Abstract
Background
Giant cell arteritis (GCA) is a systemic vasculitis of elderly individuals associated with significant morbidity, including blindness, stroke, and myocardial infarction. Previous studies have investigated whether GCA is associated with increased mortality, with conflicting results. The objective of this study is to determine whether GCA, is associated with increased mortality.
Methods
Forty-four cases with GCA were identified from the University of Utah Health Sciences Center, the major tertiary care center for the Intermountain West. The Utah Population Database, a unique biomedical information resource, selected cases and age- and gender-matched controls. Cases were defined as patients with a temporal artery biopsy-proven diagnosis of GCA (international classification of diseases [ICD]-9 code 446.5) between 1991 and 2005. Exclusion criteria included a negative biopsy, alternative diagnoses, or insufficient clinical data. For each of the 44 cases, 100 controls were identified; thus, 4,400 controls were included in the data analysis. Median survival time and 5-year cumulative survival were measured for cases and controls.
Results
The median survival time for the 44 GCA cases was 1,357 days (3.71 years) after diagnosis compared with 3,044 days (8.34 years) for the 4,400 controls (p = 0.04). Five-year cumulative survival was 67% for the control group versus 35% for the cases (p < .001). Survival rates for cases and controls converged at approximately 11.12 years.
Conclusions
Patients with GCA were more likely than age- and gender-matched controls to die within the first 5 years following diagnosis.
Keywords: Giant cell arteritis, Vasculitis, Temporal arteritis, Mortality, Utah population database
GIANT cell arteritis (GCA) is a systemic vasculitis of elderly individuals associated with significant morbidity. GCA can result in vision loss, peripheral neuropathies, scalp necrosis, altered mental status, congestive heart failure, myocardial infraction, aortitis, aortic aneurysm, and stroke (1–15). Mortality among patients with GCA may be due to cerebral arteritis, coronary arteritis, aortic aneurysm rupture, and thromboembolic events (16–18).
Previous studies have attempted to address whether GCA is associated with increased mortality, with conflicting results (Table 1). Some studies have concluded that GCA has no effect on mortality (8,18–20,22–24,27,29,30,32), whereas others suggest that GCA is associated with short- or long-term increases in mortality (21,25,26,28,31). Many of these studies had limitations such as a small sample size (19,22,25) or inclusion of cases with diagnoses not confirmed by biopsy (8,19,22,24,26,27,32).
Table 1.
Comparative Analysis of Giant Cell Arteritis Mortality Studies to Date
| Study | Year | Number of Patients | Percent Biopsy Proven | Primary Method of Analysis | Unit Time | Findings |
| Hauser and colleagues (19) | 1971 | 19 | 63 | Life table | y | No effect |
| Huston and colleagues (8) | 1978 | 42 | 90 | Kaplan-Meier | mo | No effect |
| Jonasson and colleagues (20) | 1979 | 136 | 100 | Standardized mortality ratio | mo | No effect |
| Graham and colleagues (21) | 1981 | 90 | 100 | Life table | mo | Increased mortality in women |
| Chuang and colleagues (22) | 1982 | 15 | 93 | Kaplan-Meier | mo | No effect |
| Gouet and colleagues (23) | 1985 | 87 | 100 | Life table | y | No effect |
| Andersson and colleagues (24) | 1986 | 90 | 72 | Standardized mortality ratio | mo | Increased 5-y survival, no effect at 10 and 15 y |
| Nordborg and Bengtsson (18) | 1989 | 284 | 100 | Standardized mortality ratio | mo | No effect |
| Bisgård and colleagues (25) | 1991 | 34 | 100 | Standardized mortality ratio | y | Increased mortality |
| Nesher and colleagues (26) | 1994 | 43 | 91 | Standardized mortality ratio | mo | Increased mortality |
| Matteson and colleagues (27) | 1996 | 205 | Not reported (<100) | Standardized mortality ratio | d | No effect |
| García-Porrúa and colleagues (28) | 1996 | 54 | 100 | Standardized mortality ratio | mo | Increased mortality at 5 y, no effect at 10 y |
| González-Gay and colleagues (29) | 1997 | 109 | 100 | Standardized mortality ratio | mo | No effect |
| Gran and colleagues (30) | 2001 | 42 | 100 | Cox proportional hazard | mo | No effect |
| Uddhammar and colleagues (31) | 2002 | 136 | 100 | Standardized mortality ratio | d | Increased mortality in women |
| Salvarani and colleagues (32) | 2004 | 173 | 87 | Kaplan-Meier | d | No effect |
| Crow and colleagues | 2007 | 44 | 100 | Kaplan-Meier | d | Increased mortality at 5 y |
Note: d = days; mo = months; y = years.
The purpose of this study was to rigorously evaluate the effect of GCA on mortality by conducting an analysis that included only biopsy-proven cases. Rather than relying on life tables or standardized mortality ratios, we identified 100 controls for each case matched by birth year, gender, and birthplace geography.
METHODS
This retrospective cohort study was approved by the University of Utah Institutional Review Board. Our investigation utilized data from the Utah Population Database (UPDB), a unique biomedical and health-related information source which collects all coded medical diagnoses at the University of Utah Health Sciences Center (including inpatients and outpatients), all death certificates and the majority of birth certificates for the state of Utah (33). We searched the UPDB for patients coded for GCA (international classification of diseases [ICD]-9 code 446.5) between 1991 and 2005. In addition, medical records were reviewed for inclusion and exclusion criteria of all potential study subjects initially identified by the UPDB. A subject was included only if his or her chart contained a report of a positive superficial temporal artery biopsy. Potential subjects were excluded if they were found to have a negative biopsy, an alternative diagnosis, or insufficient clinical data. Collected data also included birth date, diagnosis date, gender, date of death or last follow-up, steroid use, diagnosis of polymyalgia rheumatica (PMR), and ophthalmic findings.
Age- and gender-matched controls for each subject were identified within the UPDB. Controls were also matched to cases by geographic location. Controls were included only if they had a last follow-up date at or beyond the date of disease onset for the case. A random number generator was used to select 100 controls for each case. No control was used for more than one case. Due to the advanced age of one case, the matching birth year had to be expanded by ±2.5 years to identify 100 controls.
A biostatistician (S.C.A.) analyzed the data using the Kaplan-Meier (product limit) method with a post hoc log-rank comparison of the survival of cases and controls. SPSS 15.0 for Windows© (SPSS Inc., Chicago, IL) was used for the analysis. For statistical significance, α was set at 0.05. Five-year survival curves, median survival times, and 95% confidence intervals were calculated for each group. Survival was measured in days to maximize the sensitivity of the analysis.
RESULTS
Two hundred eighty-eight subjects were initially identified by the UPDB as having an ICD-9 diagnosis of 446.5. After medical record review, GCA diagnosis was confirmed in 44 subjects. Clinical and demographic data for these 44 cases are summarized in Table 2. From 236,217 UPDB-identified potential controls, 4,400 were randomly selected.
Table 2.
Clinical and Demographic Data for Giant Cell Arteritis Study Subjects
| Total number of patients | 44 |
| Mean age at diagnosis | 76.7 y |
| Age range at diagnosis | 57–93 y |
| Females | 38 (86.4%) |
| Males | 6 (13.6%) |
| Female:male ratio | 6.3:1 |
| Living | 23 (52.3%) |
| Deceased | 21 (47.7%) |
| Polymyalgia rheumatica diagnosis | 9 (20.5%) |
| Vision loss | 24 (54.5%) |
At the time of this analysis, 21 of the 44 patients were deceased. Analysis of cause of death information revealed that none of the patients had died as a direct result of GCA. Seven patients had died as a result of treatment (gastrointestinal hemorrhage, pneumonia, urinary tract infection, sepsis), eight patients had died as a result of diseases unrelated to GCA or its treatment, and for six patients there were insufficient data to make a determination as to the cause of death and its relationship to GCA or its treatment.
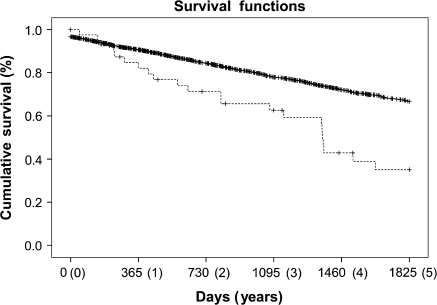
The median survival time for the 44 GCA cases was 1,357 days (3.71 years) after diagnosis, compared with 3,044 days (8.34 years) for the controls (p = .04). The 5-year cumulative survival for the 44 GCA cases was 35%, versus a 5-year survival of 67% for the 4,400 control subjects (p < .001; Figure 1). The cumulative survival in the control group reached 35% at approximately 11.12 years.
Figure 1.
Kaplan-Meier analysis to compare the cumulative survival curves of 44 biopsy-proven giant cell arteritis (GCA) patients (dashed line) to 4,400 controls (solid line). One-hundred controls were randomly selected for each of the 44 GCA patients after being matched by birth year and gender. The 5-year survival of GCA patients is significantly reduced compared with that of the control group (p < .001).
DISCUSSION
Our results indicate that a diagnosis of GCA is significantly associated with reduced 5-year survival. The survival rates for cases and controls converge at 11.12 years, suggesting that the adverse affect on survival is present only in the years immediately following diagnosis. At this time survival data beyond this point are limited, making further inferences difficult.
When compared with clinical and demographic features described in the literature, the ages of our 44 GCA cases are representative of other study populations with the disease. Although only nine of our 44 patients (20.5%) had a concurrent diagnosis of PMR, many more had symptoms that closely resembled those of PMR. Thus, PMR prevalence in our subjects is consistent with previous reports. The ratio of females to males in our study was 6.3:1, whereas most studies have found this ratio to be closer to 2:1 (6,12). We found that almost 55% of our patients had vision loss, more than other studies have reported, because as ophthalmologists we emphasize vision loss assessment. Another study has reported that GCA patients with vision loss have a reduced survival rate when compared with GCA patients with unchanged vision (34). Therefore, patients who present with new visual symptoms should receive especially careful and regular follow-up assessment and care.
Because this study is a retrospective chart review, it lacks uniformity of history taking, chart information, and treatment. An additional limitation is the variability of follow-up time and attrition during the study. Information regarding treatment regimens and duration, as well as presence or absence of standard cardiovascular risk factors, was not consistently available. Nonetheless, this investigation represents a unique approach to the assessment of GCA-associated mortality in that we have compared our GCA cases with controls matched by age, gender, and geography.
In order to optimally understand our results in the context of previous studies, it is important to compare the statistical methods previously employed. A number of prior studies relied on life tables, which demographers construct by defining an age interval (generally 1 year) and then comparing the number of subjects alive at the beginning of the interval with the number of subjects alive at the interval's end. Age-specific mortality rates are calculated if the subjects are divided by age. The 1-year time intervals typically used for life tables have limited sensitivity in an elderly population; for example, the life expectancy of an 80-year-old individual may differ significantly from that of an 80.5-year-old. In addition, the age-specific rates in the standard population are based on empirical observations, which will show random fluctuation (35). We have addressed the limitations of using life tables by defining a cohort of 100 control subjects for each study subject and then performing a survival analysis of the two groups.
Some previous studies attempted to integrate both early and late effects on longevity, to detect an initial negative effect on survival, or to identify a return to normal age-related mortality rates (23,24,28,29). One research team initially observed that patients with GCA had a significantly reduced mortality when compared with the expected mortality rate, but this finding was not observed with long-term follow-up (24,36). We found that the diagnosis of GCA significantly affects the 5-year survival rate; but by 11.12 years the mortality rates of the groups converge, suggesting that the negative impact of GCA on survival is eventually lost. Our statistical methodology enabled us to demonstrate both the initial, negative effect on longevity and the loss of this effect over time.
Although our results indicate that the 5-year survival of GCA patients is significantly less than that of a control group, our results do not address whether GCA itself or consequences of treatment of GCA is directly responsible for this increase in mortality. Elderly individuals, such as those in our study population, often have comorbidities that affect longevity. Furthermore, although GCA typically runs a self-limited course of 1–2 years, some patients experience spontaneous relapses during or after the 5 years following diagnosis (6,8,12,37). One may hypothesize that because the disease is more likely to be active during the first 5 years than during the subsequent 6–10 years, mortality from GCA and its treatment is greatest during the first 5 years following diagnosis. Based on our results, patients who survive the first 5 years after diagnosis may have a mortality rate that reflects that of other elderly patients in the general population (38–40).
We recommend that further studies be directed at identifying risk factors for early death after a diagnosis of GCA. Specific attention should be given to known vascular disorders, cardiac disease, and large vessel disease. Furthermore, genetic testing has proven to be useful in diagnosis or treatment of ophthalmic as well as vascular disorders, and development of such tests in the future may enhance our ability to identify risk factors for increased GCA-associated morbidity and mortality (41–44). Perhaps by identifying those patients at risk for early death, we can intervene before life-threatening complications arise.
FUNDING
B.J.K. is an American Geriatrics Society Dennis W. Jahnigen Career Development Scholar. This research was also supported by an unrestricted grant to the Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness, Inc., New York, NY. Partial support for all data sets within the Utah Population Database is provided by the Huntsman Cancer Institute.
CONFLICTS OF INTEREST
None declared.
Acknowledgments
The authors gratefully acknowledge Jennifer Harmon, Geraldine P. Mineau, Richard Pimentel, and Tien Y. Wong for assistance with use of the UPDB and review of the manuscript. B.J.K. thanks Mark A. Supiano, MD, Chief, Division of Geriatrics, University of Utah, for his guidance and advice on this project. B.J.K. has listed everyone who contributed significantly to the work and has obtained written consent from all contributors who are not authors and are listed as follows:
Study concept and design: R.W.C., B.J.K., J.E.A.W., and K.B.D.
Acquisition of data: R.W.C., B.J.K., J.E.A.W., K.Z., and K.B.D.
Analysis and interpretation of data: R.W.C., B.J.K., J.E.A.W., S.C.A., K.B.D., and S.S.
Preparation of the manuscript: R.W.C., B.J.K., J.E.A.W., S.C.A., K.Z., S.S., and K.B.D.
Statistical analysis: R.W.C. and S.C.A.
Administrative, technical, or material support: S.S.
Sponsor's role: The sponsors had no role in the design, methods, subject recruitment, data collection, analysis, and preparation of this article.
References
- 1.Save-Soderbergh J, Malmvall BE, Andersson R, Bengtsson BA. Giant cell arteritis as a cause of death. Report of nine cases. JAMA. 1986;255:493–496. doi: 10.1001/jama.1986.03370040067025. [DOI] [PubMed] [Google Scholar]
- 2.Lie JT, Failoni DD, Davis DC., Jr Temporal arteritis with giant cell aortitis, coronary arteritis, and myocardial infarction. Arch Pathol Lab Med. 1986;110:857–860. [PubMed] [Google Scholar]
- 3.Hupp SL, Nelson GA, Zimmerman LE. Generalized giant-cell arteritis with coronary artery involvement and myocardial infarction. Arch Ophthalmol. 1990;108:1385–1387. doi: 10.1001/archopht.1990.01070120031015. [DOI] [PubMed] [Google Scholar]
- 4.Morris CR, Scheib JS. Fatal myocardial infarction resulting from coronary arteritis in a patient with polymyalgia rheumatica and biopsy-proved temporal arteritis. A case report and review of the literature. Arch Intern Med. 1994;154:1158–1160. [PubMed] [Google Scholar]
- 5.Evans JM, O'Fallon WM, Hunder GG. Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis. A population-based study. Ann Intern Med. 1995;122:502–507. doi: 10.7326/0003-4819-122-7-199504010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Salvarani C, Cantini F, Boiardi L, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. N Engl J Med. 2002;347:261–271. doi: 10.1056/NEJMra011913. [DOI] [PubMed] [Google Scholar]
- 7.Caselli RJ, Daube JR, Hunder GG, Whisnant JP. Peripheral neuropathic syndromes in giant cell (temporal) arteritis. Neurology. 1988;38:685–689. doi: 10.1212/wnl.38.5.685. [DOI] [PubMed] [Google Scholar]
- 8.Huston KA, Hunder GG, Lie JT, Kennedy RH, Elveback LR. Temporal arteritis: a 25-year epidemiologic, clinical, and pathologic study. Ann Intern Med. 1978;88:162–167. doi: 10.7326/0003-4819-88-2-162. [DOI] [PubMed] [Google Scholar]
- 9.Missen GA. Involvement of the vertebro-carotid arterial system in giant-cell arteritis. J Pathol. 1972;106:P2–P3. [PubMed] [Google Scholar]
- 10.Wilkinson IM, Russell RW. Arteries of the head and neck in giant cell arteritis. A pathological study to show the pattern of arterial involvement. Arch Neurol. 1972;27:378–391. doi: 10.1001/archneur.1972.00490170010003. [DOI] [PubMed] [Google Scholar]
- 11.Pascuzzi RM, Roos KL, Davis TE., Jr Mental status abnormalities in temporal arteritis: a treatable cause of dementia in the elderly. Arthritis Rheum. 1989;32:1308–1311. doi: 10.1002/anr.1780321017. [DOI] [PubMed] [Google Scholar]
- 12.Unwin B, Williams CM, Gilliland W. Polymyalgia rheumatica and giant cell arteritis. Am Fam Physician. 2006;74:1547–1554. 1557–1558. [PubMed] [Google Scholar]
- 13.Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. Ann Intern Med. 2003;139:505–515. doi: 10.7326/0003-4819-139-6-200309160-00015. [DOI] [PubMed] [Google Scholar]
- 14.Gordon LK, Levin LA. Visual loss in giant cell arteritis. JAMA. 1998;280:385–386. doi: 10.1001/jama.280.4.385. [DOI] [PubMed] [Google Scholar]
- 15.Hellman DB, Hunder GG. Giant cell arteritis and polymyalgia rheumatica. In: Harris ED, Budd RC, Genovese MC, Firestein GS, Sargent JS, Sledge CB, editors. Kelley's Textbook of Rheumatology. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005. pp. 1343–1356. [Google Scholar]
- 16.Uriu SA, Reinecke RD. Temporal arteritis, steroid therapy, and pulmonary emboli. Arch Ophthalmol. 1973;90:355–357. doi: 10.1001/archopht.1973.01000050357003. [DOI] [PubMed] [Google Scholar]
- 17.Nuenninghoff DM, Hunder GG, Christianson TJ, McClelland RL, Matteson EL. Mortality of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: a population-based study over 50 years. Arthritis Rheum. 2003;48:3532–3537. doi: 10.1002/art.11480. [DOI] [PubMed] [Google Scholar]
- 18.Nordborg E, Bengtsson BA. Death rates and causes of death in 284 consecutive patients with giant cell arteritis confirmed by biopsy. BMJ. 1989;299:549–550. doi: 10.1136/bmj.299.6698.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauser WA, Ferguson RH, Holley KE, Kurland LT. Temporal arteritis in Rochester, Minnesota, 1951 to 1967. Mayo Clin Proc. 1971;46:597–602. [PubMed] [Google Scholar]
- 20.Jonasson F, Cullen JF, Elton RA. Temporal arteritis. A 14-year epidemiological, clinical and prognostic study. Scott Med J. 1979;24:111–117. doi: 10.1177/003693307902400203. [DOI] [PubMed] [Google Scholar]
- 21.Graham E, Holland A, Avery A, Russell RW. Prognosis in giant-cell arteritis. Br Med J (Clin Res Ed) 1981;282:269–271. doi: 10.1136/bmj.282.6260.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuang TY, Hunder GG, Ilstrup DM, Kurland LT. Polymyalgia rheumatica: a 10-year epidemiologic and clinical study. Ann Intern Med. 1982;97:672–680. doi: 10.7326/0003-4819-97-5-672. [DOI] [PubMed] [Google Scholar]
- 23.Gouet D, Marechaud R, Alcalay M, et al. Survival in giant cell arteritis: a 14-year survey of 87 patients. J Rheumatol. 1985;12:1209–1210. [PubMed] [Google Scholar]
- 24.Andersson R, Malmvall BE, Bengtsson BA. Long-term survival in giant cell arteritis including temporal arteritis and polymyalgia rheumatica. A follow-up study of 90 patients treated with corticosteroids. Acta Med Scand. 1986;220:361–364. doi: 10.1111/j.0954-6820.1986.tb02778.x. [DOI] [PubMed] [Google Scholar]
- 25.Bisgård C, Sloth H, Keiding N, Juel K. Excess mortality in giant cell arteritis. J Intern Med. 1991;230:119–123. doi: 10.1111/j.1365-2796.1991.tb00418.x. [DOI] [PubMed] [Google Scholar]
- 26.Nesher G, Sonnenblick M, Friedlander Y. Analysis of steroid related complications and mortality in temporal arteritis: a 15-year survey of 43 patients. J Rheumatol. 1994;21:1283–1286. [PubMed] [Google Scholar]
- 27.Matteson EL, Gold KN, Bloch DA, Hunder GG. Long-term survival of patients with giant cell arteritis in the American College of Rheumatology giant cell arteritis classification criteria cohort. Am J Med. 1996;100:193–196. doi: 10.1016/s0002-9343(97)89458-2. [DOI] [PubMed] [Google Scholar]
- 28.García-Porrúa C, Blanco FJ, Atanes A, Freire M, Graña J, Galdo F. Giant cell arteritis (GCA): survival analysis of 54 patients from Galicia, Spain. Arthritis Rheum. 1996;39:S64. [Google Scholar]
- 29.González-Gay MA, Blanco R, Abraira V, et al. Giant cell arteritis in Lugo, Spain, is associated with low longterm mortality. J Rheumatol. 1997;24:2171–2176. [PubMed] [Google Scholar]
- 30.Gran JT, Myklebust G, Wilsgaard T, Jacobsen BK. Survival in polymyalgia rheumatica and temporal arteritis: a study of 398 cases and matched population controls. Rheumatology (Oxford) 2001;40:1238–1242. doi: 10.1093/rheumatology/40.11.1238. [DOI] [PubMed] [Google Scholar]
- 31.Uddhammar A, Eriksson AL, Nystrom L, Stenling R, Rantapaa-Dahlqvist S. Increased mortality due to cardiovascular disease in patients with giant cell arteritis in northern Sweden. J Rheumatol. 2002;29:737–742. [PubMed] [Google Scholar]
- 32.Salvarani C, Crowson CS, O’Fallon WM, Hunder GG, Gabriel SE. Reappraisal of the epidemiology of giant cell arteritis in Olmsted County, Minnesota, over a fifty-year period. Arthritis Rheum. 2004;51:264–268. doi: 10.1002/art.20227. [DOI] [PubMed] [Google Scholar]
- 33.Wylie JE, Mineau GP. Biomedical databases: protecting privacy and promoting research. Trends Biotechnol. 2003;21:113–116. doi: 10.1016/S0167-7799(02)00039-2. [DOI] [PubMed] [Google Scholar]
- 34.Hachulla E, Boivin V, Pasturel-Michon U, et al. Prognostic factors and long-term evolution in a cohort of 133 patients with giant cell arteritis. Clin Exp Rheumatol. 2001;19:171–176. [PubMed] [Google Scholar]
- 35.Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. New York: Springer-Verlag; 1997. [Google Scholar]
- 36.Bengtsson BA, Malmvall BE. Prognosis of giant cell arteritis including temporal arteritis and polymyalgia rheumatica. Acta Med Scand. 1981;209:337–345. doi: 10.1111/j.0954-6820.1981.tb11604.x. [DOI] [PubMed] [Google Scholar]
- 37.Kyle V, Hazelman BL. Stopping steroids in polymyalgia rheumatica and giant cell arteritis. BMJ. 1990;300:344–345. doi: 10.1136/bmj.300.6721.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright SD. Profile of Utah's aging population. In: Wright SD, editor. Utah Sourcebook on Aging. Salt Lake City, UT: Empire Publishing; 1998. pp. 47–76. [Google Scholar]
- 39.Center for Health Data, Utah Department of Health. Indicator Profile of Life Expectancy at Birth. Available at: http://ibis.health.utah.gov/indicator/view/LifeExpect.UT_USSexYear.html Accessed June 4, 2008. [Google Scholar]
- 40.National Center for Health Statistics. Mortality Data from the National Vital Statistics System. Available at: http://www.cdc.gov/nchs/deaths.htm. Accessed June 1, 2008. [Google Scholar]
- 41.Rueda B, Roibas B, Martin J, Gonzalez-Gay MA. Influence of interleukin 10 promoter polymorphisms in susceptibility to giant cell arteritis in Northwestern Spain. J Rheumatol. 2007;34:1535–1539. [PubMed] [Google Scholar]
- 42.Gonzalez-Gay MA, Rueda B, Vilchez JR, et al. Contribution of MHC class I region to genetic susceptibility for giant cell arteritis. Rheumatology (Oxford) 2007;46:431–434. doi: 10.1093/rheumatology/kel324. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez-Gay MA, Amoli MM, Garcia-Porrua C, Ollier WE. Genetic markers of disease susceptibility and severity in giant cell arteritis and polymyalgia rheumatica. Semin Arthritis Rheum. 2003;33:38–48. doi: 10.1053/sarh.2002.50025. [DOI] [PubMed] [Google Scholar]
- 44.Sieving PA, Collins FS. Genetic ophthalmology and the era of clinical care. JAMA. 2007;297:733–736. doi: 10.1001/jama.297.7.733. [DOI] [PubMed] [Google Scholar]