Abstract
Background
Poor muscle size and function (sarcopenia) have an important role in the age-associated disability process. However, no commonly accepted index of sarcopenia exists for use in epidemiological studies.
Methods
A cohort of 998 community-dwelling African Americans 49–65 years’ old at baseline was used to construct the short portable sarcopenia measure (SPSM). SPSM was conceptualized as a measure of sarcopenia that combines estimates of muscle quantity and function into a single scale, is based on component items that can be obtained easily in the field, represents muscle status at a single time point that can be used without sex-specific adjustments, and can be used to follow change in muscle status over time with each person as his or her own control. We used exploratory factor analysis (EFA) to identify a unidimensional scale based on timed chair rises, lean mass, and grip strength divided by height. We used these three items and their EFA factor weights to construct SPSM (mean 9.0, median 9, range 0 [worst] to 18 [best] at baseline). Construct validity of the new measure, over a period of 36 months was examined.
Results
SPSM required 8.5 pounds of equipment and 12.4 minutes to complete. It showed good score distribution and convergent, discriminant, and predictive validity with measures of muscle function, body composition, physical performance, psychological factors, and functional limitation cross-sectionally and with muscle function and body composition longitudinally. Extensive sensitivity analyses confirmed SPSM's robustness.
Conclusions
SPSM is a brief, portable, and valid measure of sarcopenia for use in epidemiological research. Similar studies in other populations are needed.
Keywords: Aging, Muscles, African Americans, Disability
MUSCLE size and strength decline with age (1,2), and poor muscle size, strength, power, and function (sarcopenia) have an important role in the age-associated disability process (3). Sarcopenia has been associated with prevalent and incident disability; with declines in lower extremity performance and self-reported functional status; and with subclinical cardiovascular disease, falls, nursing home admissions, and mortality (3–10). Sarcopenia has many different aspects and can be studied at a variety of levels (4,8, 11). Some (6,12–14) have restricted sarcopenia to mean lack of muscle mass only (literally, “flesh loss”). However, others (15,16) have included muscle function in the clinical description, and several (17–19) have included muscle performance in the operational definition. Adipose tissue, connective tissue proteins, and other extracellular components increase intramuscularly with age (20), and fat infiltration into muscles has been associated with poorer muscular performance (8,21). Notably, muscle composition, strength, and function appear to be at least as important as the amount of muscle mass alone in explaining logically appropriate correlates of sarcopenia (8,22).
Many previous measures of sarcopenia have used various laboratory methods that are not field portable (8,13,23,24). In general, measures that are appropriate for field use include bioelectrical impedance and grip strength (15). A few portable epidemiological measures have been described. For example, Baumgartner and colleagues (19) proposed sex-specific equations for estimating muscle mass based on height, weight, hip circumference, grip strength, and sex, and others have recommended field measures of strength as proxies for muscle mass (17,18). We found, however, that our study participants were uncomfortable with invasive hands-on measures like hip circumferences obtained in their homes by field staff. To our knowledge, no field measure has been developed that combines estimates of muscle quantity and function.
We used data from the African American Health (AAH) project to develop a measure of sarcopenia that combined estimates of muscle quantity and function into a single scale, was based on component items that could be easily employed in epidemiological studies, used a modicum of equipment, and would not feel invasive to participants. Furthermore, we wanted a measure that (a) would represent muscle status at a single time point that could be used without sex-specific adjustments; (b) could be used to follow change in muscle status over time, with each person serving as his or her own control; and (c) would demonstrate reasonable dispersion of results with minimal ceiling or floor effects. We then examined the convergent, discriminant, and predictive validity of the new measure. Multiple sensitivity analyses using different configurations of the new measure were performed to examine robustness.
METHODS
Study Sample
AAH is a representative population-based panel study of African Americans who were born in 1936 through 1950 from two diverse socioeconomic areas of St. Louis, Missouri, with a recruitment rate of 76% (25). The 998 participants were aged 49–65 years at the baseline assessment in 2000–2001. Inclusion criteria involved self-reported Black or African American race, Mini-Mental State Examination (26) score of 16 or greater, and willingness to provide informed consent. When sample weights are applied, the AAH cohort represents the noninstitutionalized African American population in the two areas in 2000. Baseline (Wave 1) assessments were performed in participants’ homes and averaged 2.5 hours.
The 36-month follow-up (Wave 4) was also performed in the home and averaged 1.5 hours. Of the 998 participants who were assessed at baseline, 853 were successfully reassessed at Wave 4, representing 90.1% of 947 participants who survived to the 36-month follow-up. Attrition analysis indicated that dropout status was associated only with diagnoses of cancer and heart disease and better vision. Therefore, no potentially confounding attrition bias was evident. All procedures were approved by the institutional review boards at the involved institutions, and all participants provided informed consent.
The Short Portable Sarcopenia Measure
Components.—
To construct the short portable sarcopenia measure (SPSM), we first selected measures to represent lean mass (“lean body mass index [BMI],” using bioelectrical impedance body compositional measurement), upper body muscular status (grip strength/height), and physical performance related to lower body strength and power (27, 28) (timed chair stands). For lean BMI, weight was determined using the portable Tanita Ultimate Scale Model 2001 (Tanita Corporation of America, Arlington Heights, IL), with participants in bare feet and wearing their usual house clothing, and percent body fat was measured using the Tanita scale's bioelectrical impedance program. Height was measured using nonmarring tape, a plastic right angle, and a retractable tape measure while the participant stood erect without shoes against a doorframe. We defined lean BMI as [1 − percent body fat] × [body weight in kg/height in m2], which we validated using dual energy x-ray absorptiometry (DEXA, Hologic QDR 4500W; Hologic, Inc., Bedford, MA) on a subsample of the cohort in the clinical testing center. In brief, DEXA was used to estimate non-bone total lean mass (excluding the head) and non-bone appendicular skeletal mass (ASM), in grams. Tanita total lean mass was calculated as [1 − percent body fat] × [body weight in kg]; correlation with DEXA total lean was .90. Tanita percent lean was determined as [1 − percent body fat]; its correlation with DEXA percent ASM was .83. (More details are available on request.)
Grip strength was assessed as the average of three maximal effort trials using a hand-held isometric dynamometer (Baseline; Fabrication Enterprises, Inc., Irvington, NY, or Jamar; Preston Corp., Jackson, MI). Consistent with prior literature (29,30), grip strength was found to have a strong (r = .59), linear association with height. Thus, we adjusted for this relationship by dividing grip strength by height. We elected against adjusting for BMI to avoid confounding the lean BMI component contribution to SPSM. For chair stands, participants were asked to complete five rises and returns as fast as possible (60 seconds maximum). Test–retest intrarater intraclass correlation coefficients (ICCs) were .80 (95% confidence interval [CI] 0.71–0.88) for grip strength divided by height, 0.72 (0.55–0.83) for chair stands, and .86 (0.78–0.92) for lean BMI in a randomly selected subsample of 80 participants 5–45 days after baseline (31).
Wave 1 performance results were divided so that approximately one fifth of participants were in each of 5-score categories (0–4) for each component. Cut-point scores for handgrip divided by height were: 0 for unable, unsafe, too much pain, or ≤14.07 kg/m; 1 for >14.07 to ≤17.64 kg/m; 2 for >17.64 to ≤20.57 kg/m; 3 for >20.57 to ≤25.04 kg/m; and 4 for >25.04 kg/m. Cut-point scores for chair stands were: 0 for unable, unsafe, too much pain, or >23.88 seconds; 1 for >13.67 to ≤23.88 seconds; 2 for >11.29 to ≤13.67 seconds; 3 for >9.13 to ≤11.29 seconds; and 4 for ≤9.13 seconds. Cut-point scores for lean BMI were: 0 for <16.95 kg/m2, 1 for >16.95 to ≤18.31 kg/m2, 2 for >18.31 to ≤19.61 kg/m2, 3 for >19.61 to ≤21.10 kg/m2, and 4 for >21.10 kg/m2.
Component combination.—
We conducted an exploratory factor analysis of the three component items (scored 0–4) at baseline. A single-factor solution emerged, with the following factor loadings: chair stands 0.433, lean BMI 0.625, and grip strength 0.873, supporting factorial validity. We combined the three components using a weighting scheme based on these factor loadings: the chair stands score was weighted 1, the lean BMI score was weighted 1.5, and the grip strength score was weighted 2. The SPSM score was the sum of the three weighted component scores and ranged from 0 (greatest sarcopenia) to 18 (least sarcopenia), in 0.5 increments. This scoring algorithm was used to determine SPSM at both Waves 1 and 4. For participants with missing data on one component (8%, 13%, and 9%, respectively, for grip strength divided by height, lean BMI, and chair stands at baseline), the total score was calculated as the average of the other two components × 3 (32). SPSM scores were available for 912 participants at baseline and for 780 at 36-month follow-up. SPSM intrarater test–retest reliability using the ICC was .89 (95% CI 0.82–0.93) in a randomly selected subsample of 71 participants 5–45 days after baseline assessment.
Construct Validation of SPSM
Using the approach described in Carmines and Zeller (33), we examined the construct validity of SPSM. Convergent validity measures were obtained in the clinical testing center on subsamples of participants, plus self-reported and physical performance measures acquired during in-home assessments. The clinical center measures included muscle testing using a computerized isokinetic dynamometer (Biodex Medical Systems, Inc., Shirley, NY), body composition using DEXA, and two physical performance assessments. Using the Biodex set at 60° per second angular velocity, maximum strength, power, and total work were calculated as the average over two sets of the maximum peak torque, power, and total work per set of three repetitions, in foot-pounds, watts, and foot-pounds, respectively. The clinical center's physical performance measures were a 4-m usual gait speed assessment (the average of two trials in meters per second, using a preestablished course free of obstacles) and a 6-minute walk test using an unimpeded 90-ft hallway path and a standardized protocol (34). The average time delay between in-home assessment and in-center evaluation was 47 (SD 36) days for Wave 1 and 42 (SD 37) days for Wave 4.
In the home, usual gait speed was measured as the average of two trials in meters per second, using a standardized 3- or 4-m course (31,35), and the standing balance score from the Short Physical Performance Battery (SPPB) ranged from 0 to 4 (4,35). Falls efficacy was measured using Tinetti's scale (36) (total score 0 = lowest self-efficacy to 100 = highest self-efficacy). Depressive symptoms were measured using the 11-item Center for Epidemiologic Studies–Depression scale (37). We hypothesized a priori that the correlation between SPSM and the isokinetic dynamometer and DEXA measures would be higher than those with the other measures of convergent validity.
Discriminant validation measures were collected during the in-home assessments. Lower body functional limitation (continuous variable) was measured as the sum of reported difficulty for (or inability to perform) five activities (walking one-quarter mile, going up and down 10 steps, standing for 2 hours, stooping–crouching–kneeling, and lifting and carrying 10 pounds) (25). Dichotomous validation measures included self-reports of walking one-quarter mile more than once per week, falling ≥2 times in the past year, experiencing ≥1 injurious falls in the past year, having fear of falling, and fair or poor self-rated health (38). We hypothesized that SPSM would demonstrate a part correlation moderately lower than correlations with the isokinetic dynamometer and DEXA measures but higher than part correlations with the other convergent validity measures in Table 1. Furthermore, we hypothesized that SPSM would be a significant predictor of all the dichotomous discriminant measures, with the strongest relationship with fear of falling.
Table 1.
Correlation of Muscle Function, Body Composition, Physical Performance, and Psychological Variables With SPSM Using Sample-Weighted Data
| Measure | Wave 1 SPSM |
Wave 4 SPSM |
||||
| A. Muscle Function and Body Composition | n | Correlation | p Value | n | Correlation | p Value |
| Knee extension | ||||||
| Maximum peak torque* | 179 | .633 | <.001 | 309 | .621 | <.001 |
| Maximum power† | 179 | .615 | <.001 | 309 | .610 | <.001 |
| Maximum total work‡ | 179 | .650 | <.001 | 309 | .562 | <.001 |
| Knee flexion | ||||||
| Maximum peak torque* | 179 | .615 | <.001 | 309 | .653 | <.001 |
| Maximum power† | 179 | .628 | <.001 | 309 | .633 | <.001 |
| Maximum total work‡ | 179 | .625 | <.001 | 309 | .585 | <.001 |
| Total lean mass§ | 182 | .663 | <.001 | 318 | .680 | <.001 |
| Appendicular skeletal mass§ | 182 | .658 | <.001 | 318 | .613 | <.001 |
| B. Physical Performance | n | Part Correlation‖ | p Value | n | Part Correlation‖ | p Value |
| 4-m walk, in center¶ | 169 | .288 | <.001 | 312 | .263 | <.001 |
| 6-min walk, in center# | 156 | .204 | .002 | 279 | .267 | <.001 |
| Usual gait speed** | 476 | .094 | .007 | 674 | .143 | <.001 |
| Standing balance score†† | 895 | .257 | <.001 | 755 | .149 | <.001 |
| C. Psychological Variables | n | Part Correlation‖ | p Value | n | Part Correlation‖ | p Value |
| Falls efficacy scale | 911 | .213 | <.001 | 778 | .206 | <.001 |
| CES-D 11 score‡‡ | 909 | −.237 | <.001 | 776 | −.168 | <.001 |
Notes: CES-D = Center for Epidemiologic Studies–Depression scale; SPSM = short portable sarcopenia measure.
Measured as the average of the maximum peak torque from each of two sets of three repetitions at 60° per second.
Measured as the average of the maximum power for one repetition from each of two sets of three repetitions at 60° per second.
Measured as the average maximum total work for one repetition from each of two sets of three repetitions at 60° per second.
Measured by dual energy x-ray absorptiometry; bones and head excluded.
Adjusted for age and sex.
Measured as the average of two trials in meters per second using a 4-m course in testing center; participants were instructed to walk at their usual pace.
Measured using a 90-ft path in the testing center; participants were instructed to walk as far as possible in 6 minutes.
Measured as the average of two trials in meters per second using a 3- or 4-m course in home; participants were instructed to walk at their usual pace (35).
Hierarchical standing balance component of the Short Physical Performance Battery (4).
CES-D 11 denotes the 11-item short form of the CES-D.
Predictive validation involved correlations between change in SPSM from Wave 1 to Wave 4 and change over the same time in each of the isokinetic dynamometer and DEXA measures for the subsample of participants with measures at both waves. We hypothesized that SPSM would correlate significantly with change in each of the validating variables.
Statistical Analysis
All analyses were performed using SPSS, version 14.0 (SPSS, Inc., Chicago, IL), and sample weights. The relationships between SPSM and strength, power, work, and total and appendicular lean mass were examined using Pearson correlations. The associations with physical performance (Table 1, panel B), psychological variables (Table 1, panel C), and lower body functional limitation (Table 2, panel A) were assessed using multiple linear regression, adjusted for age and sex using the part correlation. The relationships with dichotomous discriminant variables (Table 2, panel B) were investigated using multivariable logistic regression, adjusted for age and sex. The associations of change in SPSM with change in the isokinetic dynamometer and DEXA measures for a period of 36 months were examined using Pearson correlations and the (Wave 1 − Wave 4) score for each variable.
Table 2.
Discriminant Validity: Association of Functional Limitations, Frequency of Walking One-Quarter Mile, Fall-Related Outcomes, and Self-rated Health With SPSM Using Sample-Weighted Data
| Measure | Wave 1 SPSM |
Wave 4 SPSM |
||||
| A. Continuous Variable* | n | Part Correlation | p Value | n | Part Correlation | p Value |
| LBFL | 910 | −.255 | <.001 | 780 | −.236 | <.001 |
| B. Dichotomous Variables† | n | Prevalence aOR‡ (95% CI) | p Value | n | Prevalence, OR‡ (95% CI) | p Value |
| Walking one-quarter mile§ | 887 | 1.123 (1.080–1.168) | <.001 | 767 | 1.117 (1.072–1.164) | <.001 |
| Frequent falling (≥2 per year) | 909 | 0.859 (0.804–0.917) | <.001 | 778 | 0.913 (0.864–0.966) | .002 |
| Any injurious fall in past year | 912 | 0.933 (0.854–1.019) | .12 | 780 | 0.914 (0.832–1.003) | .06 |
| Fear of falling (yes = 1, no = 0) | 912 | 0.888 (0.850–0.927) | <.001 | 779 | 0.892 (0.853–0.933) | <.001 |
| Self-rated health (fair–poor = 1, others = 0) | 911 | 0.868 (0.834–0.904) | <.001 | 780 | 0.867 (0.829–0.906) | <.001 |
Notes: CI = confidence interval; OR = odds ratio; LBFL = lower body functional limitation; SPSM = short portable sarcopenia measure.
Multiple linear regression analysis, adjusted for age and sex.
Multivariable logistic regression analysis, adjusted for age and sex.
Nota bene: the adjusted OR denotes the change in the validating variable per point on SPSM.
Measured as ≤1 time/wk versus greater than one time per week.
Sensitivity analyses included the following. Analyses were run using only participants with data for all three component tests, and analyses using age–sex adjustments were repeated without them. Different definitions of SPSM were examined, including: continuous variable versions of lean BMI, grip strength divided by height, and chair stands were tested to assess the need for an aggregate measure of all three; SPSM was constructed as a simple sum of items (ie, without weighting the component items); lean BMI was changed to a simple measure of percent lean [1 − percent body fat]; lean BMI was dropped from the SPSM specification; chair stands (the lowest weighted SPSM item) was dropped; and a physical performance measure of lower body functioning, the SPPB (4,35), was substituted for SPSM to examine whether SPSM essentially represents physical performance, not sarcopenia per se. (Table 1 convergent analyses were used for these examinations.) Finally, because Newman and colleagues (2) showed that measures of adiposity were associated with strength and muscle quality independently of muscle mass, we adjusted the validation analyses with strength, power, and total work for BMI and for percent body fat in separate sets of analyses.
RESULTS
Descriptive Data
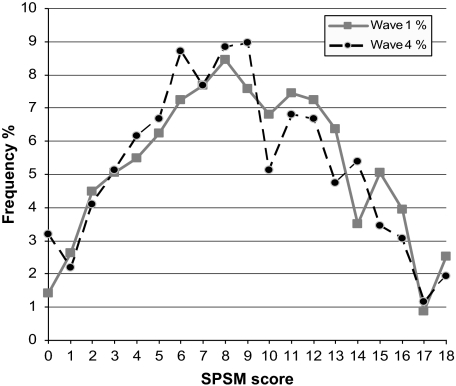
At baseline, the mean age of participants was 56.7 years, and 58% were women. The average educational attainment was 12.5 years, 19% reported less than $20,000 in annual household income, and 49% had a BMI ≥30. Mean baseline lower body functional limitation was 1.29 (SD 1.45). By the 36-month follow-up, 51 participants had died and 16 had been admitted to a nursing home. The equipment used for measuring SPSM weighed 8.5 pounds and cost $299 per assessment kit (in 2008 dollars). SPSM averaged 12.4 minutes to complete and demonstrated an approximately normal distribution (Figure 1), with 2.5% and 1.9% at ceiling (SPSM = 18) and 1.4% and 3.2% at floor (SPSM = 0) for Waves 1 and 4, respectively.
Figure 1.
SPSM unweighted score distributions for Wave 1 (baseline) and Wave 4 (36-month follow-up). n = 912, M = 9.0, SD = 4.4, range = 0–18, 1.4% with score of 0 and 2.5% with score of 18 at Wave 1, and n = 780, M = 8.6, SD = 3.9, range = 0–18, 3.2% with score of 0 and 1.9% with score of 18 at Wave 4. (For clarity of presentation, scores were rounded to the nearest integer.) SPSM = short portable sarcopenia measure.
Construct Validity
Both baseline and follow-up SPSM scores were strongly associated with knee strength, power, and total work, and with total lean and ASM. As predicted, SPSM was independently associated with the four physical performance and two psychological measures, but the associations were weaker than were those with isokinetic dynamometer and body composition measures (Table 1). SPSM demonstrated discriminant validity for the lower body functional limitation (Table 2, panel A) and the five dichotomous variables, except for a marginal relationship with injurious falls at both Waves (Table 2, panel B). Our a priori hypotheses were generally confirmed, although the strongest relationship with the dichotomous variables was with self-rated health, closely followed by the fear of falling and walking one-quarter mile. Notably, there was a 9%–14% decrease in having experienced the dichotomous validating outcome for each point in SPSM.
The predictive validation results demonstrated significant relationships between change in SPSM and change in the torque, power, and work for knee extension and more modest associations for knee flexion measures (Table 3). There was very little change in total lean mass and ASM across the cohort (data available on request) over the 36-month follow-up, which probably explained the lack of correlation with change in SPSM.
Table 3.
Predictive Validity: Correlation of 36-Month Change in Muscle Function and Body Composition for a Period of 36 Months With 36-Month Change in SPSM Using Sample-Weighted Data
| Measure | W1 SPSM Minus W4 SPSM |
||
| A. Muscle Function and Body Composition | n | Correlation | p Value |
| Knee extension | |||
| Maximum peak torque W1 minus W4* | 117 | .267 | .004 |
| Maximum power W1 minus W4† | 117 | .235 | .012 |
| Maximum total work W1 minus W4‡ | 117 | .317 | <.001 |
| Knee flexion | |||
| Maximum peak torque W1 minus W4* | 117 | .172 | .069 |
| Maximum power W1 minus W4† | 117 | .182 | .054 |
| Maximum total work W1 minus W4‡ | 117 | .236 | .012 |
| Total lean mass W1 minus W4§ | 124 | −.061 | .526 |
| Appendicular skeletal mass W1 minus W4§ | 124 | −.014 | .886 |
Notes: SPSM = short portable sarcopenia measure; W1 = Wave 1; W4 = Wave 4.
Measured as the average of the maximum peak torque from each of two sets of three repetitions at 60° per second.
Measured as the average of the maximum power for one repetition from each of two sets of three repetitions at 60° per second.
Measured as the average maximum total work for one repetition from each of two sets of three repetitions at 60° per second.
Measured by dual energy x-ray absorptiometry; bones and head excluded.
Sensitivity analyses including only participants with complete data on all three component items and those dropping age and sex adjustments made no meaningful difference in observed associations. SPSM consistently showed stronger associations with the validating measures in Table 1 than any of the individual tests, with limited, unsurprising exceptions such as stronger relationships of lean BMI with muscle mass measures, chair stands with physical performance measures, and grip strength with the 6-minute walk and the falls efficacy scale at Wave 1. Changing the lean mass component to percent lean or dropping it altogether led to sizable decreases in the associations with muscle performance by Biodex and body composition by DEXA but somewhat stronger relationships with the other measures. Dropping chair stands led to consistent decreases in associations with Biodex measures, although modest increases in associations with DEXA measures. Substituting SPPB for SPSM showed considerable decreased Biodex measure associations and no significant association with DEXA measures, although improved associations with physical performance and psychological measures. Because SPSM was designed to represent sarcopenia (represented by strong, consistent association with Table 1, panel A, measures, with more modest association with Table 1, panels B and C measures), the original definition was retained. Addition of either BMI or percent body fat did not meaningfully change the associations between SPSM and the isokinetic measurements or increase the explanatory power in the construct validation models.
DISCUSSION
The longer that older adults can maintain proper muscle mass and function, the more likely they are to avoid or delay age-associated health problems such as lower body functional limitation, disability, falls, and mortality (3,39). Efforts to understand the effects of and changes in muscle size and function over time at the population level would be greatly facilitated by a valid and reliable evaluation instrument that measures the essential features of muscle status. SPSM may serve this purpose. We believe that this study has demonstrated its reliability, validity, and definitional robustness. Our results are consistent with those of Lauretani and colleagues (17), who found that grip strength was a strong correlate of muscle mass and functional limitations but extends their work to include other factors (lean mass and chair stands) that appear to have improved the correlation of the composite with the validating variables compared with grip strength alone. Our participants were also much more willing to participate in the Tanita-derived lean BMI determination than have their hip circumference measured accurately (ie, with minimal interference from clothing). Notably, DEXA could be substituted for our bioelectrical impedance measure of lean BMI in clinic settings with easier access to DEXA. In addition to its practicality, low cost, and time efficiency, SPSM is also safe to measure. We have obtained the SPSM component measures in participants’ homes more than 1,700 times without a single complaint, fall, or other serious adverse event.
The population studied (late middle-age, urban-dwelling African Americans) is both an advantage and a potential limitation. On the one hand, African Americans are particularly susceptible to experiencing age-associated declines in physical functioning (25,40,41). On the other hand, this study involved a single race–ethnic group of limited age range from a single metropolitan area, which restricts its generalizability. Thus, replication of these analyses in other populations, in other age groups, in other geographic areas, and with different types of chronic illnesses (eg, arthritis, heart failure) is needed (15).
In summary, SPSM appears to be a brief, portable, reliable, valid, and robust sarcopenia measure for epidemiological research. As interventions are developed to prevent or ameliorate age-associated declines in muscle quantity and function and are disseminated into the community with the intent of having a long lasting impact, a validated field measure that integrates several important aspects of the muscle deterioration syndrome of sarcopenia should prove useful (3).
Acknowledgments
This study was supported by a grant from the National Institute on Aging to D.K.M. (R01 AG-10436). This article was presented in part in abstract form at the 2002 Annual Scientific Meeting of the Gerontological Society of American, Boston, November 5, 2002, and at the 2006 Annual Scientific Meeting of the American Geriatrics Society, Chicago, May 5, 2006. Dr F.D.W is Associate Director of the Center for Research in the Implementation of Innovative Strategies in Practice at the Iowa City VA Medical Center and funded through the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service.
References
- 1.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–448. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 2.Newman AB, Haggerty CL, Goodpaster B, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51:323–330. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- 3.Morley JE. Sarcopenia revisited [Editorial] J Gerontol A Biol Sci Med Sci. 2003;58:909–910. [Google Scholar]
- 4.Guralnik JM, Simonsick EM, Ferrucci L, et al. A Short Physical Performance Battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol Med Sci. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 5.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 6.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 7.Ferrucci L, Penninx BW, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 8.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 9.Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 10.Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. 2002;57:B359–B365. doi: 10.1093/gerona/57.10.b359. [DOI] [PubMed] [Google Scholar]
- 11.Yarasheski KE. Exercise, aging, and muscle protein metabolism. J Gerontol A Biol Sci Med Sci. 2003;58:M918–M922. doi: 10.1093/gerona/58.10.m918. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg IH, Roubenoff R. Stalking sarcopenia. Ann Intern Med. 1995;123:727–728. doi: 10.7326/0003-4819-123-9-199511010-00014. [DOI] [PubMed] [Google Scholar]
- 13.Melton LJ, III, Khosla S, Crowson CS, O'Connor MK, O'Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc. 2000;48:625–630. [PubMed] [Google Scholar]
- 14.Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159:413–421. doi: 10.1093/aje/kwh058. [DOI] [PubMed] [Google Scholar]
- 15.Chumlea WC, Guo SS, Vellas B, Guigoz Y. Techniques of assessing muscle mass and function (sarcopenia) for epidemiological studies of the elderly [Review] J Gerontol A Biol Sci Med Sci. 1995;50:45–51. doi: 10.1093/gerona/50a.special_issue.45. [DOI] [PubMed] [Google Scholar]
- 16.Short KR, Nair KS. Mechanisms of sarcopenia of aging. J Endocrinol Invest. 1999;22:95–105. [PubMed] [Google Scholar]
- 17.Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 18.Semba RD, Blaum C, Guralnik JM, Moncrief DT, Ricks MO, Fried LP. Carotenoid and vitamin E status are associated with indicators of sarcopenia among older women living in the community. Aging Clin Exp Res. 2003;15:482–487. doi: 10.1007/BF03327377. [DOI] [PubMed] [Google Scholar]
- 19.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 20.Heymsfield SB, Gallagher D, Visser M, Nunez C, Wang ZM. Measurement of skeletal muscle: laboratory and epidemiological methods. J Gerontol A Biol Sci Med Sci. 1995;50(Special Issue):23–29. doi: 10.1093/gerona/50a.special_issue.23. [DOI] [PubMed] [Google Scholar]
- 21.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol. 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 22.Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the Health, Aging and Body Composition Study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 23.Nair KS. Muscle protein turnover: methodological issues and the effect of aging. J Gerontol A Biol Sci Med Sci. 1995;50(Special Issue):107–112. doi: 10.1093/gerona/50a.special_issue.107. [DOI] [PubMed] [Google Scholar]
- 24.Roubenoff R, Rall LC, Veldhuis JD, et al. The relationship between growth hormone kinetics and sarcopenia in postmenopausal women: the role of fat mass and leptin. J Clin Endocrinol Metab. 1998;83:1502–1506. doi: 10.1210/jcem.83.5.4809. [DOI] [PubMed] [Google Scholar]
- 25.Miller DK, Wolinsky FD, Malmstrom TK, Andresen EM, Miller JP. Inner city middle aged African Americans have excess frank and subclinical disability. J Gerontol A Biol Sci Med Sci. 2005;60A:207–212. doi: 10.1093/gerona/60.2.207. [DOI] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L. A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? J Gerontol A Biol Sci Med Sci. 2003;58:728–733. doi: 10.1093/gerona/58.8.m728. [DOI] [PubMed] [Google Scholar]
- 28.Bean JF, Kiely DK, LaRose S, Alian J, Frontera WR. Is stair climb power a clinically relevant measure of leg power impairments in at-risk older adults? Arch Phys Med Rehabil. 2007;88:604–609. doi: 10.1016/j.apmr.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Frederiksen H, Hjelmborg J, Mortensen J, McGue M, Vaupel JW, Christensen K. Age trajectories of grip strength: cross-sectional and longitudinal data among 8,342 Danes aged 46 to 102. Ann Epidemiol. 2006;16:554–562. doi: 10.1016/j.annepidem.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Kuh D, Hardy R, Butterworth S, et al. Developmental origins of midlife grip strength: findings from a birth cohort study. J Gerontol A Biol Sci Med Sci. 2006;61:702–706. doi: 10.1093/gerona/61.7.702. [DOI] [PubMed] [Google Scholar]
- 31.Wolinsky FD, Miller DK, Andresen EM, Malmstrom TK, Miller JP. Reproducibility of physical performance and physiologic assessments. J Aging Health. 2005;17:111–124. doi: 10.1177/0898264304272784. [DOI] [PubMed] [Google Scholar]
- 32.Ostir GV, Volpato S, Fried LP, Chaves P, Guralnik JM Women's Health and Aging Study. Reliability and sensitivity to change assessed for a summary measure of lower body function: results from the Women's Health and Aging Study. J Clin Epidemiol. 2002;55:916–921. doi: 10.1016/s0895-4356(02)00436-5. [DOI] [PubMed] [Google Scholar]
- 33.Carmines EG, Zeller RA. Reliability and Validity Assessment. Quantitative Applications in the Social Sciences. Newbury Park, CA: Sage Publications; 1979. [Google Scholar]
- 34.Steele B. Timed walking tests of exercise capacity in chronic cardiopulmonary illness. J Cardiopulm Rehabil. 1996;16:25–33. doi: 10.1097/00008483-199601000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Miller DK, Malmstrom TK, Andresen EM, Miller JP, Wolinsky FD. Adverse outcomes and correlates of change in the Short Physical Performance Battery over 36 months in the African American Health project. J Gerontol A Biol Sci Med Sci. 2008;63:487–494. doi: 10.1093/gerona/63.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tinetti ME, Mendes de Leon CF, Doucette JT, Baker DI. Fear of falling and fall-related efficacy in relationship to functioning among community-living elders. J Gerontol. 1994;49:M140–M147. doi: 10.1093/geronj/49.3.m140. [DOI] [PubMed] [Google Scholar]
- 37.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 38.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 39.Fried LP, Ettinger WH, Lind B, Newman AB, Gardin J. Physical disability in older adults: a physiological approach. Cardiovascular Health Study Research Group. J Clin Epidemiol. 1994;47:747–760. doi: 10.1016/0895-4356(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 40.Miller DK, Carter ME, Miller JP, et al. Inner-city older blacks have high levels of functional disability. J Am Geriatr Soc. 1996;44:1166–1173. doi: 10.1111/j.1532-5415.1996.tb01365.x. [DOI] [PubMed] [Google Scholar]
- 41.National Center for Health Statistics. Healthy People 2000 Final Review. Hyattsville, MD: Public Health Service; 2001. [Google Scholar]