Abstract
Background
Sarcopenia, the age-related loss of muscle mass, may not be an isolated process but is associated with an increase in fat mass. The aim of this study was to estimate the mortality risk of sarcopenia in the presence or absence of obesity.
Methods
Data are from 934 participants aged 65 years or older, enrolled in the “Invecchiare in Chianti” study, and followed for 6 years. At baseline, a peripheral quantitative computerized tomography (pQCT) scan was performed on all participants to evaluate the muscle density, and the muscular and fat cross-sectional areas of the calf. Walking speed was measured on a 7-m track. Cox proportional hazard models were performed to estimate the association of pQCT measures (per 1 standard deviation increase) with mortality.
Results
Unadjusted analyses showed significant associations of muscle density (hazard ratio [HR] 0.78, 95% confidence interval [CI] 0.69–0.88), muscle area (HR 0.75, 95% CI 0.66–0.86), and fat area (HR 0.82, 95% CI 0.73–0.92) with mortality. After adjustment for potential confounders, no body composition parameter was significantly associated with mortality. Walking speed (used as a reference measure to verify whether the negative results were due to peculiarities of the study sample) confirmed its well-established association with mortality risk (HR 0.73, 95% CI 0.60–0.88). These results did not change after the analyses were stratified according to sarcopenia and body mass index groups, and restricted to participants with frailty or a high inflammatory profile.
Conclusions
Calf skeletal muscle and fat mass are not significant risk factors for mortality in community-dwelling older adults. Walking speed confirmed to be a powerful predictor of health-related events.
Keywords: Skeletal muscle, Body composition, Fat mass, Walking speed, Obesity, Sarcopenia, Mortality, InCHIANTI
SEVERAL studies have demonstrated that sarcopenia, the age-related involuntary loss of skeletal muscle (1–3), is inversely associated with muscle strength and endurance, potentially leading to dependency in older persons (4–8). In fact, the reduction of muscle mass occurring with aging is significantly correlated with physical impairment and disability (1,2,8–10).
It has been suggested that the development of sarcopenia is not an isolated process, but rather a vicious cycle, whereas loss in muscle mass is accompanied by a parallel gain in fat mass, and potentially leads to the extreme condition of sarcopenic obesity (2,11,12). Accordingly, recent studies evaluating skeletal muscle also tend to combine information on both fat and muscle mass (13).
Recently, Newman and colleagues (14) reported that muscle strength, but not muscle mass, was predictive of mortality in the Health, Aging, and Body Composition (Health ABC) study. However, as acknowledged by the authors, results might have been driven by the high functional status of the Health ABC population. It was hypothesized that lean mass measures may be more important in participants with some physical impairment because they reflect not only the level of physical impairment but also the overall health status. Consistent results were reported by Visser and colleagues (15) who showed in the same cohort that the association between low muscle mass and incident mobility limitation might be a function of lower muscle strength.
In the present study, we hypothesized that body composition, and in particular sarcopenia, may be associated with an increased risk of mortality in a nonselected population of older adults. This study aimed to evaluate whether low muscle and high fat mass, assessed using a peripheral quantitative computerized tomography (pQCT) scan of the calf, are predictors of mortality in older persons. In particular, we evaluated whether low muscle mass (when occurring in association both with and without obesity) might predict mortality. Following the hypothesis raised by previous studies (14,15), in which low muscle mass might be predictive of mortality only among the frailest older individuals, restricted analyses were performed among participants with clinical (ie, frailty) (16) and biological (ie, high inflammatory profile) markers of poor health.
METHODS
The present report is based on data from the “Invecchiare in Chianti” (Aging in the Chianti area, InCHIANTI) study (17), a prospective population-based study of older persons, designed by the Laboratory of Clinical Epidemiology of the Italian National Research Council on Aging (INRCA, Florence, Italy). The InCHIANTI study aims to identify risk factors for the onset of disability in the elderly population, study physiological subsystems critical for walking, and define critical ranges for tests evaluating the integrity of physiological subsystems important for walking.
The study population for these analyses included 1,155 participants between 65 and 102 years of age, randomly selected from residents in two towns of the Chianti geographic area (Greve in Chianti and Bagno a Ripoli, Tuscany, Italy). The data collection started in September 1998 and was completed in March 2000. A detailed description of the sampling procedure and data collection method has previously been published (17). Three and 6 years after the baseline visit (2001–2003 and 2004–2006), study participants underwent repeated phlebotomy, laboratory testing, and physical performance assessment. The Italian National Research Council on Aging Ethical Committee ratified the study protocol.
The present analyses were performed on data from 934 participants; 221 participants were excluded because of missing data on pQCT and walking speed measures (n = 153) or lost during the follow-up (n = 172).
Mortality
Mortality data were obtained from the Mortality General Registry maintained by the Tuscany Region and from death certificates filed upon death at the registry office of the municipality of residence. For the present analyses, follow-up time was defined as the period from the baseline visit till the day of death (for participants who died) or the last contact date (for those who did not have the outcome event).
pQCT Measures and Sarcopenia
A right leg pQCT scan was performed on all participants by a recent generation device (XCT 2000; Stratec, Pforzheim, Germany) to evaluate the cross-sectional muscle and fat areas of the calf. The pQCT technology, an increasingly used imaging method in research [eg, see (18–20)], has been shown to be highly reproducible for the assessment of body composition parameters (21).
Data presented here were derived from standard 2.5-mm-thick transverse scans obtained at 66% of the tibial length starting from the tibiotarsal joint. Previous studies demonstrated that this is the region with the largest outer calf diameter, with a small variability across individuals (21). The muscle density (in mg/cm3), muscle area (in cm2), and fat area (in cm2) were ascertained using the BonAlyse software version 3.1 (BonAlyse Ltd, Jyväskylä, Finland). Different tissues in the analyses were separated according to different density thresholds, using the “soft tissue” algorithm: a density value of 15 mg/mm3 was used to separate fat from muscle tissue and 180 mg/mm3 to separate muscle from bone tissue. Muscle density is calculated from x-ray attenuation and represents a measure of fatty degeneration of muscle tissue.
Sarcopenia.—
Sarcopenia was defined according to the lowest gender-specific tertile of the residuals of a linear regression model that predicted the dependent variable muscle mass area (in cm2) from height (in cm) and fat mass area (log value of cm2; independent variables). A similar approach has already been used in the literature to better estimate sarcopenia, especially in women and obese individuals (13). A positive fat-adjusted muscle area indicates a relatively muscular individual, whereas negative values would indicate relatively sarcopenic individuals.
Seven-Meter Walking Speed
Walking speed was defined as the best performance (in m/s) of two 7-m walks at usual pace along a corridor. Participants were allowed to use canes or walkers. It is well documented in the literature that walking speed is associated with body composition (22) and clinical conditions (23), and represents a powerful predictor of health-related events in older persons (24,25).
Covariates
Covariates included sociodemographic variables (age, gender, study site, smoking habit, education), height, weight, Mini-Mental State Examination score (26), Center for Epidemiological Studies-Depression scale score (27), comorbidity (adjudicated diagnoses of cancer, coronary artery disease, congestive heart failure, diabetes mellitus, hypertension, osteoarthritis, peripheral artery disease, respiratory disease, stroke), and physical activity level.
Adjudicated disease diagnoses were based on self-reported history, clinical documentation, and medication use, as well as prestandardized criteria derived from the Women's Health and Aging Study protocol (28). Physical activity was assessed using an interviewer-administered questionnaire in which participants were asked to indicate their average level of physical activity during the past year. This questionnaire has previously been described (29) and used (30,31) in the literature. In the present study, we defined as “physically active” any participant reporting moderate- to high-intensity exercise performed for at least 1–2 h/wk or light-intensity exercise performed for more than 4 h/wk. This categorization provided the closest approximation to the recommended levels of moderate physical activity defined by the U.S. Department of Health and Human Services (ie, moderate-intensity physical activity for ≥30 minutes on ≥5 d/wk or vigorous-intensity physical activity on ≥3 d/wk for ≥20 minutes per occasion) (32).
Obesity.—
Given the well-established nonlinear relationship between body mass index (BMI) and mortality (33–35), and aiming at evaluating how obesity modified the relationship of sarcopenia with the study outcome, all the sarcopenia-related analyses were stratified according to the following BMI groups: (a) BMI less than 25 kg/m2, (b) BMI 25–29.9 kg/m2, and (c) BMI 30 kg/m2 or greater. BMI was defined as body weight (in kg) divided by square height (in m2).
Frailty.—
Because body composition parameters might have a different prognostic value for mortality in individuals with poorer health status, analyses were also performed in a restricted sample of pre-frail or frail participants (those presenting at least one of the five frailty criteria defined by Fried and colleagues, ie, weight loss, exhaustion, physical inactivity, low walking speed, and low hand grip strength) (16). This frailty definition has been shown to be predictive of adverse outcomes that geriatricians tend to associate with “being frail” (such as falls, hospitalizations, disability, and death) (16). Moreover, these criteria were also found to be correlated with body composition parameters in the InCHIANTI study (36).
Inflammatory markers.—
Interleukin-6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor-α (TNF-α) were considered as additional potential explanatory covariates in the adjusted models to evaluate the relationship of body composition measures and sarcopenia with mortality. This is consistent with the hypothesis that inflammatory markers are not only able to predict major health-related adverse events (37,38) but are also associated with body composition (39,40) and physical function (41,42) modifications. Moreover, to further investigate whether body composition is an indicator for mortality among frail participants, restricted analyses were performed in a sample of participants presenting a biological marker of poor health status (ie, high inflammatory profile). Participants having two or three inflammatory markers above their median values were considered as presenting a high inflammatory profile and consequently classified as biologically “frail.”
The venipuncture was performed in the morning after a 12-hour fast and after the patient was sedentary in a sitting or supine position for at least 15 minutes. Serum was aliquoted and stored at −80°C until enzyme-linked immunosorbent assay (ELISA) tests were performed. All cytokine assays were performed at the INRCA central laboratory. IL-6 and TNF-α were quantified with immunoassay kits (BioSource Cytoscreen human IL-6 and human TNF-α UltraSensitive kits; BioSource International, Inc, Camarillo, CA). The minimum detectable concentrations were 0.10 pg/mL for IL-6 and 0.09 pg/mL for TNF-α. The interassay coefficient of variation was 7% for both kits. High-sensitivity CRP concentrations were measured using an ELISA and colorimetric competitive immunoassay (Roche Diagnostics, Mannheim, Germany). The lower detectable concentration was 0.03 mg/L, and the interassay coefficient of variation was 5%. All assays were performed in duplicate for all inflammatory markers.
Statistical Analyses
Differences in proportions and means of variables according to the vital status at the end of the follow-up were assessed using chi-square and analysis of variance statistics, respectively. Median values with interquartile ranges and p values based on Mann-Whitney U statistics were calculated and reported for nonnormally distributed variables. All variables found to be significantly different (p < .10) in univariate analyses were used as covariate to adjust subsequent multivariate analyses. For nonnormally distributed variables, log transformation was used. Pearson's correlation analyses were performed among the independent variables of interest. Cox proportional hazard models were used to evaluate the relationship of calf pQCT measures with survival (dependent variable). Hazard ratios (HRs) and 95% confidence intervals (95% CIs) are shown in the results. To permit direct comparison between pQCT measures, risks are shown per standard deviation (SD) increase. Kaplan-Meier survival curves stratified for BMI were analyzed according to sarcopenia groups. BMI-stratified adjusted Cox proportional hazard models were also performed to estimate the risk of sarcopenia on time to death. A p value less than .05 was considered as indicating statistical significance for all analyses.
RESULTS
The mean age of the sample population (N = 934) was 74.5 (SD 7.0) years at baseline; 54.9% of the sample were women. The main sociodemographic, clinical, and biological characteristics of the studied sample according to the study outcome (vital status) are presented in Table 1. At baseline, participants who died during the follow-up (n = 263 [28.2%]; mean length of the follow-up, 5.1 years) were older, more likely to be men, and had lower education and cognitive function than those who survived. Moreover, they also had a higher prevalence of many chronic diseases (congestive heart failure, coronary artery disease, hypertension, peripheral artery disease, respiratory disease, osteoarthritis, and stroke) and higher levels of inflammatory markers. Participants who died during the follow-up had significantly lower muscle density, muscle and fat areas, and walking speed compared with those who survived.
Table 1.
Sample (N = 934) Characteristics According to the Study Outcome (Mortality)
| Survived (N = 671) | Deceased (N = 263) | p | |
| Age | 72.3 ± 5.5 | 80.0 ± 7.3 | <.001 |
| Gender (women) | 57.5 | 48.3 | .01 |
| Site (Bagno a Ripoli) | 54.5 | 46.4 | .03 |
| Smoking status | |||
| Never | 59.3 | 55.1 | .38 |
| Former | 27.1 | 28.1 | |
| Current | 13.6 | 16.7 | |
| Education (y) | 5.8 ± 3.3 | 4.6 ± 2.9 | <.001 |
| MMSE score | 25.9 ± 2.8 | 22.7 ± 5.3 | <.001 |
| CES-D score | 12.3 ± 8.8 | 14.1 ± 8.6 | .005 |
| Physical activity* | 42.3 | 23.0 | <.001 |
| Body mass index (kg/m2) | 27.7 ± 4.0 | 27.0 ± 4.1 | .02 |
| <25 | 25.9 | 35.9 | .02 |
| 25–29.9 | 48.6 | 41.4 | |
| ≥30 | 25.6 | 22.7 | |
| Height (cm) | 159.1 | 156.3 | <.001 |
| Weight (kg) | 70.1 | 66.1 | <.001 |
| Cancer | 5.8 | 8.7 | .11 |
| Congestive heart failure | 1.8 | 12.2 | <.001 |
| Coronary artery disease | 6.7 | 10.6 | .04 |
| Diabetes mellitus | 10.0 | 13.7 | .10 |
| Hypertension | 34.1 | 41.1 | .05 |
| Osteoarthritis | 9.2 | 16.0 | .003 |
| Peripheral artery disease | 3.3 | 14.4 | <.001 |
| Respiratory disease | 5.5 | 14.4 | <.001 |
| Stroke | 2.8 | 9.9 | <.001 |
| Interleukin-6 (pg/mL) | 1.31 (0.78–1.99) | 1.89 (1.11–3.34) | <.001 |
| C-reactive protein (μg/mL) | 2.39 (1.25–4.99) | 3.41 (1.60–6.59) | <.001 |
| Tumor necrosis factor-α (pg/mL) | 1.90 (1.48–3.12) | 2.32 (1.69–3.65) | <.001 |
| Muscle density (mg/cm3) | 71.20 ± 3.44 | 69.64 ± 3.67 | <.001 |
| Muscle area (cm2) | 64.0 ± 12.2 | 58.0 ± 12.6 | <.001 |
| Fat area (cm2) | 17.4 (10.8–26.5) | 14.0 (8.4–21.2) | <.001 |
| Walking speed (m/s) | 1.20 ± 0.24 | 0.91 ± 0.35 | <.001 |
Notes: MMSE = Mini-Mental State Examination; CES-D = Center for Epidemiological Studies-Depression scale. Values expressed as percentage, mean ± standard deviation, or median (interquartile range).
Percent of participants reporting moderate- to high-intensity exercise performed for at least 1–2 h/wk or light-intensity exercise performed for more than 4 h/wk.
Spearman's correlation analyses showed significant and inverse correlations of fat area with muscle area (r = −.261) and muscle density (r = −.192; all p values <.001). No significant correlation was found between muscle density and muscle area (p = .90).
Results from Cox proportional hazard analyses exploring the predictive value for mortality of pQCT parameters and walking speed are reported in Table 2. Unadjusted analyses showed significant associations of muscle density (per SD increase HR 0.78, 95% CI 0.69–0.88), muscle area (per SD increase HR 0.75, 95% CI 0.66–0.86), and fat area (per SD increase HR 0.82, 95% CI 0.73–0.92) with mortality. After adjustment for potential confounders, no significant results were reported for the association between body composition parameters and mortality.
Table 2.
Results From Separate Cox Proportional Hazard Analyses Exploring the Relationship of Body Composition Parameters and Physical Performance (per Standard Deviation Increments) With Mortality
| Model 1, HR (95% CI) | Model 2, HR (95% CI) | Model 3, HR (95% CI) | Model 4, HR (95% CI) | |
| Muscle density (mg/cm3) | 0.75 (0.65–0.86)* | 0.90 (0.78–1.04) | 0.94 (0.80–1.10) | 0.94 (0.80–1.11) |
| Muscle area (cm2) | 0.83 (0.69–0.98)* | 0.82 (0.66–1.00) | 0.84 (0.67–1.05) | 0.86 (0.68–1.08) |
| Fat area (log value of cm2) | 0.74 (0.64–0.84)* | 1.00 (0.84–1.19) | 0.99 (0.82–1.19) | 0.96 (0.78–1.18) |
| Walking speed (m/s) | 0.55 (0.48–0.62)* | 0.69 (0.59–0.81)* | 0.72 (0.60–0.87)* | 0.73 (0.60–0.88)* |
Notes: Standard deviations: muscle density, 3.570 mg/cm3; muscle area, 12.568 cm2; fat area (log value of cm2), 0.63086; walking speed, 0.30370 m/s. Model 1: adjusted for height and weight. Model 2: adjusted for model 1 + age and gender. Model 3: adjusted for model 2 + site, education, Mini-Mental State Examination score, height, weight, Center for Epidemiological Studies-Depression scale score, physical activity, congestive heart failure, coronary artery disease, hypertension, peripheral artery disease, respiratory disease, osteoarthritis, and stroke. Model 3: adjusted for model 3 + interleukin-6 (log value), C-reactive protein (log value), and tumor necrosis factor-α (log value). HR = hazard ratio; CI = confidence interval.
p < .05.
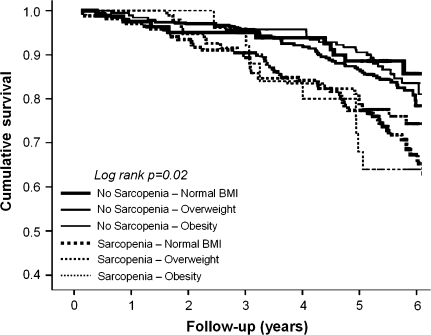
In Figure 1, Kaplan-Meier survival curves for mortality are shown according to sarcopenia and BMI groups. Borderline significances (all p values ≤.1) were obtained from pairwise comparisons between nonsarcopenic and sarcopenic participants, independent of BMI groups.
Figure 1.
Kaplan-Meier survival curves for mortality according to sarcopenia and body mass index (BMI) groups.
Results from adjusted Cox proportional hazard analyses (model 4 adjustment) are presented in Table 3. No significant difference was reported in the estimate of mortality risk across the six sarcopenia and BMI groups. No significant interactions of gender or obesity were found in the relationship between the independent variables of interest and the mortality outcome (all p values for interaction terms >.4).
Table 3.
Results From Adjusted Cox Proportional Hazard Models (Expressed as Hazard Ratio [95% Confidence Interval]) Exploring the Relationship of Sarcopenia and Low Walking Speed With Mortality According to Body Mass Index (BMI, in kg/m2) Groups
| No Sarcopenia | Sarcopenia | |
| BMI < 25 | 1 (reference group), n/N = 18/81 | 1.25 (0.69–2.25), n/N = 66/168 |
| BMI 25–29.9 | 1.17 (0.65–2.09), n/N = 63/307 | 1.24 (0.65–2.37), n/N = 30/94 |
| BMI ≥ 30 | 0.99 (0.53–1.84), n/N = 40/188 | 1.18 (0.46–3.02), n/N = 10/25 |
| No low walking speed | Low walking speed | |
| BMI < 25 | 1 (reference group), n/N = 27/163 | 2.29 (1.36–3.88)*, n/N = 52/85 |
| BMI 25–29.9 | 1.33 (0.77–2.27), n/N = 37/289 | 1.98 (1.17–3.35)*, n/N = 58/126 |
| BMI ≥ 30 | 1.09 (0.58–2.07), n/N = 20/146 | 2.15 (1.22–3.78)*, n/N = 36/79 |
Notes: n/N, number of events/total number of participants. Sarcopenia defined according to the lowest gender-specific tertile of the residuals of fat- and height-adjusted muscle mass: men, −3.38363; women, −5.75560. Low walking speed defined according to the lowest gender-specific tertile of walking speed: men, 1.1475 m/s; women, 0.9845 m/s. Analyses are adjusted for age, gender, site, education, Mini-Mental State Examination score, Center for Epidemiological Studies-Depression scale score, physical activity, congestive heart failure, coronary artery disease, hypertension, peripheral artery disease, respiratory disease, osteoarthritis, stroke, interleukin-6 (log value), C-reactive protein (log value), and tumor necrosis factor-α (log value).
p < .05.
Finally, to evaluate the hypothesis that body composition parameters may be associated with mortality in older participants with a poorer health status, restricted secondary analyses were performed (a) in a sample of participants (n = 436, n of events = 155 [35.6%]) reporting one or more frailty criteria (16), and (b) in a sample of participants having at least two inflammatory markers (ie, CRP, IL-6, TNF-α) above their median levels (n = 461, n of events = 165 [35.8%]). Results from restricted samples were consistent with those obtained in the general population. In fact, no significant association with mortality was found for body composition parameters in both restricted samples. No significant interaction was found for frailty or high inflammatory profile in the relationship between sarcopenia and mortality.
Walking Speed
Given the lack of significant results obtained for pQCT parameters, analyses were reperformed considering a physical performance measure (ie, 7-m walking speed) as an additional independent variable of interest. The aim of these ancillary analyses was to evaluate through a well-established predictor of negative health-related events in older persons (24,25) whether the negative results obtained with pQCT parameters might have been biased by abnormalities within our sample population.
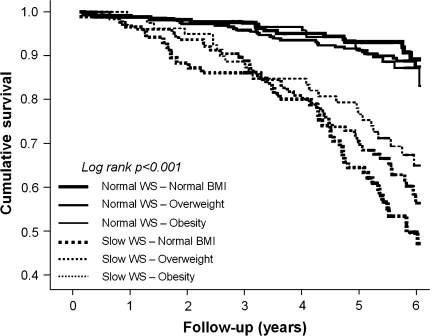
Spearman’s correlation analyses showed that walking speed was significantly correlated with muscle area (r = .417), muscle density (r = .268), and fat area (r = −.137; all p values <.001). Walking speed showed to be significantly associated with mortality (Table 2), even after adjustment for all the potential confounders (Model 4 adjustment: per SD increase, HR 0.73, 95% CI 0.60–0.88). Kaplan-Meier survival curves for mortality were performed according to slow walking speed (defined as the slowest gender-specific walking speed tertiles) and BMI groups (Figure 2). Significant differences were found when participants with slow walking speed were compared with participants with normal walking speed, independent of BMI groups (all p values <.001). These results were also confirmed by adjusted Cox proportional hazard models (Table 3). Walking speed (continuous variable) did not lose its significant association with mortality even when analyses were restricted to participants with one or more frailty criteria or high inflammatory profile (model 3 adjustment: per SD increase HR 0.68, 95% CI 0.54–0.84; and HR 0.70, 95% CI 0.54–0.90, respectively). No significant interaction was found for frailty or high inflammatory profile in the relationship between low walking speed and mortality.
Figure 2.
Kaplan-Meier survival curves for mortality according to walking speed (WS) and body mass index (BMI) groups.
DISCUSSION
In the present study, we explored the relationship between skeletal muscle parameters and mortality risk in a sample of community-dwelling older persons. Our findings showed that body composition measures (in particular, sarcopenia) are not associated with a significantly increased risk of mortality, whereas walking speed confirmed to be a powerful predictor of adverse health-related events.
In a previous study from the Health ABC database (14), no significant relationship of muscle area and mass with mortality was observed. In contrast, quadriceps and grip strength were strongly predictive of mortality. As noted by the authors, the Health ABC population was not disabled at the baseline. Therefore, the prognostic value of body composition parameters and sarcopenia in older persons with some degree of impairment could not be determined. Our results, besides being confirmatory of this previous study (14), extend these findings to a wider population of older persons. In fact, our sample is drawn from a heterogeneous, representative population enrolled with no application of exclusion criteria (17). Moreover, physical performance was the only independent variable of interest significantly associated with an increased risk of mortality, even after the analyses were restricted to participants with biological or clinical markers of frailty.
Because sarcopenia was not a risk factor for mortality, the relationship between walking speed and risk of dying requires further explanations. Sarcopenia is defined on the basis of static measurements of skeletal muscle obtained by different imaging methodologies. On the contrary, walking is the result of multiple interactions existing among several different systems (eg, nervous system, respiratory system, cardiovascular system), in addition to the skeletal muscular apparatus. The harmonic integration of these systems allows the correct explication of the critical subcomponents of walking (eg, motor programming, coordination, execution, energy production and delivery, oxygenation). Consequently, objective measures of physical performance provides a comprehensive assessment of the multiple and complex interactions among the various systems required for optimal physical functioning. Therefore, walking speed may be considered as a more thorough indicator of well-being rather than body composition measures. As a result, it is likely that the increased risk of adverse health events captured by poor physical performance may simply be the ultimate outcome of the accumulation of a broader range of age-related pathophysiological modifications and (sub)clinical conditions.
Our analyses considered the part played by inflammation as a potential explanatory and effect modifier contributing to the relationship between body composition parameters or walking speed and mortality. The irrelevant effect of inflammation on the relationship between physical performance and mortality risk further confirms that walking speed may provide information beyond functional status. Consistent with previous findings (24), after the inclusion of medical conditions and disease risk factors in the adjusted models, the strength of the relationship between physical performance and mortality remained almost unchanged. This innate link between walking speed and survival is not peculiar of humans but is also common to other species [eg, Caenorhabditis elegans (43), Drosophila melanogaster (44), mouse (45), rat (46)]. This universal feature of physical performance measures may further justify the use of walking speed as a marker of aging or as an objective indicator in the extent of cumulative age-related body changes or disease burden.
Some limitations of the present study need to be mentioned. The InCHIANTI study allows for the adjustment of our analyses for many health- and disease-related characteristics. However, there could be unmeasured factors potentially explaining the observed relationships. The muscle and fat measurements considered in the present study were obtained from tibial pQCT scans. Therefore, our findings may not be applicable to the entire body composition. In our sample population, a limited number of participants (n = 77) were frail according to the Fried and colleagues’ definition (ie, presence of three or more criteria). Therefore, to avoid the possible lack of adequate statistical power and the consequent risk of false-negative results, we chose to also consider pre-frail participants (ie, presence of one or two frailty criteria) in our restricted analyses. However, we believe this choice did not significantly affect our findings. In fact, although participants with one or two frailty criteria may not be defined as frail, their pre-frailty status still expose them at increased risk for short- and long-term negative health-related events, including mortality (16).
In conclusion, our study shows that skeletal muscle measures and sarcopenia are not associated with a significantly higher risk of mortality in community-dwelling older persons. Physical performance (in particular, walking speed) confirms to be a powerful predictor of health-related events, independent of sociodemographics, clinical conditions, and inflammation. The use of physical performance tests in the clinical and research setting should be encouraged to provide a better evaluation of older participants.
Acknowledgments
The InCHIANTI study was supported as a “targeted project” (ICS 110.1/RS97.71) by the Italian Ministry of Health and by the U.S. National Institute on Aging (contracts N01-AG-916413, N01-AG-5-0002, and N01-AG-821336, and grant R01-AG-027012). This research was supported in part by the Intramural Research Program, National Institute on Aging, National Institutes of Health. We thank Hazel Lees for editing the manuscript.
References
- 1.Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001;137:231–243. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–448. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 3.Marcell TJ. Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci. 2003;58A:911–916. doi: 10.1093/gerona/58.10.m911. [DOI] [PubMed] [Google Scholar]
- 4.Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci. 2002;57:M772–M777. doi: 10.1093/gerona/57.12.m772. [DOI] [PubMed] [Google Scholar]
- 5.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 6.Rolland Y, Lauwers-Cances V, Cournot M, et al. Sarcopenia, calf circumference, and physical function of elderly women: a cross-sectional study. J Am Geriatr Soc. 2003;5:1120–1124. doi: 10.1046/j.1532-5415.2003.51362.x. [DOI] [PubMed] [Google Scholar]
- 7.Roubenoff R, Parise H, Payette HA, et al. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med. 2003;115:429–435. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Villareal DT, Banks M, Siener C, Sinacore DR, Klein S. Physical frailty and body composition in obese elderly men and women. Obes Res. 2004;12:913–920. doi: 10.1038/oby.2004.111. [DOI] [PubMed] [Google Scholar]
- 9.Newman AB, Haggerty CL, Goodpaster B, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition study. J Am Geriatr Soc. 2003;51:323–330. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- 10.Harris T. Muscle mass and strength: relation to function in population studies. J Nutr. 1997;127:1004S–1006S. doi: 10.1093/jn/127.5.1004S. [DOI] [PubMed] [Google Scholar]
- 11.Roubenoff R. Sarcopenic obesity: does muscle loss cause fat gain? Lessons from rheumatoid arthritis and osteoarthritis. Ann N Y Acad Sci. 2000;904:553–557. doi: 10.1111/j.1749-6632.2000.tb06515.x. [DOI] [PubMed] [Google Scholar]
- 12.Roubenoff R, Hughes VA. Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci. 2000;55A:M716–M724. doi: 10.1093/gerona/55.12.m716. [DOI] [PubMed] [Google Scholar]
- 13.Newman AB, Kupelian V, Visser M, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 14.Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the Health, Aging and Body Composition Study cohort. J Gerontol A Biol Sci Med Sci. 2006;61A:72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 15.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60A:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 16.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 17.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 18.Devonshire AL, Lauretani F, Bos A, Chi C, Ling S, Ferrucci L. Metabolic correlates of aging muscle: data from the Baltimore Longitudinal Study of Aging. FASEB J. 2007;21:763.4. [Google Scholar]
- 19.Schweizer R, Martin DD, Haase M, et al. Similar effects of long-term exogenous growth hormone (GH) on bone and muscle parameters: a pQCT study of GH-deficient and small-for-gestational age (SGA) children. Bone. 2007;41:875–881. doi: 10.1016/j.bone.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 20.Valenti G, Denti L, Maggio M, et al. Effect of DHEAS on skeletal muscle over the life span: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59:M466–M472. doi: 10.1093/gerona/59.5.m466. [DOI] [PubMed] [Google Scholar]
- 21.Rittweger J, Beller G, Ehrig J, et al. Bone-muscle strength indices for the human lower leg. Bone. 2000;27:319–326. doi: 10.1016/s8756-3282(00)00327-6. [DOI] [PubMed] [Google Scholar]
- 22.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the Health, Aging, and Body Composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 23.Cesari M, Onder G, Russo A, et al. Comorbidity and physical function—results from the ilSIRENTE study. Gerontology. 2005;52:24–32. doi: 10.1159/000089822. [DOI] [PubMed] [Google Scholar]
- 24.Cesari M, Kritchevsky SB, Penninx BWJH, et al. Prognostic value of usual gait speed in well-functioning elders—results from the Health, Aging and Body Composition (Health ABC) Study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 25.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55A:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh P. Mini-mental state: a practical method for grading the state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 28.Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME. The Women's Health and Aging Study—Health and Social Characteristics of Older Women With Disability. Bethesda, MD: National Institute on Aging; 1995. NIH Pub. No. 95-4009. [Google Scholar]
- 29.Patel KV, Coppin AK, Manini TM, et al. Midlife physical activity and mobility in older age—the InCHIANTI study. Am J Prev Med. 2006;31:217–224. doi: 10.1016/j.amepre.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elosua R, Bartali B, Ordovas JM, et al. Association between physical activity, physical performance, and inflammatory biomarkers in an elderly population: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2005;60A:760–767. doi: 10.1093/gerona/60.6.760. [DOI] [PubMed] [Google Scholar]
- 31.Cesari M, Pahor M, Bartali B, et al. Antioxidants and physical performance in elderly persons: the Invecchiare in Chianti (InCHIANTI) study. Am J Clin Nutr. 2004;79:289–294. doi: 10.1093/ajcn/79.2.289. [DOI] [PubMed] [Google Scholar]
- 32.Physical Activity for Everyone: How Much Exercise Do You Need? Atlanta, GA: U.S. Department of Health and Human Services; 2008. Available at: http://www.cdc.gov/nccdphp/dnpa/physical/recommendations/index.htm. Accessed June 25, 2008. [Google Scholar]
- 33.Ajani UA, Lotufo PA, Gaziano JM, et al. Body mass index and mortality among US male physicians. Ann Epidemiol. 2004;14:731–739. doi: 10.1016/j.annepidem.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Freedman DM, Ron E, Ballard-Barbash R, Doody MM, Linet MS. Body mass index and all-cause mortality in a nationwide US cohort. Int J Obes (Lond) 2006;30:822–829. doi: 10.1038/sj.ijo.0803193. [DOI] [PubMed] [Google Scholar]
- 35.Allison DB, Faith MS, Heo M, Kotler DP. Hypothesis concerning the U-shaped relation between body mass index and mortality. Am J Epidemiol. 1997;146:339–349. doi: 10.1093/oxfordjournals.aje.a009275. [DOI] [PubMed] [Google Scholar]
- 36.Cesari M, Leeuwenburgh C, Lauretani F, et al. Frailty syndrome and skeletal muscle—results from the InCHIANTI study. Am J Clin Nutr. 2006;83:1142–1148. doi: 10.1093/ajcn/83.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen HJ, Harris T, Pieper CF. Coagulation and activation of inflammatory pathways in the development of functional decline and mortality in the elderly. Am J Med. 2003;114:180–187. doi: 10.1016/s0002-9343(02)01484-5. [DOI] [PubMed] [Google Scholar]
- 38.Reuben DB, Cheh AI, Harris TB, et al. Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr Soc. 2002;50:638–644. doi: 10.1046/j.1532-5415.2002.50157.x. [DOI] [PubMed] [Google Scholar]
- 39.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC study. J Gerontol A Biol Sci Med Sci. 2002;57A:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 40.Cesari M, Kritchevsky SB, Baumgartner RN, et al. Sarcopenia, obesity and inflammation—results from the TRAIN study. Am J Clin Nutr. 2005;82:428–34. doi: 10.1093/ajcn.82.2.428. [DOI] [PubMed] [Google Scholar]
- 41.Cesari M, Penninx BWJH, Pahor M, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59A:M242–M248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 42.Taaffe DR, Harris TB, Ferrucci L, Rowe JW, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons. J Gerontol A Biol Sci Med Sci. 2000;55A:M709–M715. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- 43.Herndon LA, Schmeissner PJ, Dudaronek JM, et al. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- 44.Le Bourg E. The rate of living theory. Spontaneous locomotor activity, aging and longevity in Drosophila melanogaster. Exp Gerontol. 1987;22:359–369. doi: 10.1016/0531-5565(87)90034-9. [DOI] [PubMed] [Google Scholar]
- 45.Duffy PH, Feuers RJ, Pipkin JL, Turturro A, Hart RW. Age and temperature related changes in behavioral and physiological performance in the Peromyscus leucopus mouse. Mech Ageing Dev. 1997;95:43–61. doi: 10.1016/s0047-6374(96)01834-9. [DOI] [PubMed] [Google Scholar]
- 46.Carter CS, Sonntag WE, Onder G, Pahor M. Physical performance and longevity in aged rats. J Gerontol A Biol Sci Med Sci. 2002;57:B193–B197. doi: 10.1093/gerona/57.5.b193. [DOI] [PubMed] [Google Scholar]