–477. [DOI] [PubMed] [Google Scholar]
- 47.Thomson MA, Yoder BA, Winter VT, Martin H, Catland D, Siler-Khodr TM, Coalson JJ. Treatment of immature baboons for 28 days with early nasal continuous positive airway pressure. Am J Respir Crit Care Med 2004;169:1054–1062. [DOI] [PubMed] [Google Scholar]
- 48.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, Bauer CR, Donovan EF, Korones SB, Laptook AR, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol 2007;196:147–148. [DOI] [PubMed] [Google Scholar]
- 49.Trotter A, Maier L, Grill HJ, Kohn T, Heckmann M, Pohlandt F. Effects of postnatal estradiol and progesterone replacement in extremely preterm infants. J Clin Endocrinol Metab 1999;84:4531–4535. [DOI] [PubMed] [Google Scholar]
- 50.Trotter A, Bokelmann B, Sorgo W, Bechinger-Kornhuber D, Heinemann H, Schmucker G, Oesterle M, Kohntop B, Brisch KH, Pohlandt F. Follow-up examination at the age of 15 months of extremely preterm infants after postnatal estradiol and progesterone replacement. J Clin Endocrinol Metab 2001;86:601–603. [DOI] [PubMed] [Google Scholar]
- 51.Trotter A, Maier L, Kron M, Pohlandt F. Effect of oestradiol and progesterone replacement on bronchopulmonary dysplasia in extremely preterm infants. Arch Dis Child Fetal Neonatal Ed 2007;92:F94–F98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones LA, Hajek RA. Effects of estrogenic chemicals on development. Environ Health Perspect 1995;103:63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trotter A, Maier L, Kohn T, Bohm W, Pohlandt F. Growth of the uterus and mammary glands and vaginal cytologic features in extremely premature infants with postnatal replacement of estradiol and progesterone. Am J Obstet Gynecol 2002;186:184–188. [DOI] [PubMed] [Google Scholar]
- 54.Studd J, Pornel B, Marton I, Bringer J, Varin C, Tsouderos Y, Christiansen C. Efficacy and acceptability of intranasal 17 beta-oestradiol for menopausal symptoms: randomised dose–response study. Aerodiol Study Group. Lancet 1999;353:1574–1578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.