Abstract
We examined how mood and activation of old age schema influenced attentional focus on physical symptoms in older adults. Seventy-one individuals aged 60 years or above participated in an experiment that manipulated mood and old age schema. They completed a modified Stroop task that measured attentional bias to physical symptoms. Controlling for baseline processing speed, the two variables had significant main and interaction effects on attentional focus on symptoms. Sad mood and old age schema independently minimized the bias to avoid attending to symptoms. When combined, these two variables contributed to greater attentional focus on symptoms. These findings highlight psychological influences on current attention to symptoms.
Keywords: Aging, Mood, Physical symptoms
STUDIES on emotion, health, and psychopathology often employ the term “attentional bias” to describe the allocation of attention to stimuli that signal potential threat (e.g., Snider, Asmundson, & Weiss, 2000; Williams, Mathews, & MacLeod, 1996). Once a stimulus is attended to and deemed important, relevant cognitive–emotional materials are activated to influence behaviors and judgments (Beck & Clark, 1997). For example, attentional bias to pain-related stimuli among chronic pain patients promotes anxiety and avoidant behaviors that exacerbate and maintain pain (Snider et al., 2000). Most studies of attentional bias to symptoms have not focused on older adults, who may attend to symptoms differently because they tend to have more chronic conditions and symptoms than younger adults (Leventhal & Crouch, 1997). It is important to study attentional bias in older adults, whose attention allocation to symptom-related stimuli may affect perception and reporting of illness symptoms. A commonly used method to assess attentional bias in the health and psychopathology literature is the modified Stroop task (Snider et al., 2000; Williams et al., 1996). Participants are instructed to name the color of each stimulus word rather than reading it aloud. A longer response latency indicates attention to word content that disrupts color naming (Williams et al., 1996). Attentional bias is calculated by subtracting the average response latency to neutral words from latency to symptom words. A positive score suggests attentional bias to symptoms.
Mood may influence an individual's attentional processes (e.g., Fox & Knight, 2005; Mor & Winquist, 2002). Stimuli with a negative emotional valence may become more salient and attract more attention when someone is in a bad mood (Williams et al., 1996). Different mood induction techniques, such as a combination of music and autobiographical recall, have effectively induced sad mood (Gerrards-Hesse, Spies, & Hesse, 1994). However, the influence of mood on attentional bias to symptoms has only been studied among college students and people with specific illnesses (Gendolla, Abele, Andrei, Spurk, & Richter, 2005; Williams et al., 1996). Few studies evaluated the moderators of the relationship between mood and attention to symptoms, with the exception of self-focus (Gendolla et al., 2005). In emotionally distressed individuals, activation of cognitive structures relevant to a stimulus has been thought to promote attentional bias to that stimulus (Beck & Clark, 1997). Aging-related cognitions may enhance attentional bias to symptoms among older adults, who tend to attribute physical symptoms to old age (Leventhal & Crouch, 1997). The relevance of aging-related cognitions to health decline has been illustrated in the aging stereotype literature. When primed with aging-related words, older adults had slower walking speed and poorer gait (Hausdorff, Levy, & Wei, 1999). This behavioral consequence of aging stereotypes suggests that physical decline can be activated via an old age schema. No study has explored how the old age schema influences attentional bias to symptoms among older adults. In light of the role of cognition in emotional distress (Beck & Clark, 1997), this study explored whether the cognitive schema of old age would enhance the influence of sad mood on older adults’ attentional bias to symptoms.
To examine how sad mood and the old age schema affect attentional bias to symptoms in older adults, we conducted an experiment with a 2 × 2 between-subjects design manipulating mood (sad, neutral) and old age schema (activated, not activated). We hypothesized (a) a main effect of mood, in which older adults in a sad mood had stronger attentional bias to symptoms, (b) a main effect of old age schema, in which older adults with an activated old age schema had stronger attentional bias to symptoms, and (c) a Mood × Schema interaction on attentional bias to symptoms.
METHODS
Participants
Seventy-six community-dwelling older adults aged 60 years and above participated in the study. Five were excluded because of a self-reported diagnosis of depression, poor English comprehension, or visual impairment. The final sample included 71 participants (mean age = 73.79, SD = 7.68). Sixty-one percent were women. Over half of the participants were Caucasian (58%; Asian: 23%, African American: 10%, other: 9%). Participants’ average years of education was 16.65 (SD = 2.72).
Measures
Sad mood.—
The 20-item Center for Epidemiological Studies–Depression scale (CES-D; Radloff, 1977) was modified to measure current mood state (Fox, Knight, & Zelinski, 1998). Internal consistencies of the present tense CES-D ranged from .85 to .93 in older participants (Fox et al., 1998; Knight, Maines, & Robinson, 2002).
English proficiency.—
We used 20 items from the Shipley (1986) vocabulary test as a measure of cognitive ability and English proficiency. Each correct response was given one point. Average prorated score of our sample was 35.9, similar to previous findings (Knight et al., 2002).
Procedure
Participants completed a background questionnaire and the vocabulary test. They were randomly assigned to one of the four groups: (a) control, (b) old age schema, (c) sad mood, and (d) sad mood and old age schema. In the sad mood conditions, participants wrote about a personal event that made them feel very sad. In the neutral mood conditions, participants wrote about an event that made them neither happy nor sad. Sad or neutral music was played during the study for mood retention (see Fox et al., 1998). Participants in the age schema conditions then answered eight aging-related questions, such as “At what age do you think the average person becomes old?” Participants in the no age schema conditions answered eight similarly structured questions about population density, such as “How many people per square mile do you think a crowded city has?” We administered the CES-D as a manipulation check.
Participants then completed the modified Stroop task. Eighteen symptom words and 18 neutral words were presented at the center of a 640 × 480 pixel screen, each preceded by the presentation of a white fixation cross for 1,000 ms. Words were matched in frequency and length (Kucera & Francis, 1967; see Appendix). To ascertain that differences in response were not due to schematic relatedness among symptom words, all neutral words were selected from the semantic category, work. Words were presented in red, yellow, green, and blue fonts. The presentation order was randomized, but the same word would not appear in succession. Participants pressed one of the four keys to identify word color. Twenty practice trials were given to assess baseline processing speed. Response accuracy and reaction time (RT) from stimulus onset to pressing of any colored key were recorded. The task also included five aging-related words (see Appendix) as a manipulation check for age schema activation. Finally, participants answered a health questionnaire and completed the poststudy CES-D.
RESULTS
Chi-square and analysis of variance (ANOVA) values did not reveal significant differences in demographic background and health across the four groups.
Manipulation Checks
To examine the effectiveness of our mood induction, we conducted a Mood × Schema repeated measures ANOVA with time as a within-subject factor and CES-D scores as the dependent variable. There was a significant main effect of time on CES-D scores, F(2, 66) = 9.94, p < .001, η2 = 0.23, and significant Time × Mood interaction, F(2, 66) = 11.46, p < .001, η2 = 0.26. Multiple comparisons revealed that preinduction scores did not significantly differ across groups. However, there was a significant increase in postinduction scores in the two sad mood conditions: t(18) = 2.42, p < .05 and t(17) = 3.95, p < .01, but not the control group: t(15) = –1.33, ns. End-of-study scores were not significantly different from preinduction scores: t = –1.21 to –0.75, ns. In other words, mood induction was effective in inducing sad mood, and the effect of mood induction dissipated by the end of the study. Excluding participants who were not individually induced into sad mood did not create significant changes in our findings.
To determine whether old age schema was activated, we conducted a Mood × Schema analysis of covariance with attentional bias to aging-related words as our dependent variable, covarying processing speed. RT data may be susceptible to the influence of outliers. However, no values were three standard deviations or more from the mean. Thus, all values were retained for analysis. Mean RTs are reported in Table 1. A significant main effect of old age schema emerged, F(1, 66) = 45.89, p < .001, η2 = 0.41. Main effect of mood and Mood × Schema interaction were nonsignificant, F(1, 67) = 3.01, p = .09, η2 = 0.04 and F(1, 67) = 0.44, p = .51, η2 = 0.01, respectively. Compared with the non age schema groups, the old age schema groups paid more attention to aging-related words, and this was not due to their induced mood state.
Table 1.
Mean Reaction Times (SD) to Different Word Stimuli in Milliseconds
| Word Type |
|||
| Group | Neutral | Illness | Aging Related |
| Control | 916.08 (145.85) | 904.46 (148.62) | 910.34 (145.76) |
| Old age schema | 930.53 (121.46) | 928.40 (122.16) | 934.92 (120.87) |
| Sad mood | 935.98 (134.56) | 933.92 (135.94) | 933.35 (134.51) |
| Old age schema and sad mood | 904.75 (102.80) | 919.57 (103.09) | 910.62 (102.54) |
| All participants | 922.20 (124.58) | 922.24 (125.74) | 922.80 (124.29) |
Attentional Bias to Symptoms
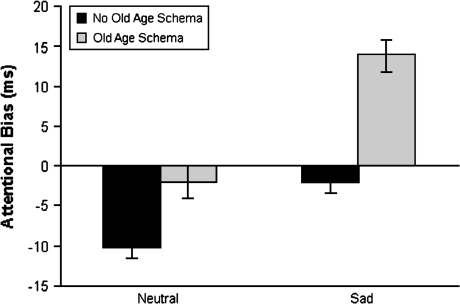
Controlling for baseline processing speed, significant main effects of mood and old age schema on attentional bias to symptom words emerged, F(1, 66) = 57.66, p < .001, η2 = 0.47 and F(1, 66) = 58.14, p < .001, η2 = 0.47, respectively. The Mood × Schema interaction was also significant, F(1, 66) = 5.86, p < .05, η2 = 0.08. Post hoc analysis showed that the control group focused significantly less attention on symptom words than participants whose old age schema was activated (mean difference = −9.44, SE = 2.44, p < .05). The control group also attended less to symptoms compared with the sad mood group (mean difference = −9.50, SE = 2.41, p < .05). As Figure 1 illustrates, sad mood and old age schema independently minimized the tendency to avoid attending to symptoms. When both sad mood and old age schema were induced, participants showed a net attentional bias toward symptoms.
Figure 1.
Attentional bias to symptom words in milliseconds. Error bars represent standard errors.
DISCUSSION
To our knowledge, this is the first experimental study to examine how mood and old age schema influence older adults’ attention to physical symptoms. Our hypotheses were supported with regard to the effects of sad mood and old age schema on attentional bias to symptoms. However, although sad mood and old age schema independently eliminated older adults’ tendency to direct attention away from symptoms, neither one alone resulted in a net attentional bias toward symptoms. Attentional bias toward symptoms occurred in the presence of only both sad mood and old age schema.
In previous research, it has been found that negative mood heightened attention to physical symptoms only when there was a simultaneous focus on the self (Gendolla et al., 2005). Our study did not deliberately elicit self-focus, but the self-relevance of old age schema may produce a type of self-focus. Old age schema also could have promoted attention to symptoms because poor health is a common theme within aging stereotypes. Future research on the impact of old age schema on older adults’ attention to physical symptoms should explore the underlying mechanisms.
Our study is limited in the following ways: Using aging-related words in the modified Stroop task as a manipulation check could have facilitated attentional bias to symptoms because of their conceptual relevance. However, participants whose old age schema was not activated before the Stroop task did not demonstrate an attentional bias to symptoms. Inclusion of aging-related words did not seem to reduce the effect of sad mood and old age schema on attentional bias to symptoms. Nevertheless, future studies may benefit from a different manipulation check. Although our sample was ethnically diverse, most participants were highly educated, and their depressed mood scores were low. Research is needed to replicate these findings in less educated older adults and those who are experiencing more distress.
Overall, our study shows that both sad mood and age-related cognitions influence older adults’ symptom-related information processing. Understanding influences on older adults’ attention to symptoms is important because attention toward or away from symptom-related information may influence symptom-related distress and behaviors (Cioffi, 1991; Snider et al., 2000). Clinically, this implies that health interventions need to consider both older adults’ mood state, as well as broader beliefs about aging, and the combination of the two as influences on symptom perception and related decisions and behaviors.
Acknowledgments
Portions of this research were presented at the 2007 meeting of the Gerontological Society of America in San Francisco, CA.
APPENDIX
Word stimuli for the Modified stroop task
Symptom words: vertigo, stiffness, dizziness, indigestion, cramp, numbness, heaviness, congested, nausea, breathless, headache, lump, cough, bruised, sore, fatigue, swollen, pain.
Neutral words: florist, salesgirl, bodyguard, interviewer, crews, artisans, comedians, announcer, keeper, ballplayer, laborers, heir, chore, peasant, tech, athlete, pirates, king.
Aging-related words: aging, elderly, seniors, slowness, wrinkles.
References
- Beck AT, Clark DA. An information processing model of anxiety: Automatic and strategic processes. Behaviour Research and Therapy. 1997;35:49–58. doi: 10.1016/s0005-7967(96)00069-1. [DOI] [PubMed] [Google Scholar]
- Cioffi D. Beyond attentional strategies: A cognitive-perceptual model of somatic interpretation. Psychological Bulletin. 1991;109:25–41. doi: 10.1037/0033-2909.109.1.25. [DOI] [PubMed] [Google Scholar]
- Fox LS, Knight BG. The effects of anxiety on attentional processes in older adults. Aging and Mental Health. 2005;9:585–593. doi: 10.1080/13607860500294282. [DOI] [PubMed] [Google Scholar]
- Fox LS, Knight BG, Zelinski E. Mood induction with older adults: A tool for investigating effects of depressed mood. Psychology and Aging. 1998;13:519–523. doi: 10.1037//0882-7974.13.3.519. [DOI] [PubMed] [Google Scholar]
- Gendolla GHE, Abele AE, Andrei A, Spurk D, Richter M. Negative mood, self-focused attention, and the experience of physical symptoms: The joint impact hypothesis. Emotion. 2005;5:131–144. doi: 10.1037/1528-3542.5.2.131. [DOI] [PubMed] [Google Scholar]
- Gerrards-Hesse A, Spies K, Hesse FW. Experimental inductions of emotional states and their effectiveness. British Journal of Psychology. 1994;85:55–78. [Google Scholar]
- Hausdorff JM, Levy BR, Wei JY. The power of ageism on physical function of older persons: Reversibility of age related gait changes. Journal of the American Geriatrics Society. 1999;47:1346–1349. doi: 10.1111/j.1532-5415.1999.tb07437.x. [DOI] [PubMed] [Google Scholar]
- Knight BG, Maines M, Robinson GS. The effects of sad mood on memory in older adults: A test of the mood congruence effect. Psychology and Aging. 2002;17:653–661. doi: 10.1037//0882-7974.17.4.653. [DOI] [PubMed] [Google Scholar]
- Kucera H, Francis WN. Computational analysis of present-day English. Providence, RI: Brown University Press; 1967. [Google Scholar]
- Leventhal EA, Crouch M. Are there differences in perceptions of illness across the lifespan? In: Petrie KJ, Weinman JA, editors. Perceptions of health and illness. Amsterdam: Harwood Academic Press; 1997. pp. 77–101. [Google Scholar]
- Mor N, Winquist J. Self-focused attention and negative affect: A meta-analysis. Psychological Bulletin. 2002;128:638–662. doi: 10.1037/0033-2909.128.4.638. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Shipley WC. Shipley Institute of Living Scale. Los Angeles: Western Psychological Services; 1986. [Google Scholar]
- Snider BS, Asmundson GJG, Weiss KC. Automatic and strategic processing of threat cues in patients with chronic pain: A modified-Stroop evaluation. Clinical Journal of Pain. 2000;16:144–154. doi: 10.1097/00002508-200006000-00008. [DOI] [PubMed] [Google Scholar]
- Williams JM, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychological Bulletin. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]