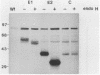
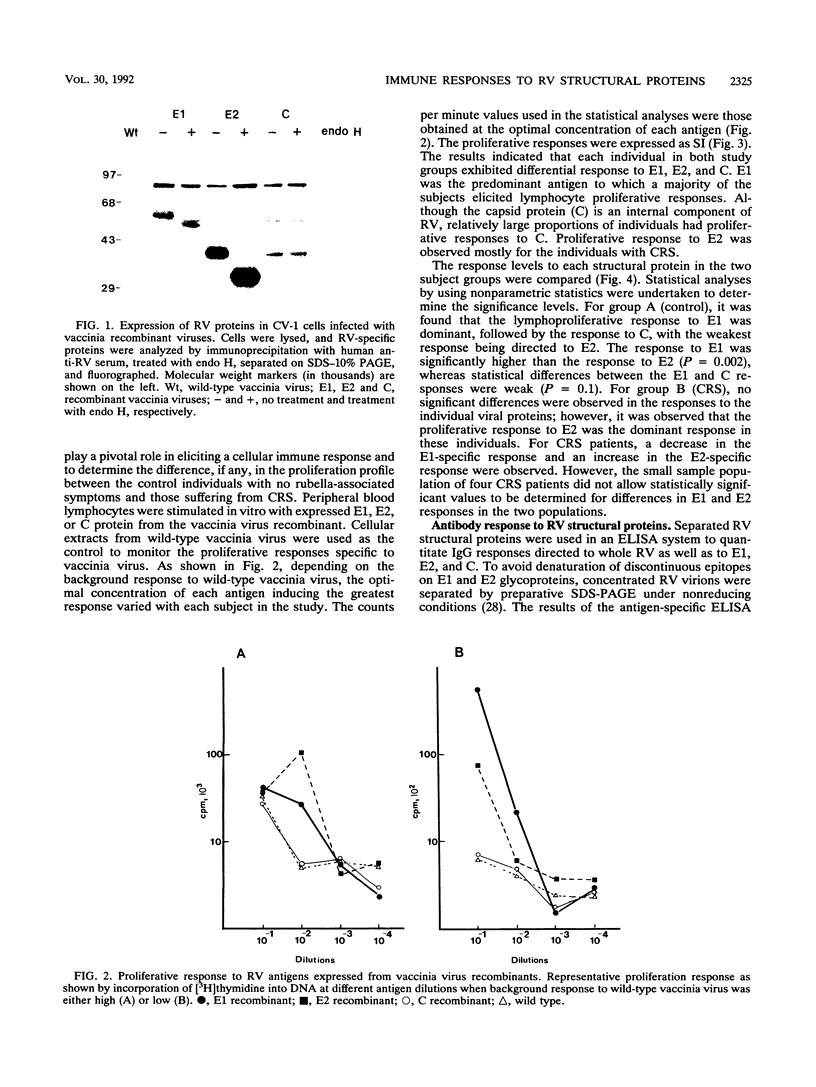
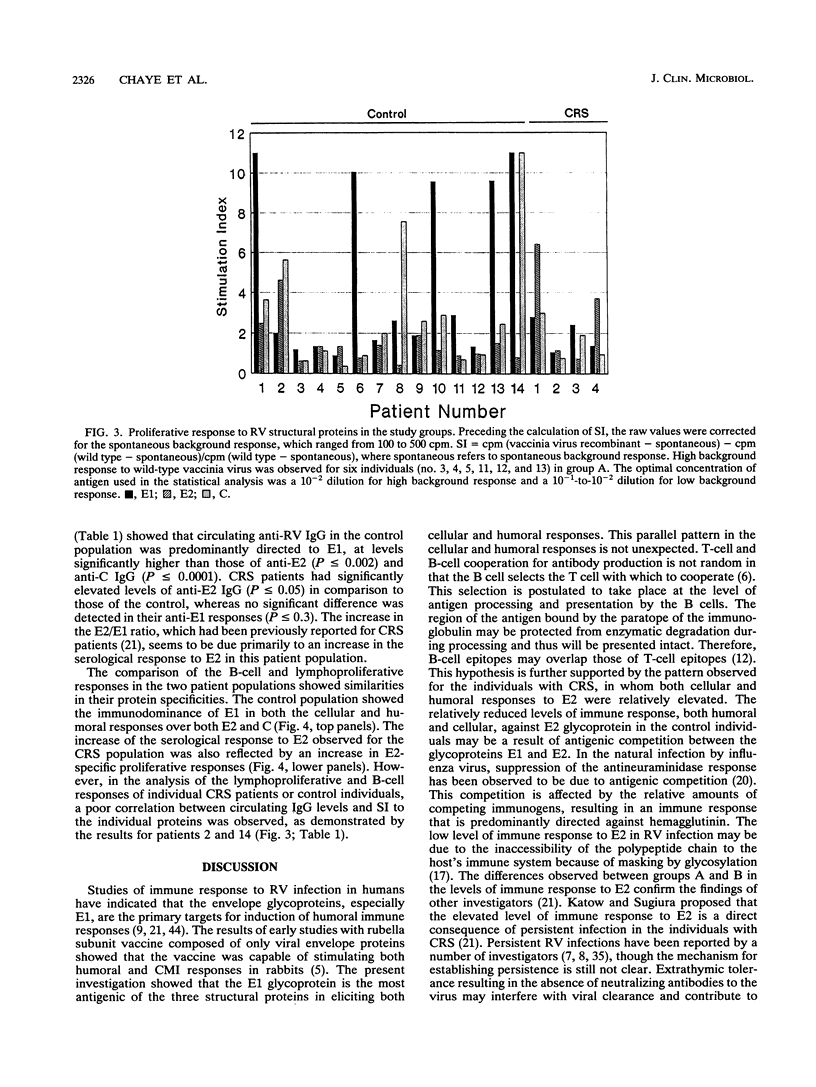
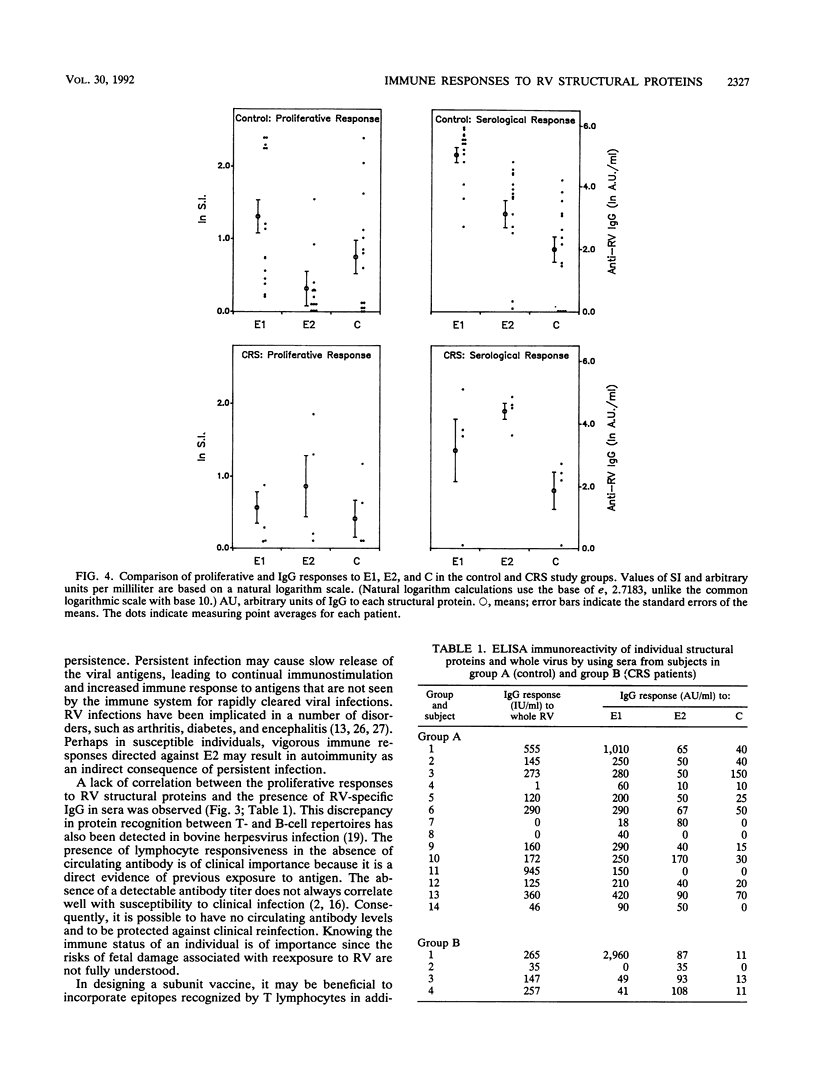
Abstract
Better understanding of cell-mediated immune responses to rubella virus would provide the basis for the development of safe and effective vaccines against rubella and would aid in analysis of the pathophysiology of congenital rubella syndrome. We have expressed individual rubella virus structural proteins, E1, E2 and C, via vaccinia virus recombinants. Using the expressed recombinant proteins as antigens, we were able to demonstrate antigen-specific lymphocyte proliferative responses in control individuals and individuals with congenital rubella syndrome. Among the two human groups studied, E1 glycoprotein proved to be a better immunogen than E2 or C. For the control individuals, significant differences in proliferative responses to the structural proteins E1, E2, and C were observed. These differences were not significant in individuals with congenital rubella syndrome. In parallel to the lymphoproliferative responses, immunoglobulin G responses were also found directed mainly to the E1 glycoprotein. These results suggest that E1 may be the most important rubella virus antigen to study in determining the domains required for constructing subunit vaccines against rubella.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brody J. A. The infectiousness of rubella and the possibility of reinfection. Am J Public Health Nations Health. 1966 Jul;56(7):1082–1087. doi: 10.2105/ajph.56.7.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buimovici-Klein E., Cooper L. Z. Cell-mediated immune response in rubella infections. Rev Infect Dis. 1985 Mar-Apr;7 (Suppl 1):S123–S128. doi: 10.1093/clinids/7.supplement_1.s123. [DOI] [PubMed] [Google Scholar]
- Buimovici-Klein E., Lang P. B., Ziring P. R., Cooper L. Z. Impaired cell-mediated immune response in patients with congenital rubella: correlation with gestational age at time of infection. Pediatrics. 1979 Nov;64(5):620–626. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cappel R., De Cuyper F. Efficacy and immune response to rubella subunits vaccines. Arch Virol. 1976;50(3):207–213. doi: 10.1007/BF01320574. [DOI] [PubMed] [Google Scholar]
- Celada F., Sercarz E. E. Preferential pairing of T-B specificities in the same antigen: the concept of directional help. Vaccine. 1988 Apr;6(2):94–98. doi: 10.1016/s0264-410x(88)80006-9. [DOI] [PubMed] [Google Scholar]
- Chantler J. K., Ford D. K., Tingle A. J. Persistent rubella infection and rubella-associated arthritis. Lancet. 1982 Jun 12;1(8285):1323–1325. doi: 10.1016/s0140-6736(82)92398-4. [DOI] [PubMed] [Google Scholar]
- Cunningham A. L., Fraser J. R. Persistent rubella virus infection of human synovial cells cultured in vitro. J Infect Dis. 1985 Apr;151(4):638–645. doi: 10.1093/infdis/151.4.638. [DOI] [PubMed] [Google Scholar]
- Dorsett P. H., Miller D. C., Green K. Y., Byrd F. I. Structure and function of the rubella virus proteins. Rev Infect Dis. 1985 Mar-Apr;7 (Suppl 1):S150–S156. doi: 10.1093/clinids/7.supplement_1.s150. [DOI] [PubMed] [Google Scholar]
- Ffrench R. A., Tang X. L., Anders E. M., Jackson D. C., White D. O., Drummer H., Wade J. D., Tregear G. W., Brown L. E. Class II-restricted T-cell clones to a synthetic peptide of influenza virus hemagglutinin differ in their fine specificities and in the ability to respond to virus. J Virol. 1989 Jul;63(7):3087–3094. doi: 10.1128/jvi.63.7.3087-3094.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleet W. F., Jr, Benz E. W., Jr, Karzon D. T., Lefkowitz L. B., Herrmann K. L. Fetal consequences of maternal rubella immunization. JAMA. 1974 Feb 11;227(6):621–627. [PubMed] [Google Scholar]
- Ginsberg-Fellner F., Witt M. E., Fedun B., Taub F., Dobersen M. J., McEvoy R. C., Cooper L. Z., Notkins A. L., Rubinstein P. Diabetes mellitus and autoimmunity in patients with the congenital rubella syndrome. Rev Infect Dis. 1985 Mar-Apr;7 (Suppl 1):S170–S176. doi: 10.1093/clinids/7.supplement_1.s170. [DOI] [PubMed] [Google Scholar]
- Green K. Y., Dorsett P. H. Rubella virus antigens: localization of epitopes involved in hemagglutination and neutralization by using monoclonal antibodies. J Virol. 1986 Mar;57(3):893–898. doi: 10.1128/jvi.57.3.893-898.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho-Terry L., Cohen A. Rubella virus haemagglutinin: association with a single virion glycoprotein. Arch Virol. 1985;84(3-4):207–215. doi: 10.1007/BF01378973. [DOI] [PubMed] [Google Scholar]
- Ho-Terry L., Cohen A. The role of glycosylation on haemagglutination and immunological reactivity of rubella virus. Arch Virol. 1984;79(3-4):139–146. doi: 10.1007/BF01310807. [DOI] [PubMed] [Google Scholar]
- Hobman T. C., Lundstrom M. L., Gillam S. Processing and intracellular transport of rubella virus structural proteins in COS cells. Virology. 1990 Sep;178(1):122–133. doi: 10.1016/0042-6822(90)90385-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann D. M., Liebhaber H., Le Bouvier G. L., Rosenberg D. A., Halstead S. B. Rubella: reinfection of vaccinated and naturally immune persons exposed in an epidemic. N Engl J Med. 1970 Oct 8;283(15):771–778. doi: 10.1056/NEJM197010082831501. [DOI] [PubMed] [Google Scholar]
- Hutchings D. L., van Drunen Littel-van den Hurk S., Babiuk L. A. Lymphocyte proliferative responses to separated bovine herpesvirus 1 proteins in immune cattle. J Virol. 1990 Oct;64(10):5114–5122. doi: 10.1128/jvi.64.10.5114-5122.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B. E., Moran T. M., Kilbourne E. D. Antigen-presenting B cells and helper T cells cooperatively mediate intravirionic antigenic competition between influenza A virus surface glycoproteins. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6869–6873. doi: 10.1073/pnas.84.19.6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katow S., Sugiura A. Antibody response to individual rubella virus proteins in congenital and other rubella virus infections. J Clin Microbiol. 1985 Mar;21(3):449–451. doi: 10.1128/jcm.21.3.449-451.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman C. A., Phair J. P., Linnemann C. C., Jr, Schiff G. M. Cell-mediated immunity in humans during viral infection. I. Effect of rubella on dermal hypersensitivity, phytohemagglutinin response, and T lymphocyte numbers. Infect Immun. 1974 Jul;10(1):212–215. doi: 10.1128/iai.10.1.212-215.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo T. W., MacDonald I., Clarke D. M., Trudel M., Tingle A., Gilam S. Detection of antibodies to individual proteins of rubella virus. J Virol Methods. 1986 May;13(2):149–159. doi: 10.1016/0166-0934(86)90083-2. [DOI] [PubMed] [Google Scholar]
- Lundström M. L., Mauracher C. A., Tingle A. J. Characterization of carbohydrates linked to rubella virus glycoprotein E2. J Gen Virol. 1991 Apr;72(Pt 4):843–850. doi: 10.1099/0022-1317-72-4-843. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R., Marquardt P., O'Shea S., Borkenstein M., Kreth H. W. Virus-specific and autoreactive T cell lines isolated from cerebrospinal fluid of a patient with chronic rubella panencephalitis. J Neuroimmunol. 1989 Jun;23(1):1–10. doi: 10.1016/0165-5728(89)90065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauracher C. A., Mitchell L. A., Tingle A. J. Differential IgG avidity to rubella virus structural proteins. J Med Virol. 1992 Mar;36(3):202–208. doi: 10.1002/jmv.1890360310. [DOI] [PubMed] [Google Scholar]
- McDonald H., Hobman T. C., Gillam S. The influence of capsid protein cleavage on the processing of E2 and E1 glycoproteins of rubella virus. Virology. 1991 Jul;183(1):52–60. doi: 10.1016/0042-6822(91)90117-t. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Michie C. A., Gotch F. M., Smith G. L., Moss B. Recognition of influenza A virus nucleoprotein by human cytotoxic T lymphocytes. J Gen Virol. 1986 Apr;67(Pt 4):719–726. doi: 10.1099/0022-1317-67-4-719. [DOI] [PubMed] [Google Scholar]
- Milich D. R. T- and B-cell recognition of hepatitis B viral antigens. Immunol Today. 1988 Dec;9(12):380–386. doi: 10.1016/0167-5699(88)91239-X. [DOI] [PubMed] [Google Scholar]
- Oker-Blom C., Kalkkinen N., Käriäinen L., Pettersson R. F. Rubella virus contains one capsid protein and three envelope glycoproteins, E1, E2a, and E2b. J Virol. 1983 Jun;46(3):964–973. doi: 10.1128/jvi.46.3.964-973.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oker-Blom C. The gene order for rubella virus structural proteins is NH2-C-E2-E1-COOH. J Virol. 1984 Aug;51(2):354–358. doi: 10.1128/jvi.51.2.354-358.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oker-Blom C., Ulmanen I., Käriäinen L., Pettersson R. F. Rubella virus 40S genome RNA specifies a 24S subgenomic mRNA that codes for a precursor to structural proteins. J Virol. 1984 Feb;49(2):403–408. doi: 10.1128/jvi.49.2.403-408.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford J. S., Potter C. W. Persistent rubella virus infection in laboratory animals. Arch Gesamte Virusforsch. 1971;34(1):75–81. doi: 10.1007/BF01250247. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Townsend A. R., Gotch F. M., Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985 Sep;42(2):457–467. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Vesikari T., Oker-Blom N., Seppala M., Parkman P. D., Veronelli J., Robbins F. C. Isolation of attenuated rubella-vaccine virus from human products of conception and uterine cervix. N Engl J Med. 1972 May 18;286(20):1071–1074. doi: 10.1056/NEJM197205182862002. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Buimovici-Klein E. Lymphocyte responses to rubella antigen and phytohemagglutinin after administration of the RA 27/3 strain of live attenuated rubella vaccine. Infect Immun. 1975 Apr;11(4):748–753. doi: 10.1128/iai.11.4.748-753.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxham M. N., Wolinsky J. S. Detailed immunologic analysis of the structural polypeptides of rubella virus using monoclonal antibodies. Virology. 1985 May;143(1):153–165. doi: 10.1016/0042-6822(85)90104-7. [DOI] [PubMed] [Google Scholar]
- Weil M. L., Itabashi H., Cremer N. E., Oshiro L., Lennette E. H., Carnay L. Chronic progressive panencephalitis due to rubella virus simulating subacute sclerosing panencephalitis. N Engl J Med. 1975 May 8;292(19):994–998. doi: 10.1056/NEJM197505082921903. [DOI] [PubMed] [Google Scholar]
- Zhang T., Mauracher C. A., Mitchell L. A., Tingle A. J. Detection of rubella virus-specific immunoglobulin G (IgG), IgM, and IgA antibodies by immunoblot assays. J Clin Microbiol. 1992 Apr;30(4):824–830. doi: 10.1128/jcm.30.4.824-830.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mazancourt A., Waxham M. N., Nicolas J. C., Wolinsky J. S. Antibody response to the rubella virus structural proteins in infants with the congenital rubella syndrome. J Med Virol. 1986 Jun;19(2):111–122. doi: 10.1002/jmv.1890190203. [DOI] [PubMed] [Google Scholar]