Abstract
Aging is known to alter the circadian rhythms of melatonin, serotonin, thermoregulatory responses, cytokine production, and sleep/wakefulness which affect sleep quality. We tested the possible palliative effects of a 3-day administration of melatonin (0.25 or 2.5 mg/kg of body weight [b.w.] to young and old ringdoves, respectively) or tryptophan (300 mg/kg of b.w. to old ringdoves) on these rhythms. Doves are a monophasic, diurnal species; these characteristics are similar in humans. Old animals presented lower melatonin and serotonin levels; higher interleukin (IL)-1β, IL-6, and tumor necrosis factor alpha values; and reductions in the Midline-Estimating Statistic of Rhythm and amplitude of activity–rest rhythm and in the amplitude of the core temperature rhythm. Melatonin raised serum melatonin levels; tryptophan increased both melatonin and serotonin levels. Melatonin and tryptophan lowered nocturnal activity, core temperature, and cytokine levels and increased peripheral temperature in both groups. Melatonin or tryptophan may limit or reverse some of the changes that occur in sleep–wake rhythms and temperature due to age.
Keywords: Melatonin, Tryptophan, Temperature, Interleukins, Aging
AGING is a complex multifactorial process associated with internal desynchronization of circadian rhythms. Among them, increased sleep fragility and reductions in waking efficiency are particularly salient (1–3). The thermoregulatory responses and the capacity for thermal comfort are also reduced in elderly humans (4). This alteration is seemingly of importance during aging because temperature changes before and after sleep onset may act in a positive feedback loop to maintain a consolidated sleep bout (5).
The age-related disturbances in sleep–wake and temperature rhythms have been correlated with the age-related reductions in the amplitude of the nocturnal melatonin peak (6). In fact, rhythms of body temperature, sleep–wake, and responsiveness to light–dark are closely related to the secretion of melatonin, which the body uses as a time cue and sleep-promoting signal (7,8). As an example, melatonin has hypothermic effects (9) that are independent of the species chronotype (the period where a given species is more active) (10–12), but the environmental temperature may also be used as a zeitgeber regulating the secretion of melatonin in some animal species (13). Moreover, sleep efficiency, measured as the total delta power (an indicator of the depth or intensity of sleep) and the total duration of sleep, also increases in proportion to the duration of elevated melatonin levels (14). Tryptophan, the precursor of melatonin and also of the neurotransmitter serotonin, is known to increase brain serotonin and melatonin levels during light and dark, respectively (15) as well as the delta power and the amount of nonrapid eye movement sleep (16). These results support the use of dietary tryptophan supplementation for the treatment of age-related sleep disturbances (17–19).
In previous investigations in the ringdove (Streptopelia risoria), we found that the administration of melatonin led to an improvement of nocturnal rest as well as an increase in both nocturnal and diurnal melatonin levels in both young and old ringdoves (20). The same occurred with tryptophan in old birds, whose administration also raised circulating levels of serotonin (21). We hypothesized that the reduced activity observed following both melatonin and tryptophan administration may be related to the acute hypothermic effects exerted by the pineal indole (22). But this question remains unanswered.
Another age-related disturbance is the depression of the immune system, a process that has been called immunosenescence (23). In the ringdove, melatonin and tryptophan treatments have been shown to enhance the phagocytic function of heterophils (the equivalent of neutrophils in humans), which is impaired in the old animals, as well as neutralizing oxidative stress deriving from elevated immune function both in vivo and in vitro (24–28). Immune senescence is also associated with increases in the plasma levels of cytokines (29), a diverse group of nonantibody intercellular signaling proteins that regulate local and systemic immune and inflammatory responses as well as many other biological processes. Cytokines may represent a link between the immune and inflammatory systems (30) and have an important role in the regulation of sleep (31). Among the cytokines, interleukin (IL)-1β, IL-6, and tumor necrosis factor alpha (TNF-α) have been described as important factors that mediate sleep processes (32–34). Taking into account the cytokine–sleep relationship and the regulation exerted by tryptophan and melatonin on the immune response of the ringdove, an evaluation of the levels of these immune molecules and the possible effects that the amino acid and the indole may possess on them is required.
Accordingly, the present report aimed to determine age-related changes in the activity–rest, body temperature, and IL-1β, IL-6, and TNF-α levels of young and old ringdoves, as well as examining their eventual reversion as a consequence of tryptophan and melatonin administration. Furthermore, circulating levels of melatonin and serotonin were measured to evaluate the possible differences existing between animals of different ages and establish potential correlations between these parameters and activity–rest and temperature values.
METHODS
Animals
Male and female ringdoves (S. risoria) of 4–5 years of age (young) and 12–14 years of age (old; average life span of 15 years) weighing 150 ± 20 g were used in the study (n = 10, per age group). The animals were bred in our department and individually housed in 40 × 40 × 45 cm cages under controlled environmental conditions (22°C; 70% humidity), kept under a 12/12-hour light/dark photoperiod (darkness from 8 PM to 8 AM), and fed ad libitum (food and water). For the activimetry studies, the animals were transferred to specially designed cages (see Measurement of the activity–rest rhythms) and maintained under the same conditions as described previously. S. risoria is a species characterized by being diurnal and monophasic with sleep–wake cycles similar to those of humans and is therefore a good model to investigate impairments in the circadian system due to age.
The study was approved by the Ethical Committee of the University of Extremadura (Badajoz, Spain) in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Animal Treatment
Young animals were treated for 3 consecutive days with a single daily oral dose (0.25 mg/0.1 mL phosphate-buffered saline [PBS] per animal per day) of melatonin (Sigma, St Louis, MO) 1 hour before lights off (7 PM). Old animals were treated for 3 consecutive days with either a single daily oral dose (2.5 mg/0.1 mL PBS per animal per day) of melatonin 1 hour before lights off or a single oral capsule of 300 mg/kg of body weight (b.w.) of L-tryptophan (Sigma) at 9 AM (1 hour after the onset of the light period). The choice of these concentrations and of the time of administration were based on preliminary studies of administration of melatonin and L-tryptophan, which indicated that these doses induced an improvement of nocturnal rest in this species, with minimal effects on diurnal activity (20,21). Control birds received only 0.1 mL of PBS or capsules containing the excipient—methylcellulose (Sigma)—with the same schedule as the melatonin- or tryptophan-treated animals, respectively. Basal values were obtained before treatment from animals that had not been given PBS, methylcellulose, melatonin, or tryptophan. As no significant variations were observed in these values with respect to the controls, they will not be reported in the results section.
Measurement of the Temperature Rhythms
Core temperature was measured in the cloaca using a thermocouple with digital readout. The lubricated probe was inserted a distance of 3 cm in lightly hand-restrained animals, for approximately 20 seconds until reaching a steady reading. Peripheral body temperature was measured on the naked skin under the wings using an infrared thermometer (DT-8818; PCE Group, Tobarra (Albacete), Spain). Periodic (1- to 3-hour intervals) temperature measurements were performed to record the time course of body temperature.
Measurement of the Activity–Rest Rhythms
Each cage was equipped with an infrared activimetry system using two crossed perpendicular beams situated in a plane 70 mm above the sustenance plane to detect activity. Motor activity counts were automatically recorded every 15 minutes on a computer by means of a data-acquisition system. An activity pulse was recorded each time there was a change of state in the receivers, that is, the receivers activated a Transistor-transistor logic signal (5–0 V) when the beam was interrupted or restored. The change of state of the beam was interpreted as a digital signal (activity pulses), which was acquired by the computer and automatically stored using the DAS16 program (35) for further analysis. The results of the activity assessment in both young and old animals are expressed as the average of the total diurnal and nocturnal activities.
Serum Collection
Blood samples were drawn from all 10 birds at each sampling time, allowing at least 1 week between consecutive extractions. The collections (1 mL per animal per week) were taken from the brachial vein with a 25-gauge needle and a syringe at 2- or 3-hour (diurnal) and 1-hour (nocturnal) intervals and then transferred unheparinized to a pre-prepared tube containing serum-separating gel. The samples were centrifuged at room temperature for 15 minutes at 300g. The serum was then divided into aliquots in Eppendorf vials and kept frozen at −30°C until the time of assay.
Measurement of Serotonin, Melatonin, and IL-1β, IL-6, and TNF-α in Serum
Serotonin and melatonin were determined by means of commercial enzyme-linked immunosorbent assay (ELISA) (IBL, Hamburg, Germany) and radioimmunoassay (IBL) kits, respectively. Cytokine (IL-1β, IL-6, and TNF-α) levels were measured using commercial EASIA (Enzyme Amplified Sensitivity Immunoassay) kits (Biosource, Nivelles, Belgium), a modified ELISA that uses monoclonal antibodies directed against distinct antibodies for sensitivity amplification. Determinations were made in duplicate. Melatonin and cytokine results are expressed in picograms per milliliter. Serotonin values are given in nanograms per milliliter.
Chronobiological Analysis
For the study of activity–rest and temperature in the animals, a mean population cosinor analysis was performed, using the integrated computer software packages Cosinor and El Temps (Antoni Díez-Noguera, University of Barcelona, Spain). The data were fitted to the cosine function Y(t) = M + A cos(ωt − ϕ), where M is the MESOR (Midline-Estimating Statistic of Rhythm), or mean value of the fitting function; A is the amplitude or difference between the maximum or minimum value of the fitting function and the MESOR; ω is 2π times the frequency or number of oscillations per unit time; and ϕ (acrophase) is a phase angle (a measure of the timing of the peak of the variable), expressed as the lag from the reference time (in our study lights off) to the crest time of the cosine function best approximating the data. The value of ω was fixed to correspond to a 24-hour period (ω = 0.2618 rad/h).
Statistical Analysis
Data are expressed as mean ± SD of the number of determinations. The results were analyzed by using Friedman ranges for paired samples, followed by Kruskal–Wallis multiple contrasts. Correlations by multiple regression of different activity–rest and temperature values with the melatonin and serotonin data at the hours studied were taken as significant if the R2 met the criterion of significance at p < .01.
RESULTS
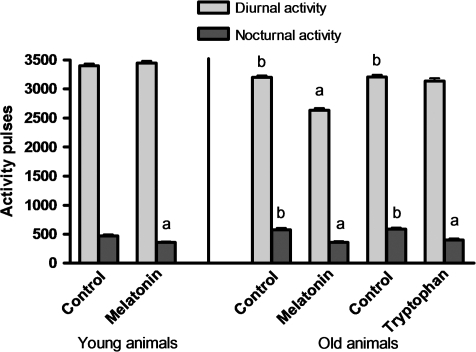
As expected, diurnal activity was always higher than nocturnal activity (p < .01), although the difference was less for older birds. Melatonin treatment reduced nocturnal activity in both old and young birds (p < .01) and also decreased diurnal activity in old birds (Figure 1). Tryptophan had similar effects on the nocturnal activity of old birds.
Figure 1.
Total diurnal and nocturnal activities in young ringdoves treated with 0.25 mg melatonin/kg of body weight (b.w.) 1 hour before lights off (7 PM) and in old ringdoves treated with 2.5 mg melatonin/kg of b.w. 1 hour before lights off (7 PM) or with 300 mg tryptophan/kg of b.w. 1 hour after lights on (9 AM). Each value represents the mean ± SD of 10 determinations. (a) p < .01 with respect to the values obtained in their respective control groups; (b) p < .01 with respect to the values obtained in the young animals.
The aforementioned results were confirmed (Table 1) by the cosinor analysis—amplitude, MESOR, and acrophase—for the locomotor activity of control and treated birds. Age caused a reduction in MESOR and amplitude (p < .05), but both parameters increased after the treatment with both melatonin and tryptophan, although the effect was only significant when the amplitude of the rhythm of old animals was compared with their respective controls.
Table 1.
Cosinor Parameters for the Locomotor Activities of the Control Groups and after a 3-Day Treatment With 0.25 and 2.5 mg of Melatonin/kg of Body Weight (b.w.) 1 Hour Before Lights Off (7 PM) in Young and Old Ringdoves, respectively, or 300 mg of Tryptophan/kg of b.w. 1 Hour After Lights on (9 AM) in Old Ringdoves
| Activity | ||||||
| Young Animals | Old Animals | |||||
| Control | Melatonin Treated | Control | Melatonin Treated | Control | Tryptophan Treated | |
| MESOR (No. of movements) | 161.4 ± 10.4 | 158.6 ± 15.2 | 141.9 ± 7.2* | 157.3 ± 12.1 | 141.5 ± 6.0* | 157.2 ± 15.2 |
| Amplitude (No. of movements) | 155.7 ± 28.0 | 159.6 ± 27.2 | 105.9 ± 13.0* | 140.4 ± 10.8† | 108.7 ± 12.4* | 140.1 ± 13.6† |
| Acrophase (h) | 17.4 ± 1.2 | 17.2 ± 1.3 | 16.8 ± 0.9 | 17.3 ± 1.2 | 16.6 ± 1.0 | 17.5 ± 1.5 |
Notes: Each value represents the mean ± SD of 10 determinations.
p < .05 with respect to their respective values in the young animals. The acrophase reference time is lights off (8 PM).
p < .05 with respect to the values obtained in the controls.
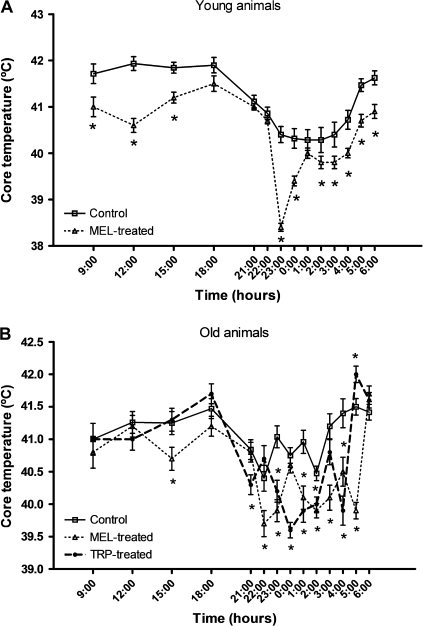
In both age groups, the control core temperature was always higher during daytime than during night (Figure 2). However, the difference between the diurnal and nocturnal values was smaller in the old birds that also showed an earlier dawn rise in temperature. Also, in both age groups, the melatonin and tryptophan treatments led to a significant decline (p < .01) in core temperature with respect to the controls. It is noteworthy that the maximum temperature decline was at around 11 PM in the melatonin-treated young animals and at around 10 PM and at midnight in the melatonin- and tryptophan-treated old animals, respectively.
Figure 2.
Core temperature values at different times over a 24-hour period in young ringdoves treated with 0.25 mg melatonin/kg of body weight (b.w.) 1 hour before lights off (7 PM) (A) and in old ringdoves treated with 2.5 mg melatonin/kg of b.w. 1 hour before lights off (7 PM) or with 300 mg tryptophan/kg of b.w. 1 hour after lights on (9 AM) (B). Each value represents the mean ± SD of 10 determinations. *p < .01 with respect to the values obtained in their respective control groups.
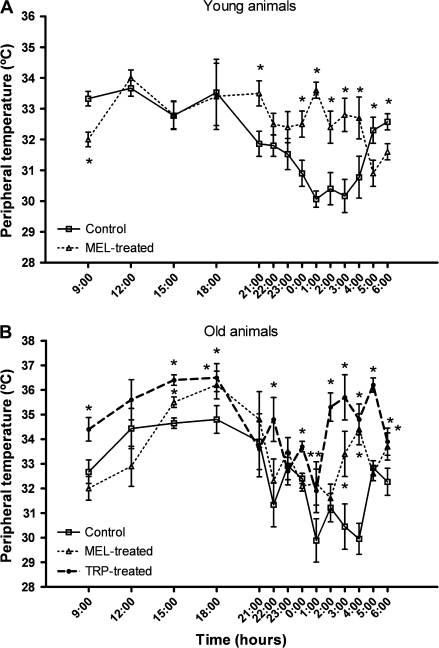
Similar to the core temperature, it was found that the diurnal values of peripheral temperature were generally higher than the nocturnal data (Figure 3). Nevertheless, contrary to the effect of melatonin and tryptophan on core temperature, their administration provoked a significant rise (p < .02) of the peripheral temperature in both young and old animals at most hours studied.
Figure 3.
Peripheral temperature values at different times over a 24-hour period in young ringdoves treated with 0.25 mg melatonin/kg of body weight (b.w.) 1 hour before lights off (7 PM) (A) and in old ringdoves treated with 2.5 mg melatonin/kg of b.w. 1 hour before lights off (7 PM) or with 300 mg tryptophan/kg of b.w. 1 hour after lights on (9 AM) (B). Each value represents the mean ± SD of 10 determinations. *p < .02 with respect to the values obtained in their respective control groups.
The control old animals presented significantly lower (p < .005) core temperature amplitudes than the young individuals, as well as a loss of core temperature rhythm (Table 2). The treatments with either melatonin or tryptophan produced a significant rise (p < .01) of the core temperature amplitude of old animals, reaching values comparable to those obtained in both the control and the melatonin-treated young birds, and a significant decrease of the core amplitude MESORs in both young and old birds. There were no significant differences between the peripheral amplitudes of the young and old controls and neither between the different treatment groups and their respective controls. However, the treatments with either melatonin or tryptophan significantly raised (p < .01) the peripheral temperature MESORs in both age groups.
Table 2.
Cosinor Parameters for the Core (A) and Peripheral (B) Temperatures of the Control Groups and After a 3-Day Treatment With 0.25 and 2.5 mg of Melatonin/kg of Body Weight (b.w.) 1 Hour Before Lights Off (7 PM) in Young and Old Ringdoves, Respectively, or 300 mg of Tryptophan/kg of b.w. 1 Hour After Lights on (9 AM) in Old Ringdoves
| Young Animals | Old Animals | |||||
| Control | Melatonin Treated | Control | Melatonin Treated | Control | Tryptophan Treated | |
| A. Core temperature | ||||||
| MESOR (°C) | 41.4 ± 0.2 | 40.6 ± 0.2* | 41.1 ± 0.1 | 40.7 ± 0.2* | 41.3 ± 0.1 | 40.9 ± 0.2* |
| Amplitude (°C) | 0.7 ± 0.3 | 0.7 ± 0.2 | 0.1 ± 0.2† | 0.5 ± 0.1* | 0.2 ± 0.1† | 0.6 ± 0.2* |
| Acrophase (h) | 17.5 ± 1.4 | 18.0 ± 2.6 | 17.5 ± 4.3 | 15.9 ± 2.7 | 19.4 ± 2.5 | 16.5 ± 3.0 |
| B. Peripheral temperature | ||||||
| MESOR (°C) | 32.2 ± 0.2 | 32.8 ± 0.3* | 32.8 ± 0.4 | 33.7 ± 0.4* | 32.9 ± 0.4 | 34.9 ± 0.4* |
| Amplitude (°C) | 1.1 ± 0.6 | 0.7 ± 0.4 | 1.4 ± 0.7 | 1.5 ± 0.8 | 1.2 ± 0.7 | 1.0 ± 0.8 |
| Acrophase (h) | 18.2 ± 2.3 | 21.1 ± 2.6 | 19.1 ± 2.0 | 21.2 ± 2.0 | 19.2 ± 2.5 | 17.9 ± 3.3 |
Notes: Each value represents the mean ± SD of 10 determinations. MESOR = Midline-Estimating Statistic of Rhythm.
p < .01 with respect to the values obtained in the controls.
p < .005 with respect to their respective values in the young animals. The acrophase reference time is lights off (8 PM).
The treatment with both melatonin and tryptophan reduced significantly the MESOR differences between the core and peripheral values (p < .01) (Figure 4). This was also the case for the amplitudes (p < .01).
Figure 4.
The Midline-Estimating Statistic of Rhythms (A) and amplitudes (B) of the core and peripheral temperatures and the differences between these temperatures for the values obtained for each parameter in young and old ringdoves treated with melatonin (0.25 and 2.5 mg/kg of b.w. 1 hour before lights off, respectively) or old ringdoves treated with tryptophan (300 mg/kg of b.w. 1 hour after lights on). Each value represents the mean ± SD of 10 determinations. *p < .05 with respect to the values obtained in their respective control groups.
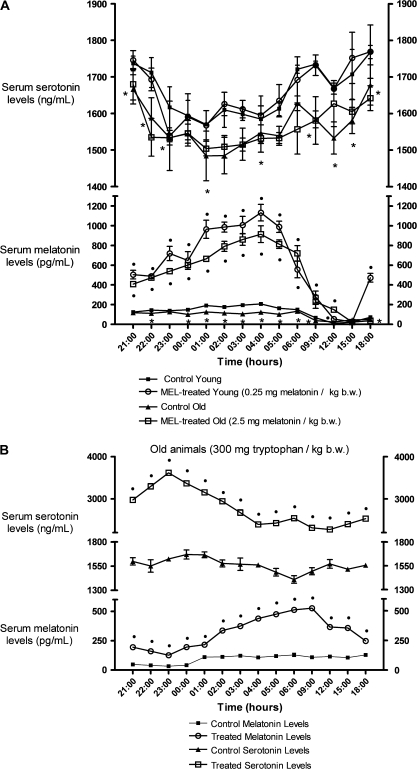
The melatonin and serotonin values were significantly higher (p < .001) in the young than in the old animals at most hours studied (Figure 5). The exceptions were noon and 3 PM when the old animals’ melatonin levels were significantly higher (p < .001), and at midnight, 1 AM, 3 AM, and 4 AM when young and old animals had similar serotonin levels. The melatonin and tryptophan treatments produced a significant rise (p < .001) of the circulating levels of melatonin in young and old animals, except for the old animals at 3 PM and 6 PM. However, only the administration with tryptophan produced a significant increase of the serotonin levels at all hours studied (p < .001).
Figure 5.
Serum melatonin (pg/mL) and serotonin (ng/mL) levels at different times over a 24-hour period in control conditions and after a 3-day administration of 0.25 mg melatonin/kg of body weight (b.w.) and 2.5 mg melatonin/kg of b.w. to young and old ringdoves, respectively, 1 hour before lights off (7 PM) (A), or 300 mg tryptophan/kg of b.w. to old ringdoves 1 hour after lights on (9 AM) (B). Each value represents the mean ± SD of 10 determinations performed in duplicate. •p < .001 with respect to the values obtained in their respective control groups; *p < .001 with respect to their respective values in the young animals.
Serum IL-1β, IL-6, and TNF-α levels are listed in Table 3. In general, the IL levels were maximal at dusk to early night and dawn to late night, with the old animals presenting higher values than the young (p < .01). In particular, there was a significant increase of IL-6 secretion in the control old animals with respect to their respective values in the young birds at 3 PM (p < .01). The treatments led in general to decreased (p < .01) IL-1β, IL-6, and TNF-α levels in both age groups. No levels of TNF-α were detected in the young group, either under control conditions or after the administration of melatonin or tryptophan.
Table 3.
Serum IL-1β, IL-6, and TNF Levels (pg/mL) at Different Times Over a 24-Hour Period in Control Conditions and After a 3-Day Administration of 0.25 mg Melatonin/kg of Body Weight (b.w.) and 2.5 mg Melatonin/kg of b.w. to Young and Old Ringdoves, respectively, 1 Hour Before Lights Off (7 PM) (A), or 300 mg Tryptophan/kg of b.w. to Old Ringdoves 1 Hour After Lights on (9 AM) (B)
| Young Animals (Mel 0.25 mg/kg of b.w.) | Old Animals (Mel 2.5 mg/kg of b.w.) | Old animals (Trp 300 mg/kg of b.w.) | |||||||||||||||||
| IL-1β | IL-6 | TNF | IL-1β | IL-6 | TNF | IL-1β | IL-6 | TNF | |||||||||||
| A | Control | Treated | Control | Treated | Control | Treated | Control | Treated | Control | Treated | Control | Treated | B | Control | Treated | Control | Treated | Control | Treated |
| 9:00 PM | — | — | 2.3 ± 1.0 | 4.4 ± 1.0 | — | — | 54.4 ± 5.4* | 33.0 ± 5.4† | 3.3 ± 1.0 | 6.5 ± 1.0 | 14.3 ± 1.0* | 10.5 ± 1.0† | 10:00 AM | — | — | 1.7 ± 1.5 | 3.0 ± 1.0 | 11.0 ± 1.0* | — |
| 10 PM | 8.9 ± 2.5 | — | 2.8 ± 1.0 | 5.8 ± 1.0† | — | — | 42.1 ± 2.1* | 43.0 ± 2.1 | 1.2 ± 1.5 | 5.5 ± 2.5 | 12.5 ± 1.5* | — | Noon | 12.5 ± 2.5* | 6.3 ± 3.5 | 3.8 ± 1.0 | 3.0 ± 1.0 | — | — |
| 11:00 PM | — | — | 5.2 ± 2.0 | 3.0 ± 0.5† | — | — | 30.3 ± 5.4* | 14.4 ± 5.4* | 1.2 ± 1.5 | 4.0 ± 1.5 | 10.9 ± 1.5* | — | 3:00 PM | — | — | 6.5 ± 2.0† | 4.4 ± 2.0 | 5.5 ± 0.5* | — |
| Midnight | 9.4 ± 3.6 | 4.5 ± 1.5 | 4.6 ± 1.5 | 3.0 ± 0.5† | — | — | 18.5 ± 4.5* | 13.0 ± 4.5 | 0.7 ± 1.5 | 3.4 ± 2.0 | 2.0 ± 0.2* | — | 6:00 PM | 1.6 ± 0.5* | 1.6 ± 0.5 | 2.3 ± 0.5 | 7.9 ± 4.0 | 4.3 ± 2.0* | — |
| 1:00 AM | — | — | 5.7 ± 1.0 | 3.4 ± 0.5† | — | — | — | — | 2.3 ± 2.0 | 3.6 ± 1.0 | 6.5 ± 1.0* | 1.75 ± 0.5† | 9:00 PM | 54.4 ± 5.4* | — | 3.3 ± 1.0 | 4.1 ± 1.0 | 14.3 ± 1.0* | 7.5 ± 1.0* |
| 2:00 AM | — | — | 5.7 ± 2.0 | 1.6 ± 0.5† | — | — | — | — | 2.3 ± 1.5 | 0.2 ± 1.0 | 5.3 ± 1.0* | — | 10:00 PM | 42.1 ± 2.1* | 10.3 ± 2.1† | 1.2 ± 1.5 | 5.6 ± 2.5 | 12.5 ± 1.5* | 9.0 ± 0.5* |
| 3:00 AM | 18.9 ± 4.5 | — | 6.2 ± 1.0 | 3.0 ± 1.0† | — | — | — | — | 3.3 ± 2.0 | 4.8 ± 1.0 | 7.5 ± 1.0* | — | 11:00 PM | 30.3 ± 5.4* | — | 1.2 ± 1.5 | 3.7 ± 0.5 | 10.9 ± 1.5* | — |
| 4:00 AM | — | — | 6.5 ± 1.0 | 5.4 ± 1.0 | — | — | 12.6 ± 2.5* | — | 3.1 ± 2.0 | 3.4 ± 1.0 | — | 4.3 ± 1.0† | Midnight | 18.5 ± 4.5* | — | 0.7 ± 1.5 | 2.7 ± 2.0 | 2.0 ± 0.2* | — |
| 5:00 AM | — | — | 2.8 ± 1.0 | 5.1 ± 1.0† | — | — | 33.5 ± 3.4* | 10.3 ± 3.4† | 3.3 ± 1.0 | 5.1 ± 1.0 | — | — | 1:00 AM | — | — | 2.3 ± 2.0 | 3.7 ± 1.0 | 6.5 ± 1.0* | — |
| 6:00 AM | 63.9 ± 3.9 | — | 5.2 ± 1.0 | 2.3 ± 1.0† | — | — | 81.2 ± 6.8* | 31.2 ± 6.8† | 3.6 ± 1.0 | 4.1 ± 1.0 | — | — | 2:00 AM | — | — | 2.3 ± 1.5 | 3.2 ± 1.0 | 5.3 ± 1.0* | — |
| 9:00 AM | — | — | 4.1 ± 2.0 | 5.8 ± 2.0 | — | — | — | — | 1.7 ± 1.5 | 5.1 ± 2.0 | 11.0 ± 1.0* | 9.3 ± 1.0 | 3:00 AM | — | — | 3.3 ± 2.0 | 3.4 ± 1.0 | 7.5 ± 1.0* | — |
| Noon | — | — | 3.1 ± 2.0 | 5.0 ± 2.0 | — | — | 12.5 ± 2.5* | 10.6 ± 2.5 | 3.8 ± 1.0 | 2.0 ± 1.0 | — | — | 4:00 AM | 12.6 ± 2.5* | — | 3.1 ± 2.0 | 3.0 ± 1.0 | — | — |
| 3:00 PM | — | 3.5 ± 0.5 | 2.3 ± 1.5 | 4.9 ± 1.5 | — | — | — | — | 6.5 ± 2.0* | 7.9 ± 2.0 | 5.5 ± 0.5* | 3.3 ± 1.0† | 5:00 AM | 33.5 ± 3.4* | — | 3.3 ± 1.0 | 5.4 ± 1.0 | — | — |
| 6:00 PM | 19.8 ± 6.8 | 3.4 ± 0.4† | 3.3 ± 1.5 | 4.1 ± 1.5 | — | — | 1.6 ± 0.5* | — | 2.3 ± 0.5 | 5.8 ± 2.0 | 4.3 ± 2.0* | — | 6:00 AM | 81.2 ± 6.8* | — | 3.6 ± 1.0 | 5.6 ± 1.0 | — | — |
Notes: Each value represents the mean ± SD of 10 determinations performed in duplicate. IL = interleukin; TNF = tumor necrosis factor.
p < .01 with respect to their respective values in the young animals. (—) Undetected values.
p < .01 with respect to the values obtained in their respective control groups.
Appendix 1 presents the correlations between the serum melatonin and serotonin levels and the locomotor activity (pulses), and core and peripheral temperatures. In the control groups, the results of the serum melatonin levels were negatively correlated with the circadian rhythms of activity and core and peripheral temperatures (p < .01, except for the relation between old control melatonin and core temperature values where p was <.02). The equivalent correlations for serotonin were positive (p < .01, except for the relations between old control serotonin and core temperature values and serotonin and peripheral temperature values from young melatonin-treated animals, where p was <.021 and <.041, respectively). The treatments either maintained or strengthened these correlations.
DISCUSSION
It is thought that the deterioration of the circadian rhythms in the elderly adults contributes to the appearance of sleep disorders, including reduced slow wave sleep, increased number and duration of night-time awakenings, shorter sleep duration, and reduced daytime activity function (36). Sleep disorders are believed to be caused, at least partly, by changes in the circadian system (37). Among these changes, the alterations in the circadian rhythms of temperature and melatonin may have a relevant role (38). In this sense, we found in our animal model a significant increase in the nocturnal activity of the old birds, together with significant reductions in the MESOR and amplitude of their activity–rest rhythm with respect to the young birds. In addition, in comparison with the young individuals the circulating levels of melatonin were significantly reduced as was the amplitude of the core temperature, with also an earlier rise in the latter hours of darkness. This is especially important because it is known that maximal sleep propensity occurs during the descending part of the temperature cycle (39). This could mean that the second half of nocturnal sleep in our animal model, and, by extension, in the elderly adults, corresponds to the ascending phase of temperature, which would hinder the maintenance of sleep even though it would not be the only factor involved.
The administration of melatonin or tryptophan not only provoked a significant decline in the nocturnal activity of both age groups but also decreased the core temperature values with respect to the controls. This is consistent with previous reports showing a significant hypothermic response to melatonin in other birds such as laying hens, sparrows, and chicks (9) and in several mammalian species, including humans (40,41). Moreover, we found a significant rise of the amplitude of the core temperature in the old animals, reaching values comparable to those obtained in both the control and the melatonin-treated young birds. This is particularly relevant given the reported decline in aging populations of core body temperature and other circadian markers, including melatonin (42).
Based on the precise phase relationship between the onset of nocturnal secretion of melatonin, the “forbidden zone” for sleep, and core body temperature, it has been proposed that melatonin may act as an internal sleep “facilitator” in diurnally active animals, and therefore be useful in the treatment of insomnia and the readjustment of impaired circadian rhythms (43), as is the case for elderly individuals. Nevertheless, it has been pointed out that a reduction in core body temperature per se does not always induce drowsiness, that is, drowsiness is associated with heat loss and not primarily with a decrease in core body temperature (22). In this regard, we tested whether there existed in the old birds an age-related alteration in the thermoregulatory process using the measurement of peripheral temperature as an indicator of heat loss. It was observed that there were no significant differences in peripheral temperatures between young and old ringdoves under control conditions. This does not mean, however, that the heat losses were similar. The differences between the control core and peripheral temperature amplitudes were greater in the old birds. This could indicate either increased losses or a greater influence of the environment and hence poorer homeostatic control of their losses (44). Melatonin or tryptophan treatments induced a significant increase in the peripheral temperature in both groups, the MESORs being significantly higher than their corresponding values in the untreated animals. This, together with the aforementioned decrease in the core temperature MESORs and the significant reduction in the differences between the core and peripheral temperature MESORs, reflects an increase in losses and the typical hypothermic action of melatonin and tryptophan. Melatonin itself or via conversion from its precursor, the amino acid tryptophan, may act by lowering core temperature, thereby causing an immediate stimulation of heat-loss mechanisms, as has been proposed to be the case for other birds (9). Thus, in the old birds, melatonin or tryptophan improves the rhythm by increasing the amplitude of the core temperature. Because the amplitude of the peripheral temperature is maintained, the difference between the two is significantly reduced, with improved homeostatic control of the losses.
Cytokines are a diverse group of signaling proteins that are produced by lymphocytes, macrophages, and other systemic cells and are involved in immune and inflammatory responses. Among cytokines, IL-1β and TNF-α have been determined to be important sleep-promoting substances (30). Furthermore, IL-6 levels have been associated with aging-related sleep problems (45). Here, we investigated whether treating the birds with either the pineal indole or its precursor may modify the secretion of IL-1β, IL-6, or TNF-α due to the aforementioned implication of these elements in sleep regulation. We observed in general that the IL-1β, IL-6, and TNF-α levels were maximal before and after lights on or lights off, with the old animals presenting higher values than the young and that the treatments led to decreased cytokine levels in both age groups. In particular, we observed a significant rise in the IL-6 values in the diurnal period in the control old animals with respect to the young. This is particularly relevant because better sleep has been associated with reduced daytime secretion of IL-6, whereas disturbed nocturnal sleep has been associated with increased daytime IL-6 levels (46).
It has been shown previously that, however, despite its somnogenic properties, IL-6 administration or elevation of its endogenous levels seems to result in sleep disturbance when associated with activation of the hypothalamic–pituitary–adrenocortical (HPA) axis, at least in humans (45). On the other hand, it has been proposed that elevated daytime secretion of somnogenic and fatigue-inducing cytokines, such as IL-6 and TNF, unassociated with HPA axis activation, leads to drowsiness and deeper sleep (46). Also, sleep deprivation increases the incidence and concentration of IL-1β (47), whose levels were here elevated in the old birds. Finally, TNF-α levels were only detected in the old birds, which could be related to the fact that the presence in serum of certain cytokines seems to be linked to potential disease processes and to cell damage (48), phenomena most likely to be occurring in the old animals. Thus, further investigations are needed to elucidate the mechanisms through which ILs work in our animal model, taking into account that aging has also been associated with increased plasma levels of these inflammatory products (49). In this respect, recent work has shown that melatonin is implicated in the reduction of proinflammatory cytokines (50), an effect also observed here in both young and old ringdoves after treatment with either melatonin or tryptophan.
Because serotonin is known to increase the proportion of slow wave sleep (51), as well as being a waking neurotransmitter (52), and tryptophan intake acts to increase circulating levels of this neurotransmitter in both brain and serum in the ringdove (16,21), it is important to understand the relationship of this molecule with temperature and locomotor activity. The present results showed positive correlations of serum tryptophan levels and the circadian rhythms of activity and core and peripheral temperatures. The equivalent correlations for melatonin were negative, that is, as the serum melatonin levels rose, the activity pulses became less frequent and the temperature recordings were lower. As serum serotonin levels rose, the activity pulses became more frequent and the temperature recordings higher. The negative correlations that were observed between melatonin levels and the rhythms of activity and temperature could be a reflection of the endogenous sleep-regulating properties of the indoleamine. In this sense, in humans a characteristic increase of melatonin that takes place during the night has been reported to be directly related to an increase in drowsiness and a reduction in body temperature (53). The positive correlations found for serotonin in the two age groups are consistent with previous findings (54).
In summary, in the ringdove the nocturnal secretion of the pineal melatonin seems to have a crucial role in the endogenous regulation of temperature in the evening, which in turn may be implicated in the initiation of sleep. Old birds displayed impairments in their circadian rhythms of locomotor activity, temperature, and circulating levels of melatonin and serotonin, and differences in the pattern of secretion of IL-1β, IL-6, and TNF-α. Exogenous melatonin and tryptophan increased the circulating levels of the pineal indole; reduced nocturnal locomotor activity, core temperature, and IL serum values; and stimulated heat-loss mechanisms while maintaining or reinforcing the correlations between the core and peripheral temperatures and circulating melatonin and serotonin. This could imply that improving the tryptophan supply in the diet or a melatonin treatment in accordance with the necessities of the circadian cycle in populations with sleep disorders would probably result in a better adjustment of their circadian rhythms, with a general improvement of their health. The present results could serve as a basis for future studies on the application of melatonin or tryptophan as “replacement therapies” to limit or reverse some of the effects of the changes that occur in sleep–wake rhythms and temperature due to age.
FUNDING
This investigation was supported by a research grant from Consejería de Infraestructuras y Desarrollo Tecnológico (Junta de Extremadura, 3PR05A053). S.D.P. was the beneficiary of a grant from Consejería de Economía, Comercio e Innovación—Fondo Social Europeo (Junta de Extremadura, POS07012).
Acknowledgments
The authors would like to express their thanks to Elena Circujano Vadillo, Ricardo Megías Cebrino, and Ana Royano Sánchez for their technical assistance and to Professors Manuel Molina and Manuel Mota, members of the Department of Mathematics (Section of Statistics and Operations Research) of the Faculty of Science, for their advice and collaboration in the statistical study.
Appendix 1.
Correlations Between the Serum Melatonin (pg/mL) and Serotonin (ng/mL) Levels and the Locomotor Activity (pulses), and Core and Peripheral Temperatures Under Control Conditions and After a 3-day Administration of 0.25 mg Melatonin/kg of Body Weight (b.w.) and 2.5 mg Melatonin/kg of b.w. to Young and Old Ringdoves, Respectively, 1 Hour Before Lights Off (7 PM), or 300 mg Tryptophan/kg of b.w. to Old Ringdoves 1 hour After Lights On (9 AM)
| Pulses of Activity | Core Temperature | Peripheral Temperature | ||
| Serum melatonin (pg/mL) | Control young animals | y = −0.4x + 176.4; R2 = .77 (–); p < .000; f.d. = 12 | y = −73.8x + 3,162.5; R2 = .62 (–); p < .001; f.d. = 12 | y = −42.9x + 1493.6; R2 = .73 (–); p < .000; f.d. = 12 |
| Melatonin-treated young animals | y = −2.6x + 1,006.9; R2 = .78 (–); p < .001; f.d. = 8 | y = −496.3x + 20,822; R2 = .68 (–); p < .002; f.d. = 9 | y = −215.9 + 7,592.3; R2 = .70 (–); p < .001; f.d. = 6 | |
| Control old animals | y = −0.4x + 129.2; R2 = .90 (–); p < .000; f.d. = 12 | y = −84.4x + 3,536.5; R2 = .56 (–); p < .02; f.d. = 7 | y = −17.4 + 650.7; R2 = .84 (–); p < .000; f.d. = 8 | |
| Melatonin-treated old animals | y = −3.9x + 856.7; R2 = .90 (–); p < .000; f.d. = 8 | y = −487.4x + 2,0262; R2 = .66 (–); p < .002; f.d. = 9 | y = −152.6x + 5,612.9; R2 = .73 (–); p < .002; f.d. = 8 | |
| Tryptophan-treated old animals | y = −1.0x + 415.3; R2 = .84 (–); p < .000; f.d. = 7 | y = −146.5x + 6,249.9; R2 = .64 (–); p < .002; f.d. = 10 | y = −68.1x + 2,626.9; R2 = .71 (–); p < .004; f.d. = 7 | |
| Serum serotonin (ng/mL) | Control young animals | y = 0.4x + 1,601.7; R2 = .62 (+); p < .003; f.d. = 10 | y = 77.3x − 1,514.3; R2 = .57 (+); p < .002; f.d. = 12 | y = 43.1x + 287.5; R2 = .62 (+); p < .001; f.d. = 12 |
| Melatonin-treated young animals | y = 0.5x + 1,585.5; R2 = .77 (+); p < .000; f.d. = 9 | y = 80.6x − 1,593; R2 = .83 (+); p < .000; f.d. = 12 | y = 46.5x + 196.5; R2 = .51 (+); p < .41; f.d. = 5 | |
| Control old animals | y = 0.4x + 1,506.5; R2 = .62 (+); p < .007; f.d. = 8 | y = 143.6x − 4,343.9; R2 = .51 (+); p < .021; f.d. = 8 | y = 33.6x + 495.4; R2 = .64 (+); p < .003; f.d. = 9 | |
| Melatonin-treated old animals | y = 0.5x + 1,516.7; R2 = .84 (+); p < .000; f.d. = 10 | y = 88.2x − 2,010.9; R2 = .82 (+); p < .000; f.d. = 9 | y = 24.1x + 744.6; R2 = .76 (+); p < .001; f.d. = 8 | |
| Tryptophan-treated old animals | y = 4.2x + 2,348.5; R2 = .88 (+); p < .000; f.d. = 9 | y = 593.6x − 2,1168; R2 = .80 (+); p < .000; f.d. = 9 | y = 235.3x − 5,144.5; R2 = .70 (+); p < .002; f.d. = 8 |
Note: (+) and (−) indicate that there exists a positive or negative correlation, respectively, between the two variables; df = degrees of freedom.
References
- 1.Pandi-Perumal SR, Seils LK, Kayumov L, et al. Senescence, sleep, and circadian rhythms. Ageing Res Rev. 2002;1:559–604. doi: 10.1016/s1568-1637(02)00014-4. [DOI] [PubMed] [Google Scholar]
- 2.Yoon IY, Kripke DF, Elliott JA, Youngstedt SD, Rex KM, Hauger RL. Age-related changes of circadian rhythms and sleep-wake cycles. J Am Geriatr Soc. 2003;51:1085–1091. doi: 10.1046/j.1532-5415.2003.51356.x. [DOI] [PubMed] [Google Scholar]
- 3.Kripke DF, Youngstedt SD, Elliott JA, et al. Circadian phase in adults of contrasting ages. Chronobiol Int. 2005;22:695–709. doi: 10.1080/07420520500180439. [DOI] [PubMed] [Google Scholar]
- 4.Campbell SS, Murphy PJ. Relationships between sleep and body temperature in middle-aged and older subjects. J Am Geriatr Soc. 1998;46:458–462. doi: 10.1111/j.1532-5415.1998.tb02466.x. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert SS, van den Heuvel CJ, Ferguson SA, Dawson D. Thermoregulation as a sleep signalling system. Sleep Med Rev. 2004;8:81–93. doi: 10.1016/S1087-0792(03)00023-6. [DOI] [PubMed] [Google Scholar]
- 6.Pandi-Perumal SR, Zisapel N, Srinivasan V, Cardinali DP. Melatonin and sleep in aging population. Exp Gerontol. 2005;40:911–925. doi: 10.1016/j.exger.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Zisapel N. Sleep and sleep disturbances: biological basis and clinical implications. Cell Mol Life Sci. 2007;64:1174–1186. doi: 10.1007/s00018-007-6529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinert D, Waterhouse J. The circadian rhythm of core temperature: effects of physical activity and aging. Physiol Behav. 2007;90:246–256. doi: 10.1016/j.physbeh.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Rozenboim I, Miara L, Wolfenson D. The thermoregulatory mechanism of melatonin-induced hypothermia in chicken. Am J Physiol. 1998;274:232–236. doi: 10.1152/ajpregu.1998.274.1.R232. [DOI] [PubMed] [Google Scholar]
- 10.Cagnacci A, Elliott JA, Yen SS. Melatonin: a major regulator of the circadian rhythm of core temperature in humans. J Clin Endocrinol Metab. 1992;75:447–452. doi: 10.1210/jcem.75.2.1639946. [DOI] [PubMed] [Google Scholar]
- 11.Underwood H. Endogenous rhythms. In: Gans C, Crews D, editors. Biology of the Reptilian. Hormones Brain and Behavior. Chicago, IL: University of Chicago Press; 1992. pp. 229–297. [Google Scholar]
- 12.Lin MT, Chuang JI. Melatonin potentiates 5-HT(1A) receptor activation in rat hypothalamus and results in hypothermia. J Pineal Res. 2002;33:14–19. doi: 10.1034/j.1600-079x.2002.01867.x. [DOI] [PubMed] [Google Scholar]
- 13.Rial RV, Akaârir M, Gamundi A, et al. Wake and sleep hypothalamic regulation in diurnal and nocturnal chronotypes. J Pineal Res. 2008;45:225–226. doi: 10.1111/j.1600-079X.2008.00571.x. [DOI] [PubMed] [Google Scholar]
- 14.Kunz D, Mahlberg R, Müller C, Tilmann A, Bes F. Melatonin in patients with reduced REM sleep duration: two randomized controlled trials. J Clin Endocrinol Metab. 2004;89:128–134. doi: 10.1210/jc.2002-021057. [DOI] [PubMed] [Google Scholar]
- 15.Esteban S, Nicolaus C, Garmundi A, et al. Effect of orally administered L-tryptophan on serotonin, melatonin, and the innate immune response in the rat. Mol Cell Biochem. 2004;267:39–46. doi: 10.1023/b:mcbi.0000049363.97713.74. [DOI] [PubMed] [Google Scholar]
- 16.Garau C, Aparicio S, Rial RV, Nicolau MC, Esteban S. Age-related changes in circadian rhythm of serotonin synthesis in ring doves: effects of increased tryptophan ingestion. Exp Gerontol. 2006;41:40–48. doi: 10.1016/j.exger.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Cubero J, Narciso D, Aparicio S, et al. Improved circadian sleep-wake cycle in infants fed a day/night dissociated formula milk. Neuro Endocrinol Lett. 2006;27:373–380. [PubMed] [Google Scholar]
- 18.Riemann D, Vorderholzer U. Treatment of depression and sleep disorders. Significance of serotonin and L-tryptophan in pathophysiology and therapy. Fortschr Med. 1998;116:40–42. [PubMed] [Google Scholar]
- 19.Cubero J, Narciso D, Valero V, et al. The oral administration of tryptophan improves nocturnal rest in young animals: correlation with melatonin. Biog Amines. 2006;20:53–62. [Google Scholar]
- 20.Paredes SD, Terrón MP, Valero V, Barriga C, Reiter RJ, Rodríguez AB. Orally administered melatonin improves nocturnal rest in young and old ringdoves (Streptopelia risoria) Basic Clin Pharmacol Toxicol. 2007;100:258–268. doi: 10.1111/j.1742-7843.2006.00032.x. [DOI] [PubMed] [Google Scholar]
- 21.Paredes SD, Terrón MP, Cubero J, et al. Tryptophan increases nocturnal rest and affects melatonin and serotonin serum levels in old ringdove. Physiol Behav. 2007;90:576–582. doi: 10.1016/j.physbeh.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Kräuchi K, Cajochen C, Pache M, Flammer J, Wirz-Justice A. Thermoregulatory effects of melatonin in relation to sleepiness. Chronobiol Int. 2006;23:475–484. doi: 10.1080/07420520500545854. [DOI] [PubMed] [Google Scholar]
- 23.Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120:435–446. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodríguez AB, Nogales G, Marchena JM, Ortega E, Barriga C. Suppression of both basal and antigen-induced lipid peroxidation in ring dove heterophils by melatonin. Biochem Pharmacol. 1999;58:1301–1306. doi: 10.1016/s0006-2952(99)00207-5. [DOI] [PubMed] [Google Scholar]
- 25.Terrón MP, Cubero J, Marchena JM, Barriga C, Rodríguez AB. Melatonin and aging: in vitro effect of young and mature ring dove physiological concentrations of melatonin on the phagocytic function of heterophils from old ring dove. Exp Gerontol. 2002;37:421–426. doi: 10.1016/s0531-5565(01)00209-1. [DOI] [PubMed] [Google Scholar]
- 26.Paredes SD, Terrón MP, Marchena AM, et al. Effect of exogenous melatonin on viability, ingestion capacity, and free-radical scavenging in heterophils from young and old ringdoves (Streptopelia risoria) Mol Cell Biochem. 2007;304:305–314. doi: 10.1007/s11010-007-9513-7. [DOI] [PubMed] [Google Scholar]
- 27.Paredes SD, Terrón MP, Marchena AM, et al. Tryptophan modulates cell viability, phagocytosis and oxidative metabolism in old ringdoves. Basic Clin Pharmacol Toxicol. 2007;101:56–62. doi: 10.1111/j.1742-7843.2007.00076.x. [DOI] [PubMed] [Google Scholar]
- 28.Paredes SD, Barriga C, Rodríguez AB. Melatonin and tryptophan as therapeutic agents against the impairment of the sleep-wake cycle and immunosenescence due to aging in Streptopelia risoria. Neuro Endocrinol Lett. 2007;28:757–760. [PubMed] [Google Scholar]
- 29.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Kapsimalis F, Basta M, Varouchakis G, Gourgoulianis K, Vgontzas A, Kryger M. Cytokines and pathological sleep. Sleep Med. 2008;9:603–614. doi: 10.1016/j.sleep.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 31.Kapsimalis F, Richardson G, Opp MR, Kryger M. Cytokines and normal sleep. Curr Opin Pulm Med. 2005;11:481–484. doi: 10.1097/01.mcp.0000183062.98665.6b. [DOI] [PubMed] [Google Scholar]
- 32.Chen L, Taishi P, Majde JA, Peterfi Z, Obal F, Jr, Krueger JM. The role of nitric oxide synthases in the sleep responses to tumor necrosis factor-alpha. Brain Behav Immun. 2004;18:390–398. doi: 10.1016/j.bbi.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Baker FC, Shah S, Stewart D, et al. Interleukin 1beta enhances non-rapid eye movement sleep and increases c-Fos protein expression in the median preoptic nucleus of the hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2005;288:R998–R1005. doi: 10.1152/ajpregu.00615.2004. [DOI] [PubMed] [Google Scholar]
- 34.Morrow JD, Opp MR. Sleep-wake behavior and responses of interleukin-6-deficient mice to sleep deprivation. Brain Behav Immun. 2005;19:28–39. doi: 10.1016/j.bbi.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 35.De la Iglesia HO, Cambras T, Schwart WJ, Diez-Noguera A. Forced desynchronization of dual circadian oscillators within the rat suprachiasmatic nucleus. Curr Biol. 2004;14:796–800. doi: 10.1016/j.cub.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 36.Huang YL, Liu RY, Wang QS, Van Someren EJ, Xu H, Zhou JN. Age-associated difference in circadian sleep-wake and rest-activity rhythms. Physiol Behav. 2002;17:597–603. doi: 10.1016/s0031-9384(02)00733-3. [DOI] [PubMed] [Google Scholar]
- 37.Van Someren EJ. Circadian and sleep disturbances in the elderly. Exp Gerontol. 2000;35:1229–1237. doi: 10.1016/s0531-5565(00)00191-1. [DOI] [PubMed] [Google Scholar]
- 38.Barriga-Ibars C, Rodríguez-Moratinos AB, Esteban S, Rial RV. Interrelations between sleep and the immune status. Rev Neurol. 2005;40:548–556. [PubMed] [Google Scholar]
- 39.Dijk DJ, Lockley SW. Integration of human sleep-wake regulation and circadian rhythmicity. J Appl Physiol. 2002;92:852–862. doi: 10.1152/japplphysiol.00924.2001. [DOI] [PubMed] [Google Scholar]
- 40.Nave R, Herer P, Haimov I, Shlitner A, Lavie P. Hypnotic and hypothermic effects of melatonin on daytime sleep in humans: lack of antagonism by flumazenil. Neurosci Lett. 1996;214:123–126. doi: 10.1016/0304-3940(96)12899-8. [DOI] [PubMed] [Google Scholar]
- 41.Reid K, Van den Heuvel C, Dawson D. Day-time melatonin administration: effects on core temperature and sleep onset latency. J Sleep Res. 1996;5:150–154. doi: 10.1046/j.1365-2869.1996.t01-1-00006.x. [DOI] [PubMed] [Google Scholar]
- 42.Reiter RJ. The pineal gland and melatonin in relation to aging: a summary of the theories and of the data. Exp Gerontol. 1995;30:199–212. doi: 10.1016/0531-5565(94)00045-5. [DOI] [PubMed] [Google Scholar]
- 43.Cajochen C, Kräuchi K, Wirz-Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol. 2003;15:432–437. doi: 10.1046/j.1365-2826.2003.00989.x. [DOI] [PubMed] [Google Scholar]
- 44.Helwig BG, Parimi S, Ganta CK, Cober R, Fels RJ, Kenney MJ. Aging alters regulation of visceral sympathetic nerve responses to acute hypothermia. Am J Physiol Regul Integr Comp Physiol. 2006;291:R573–R579. doi: 10.1152/ajpregu.00903.2005. [DOI] [PubMed] [Google Scholar]
- 45.Vgontzas AN, Zoumakis M, Bixler EO, et al. Impaired nighttime sleep in healthy old versus young adults is associated with elevated plasma interleukin-6 and cortisol levels: physiologic and therapeutic implications. J Clin Endocrinol Metab. 2003;88:2087–2095. doi: 10.1210/jc.2002-021176. [DOI] [PubMed] [Google Scholar]
- 46.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab. 1999;84:2603–2607. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- 47.Everson CA. Clinical assessment of blood leukocytes, serum cytokines, and serum immunoglobulins as responses to sleep deprivation in laboratory rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1054–R1063. doi: 10.1152/ajpregu.00021.2005. [DOI] [PubMed] [Google Scholar]
- 48.Remick DG. Protein analysis and bioassays of cytokines and cytokine receptors. In: Rose NR, Hamilton RG, Detrick B, editors. Manual of Clinical Laboratory Immunology. 6th ed. Washington, DC: American Society of Microbiology;; 2002. pp. 320–337. [Google Scholar]
- 49.Pedersen BK, Bruunsgaard H, Ostrowski K, et al. Cytokines in aging and exercise. Int J Sports Med. 2000;21(suppl 1):S4–S9. doi: 10.1055/s-2000-1444. [DOI] [PubMed] [Google Scholar]
- 50.Rodríguez MI, Escames G, López LC, et al. Chronic melatonin treatment reduces the age-dependent inflammatory process in senescence-accelerated mice. J Pineal Res. 2007;42:272–279. doi: 10.1111/j.1600-079X.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 51.Jouvet M. Sleep and serotonin: an unfinished story. Neuropsychopharmacology. 1999;21(suppl 2):24S–27S. doi: 10.1016/S0893-133X(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 52.Ursin R. Serotonin and sleep. Sleep Med Rev. 2002;6:55–69. doi: 10.1053/smrv.2001.0174. [DOI] [PubMed] [Google Scholar]
- 53.Dijk DJ, Cajochen C. Melatonin and the circadian regulation of sleep initiation, consolidation, structure, and the sleep EEG. J Biol Rhythms. 1997;12:627–635. doi: 10.1177/074873049701200618. [DOI] [PubMed] [Google Scholar]
- 54.Paredes SD, Terrón MP, Cubero J, et al. Comparative study of the activity/rest rhythms in young and old ringdove (Streptopelia risoria): correlation with serum levels of melatonin and serotonin. Chronobiol Int. 2006;23:779–793. doi: 10.1080/07420520600827145. [DOI] [PubMed] [Google Scholar]